Abstract
Glial cells orchestrate immunocyte recruitment to focal areas of viral infection within the brain and synchronize immune cell functions through a regulated network of cytokines and chemokines. Since recruitment of T lymphocytes plays a critical role in resolving cytomegalovirus (CMV) infection, we investigated the production of a T-cell chemoattractant, CXCL10 (gamma interferon-inducible protein 10) in response to viral infection of human glial cells. Infection with CMV was found to elicit the production of CXCL10 from primary microglial cells but not from astrocytes. This CXCL10 expression was not dependent on secondary protein synthesis but did require the phosphorylation of p38 mitogen-activated protein (MAP) kinase. In addition, migration of activated lymphocytes toward supernatants from CMV-stimulated microglial cells was partially suppressed by anti-CXCL10 antibodies. Since regulation of central nervous system inflammation is essential to allow viral clearance without immunopathology, microglial cells were then treated with anti-inflammatory cytokines. CMV-induced CXCL10 production from microglial cells was suppressed following treatment with interleukin-10 (IL-10) and IL-4 but not following treatment with transforming growth factor β. The IL-10-mediated inhibition of CXCL10 production was associated with decreased CMV-induced NF-κB activation but not decreased p38 MAP kinase phosphorylation. Finally, CMV infection of fully permissive astrocytes resulted in mRNA expression for the viral homologue to human IL-10 (i.e., cmvIL-10 [UL111a]) in its spliced form and conditioned medium from CMV-infected astrocytes inhibited virus-induced CXCL10 production from microglial cells through the IL-10 receptor. These findings present yet another mechanism through which CMV may subvert host immune responses.
Microglial cells, the resident macrophages of the brain, are sensors of viral infection within the central nervous system (CNS; 28). In a healthy brain, microglial cells exist in a quiescent (ramified) state lacking many of the effector functions and receptor expression patterns observed in activated tissue macrophages. However, in response to CNS infections, microglial cells can quickly transform into an activated (amoeboid) state, acquiring macrophage markers and critical effector functions required to launch effective immune responses (1). Microglial cells respond to viral infections through a highly regulated network of cytokines and chemokines, which subsequently orchestrate a multicellular immune response against the infectious agent. The nature of this immune response is greatly dependent on the nature of the immune stimulus provided by the infecting agent.
Human cytomegalovirus (CMV) induces a specific cytokine and chemokine production profile in glial cells (38). We have previously shown that astrocytes, which are fully permissive for CMV replication (37), produce chemokines that induce chemotaxis of microglial cells, such as monocyte chemoattractant protein 1 (MCP-1 [also known as CCL2]), in response to viral infection. Microglial cell-derived antiviral cytokines like tumor necrosis factor alpha (TNF-α) suppress CMV replication in astrocytes (9), and gamma interferon (IFN-γ), a potent T-cell-derived cytokine (13), also inhibits CMV replication in these glial cells (8). The importance of T-cell responses in CMV neuropathogenesis can be appreciated by the fact that CMV encephalitis is observed commonly during advanced AIDS, when CD4+ lymphocyte counts are lower than 50/mm3 (2). Moreover, previous work in our laboratory with in vitro systems has demonstrated that T cells obtained from CMV-seropositive donors inhibit viral replication in permissive astrocytes (7). Lack of protective lymphocyte responses in patients with advanced AIDS, because of the destruction of lymphocytes or dysregulation of microglial cell responses by human immunodeficiency virus type 1, may culminate in the development of CMV encephalitis. Still, very little is known about the chemotactic signals that recruit protective lymphocytes into the brain during CMV infection.
Recruitment of leukocytes into the brain parenchyma is precisely regulated by chemokine expression from glial cells responding to particular noxious stimuli (22, 51). CXC chemokine ligand 10 (CXCL10; gamma interferon-inducible protein 10 [IP-10]), which is encoded by a gene initially identified as an early IFN-γ response gene (40), has been demonstrated to be critical in providing host defense against viral infection of the CNS (32). Apart from its antiviral (39) and angiostatic properties (48), CXCL10 has been shown to be involved in the recruitment of IFN-γ-producing lymphocytes into the brain (16, 31). Both astrocytes and microglial cells respond to various antigenic stimuli to produce CXCL10 (18, 66). Astrocytes produce CXCL10 in response to IFN-γ, TNF-α (42, 49), and viral proteins like human immunodeficiency virus type 1 gp120 (4) and during viral infections (10, 50, 53, 54, 58). Microglial cells and other cells of the monocyte/macrophage lineage also produce CXCL10 in response to bacterial lipopolysaccharide (LPS) (26) and IFN-γ (66). For these reasons, we postulated in this study that CMV infection of glial cells would lead to the production of CXCL10.
Although initiation of an immune response by glial cells is an important protective mechanism in the CNS, unrestrained or overzealous inflammatory responses may result in irreparable brain damage. The mechanisms that regulate CNS inflammation are poorly understood. The unique immunologic microenvironment of the CNS has been proposed to alter lymphocyte functions and inhibit their proliferation but maintain antiviral cytokine production (23). Anti-inflammatory cytokines like interleukin-10 (IL-10), IL-4, and transforming growth factor β (TGF-β) are also produced during viral CNS infection (69). These cytokines have been demonstrated to inhibit the production of proinflammatory factors, thereby counteracting potentially deleterious consequences of the inflammatory response (15, 21, 45, 52). Viruses have also evolved homologues to these anti-inflammatory cytokines, possibly as a mechanism by which to evade host immune responses. A CMV homologue to human IL-10 has recently been identified (27) that is able to inhibit proliferation, suppress major histocompatibility complex expression, and downregulate cytokine production from activated lymphocytes (64). In this study, we examined astrocytes and microglial cells, two glial cell types involved in host defense against CMV infection of the brain, for the ability to produce CXCL10 in response to the virus. We also examined the effects of anti-inflammatory cytokines and the viral IL-10 homologue cmvIL-10 on CMV-induced CXCL10 production.
MATERIALS AND METHODS
Brain cell cultures.
Primary human microglial cells were prepared as described previously (6) under a protocol approved by our Institutional Human Research Subjects Committee. Briefly, fetal brain tissues obtained from human abortuses at 16 to 22 weeks of gestation were cleared of meninges and dissociated by repeated passage through a pipette under sterile conditions. The triturated tissue was incubated with 0.125% trypsin for 45 min at 37°C to make a single-cell suspension. Trypsin digestion was stopped by using 10% fetal bovine serum (FBS). The cells were washed and resuspended in Dulbecco's modified Eagle's medium (DMEM; with 10% heat-inactivated FBS, 100 U of penicillin per ml, and 100 μg of streptomycin per ml). The cell suspension was seeded at 75 × 106 to 100 × 106 cells in 75-cm2 tissue culture flasks and incubated in a humidified incubator at 37°C with 10% CO2. Cultures were grown for 2 weeks with weekly changes of medium. Microglial cells floating in the medium and those loosely attached to the monolayer were harvested by gentle shaking. The harvested cells were seeded into tissue culture plates (2 × 105 cells/well in a 48-well tissue culture plate or 2 × 106 cells/well in a 6-well tissue culture plate). The plated cells were washed after 60 min of incubation at 37°C. The microglial cells used in these experiments were ≥99% pure, as determined by CD68 antibody staining. Less that 1% of the cells stained with antibodies to glial fibrillary acidic protein, an astrocyte marker.
Primary astrocyte cultures were prepared by shaking the flasks, after 21 days in culture, at 180 to 200 rpm for 16 to 18 h. The monolayer was then washed with Hanks buffer to remove any floating nonastroglial cells. The adherent cells were trypsinized and seeded into fresh flasks with a medium change 24 h after plating. This procedure was repeated three or four times at weekly intervals. The final cultures, which contained ≥99% astrocytes (glial fibrillary acidic protein-positive cells), were grown in tissue culture plates for 3 to 5 days prior to use.
Virus.
Sucrose-purified human CMV AD169 (American Type Culture Collection, Manassas, Va.) was used in this study. Human foreskin fibroblasts (HFF; CRL-1635 [American Type Culture Collection]) were used to propagate viral stocks. Infected HFF cultures were harvested at 80 to 100% cytopathic effect and subjected to three freeze-thaw cycles. Cellular debris was removed by centrifugation (1,000 × g) at 4°C, and the virus was pelleted through a 35% sucrose cushion (in Tris-buffered saline [50 mM Tris-HCl, 150 mM NaCl, pH 7.4]) at 23,000 × g for 2 h at 4°C. The pellet was resuspended in DMEM containing 10% FBS. Viral stock titers were determined on HFF cells as 50% tissue culture infective doses (TCID50) per milliliter. A multiplicity of infection (MOI) of 5 TCID50 was used in all experiments unless indicated otherwise. Mock-infected HFF cultures were processed in exactly the same manner as viral stocks. Glial cell cultures treated with mock-infected culture preparations were used to control for nonspecific cell stimulation in all experiments.
UV inactivation was achieved by placing sucrose-purified viral stocks or virus-infected cell culture supernatants in a tissue culture dish at a distance of 8 cm from a 256-nm UV light source, on ice, for 30 to 45 min. Titers of sucrose-purified viral stock inactivated in this manner were 4 log TCID50 lower than those of replication-competent virus.
ELISA.
A previously described sandwich enzyme-linked immunosorbent assay (ELISA)-based system (52) was used to measure levels of CXCL10 production from glial cell cultures. ELISA plates (96 wells) were coated with a mouse anti-human CXCL10 capture antibody (Pharmingen, San Diego, Calif.) at 2 μg/ml overnight at 4°C. The plates were washed (0.05% Tween 20 in phosphate-buffered saline) and blocked with 1% BSA in phosphate-buffered saline for 1 h at 37°C. Serial dilutions of recombinant human CXCL10 were used to generate a standard concentration curve. CMV-infected, uninfected, or mock-treated culture supernatants were incubated in capture antibody-coated wells for 2 h at 37°C. Biotin-labeled mouse anti-human CXCL10 antibody (1 μg/ml; Pharmingen) was added, the mixture was incubated for 90 min at 37°C, and then horseradish peroxidase-conjugated streptavidin (1:4,000; Jackson Immunoresearch, West Grove, Pa.) was added and the mixture was incubated for another 45 min. A chromogenic substrate (K-blue; Neogen Corporation, Lexington, Ky.) was then added, and the mixture was incubated for 10 to 20 min at room temperature. Color development was stopped with 1 M H2SO4. Levels of CXCL10 in the culture supernatants were estimated from the standard concentration curve by using A450 values. The sensitivity of this CXCL10 ELISA was 10 pg/ml.
RPA.
To determine the levels of CXCL10 mRNA expression, total RNA was extracted (Qiagen, Valencia, Calif.) from CMV-stimulated microglial cells or astrocytes and assayed by using RiboQuant (Pharmingen), a multiprobe RNase protection assay (RPA). Briefly, total RNA was extracted from uninfected or CMV-infected cells at various time points postinfection (p.i.). The RNA was hybridized to 32P-labeled RPA probes specific for the indicated chemokines. The protected probes were then resolved on a 5% denaturing polyacrylamide gel (19:1 40% acrylamide-bisacrylamide, 10× Tris-borate-EDTA, urea) and analyzed with a phosphorimager (Molecular Dynamics, Sunnyvale, Calif.). All chemokine gene expression data were normalized to glyceraldehyde-3-phosphate dehydrogenase levels from the same sample.
Chemotaxis assay.
Lymphocyte migration toward infected cell supernatants was assessed in a 96-well chemotaxis chamber (Neuro Probe, Gaithersburg, Md.). Peripheral blood mononuclear cells were isolated from healthy donor blood and incubated for 1 h at 37°C in a tissue culture flask. After three rounds of incubation at 37°C, nonadherent cells were cultured for 21 days in RPMI 1640 medium supplemented with FBS (10%), 50 μM 2-mercaptoethanol, and recombinant IL-2 (10 ng/ml; R&D Systems, Minneapolis, Minn.). Among white blood cells, the CXCL10 receptor, CXCR3, is mainly expressed on T lymphocytes. Activation with IL-2 is required for maximal expression of this receptor on T cells (35, 36). After 3 weeks in culture with IL-2, these activated lymphocytes were washed and resuspended in fresh RPMI 1640 medium, supplemented with 0.001% bovine serum albumin, before use. Supernatants from CMV-stimulated microglial cells (48 h p.i.) were added to the lower wells, and 200 μl of the lymphocyte suspension (2 × 106 cells/well) was added to the upper wells of the chemotaxis chamber. The upper and lower wells were separated by a polyvinylpyrrolidone-free polycarbonate filter with a 3-μm pore size. Lymphocytes that had migrated through the filter into the lower wells after 3 h of incubation at 37°C were collected by centrifugation. The number of migrated cells was quantitated by spectrophotometric analysis with a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described previously (62).
Western blot analysis.
Standard procedures were followed for preparation of cell lysates, electrophoresis, protein transfer, and analysis by immunodetection. Briefly, 5 × 105 microglial cells were plated in DMEM (with 1% FBS) and infected with CMV at an MOI of 5 TCID50. The cells were lysed in 2× Laemmli sodium dodecyl sulfate (SDS) sample preparation buffer (10% SDS, 0.015 mM Tris-HCl, 0.1% bromophenol blue, glycerol), boiled, and separated on an SDS-12% polyacrylamide gel. The separated proteins were transferred to a nitrocellulose membrane (Micron Separations, Westborough, Mass.) and blocked for 2 h in 6% Blotto (Santa Cruz Biotechnology Inc., Santa Cruz, Calif.) in Tris-buffered saline with 0.1% Tween 20. The blots were then probed (overnight at 4°C) for p38 mitogen-activated protein (MAP) kinase by using antibodies specific for phosphorylated p38 (28B10 monoclonal antibody; 1:1,500) or total p38 (polyclonal rabbit antibody, 1:1,000; Cell Signaling Technology Inc., Beverly Mass.).
NF-κB ELISA.
A recently described colorimetric ELISA for nuclear factor κB (NF-κB) (56) was used to assess the effects of anti-inflammatory cytokines on CMV-induced activation of this transcription factor in microglial cells. Briefly, cells were lysed at 2 h p.i. and assayed for NF-κB p65 activation by using a transcription factor assay kit in accordance with the manufacturer's (Active Motif, Carlsbad, Calif.) protocol. Cell lysates were added to microplates coated with oligonucleotides containing the NF-κB consensus sequence. Primary antibody to NF-κB p65 was added, followed by horseradish peroxidase-conjugated detection antibody. A chromogenic substrate is used to quantitate the activated NF-κB p65 subunit by using A450 (optical density) values. The values were normalized to total protein content in the lysates as measured with a commercially available protein assay (Bio-Rad Laboratories, Hercules, Calif.).
Analysis of cmvIL-10 gene expression.
Reverse transcriptase PCR (RT-PCR) was performed on RNA extracted from uninfected astrocytes and CMV AD169-infected astrocytes at 3, 8, 24, 32, and 48 h p.i. by using a previously described protocol (9). The forward (5′-AGGCGGTATCTGGAGATCGTGTTT-3′) and reverse (5′-TGCAGATACTCTTCGAGACGGCTA-3′) primers were derived from the first and second exons of cmvIL-10 sequences, respectively (nucleotides 160018 to 160041 and 160351 to 160328 of the CMV genome sequence [GenBank accession no. X17403] [27]). The primers were designed to amplify across the second intron of the spliced mRNA product. The calculated size of the genomic-length PCR product is 348 bp, and that of the product corresponding to the spliced mRNA is 251 bp. Identical samples were also subjected to PCR amplification without RT treatment (negative RT controls).
Statistical analysis.
Data are represented as the mean ± the standard error of the mean (SEM) of pooled samples from different experiments. Student's t test was applied to pooled data from multiple experiments with cultures derived from at least two different donors. Differences between sample groups with P < 0.05 were considered significant.
RESULTS
Microglial cells produce CXCL10 in response to stimulation with CMV.
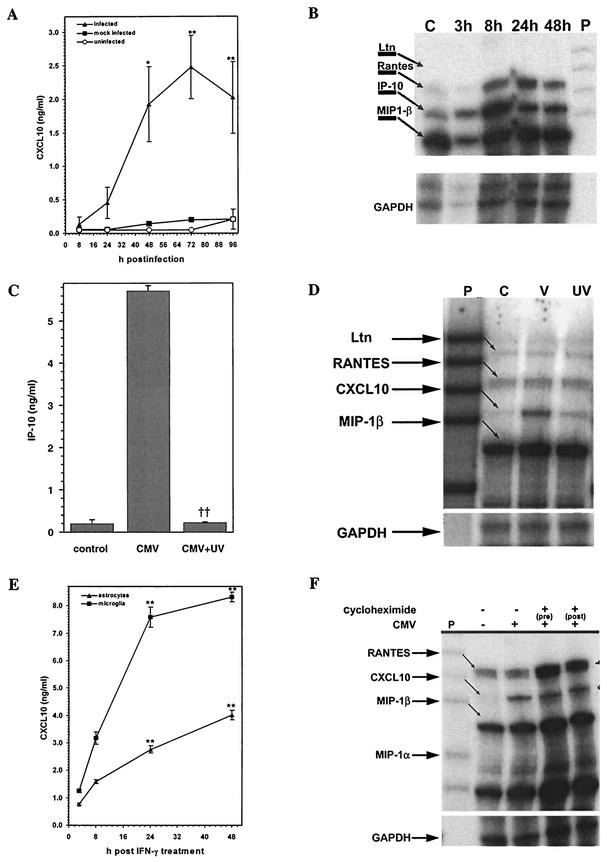
Supernatants from CMV-infected, mock-infected (virus-free HFF cell extracts), and uninfected primary human glial cell cultures were examined for CXCL10, a chemokine that recruits activated T lymphocytes and NK cells (4, 14). Purified microglial cells (2 × 105) were exposed to CMV AD169 (MOI of 5 TCID50) for 8, 24, 48, 72, or 96 h prior to measurement of supernatant CXCL10 levels by ELISA. Supernatants from infected cultures showed a significant increase in CXCL10 production compared to mock-infected or uninfected cultures (Fig. 1A). Levels of CXCL10 production peaked between 48 and 72 h p.i. The mean levels of CXCL10 production by infected microglial cultures at 48 and 72 h p.i. were 1.93 ± 0.57 ng/ml (P < 0.05 versus mock-infected cultures; n = 7) and 2.93 ± 0.47 ng/ml (P < 0.01 versus mock-infected cultures; n = 7), respectively. Mock-infected cultures demonstrated minimal CXCL10 production (0.14 ± 0.02 and 0.20 ± 0.02 ng/ml) at the same time points. This increase in CXCL10 protein from infected microglial cell culture supernatants was coupled with an increase in cellular CXCL10 mRNA synthesis, which was upregulated by 29 and 59% at 24 and 48 h p.i., respectively (Fig. 1B).
FIG.1.
Microglial cells produce CXCL10 in response to stimulation with CMV. (A) Induction of CXCL10 protein. Microglial cells (2 × 105) were exposed to CMV AD169 at an MOI of 5 TCID50 (infected) or to virus-free HFF cell extracts (mock infected) or medium alone (uninfected) for 8, 24, 48, 72, and 96 h prior to measurement of supernatant CXCL10 levels by ELISA. Data are expressed as mean (± SEM) CXCL10 levels from pooled data obtained during three separate experiments with microglial cells isolated from three different brain specimens. *, P < 0.05; **, P < 0.01 (versus mock-infected control cells). (B) Kinetics of chemokine mRNA induction in response to CMV. Total RNA was extracted from CMV-exposed microglial cells at 3, 8, 24, and 48 h, and 4 μg was used in the multiprobe chemokine RPA in accordance with the manufacturer's (Pharmingen) instructions. Lane C, uninfected microglial cell RNA; lane P, probe alone. Ltn, lymphotactin. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (C) UV inactivation decreases CXCL10 induction in microglial cells. Microglial cells (2 × 105) were stimulated with UV-inactivated CMV AD169 (equivalent to an MOI of 5 TCID50). CXCL10 levels were measured by ELISA at 72 h p.i.. Data are presented as the mean ± the SEM of pooled samples from three separate experiments. ††, P < 0.01 versus infected cells. (D) Decreased CXCL10 mRNA expression in microglial cells by UV-inactivated CMV. Total RNA from microglial cells stimulated with replication-competent (V) or UV-inactivated (UV) CMV or from uninfected cells (C) was analyzed by RPA at 24 h p.i. (E) IFN-γ (200 U/ml)-exposed microglial cells (microglia) were used as a positive control. CXCL10 production was also examined following IFN-γ treatment of astrocytes (2 × 105 astrocytes). The data shown are the mean ± the SEM of pooled data from three separate experiments with glial cells obtained from two different brain specimens. **, P < 0.01 versus untreated cells. (F) Microglial cells were treated with cycloheximide (10 μg/ml) 30 min before (pre) or 6 h after (post) the addition of CMV. Total RNA was extracted from cycloheximide-treated and control cultures at 18 h p.i. and analyzed for CXCL10 mRNA by RPA.
To determine if replication-competent virus is essential for induction of CXCL10, microglial cell cultures were stimulated with UV-inactivated CMV AD169. UV inactivation significantly decreased the ability of CMV to induce CXCL10 production in microglial cells (Fig. 1C and D). CXCL10 induction was sevenfold lower (P < 0.01) with UV-inactivated virus than with replication-competent virus (4.77 ± 0.32 ng/ml versus 0.65 ± 0.17 ng/ml; n = 8 from three different donor specimen). CXCL10 mRNA expression was also decreased subsequent to CMV inactivation (Fig. 1D).
In contrast to microglial cells, CMV infection failed to induce CXCL10 production in astrocytes. CXCL10 production levels measured in CMV-infected astrocyte culture supernatants were consistently below the limit of detection of the ELISA (<10 pg/ml; n = 3). Additionally, CXCL10 mRNA expression was not detected by RPA analysis of infected astrocytes at 3, 8, and 24 h p.i. To determine if the failure to produce CXCL10 was inherent to primary astrocyte cultures, we stimulated both astrocytes and microglial cells with IFN-γ, the signature stimulus for CXCL10 production. We found that both astrocytes and microglial cells produced CXCL10 in response to IFN-γ, achieving peak levels between 24 and 48 h poststimulation (Fig. 1E). To determine whether CXCL10 regulates viral expression in CMV-infected astrocytes, we treated primary astrocyte cultures with recombinant CXCL10 either 24 or 48 h prior to infection with RC256, a recombinant virus expressing β-galactosidase from the viral early promoter, and determined β-galactosidase expression by a colorimetric assay using CPRG as a substrate (63). Viral expression from CXCL10-treated astrocytes was not different from that from untreated cells, indicating that this chemokine had no effect on viral expression in primary astrocytes (data not shown).
Induction of CXCL10 is not dependent on de novo protein synthesis.
TNF-α and IFN-γ have been shown to induce CXCL10 production from astroglial cells (41). Previous work in our laboratory has shown that TNF-α, but not IFN-γ, is induced in microglial cells following stimulation with CMV (9). To determine if CMV-induced CXCL10 production is consequent to secondary cytokine production, microglial cells were treated with neutralizing antibodies to TNF-α, IFN-γ, or TGF-β 30 min prior to addition of the virus. Treatment with antibodies to TNF-α, TGF-β, or IFN-γ did not eliminate the production of CXCL10 from CMV-stimulated microglial cells (data not shown). To further examine if secondary protein synthesis is required for CMV-stimulated CXCL10 production in microglial cells, we treated the cultures with cycloheximide (10 μg/ml) 30 min before and 6 h after stimulation with CMV. Total RNA was examined by RPA to determine whether inhibition of protein synthesis leads to decreased CXCL10 mRNA synthesis 18 h poststimulation with CMV. This cycloheximide treatment did not decrease CXCL10 mRNA synthesis relative to that of glyceraldehyde-3-phosphate dehydrogenase mRNA (Fig. 1F).
Inhibitors of signal transduction eliminate CMV-induced CXCL10 production in microglial cells.
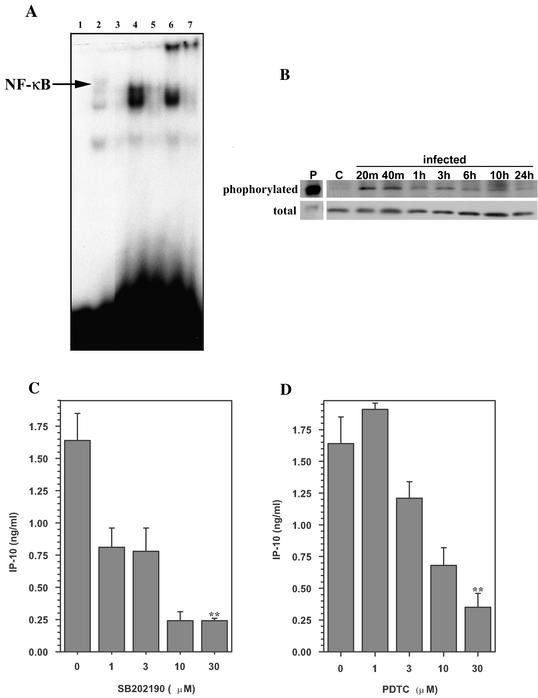
CMV binding to the host cell surface is known to activate signaling molecules and transcription factors, such as p38 MAP kinase and NF-κB (57), in various cell types (24), including macrophages (70). Therefore, we examined CMV-stimulated microglial cells for nuclear translocation of activated NF-κB and activation of p38 MAP kinase. Nuclear extracts from CMV-stimulated and untreated microglial cells were probed by using a gel shift assay for NF-κB binding at 2 and 24 h p.i. NF-κB binding to the consensus oligonucleotide sequence was demonstrated in nuclear extracts obtained from CMV-stimulated microglial cell at 2 h p.i. (Fig. 2A). NF-κB binding resulted in a mobility shift of the probe during electrophoresis, and it was further supershifted when a specific antibody against the p65 subunit was used (Fig. 2A). Nuclear extracts from unstimulated microglial cells did not possess any NF-κB binding activity when tested by the gel shift assay. In addition to NF-κB, we also examined the phosphorylation of p38 MAP kinase in CMV-stimulated microglial cells by Western blotting. Compared to unstimulated microglial cells, phosphorylation of p38 MAP kinase in stimulated cells was increased following the addition of CMV and could be detected as early as 20 min p.i. Cellular p38 MAP kinase appears to persist in the activated form for at least 10 h post CMV infection (Fig. 2B). However, total p38 MAP kinase levels in the cell remained unaffected by CMV stimulation (Fig. 2B).
FIG. 2.
Inhibitors of signal transduction block CMV-induced CXCL10 production. (A) Mobility shift assay with nuclear extracts from microglial cells stimulated with CMV (MOI of 5 TCID50). Nuclear extracts (2 μg) were probed for NF-κB binding activity. Lanes: 1, probe alone; 2, HeLa cell extract (positive control for NF-κB binding); 3, uninfected microglial cell extract; 4, CMV-stimulated microglial cell extract, 2 h p.i.; 5, CMV-stimulated microglial cell extract, 24 h p.i.; 6, same extract as lane 4 supershifted with anti-p65 antibody; 7, same extract as lane 5 supershifted with anti-p65 antibody. (B) Kinetics of phosphorylation of microglial cell p38 MAP kinase in response to CMV. At the indicated times p.i., whole-cell lysates were obtained for analysis of phosphorylated p38 MAP kinase and total p38 by Western blot assay. Lane P, positive control for phosphorylated p38; lane C, extract obtained from uninfected microglial cells. (C and D) Microglial cells were treated with SB202190 (a p38 MAP kinase inhibitor) (C) or PDTC (an inhibitor of transcription factors) (D) at the indicated concentrations (1 to 30 μM) for 30 min and 2 h prior to addition of CMV, respectively. Culture supernatants were harvested 72 h p.i., and CXCL10 levels were quantified by ELISA. Data are presented as the mean ± the SEM of triplicate samples and are representative of three or more experiments performed with cells derived from different brain specimens. **, P < 0.01 versus the untreated control.
To determine whether activation of p38 MAP kinase is essential for the CMV-induced production of CXCL10, we treated microglial cell cultures with SB202190 (a p38 MAP kinase inhibitor) 30 min prior to stimulation. These studies demonstrated that the p38 inhibitor dose dependently suppressed CMV-stimulated CXCL10 production in microglial cells (Fig. 2C). At a concentration of 30 μM, SB202190 decreased CXCL10 production by 88% compared to that of untreated cultures (untreated average of 2.41 ± 0.32 ng/ml versus treated average of 1.17 ± 0.34 ng/ml; mean suppression, 52%; n = 3; P < 0.05). Treatment with SB202474, a negative control for SB20190, did not suppress CXCL10 production (3.13 ± 0.05 versus 3.73 ± 0.20 ng/ml) in CMV-stimulated microglial cells. In addition, treatment of microglial cells with pyrrolidine dithiocarbamate (an inhibitor of NF-κB) also resulted in suppression of CXCL10 production (2.41 ± 0.32 versus 0.59 ± 0.14 ng/ml for untreated and treated cells, respectively; mean suppression, 78%; n = 3; P < 0.01) in microglial cells (Fig. 2D). RPA analysis indicated that treatment with SB202190 inhibited CXCL10 mRNA expression in CMV-stimulated microglial cells but treatment with PDTC did not (data not shown).
CXCL10 induces migration of activated lymphocytes toward supernatants from CMV-stimulated microglial cells.
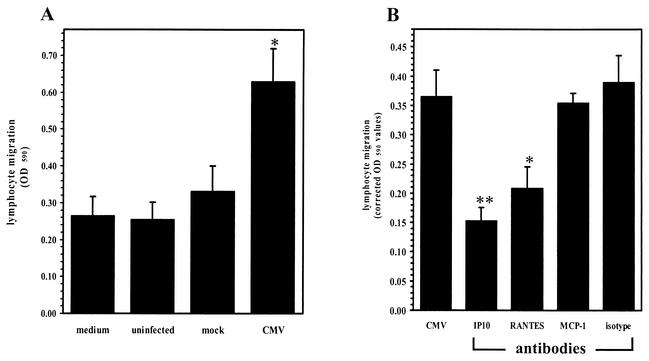
Since CXCL10 produced in response to CMV stimulation of microglial cells could hypothetically serve as a signal for T-cell chemotaxis toward foci of viral infection within the brain, we assessed the ability of activated lymphocytes to migrate toward CMV-stimulated microglial cell supernatants placed in the lower wells of a chemotaxis chamber. Activated lymphocytes were added to the upper wells of the chemotaxis chamber and incubated for 3 h at 37°C. The number of lymphocytes that migrated into the lower wells was quantified with an MTT assay (62). The migration of activated lymphocytes toward CMV-stimulated microglial cell supernatants was significantly greater (2.5-fold; P < 0.05) than chemotaxis toward mock-infected culture supernatants (Fig. 3A). Pretreatment of the infected microglial cell supernatants with neutralizing antibodies to CXCL10 (10 μg/ml) decreased lymphocyte migration by approximately 50% (P < 0.01; Fig. 3B). Neutralizing antibodies to RANTES also suppressed (42%; P < 0.05) migration toward CMV-stimulated supernatants, while antibodies to MCP-1 and isotype control antibody (Fig. 3B) had no effect on CMV-induced chemotaxis.
FIG. 3.
Activated lymphocytes migrate toward supernatants from CMV-infected microglial cells. (A) Cell migration was assessed in a 96-well chemotaxis chamber (Neuro Probe). Supernatants from CMV-stimulated microglial cells (48 h p.i.) were added to the lower wells, and activated lymphocytes (2 × 106 cells/well) in RPMI were added to the upper wells of the chemotaxis chamber. After 3 h of incubation at 37°C, the cells that had migrated to the lower plate were collected by centrifugation and quantified by spectrophotometry with an MTT assay. Medium, medium alone; uninfected, supernatants from uninfected microglial cells; mock, supernatants from mock-infected microglial cells; CMV, supernatants from CMV-infected microglial cells. Data are presented as the mean (± the SEM) of pooled data from three separate experiments utilizing glial cells from different donor specimens. *, P < 0.05 versus mock-infected supernatants. OD 590, optical density at 590 nm. (B) Inhibition of T-cell migration by anti-CXCL10 antibodies. Supernatants collected from CMV-infected microglial cells were treated (30 min) with 10 μg of specific antichemokine antibody per ml prior to assessment of chemotaxis. IP10, anti-CXCL10 antibody; RANTES, anti-RANTES antibody; isotype, isotype-matched control antibody. Data are presented as the mean (± the SEM) corrected optical density at 590 nm (migration toward sample supernatants minus random migration toward medium) from pooled data of three separate experiments with glial cells obtained from three different brain cell specimens. *, P < 0.05; **, P < 0.01 (versus untreated CMV-stimulated supernatant).
Select anti-inflammatory cytokines inhibit CMV-induced CXCL10 production.
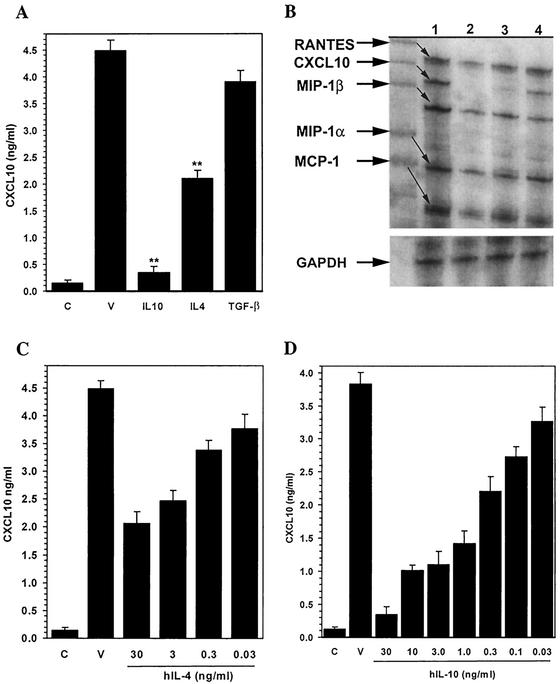
Anti-inflammatory cytokines are important regulators of potentially damaging inflammatory responses within the CNS. Previous work in our laboratory has demonstrated that anti-inflammatory cytokines such as IL-10, IL-4, and TGF-β inhibit chemokine production from stimulated microglial cells (15, 21). We next assessed the effects of IL-10, IL-4, and TGF-β on CMV-induced CXCL10 expression. In these experiments, microglial cells were treated for various time periods (30 min to overnight) with each anti-inflammatory cytokine prior to the addition of CMV. Culture supernatants were assayed by ELISA at 72 h p.i. for CXCL10 production. Pretreatment with both IL-4 and IL-10 was found to markedly suppress CXCL10 production by the CMV-stimulated microglial cells. IL-4 (30 ng/ml) pretreatment inhibited CXCL10 production (53.14%) in CMV-stimulated cultures, with levels dropping from 4.48 ± 0.16 ng/ml in untreated cells to 2.10 ± 0.18 ng/ml (n = 6; P < 0.01) following cytokine treatment (Fig. 4A). A more profound inhibition of CXCL10 was observed following IL-10 (30 ng/ml) treatment, where production decreased from 3.83 ± 0.18 ng/ml in untreated cultures to 0.35 ± 0.08 ng/ml (90%; n = 6; P < 0.01) in IL-10-treated microglial cells (Fig. 4A). Pretreatment with TGF-β was found to have no effect on CMV-induced CXCL10 production (Fig. 4A). In addition, pretreatment with IL-4 and IL-10 decreased CMV-induced CXCL10 mRNA expression (Fig. 4B). The mean levels of inhibition (n = 3) of CXCL10 mRNA by IL-4 and IL-10, quantified by densitometry analysis of RPA gels, were 34 and 59%, respectively. This IL-4- and IL-10-mediated inhibition of CMV-induced CXCL10 production in microglial cells also varied in proportion to the concentration of the cytokines (Fig. 4C and D, respectively). The average inhibition of CMV-induced CXCL10 production by IL-4 pretreatment at the highest dose (30 ng/ml) was 53%, which decreased to 44, 24, and 15% at concentrations of 3, 0.3, and 0.03 ng/ml, respectively. Similarly, inhibition of CMV-induced CXCL10 production by IL-10 pretreatment was dose dependent and decreased with declining IL-10 concentrations (30 ng/ml, 91%; 10 ng/ml, 73%; 3.0 ng/ml, 71%; 1.0 ng/ml, 63%; 0.3 ng/ml, 42%; 0.1 ng/ml, 29%; 0.03 ng/ml, 15%). Treatment with recombinant IL-10 (30 ng/ml) at either the same time or 3 h prior to CMV stimulation also suppressed (80 and 88%, respectively; P < 0.01) CMV-induced CXCL10 production. Finally, IL-10-mediated inhibition of CXCL10 production from CMV-stimulated microglial cells (3.38 ± 0.20 versus 0.78 ± 0.12 ng/ml) was eliminated (2.31 ± 0.33 ng/ml; P < 0.01 versus IL-10-treated cultures) by treatment with human IL-10 receptor-specific neutralizing antibodies (15 μg/ml).
FIG. 4.
Select anti-inflammatory cytokines regulate CMV-induced CXCL10 production by human microglial cells. (A) Anti-inflammatory cytokine-mediated inhibition of CXCL10 production as measured in microglial cell supernatants by ELISA. C, uninfected microglial cell control; V, supernatants obtained from CMV-infected microglial cells; IL-10, supernatants from cells treated with IL-10 (30 ng/ml overnight) prior to CMV infection; IL-4, IL-4 treatment prior to infection; TGF-β, supernatants from cells treated with TGF-β (30 ng/ml overnight) prior to CMV infection. **, P < 0.01 versus untreated, CMV-infected microglial cells. (B) IL-10- and IL-4-mediated inhibition of CXCL10 mRNA. A chemokine RPA was performed. Lanes: 1, RNA extracted from CMV-infected microglial cells; 2, uninfected microglial cell RNA; 3, RNA extracted from microglial cells treated with IL-10 (30 ng/ml overnight) prior to CMV infection; 4, RNA extracted from microglial cells treated with IL-4 (30 ng/ml overnight) prior to CMV infection. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (C and D) Concentration-response effects of IL-4 (C) and IL-10 (D) on CMV-induced CXCL10 production. Microglial cells were treated overnight with the indicated concentrations of IL-4 or IL-10 (0.03 to 30 ng/ml) prior to CMV infection. C, uninfected microglial cell control; V, CMV-infected microglial cells. Data are expressed as mean (± SEM) CXCL10 levels from pooled data obtained from three separate experiments with microglial cells isolated from three different brain cell specimens.
Treatment of microglial cells with anti-inflammatory cytokines is associated with decreased CMV-induced activation of NF-κB but not phosphorylation of p38 MAP kinase.
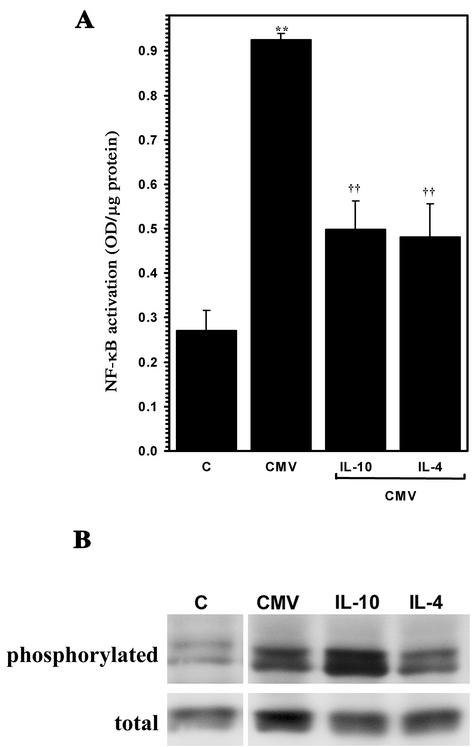
We next examined CMV-stimulated microglial cells for alterations in virus-induced activation of NF-κB subsequent to treatment with IL-10 and IL-4. Treatment with these anti-inflammatory cytokines decreased the levels of activated NF-κB, quantified with a colorimetric assay for the activated molecule (56). CMV-stimulated microglial cells had 2.4-fold greater levels of activated NF-κB than untreated control cells measured at 3 h poststimulation (Fig. 5A). Treatment of microglial cells with recombinant IL-10 (30 ng/ml) or IL-4 (30 ng/ml) prior to CMV stimulation significantly decreased virus-induced activation of NF-κB by 56% ± 13.6%, (P < 0.01) or 42% ± 8.1% (P < 0.01), respectively (Fig. 5A). NF-κB activation was also decreased by TGF-β treatment, although this cytokine had no effect on CXCL10 induction (data not shown). Interestingly, none of the anti-inflammatory cytokines tested had any inhibitory effect on CMV-induced p38 MAP kinase phosphorylation (Fig. 5B).
FIG. 5.
IL-10 treatment of microglial cells is associated with decreased CMV-induced activation of NF-κB but not phosphorylation of p38 MAP kinase. (A) Primary human microglial cells were treated overnight with IL-10 (30 ng/ml) or IL-4 (30 ng/ml) and then stimulated with sucrose-purified CMV. Activation of microglial cell NF-κB was quantified 1.5 h p.i. with an NF-κB ELISA. **, P < 0.01 versus unstimulated microglial cells; ††, P < 0.01 versus stimulated microglial cells. OD, optical density. (B) Microglial cells maintained in serum-free medium were treated with the indicated anti-inflammatory cytokine prior to viral (CMV) infection or addition of medium alone (uninfected). Whole-cell lysates were obtained for analysis of phosphorylated p38 MAP kinase (phosphorylated) and total p38 (total) by Western blot assay. C, extract obtained from uninfected microglial cells.
CMV IL-10 suppresses virus-induced CXCL10 production in microglial cells.
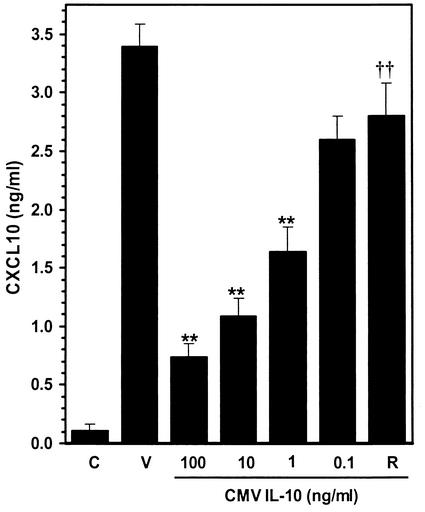
CMV is known to modulate host immune responses by expressing proteins that interfere with or mimic cellular products (44). A recently described viral homologue to human IL-10 has been shown to interact with the IL-10 receptor (27) and modulate proliferative responses and cytokine production in blood cells (64). We investigated whether this virus-encoded homologue of IL-10 could also suppress CXCL10 production by CMV-stimulated microglial cells. Microglial cells were treated overnight with recombinant CMV IL-10 (kindly provided by R&D Systems) prior to stimulation with CMV. Similar to our findings with human IL-10, CXCL10 induction (72 h poststimulation) was markedly suppressed following treatment with CMV IL-10 (100 ng/ml) (Fig. 6), and this suppression was dependent on the concentration of CMV IL-10. CXCL10 production decreased from 3.38 ng/ml with no CMV IL-10 treatment to 0.73 ± 0.12 ng/ml (78% suppression; P < 0.01), 1.08 ± 0.16 ng/ml (68% suppression; P < 0.01), 1.64 ± 0.21 ng/ml (52% suppression; P < 0.01), and 2.60 ± 0.20 ng/ml (28% suppression; P < 0.05) by CMV IL-10 at 100, 10, 1.0, and 0.1 ng/ml, respectively. Treatment with a neutralizing antibody to the human IL-10 receptor (15 μg/ml) 30 min prior to the addition of CMV IL-10 (100 ng/ml) eliminated its suppressive effect (2.81 ± 0.28 ng/ml; 17% suppression; P < 0.01 versus CMV IL-10-treated cultures; n = 8) on CMV-induced CXCL10 production. Treatment of CMV-stimulated microglial cells with the IL-10 receptor-specific antibody alone had no effect on virus-induced CXCL10 production (3.67 ± 0.20 versus 3.11 ± 0.16 ng/ml; n = 3).
FIG. 6.
Microglial cell-mediated CXCL10 production is downregulated by CMV IL-10. Concentration-response effect of CMV IL-10 on virus-induced CXCL10 production by microglial cells. Microglial cells were treated overnight with the indicated concentration of recombinant CMV IL-10 (0.1 to 100 ng/ml) prior to infection with CMV AD169. ELISA was used to quantify CXCL10 levels in the infected-cell supernatants. C, uninfected microglial cell control; V, CMV-infected microglial cells; R, microglial cells treated with an antibody to the human IL-10 receptor (15 μg/ml) prior to CMV IL-10 (100 ng/ml) treatment. Data are expressed as mean (± SEM) CXCL10 levels from pooled data obtained from three separate experiments with microglial cells isolated from three different brain specimens. **, P < 0.01 versus untreated CMV-infected microglial cells; ††, P < 0.01 versus microglial cells treated with 100 ng of CMV IL-10 per ml.
Conditioned medium from productively infected human astrocytes suppresses CMV-induced CXCL10 production by microglial cells through the IL-10 receptor.
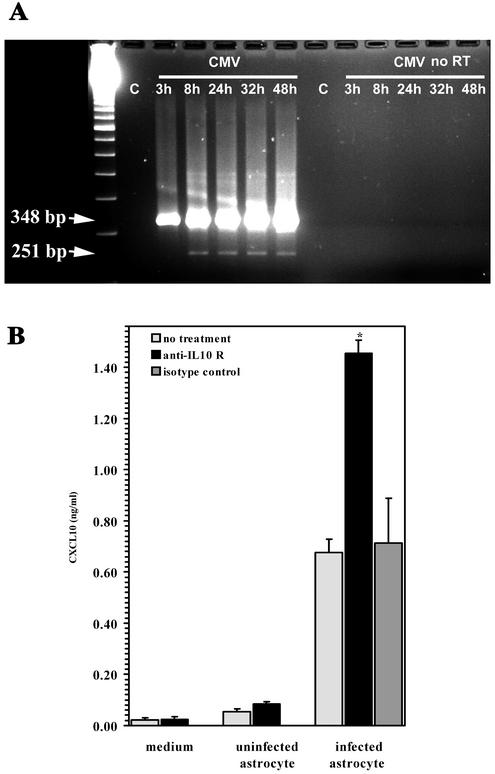
CMV infection of primary human astrocytes yields a 3- to 4-log increase in the viral titer within 5 to 7 days p.i., while cultured primary human microglial cells do not support productive viral replication (37). To determine if productive viral replication in astrocytes results in expression of the CMV IL-10 gene product, we examined infected astrocytes for cmvIL-10 mRNA expression by using PCR analysis of infected astrocyte cultures at 3, 8, 24, 32, and 48 h p.i. In these experiments, two distinct bands were resolved from infected astrocytes that were absent in the uninfected controls (Fig. 7A). The size of the smaller RT-PCR product (251 bp) corresponds to that of the spliced cmvIL-10 mRNA, while the larger RT-PCR product (348 bp) was presumably derived from unspliced RNA because no product was resolved from PCRs of RNA samples without RT (Fig. 7A). This spliced form of cmvIL-10 mRNA was not detected at 3 h p.i. but was seen 8 h p.i. (Fig. 7A).
FIG. 7.
cmvIL-10 is expressed during viral replication in human astrocytes. (A) mRNA expression. PCR analysis was performed on RNA extracted from uninfected astrocytes (lane C) and astrocytes at 3, 8, 24, 32, and 48 h following infection with CMV AD169 (MOI of 1 TCID50). Identical samples were also subjected to PCR amplification without RT treatment (no RT). The calculated size of the genomic-length PCR product is 348 bp, and that of the product corresponding to the spliced mRNA is 251 bp. cmvIL-10 mRNA was not present at 3 h p.i. but was detected by 8 h p.i. The gel shown is representative of three independent experiments with astrocytes from different brain specimens. (B) Conditioned medium from CMV-infected astrocytes inhibits microglial cell CXCL10 production through the IL-10 receptor. Microglial cells were treated with anti-IL-10 receptor antibody or isotype control antibody (15 μg/ml, 30 min) prior to the addition of medium alone or medium from uninfected or CMV-infected astrocytes (MOI of 5 TCID50). CXCL10 production was quantified by ELISA 72 h post supernatant addition. Data, expressed as the mean ± the SEM, are representative of four independent experiments with glial cells from different brain specimens. *, P < 0.05 versus untreated astrocyte supernatants.
Having demonstrated the presence of CMV IL-10 mRNA in infected astrocytes, we hypothesized that a biologically active cmvIL-10 gene product expressed in infected astrocytes would suppress CXCL10 production from microglial cells and that an antibody that inhibits human IL-10 receptor activity would fully restore chemokine production. To test this hypothesis, CMV-infected primary astrocyte cultures were frozen and thawed in the spent culture medium 3 to 4 days p.i. These lysates were then clarified to remove cellular debris and obtain CMV-infected astrocyte-conditioned medium. Conditioned media from infected and uninfected astrocyte cultures were used to test their ability to suppress CXCL10 production in microglial cells. Microglial cells were treated with an anti-IL-10 receptor antibody or an isotype control antibody (15 μg/ml) 30 min prior to the addition of conditioned medium obtained from CMV-infected astrocytes. The levels of CXCL10 production from anti-IL-10 receptor antibody-treated cultures were twofold greater than those from cultures treated with isotype antibody (Fig. 7B). The mean CXCL10 production from microglial cells treated with the infected-astrocyte medium (441.33 ± 22.27 pg/ml) increased to 914.67 ± 28.76 pg/ml (n = 12 with cells from four different specimens; P = 0.02) following anti-IL-10 receptor antibody treatment but was not significantly changed (397.78 ± 97.57 pg/ml) subsequent to treatment with an isotype control antibody.
To determine if virus present in the CMV-infected astrocyte-conditioned medium was responsible for the induction of CXCL10 in microglial cells, replication-competent virus in the medium was inactivated by UV treatment. UV inactivation decreased CXCL10 levels by 3.4-fold (207.33 ± 31.43 versus 61.0 ± 21.0 pg/ml; n = 6 from two different donors). Additionally, UV-inactivated infected astrocyte-conditioned medium suppressed CXCL10 production subsequent to CMV stimulation (MOI of 2.5 TCID50) by 2.5-fold (198.0 ± 20.22 versus 81.50 ± 11.44 pg/ml; n = 3), and this effect could be reversed by prior treatment with antibodies to the IL-10 receptor (167.50 ± 8.2 pg/ml). In addition, conditioned medium showed no detectable levels of TNF-α, which is known for its ability to induce CXCL10, nor did antibodies to this cytokine affect the induction of CXCL10.
DISCUSSION
Recruitment of T cells into the CNS is usually preceded by chemokine production from activated glial cells (3, 30), which is a critical defense mechanism against neurotropic viruses (60). Both microglial cells and astrocytes are potential sources of CXCL10 within the CNS during viral infections (3, 4, 30). In the present study, we have shown that human microglial cells produce CXCL10 in response to nonproductive infection with CMV, reaching peak levels between 48 and 72 h p.i. However, productively infected astrocytes were not found to produce this chemokine. Previous studies performed in our laboratory have shown that astrocytes are proficient at producing other chemokines, like MCP-1, in response to the same CMV infection (9). The lack of production of CXCL10 from astrocytes infected with CMV was not due to an inability of these glial cells to produce this chemokine, since stimulation with IFN-γ yielded significant amounts of CXCL10. These differences reflect the ability of glial cells to generate a complement of immune mediators specific to the type of stimulus toward which the response is directed. During CNS viral infections, CXCL10 production occurs early and correlates with the intensity of infection (3, 30). In all likelihood, these temporal and cellular patterns of glial cell responses to viral infections mold the clinical outcomes of disease.
Lymphocytes play an important role in host defense against CMV disease (55). Although there is ample experimental evidence to confirm the ability of CMV to infect human brain cells (11, 37, 43), encephalitis is rare in immunocompetent hosts and in vitro studies have shown that lymphocytes suppress CMV replication in primary astrocytes (7). It is only during advanced AIDS or in congenital infections, when T lymphocytes are deficient, that CNS disease due to CMV emerges (2). In this study, we have shown that supernatants from CMV-stimulated microglial cells induce migration of activated lymphocytes and that this ability is eliminated, in part, by antibodies to CXCL10. Disabling the effects of CXCL10 in vivo, by using either neutralizing antibodies or CXCL10 knockout animals, has been shown to inhibit the recruitment of T cells and alter the pathogenesis of inflammatory diseases of the CNS (14, 33). It can be postulated that intrinsic glial cell responses, along with subsequent trafficking of activated lymphocytes into the CNS, prevents development of CMV brain disease in an immunocompetent host. Dysfunction in either arm of protection could lead to development of encephalitis.
Control of cellular responses to environmental stimuli is mediated by activation of distinct signaling pathways that regulate gene expression (5). Binding of CMV to the host cell activates a variety of signal-transducing molecules, including NF-κB (57) and p38 MAP kinase (24, 70) in many cell types, including macrophages (70). Both p38 MAP kinase (12, 29) and NF-κB (42) have been implicated in the regulation of CXCL10 production. The CMV-induced CXCL10 production described here was not dependent on viral immediate-early protein synthesis or secondary cytokine production but rather appears to be a direct effect of viral activation of signaling pathways. This finding is significant because microglial cells produce TNF-α in response to CMV (9), a known stimulus for CXCL10 production (42) and NFκB activation (47). In our study, we found that signaling via p38 MAP kinase is involved in CXCL10 production by microglial cells. Activation of p38 MAP kinase is critical for viral replication in permissive cells (24). However, in a nonpermissive cell type like microglial cells (37), this CMV-induced p38 MAP kinase phosphorylation may lead to initiation of protective immune responses.
The mechanisms that control inflammatory responses within the CNS are only beginning to be understood. The immunosuppressive microenvironment of the CNS (23), as well as the upregulation of anti-inflammatory cytokines during various insults within the brain, appears to be required to maintain or restore homeostasis to this vital organ (67). It has been postulated that the anti-inflammatory microenvironment of the brain is maintained through constitutive expression of low levels of TGF-β, while other anti-inflammatory cytokines, such as IL-10 and IL-4, execute a more acute quenching of inflammatory processes (21). These anti-inflammatory cytokines are known to suppress chemokine production by activated microglial cells through a unique directed regulation of specific inflammatory processes rather than global suppression of cellular function (15, 19, 21, 59). Although the production of CXCL10 has been shown to be protective in some models of CNS infection (14), chronic expression of CXCL10 results in sustained neuroinflammation and consequent neurodegeneration (46). We studied the effects of IL-10, IL-4, and TGF-β on CMV-induced CXCL10 production as a potential mechanism by which to control neuroinflammation in response to viral infection. We found that IL-10 and IL-4, but not TGF-β, suppressed CVM-induced CXCL10 production. Both IL-10 and IL-4 have been shown to inhibit CXCL10 production in peritoneal macrophages, but the immune quenching effect of these cytokines is most likely dependent on the inducing stimulus. For example, while IL-4 inhibits IFN-induced CXCL10 production, IL-10 suppresses LPS- but not IFN-induced chemokine production (17, 65). The inhibitory effect of IL-10 reported in this study was greater than that of IL-4 under the same conditions. In addition, these effects were concentration dependent and associated with suppression of CMV-induced CXCL10 mRNA synthesis.
We also found that inhibition of CMV-induced CXCL10 by IL-4 and IL-10 is associated with a concomitant decrease in the activation of NF-κB. The mechanism by which this decreased activation of NF-κB results in the down-regulation of proinflammatory gene expression remains poorly understood. In monocytes, IL-4 and IL-10 utilize different mechanisms to suppress LPS-induced cytokine production. IL-10 inhibits the nuclear translocation of NF-κB without affecting other transcription factors, such as NF-IL-6, AP-1, AP-2, or CREB (68). Recent studies with monocytes have shown that IL-10 prevents the degradation of I-κB by inhibiting I-κB kinase activity and decreases the DNA-binding capacity of NF-κB (61). On the other hand, IL-4 increases the degradation of cytokine mRNA in human monocytes without affecting NF-κB translocation (68). However, in murine and porcine macrophages, IL-4 affects gene transcription rather than accelerating RNA degradation (17, 71). Taken together, these findings suggest that anti-inflammatory cytokines regulate inflammation through distinct pathways and that these pathways are dependent on both the inflammatory stimulus and the cell type involved.
CMV has evolved numerous strategies by which to counteract host immune responses that effectively aid in its sustenance of a lifelong subclinical or latent infection in immunocompetent hosts. Among the many virus-encoded gene products that mimic host immune molecules (reviewed in references 20, 34, and 44), CMV encodes a homologue to human IL-10, which serves as a ligand for the human IL-10 receptor (27). In this study, we demonstrated that CMV IL-10 is expressed during viral replication in astrocytes and that it suppresses CXCL10 production from microglial cells as efficiently as human IL-10. CMV IL-10 has also been shown to suppress lymphocyte proliferation, proinflammatory cytokine production, and cell surface expression of major histocompatibility complex molecules (64). All of these inhibitory functions are mediated through the IL-10 receptor. Although this viral protein shares only 27% homology with human IL-10, the two molecules possess similar binding affinities for the IL-10 receptor (25). The specific role of CMV IL-10 in inhibiting lymphocyte recruitment and proliferation remains speculative, but we can postulate that the virus acquired such robust mechanisms to subvert host immune responses.
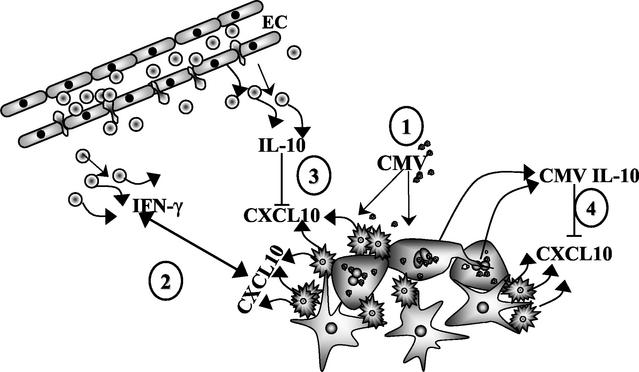
Taken together, the results of this study and of prior research by other groups support the concept of the role of microglial cell-produced CXCL10 and its regulation by human and viral IL-10 depicted in Fig. 8. Initially, CMV productively infects astrocytes and induces production of chemokines, such as MCP-1, that recruit microglial cells to the site of infection (38). Upon stimulation with CMV, microglial cells produce CXCL10, which serves as a chemotactic signal for activated T lymphocytes to enter the brain and migrate toward foci of infection. Once upon the scene of infection, activated lymphocytes produce cytokines such as IFN-γ, which proceed to control viral replication and spread. Once CMV infection is controlled, anti-inflammatory cytokines, such as IL-10, are needed to suppress the local inflammatory response, thereby preventing brain damage. These anti-inflammatory cytokines may be derived from surrounding glial cells or from additional lymphocytes that migrate into the region. CMV exploits the immunosuppressive effects of IL-10 by expressing a viral homologue of the protein that dampens CXCL10 production and most likely hampers subsequent viral clearance. As long as immunocompetency can be maintained, CMV will be readily contained. However, if this host-virus interaction is tipped in favor of CMV by severe depletion of T cells, then the virus may spread unrestrained throughout the brain.
FIG. 8.
Model for CMV subversion of neuroimmune responses through inhibition of CXCL10 production. (Step 1) CMV productively infects astrocytes and stimulates microglial cells to produce CXCL10. (Step 2) Activated T lymphocytes respond to this microglial cell-produced CXCL10 and migrate toward foci of infection in the CNS. Through the production of soluble factors such as IFN-γ, these lymphocytes work to control viral replication and spreading. (Step 3) Additional lymphocytes make available anti-inflammatory cytokines such as IL-10 that suppress CXCL10 production by microglial cells and prevent excessive inflammatory brain damage. (Step 4) CMV exploits these immunosuppressive effects by expressing a viral homologue of IL-10 (i.e., CMV IL-10) that functions to dampen CXCL10 production and possibly reduce lymphocyte-mediated viral clearance.
Acknowledgments
This study was financed in part by U.S. Public Health Service grant NS-38836.
REFERENCES
- 1.Aloisi, F. 2001. Immune function of microglia. Glia 36:165-179. [DOI] [PubMed]
- 2.Arribas, J. R., G. A. Storch, D. B. Clifford, and A. C. Tselis. 1996. Cytomegalovirus encephalitis. Ann. Intern. Med. 125:577-587. [DOI] [PubMed] [Google Scholar]
- 3.Asensio, V. C., and I. L. Campbell. 1997. Chemokine gene expression in the brains of mice with lymphocytic choriomeningitis. J. Virol. 71:7832-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asensio, V. C., J. Maier, R. Milner, K. Boztug, C. Kincaid, M. Moulard, C. Phillipson, K. Lindsley, T. Krucker, H. S. Fox, and I. L. Campbell. 2001. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. J. Virol. 75:7067-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 6.Chao, C. C., S. Hu, W. S. Sheng, D. Bu, M. I. Bukrinsky, and P. K. Peterson. 1996. Cytokine-stimulated astrocytes damage human neurons via a nitric oxide mechanism. Glia 16:276-284. [DOI] [PubMed] [Google Scholar]
- 7.Cheeran, M. C., G. Gekker, S. Hu, S. L. Yager, P. K. Peterson, and J. R. Lokensgard. 2000. CD4+ lymphocyte-mediated suppression of cytomegalovirus expression in human astrocytes. Clin. Diagn. Lab. Immunol. 7:710-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheeran, M. C., S. Hu, G. Gekker, and J. R. Lokensgard. 2000. Decreased cytomegalovirus expression following proinflammatory cytokine treatment of primary human astrocytes. J. Immunol. 164:926-933. [DOI] [PubMed] [Google Scholar]
- 9.Cheeran, M. C., S. Hu, S. L. Yager, G. Gekker, P. K. Peterson, and J. R. Lokensgard. 2001. Cytomegalovirus induces cytokine and chemokine production differentially in microglia and astrocytes: antiviral implications. J. Neurovirol. 7:135-147. [DOI] [PubMed] [Google Scholar]
- 10.Cheng, G., A. S. Nazar, H. S. Shin, P. Vanguri, and M. L. Shin. 1998. IP-10 gene transcription by virus in astrocytes requires cooperation of ISRE with adjacent κB site but not IRF-1 or viral transcription. J. Interferon Cytokine Res. 18:987-997. [DOI] [PubMed] [Google Scholar]
- 11.Cinque, P., R. Marenzi, and D. Ceresa. 1997. Cytomegalovirus infections of the nervous system. Intervirology 40:85-97. [DOI] [PubMed] [Google Scholar]
- 12.D'Aversa, T. G., K. M. Weidenheim, and J. W. Berman. 2002. CD40-CD40L interactions induce chemokine expression by human microglia: implications for human immunodeficiency virus encephalitis and multiple sclerosis. Am. J. Pathol. 160:559-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davignon, J. L., P. Castanie, J. A. Yorke, N. Gautier, D. Clement, and C. Davrinche. 1996. Anti-human cytomegalovirus activity of cytokines produced by CD4+ T-cell clones specifically activated by IE1 peptides in vitro. J. Virol. 70:2162-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dufour, J. H., M. Dziejman, M. T. Liu, J. H. Leung, T. E. Lane, and A. D. Luster. 2002. IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168:3195-3204. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlich, L. C., S. Hu, W. S. Sheng, R. L. Sutton, G. L. Rockswold, P. K. Peterson, and C. C. Chao. 1998. Cytokine regulation of human microglial cell IL-8 production. J. Immunol. 160:1944-1948. [PubMed] [Google Scholar]
- 16.Farber, J. M. 1997. Mig and IP-10: CXC chemokines that target lymphocytes. J. Leukoc. Biol. 61:246-257. [PubMed] [Google Scholar]
- 17.Gautam, S., J. M. Tebo, and T. A. Hamilton. 1992. IL-4 suppresses cytokine gene expression induced by IFN-γ and/or IL-2 in murine peritoneal macrophages. J. Immunol. 148:1725-1730. [PubMed] [Google Scholar]
- 18.Glabinski, A. R., M. Krakowski, Y. Han, T. Owens, and R. M. Ransohoff. 1999. Chemokine expression in GKO mice (lacking interferon-gamma) with experimental autoimmune encephalomyelitis. J. Neurovirol. 5:95-101. [DOI] [PubMed] [Google Scholar]
- 19.Guo, H., Y. X. Jin, M. Ishikawa, Y. M. Huang, P. H. van der Meide, H. Link, and B. G. Xiao. 1998. Regulation of beta-chemokine mRNA expression in adult rat astrocytes by lipopolysaccharide, proinflammatory and immunoregulatory cytokines. Scand. J. Immunol. 48:502-508. [DOI] [PubMed] [Google Scholar]
- 20.Hengel, H., W. Brune, and U. H. Koszinowski. 1998. Immune evasion by cytomegalovirus—survival strategies of a highly adapted opportunist. Trends Microbiol. 6:190-197. [DOI] [PubMed] [Google Scholar]
- 21.Hu, S., C. C. Chao, L. C. Ehrlich, W. S. Sheng, R. L. Sutton, G. L. Rockswold, and P. K. Peterson. 1999. Inhibition of microglial cell RANTES production by IL-10 and TGF-β. J. Leukoc. Biol. 65:815-821. [DOI] [PubMed] [Google Scholar]
- 22.Huang, D., Y. Han, M. R. Rani, A. Glabinski, C. Trebst, T. Sorensen, M. Tani, J. Wang, P. Chien, S. O'Bryan, B. Bielecki, Z. L. Zhou, S. Majumder, and R. M. Ransohoff. 2000. Chemokines and chemokine receptors in inflammation of the nervous system: manifold roles and exquisite regulation. Immunol. Rev. 177:52-67. [DOI] [PubMed] [Google Scholar]
- 23.Irani, D. N., K. I. Lin, and D. E. Griffin. 1997. Regulation of brain-derived T cells during acute central nervous system inflammation. J. Immunol. 158:2318-2326. [PubMed] [Google Scholar]
- 24.Johnson, R. A., S. M. Huong, and E. S. Huang. 2000. Activation of the mitogen-activated protein kinase p38 by human cytomegalovirus infection through two distinct pathways: a novel mechanism for activation of p38. J. Virol. 74:1158-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, B. C., N. J. Logsdon, K. Josephson, J. Cook, P. A. Barry, and M. R. Walter. 2002. Crystal structure of human cytomegalovirus IL-10 bound to soluble human IL-10R1. Proc. Natl. Acad. Sci. USA 99:9404-9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopydlowski, K. M., C. A. Salkowski, M. J. Cody, N. van Rooijen, J. Major, T. A. Hamilton, and S. N. Vogel. 1999. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J. Immunol. 163:1537-1544. [PubMed] [Google Scholar]
- 27.Kotenko, S. V., S. Saccani, L. S. Izotova, O. V. Mirochnitchenko, and S. Pestka. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. USA 97:1695-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreutzberg, G. W. 1996. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19:312-318. [DOI] [PubMed] [Google Scholar]
- 29.Kutsch, O., J.-W. Oh, A. Nath, and E. N. Benveniste. 2000. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 Tat in astrocytes. J. Virol. 74:9214-9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lane, T. E., V. C. Asensio, N. Yu, A. D. Paoletti, I. L. Campbell, and M. J. Buchmeier. 1998. Dynamic regulation of alpha- and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol. 160:970-978. [PubMed] [Google Scholar]
- 31.Liu, M. T., B. P. Chen, P. Oertel, M. J. Buchmeier, D. Armstrong, T. A. Hamilton, and T. E. Lane. 2000. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J. Immunol. 165:2327-2330. [DOI] [PubMed] [Google Scholar]
- 32.Liu, M. T., B. P. Chen, P. Oertel, M. J. Buchmeier, T. A. Hamilton, D. A. Armstrong, and T. E. Lane. 2001. The CXC chemokines IP-10 and Mig are essential in host defense following infection with a neurotropic coronavirus. Adv. Exp. Med. Biol. 494:323-327. [DOI] [PubMed] [Google Scholar]
- 33.Liu, M. T., H. S. Keirstead, and T. E. Lane. 2001. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J. Immunol. 167:4091-4097. [DOI] [PubMed] [Google Scholar]
- 34.Loenen, W. A., C. A. Bruggeman, and E. J. Wiertz. 2001. Immune evasion by human cytomegalovirus: lessons in immunology and cell biology. Semin. Immunol. 13:41-49. [DOI] [PubMed] [Google Scholar]
- 35.Loetscher, M., B. Gerber, P. Loetscher, S. A. Jones, L. Piali, I. Clark-Lewis, M. Baggiolini, and B. Moser. 1996. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J. Exp. Med. 184:963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loetscher, M., P. Loetscher, N. Brass, E. Meese, and B. Moser. 1998. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur. J. Immunol. 28:3696-3705. [DOI] [PubMed] [Google Scholar]
- 37.Lokensgard, J. R., M. C. Cheeran, G. Gekker, S. Hu, C. C. Chao, and P. K. Peterson. 1999. Human cytomegalovirus replication and modulation of apoptosis in astrocytes. J. Hum. Virol. 2:91-101. [PubMed] [Google Scholar]
- 38.Lokensgard, J. R., M. C.-J. Cheeran, S. Hu, G. Gekker, and P. K. Peterson. 2000. Microglia: defenders of the brain against viral infection, p. 39-50. In P. Peterson, and J. S. Remington (ed.), New concepts in immunopathogenesis of CNS infections, 2nd ed., in press. Blackwell Science, Malden, Mass.
- 39.Lokensgard, J. R., S. Hu, W. Sheng, M. vanOijen, D. Cox, M. C. Cheeran, and P. K. Peterson. 2001. Robust expression of TNF-α, IL-1β, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplex virus. J. Neurovirol. 7:208-219. [DOI] [PubMed] [Google Scholar]
- 40.Luster, A. D., J. C. Unkeless, and J. V. Ravetch. 1985. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 315:672-676. [DOI] [PubMed] [Google Scholar]
- 41.Majumder, S., L. Z. Zhou, P. Chaturvedi, G. Babcock, S. Aras, and R. M. Ransohoff. 1998. p48/STAT-1α-containing complexes play a predominant role in induction of IFN-γ-inducible protein, 10 kDa (IP-10) by IFN-γ alone or in synergy with TNF-α. J. Immunol. 161:4736-4744. [PubMed] [Google Scholar]
- 42.Majumder, S., L. Z. Zhou, P. Chaturvedi, G. Babcock, S. Aras, and R. M. Ransohoff. 1998. Regulation of human IP-10 gene expression in astrocytoma cells by inflammatory cytokines. J. Neurosci. Res. 54:169-180. [DOI] [PubMed] [Google Scholar]
- 43.McCarthy, M., D. Auger, and S. R. Whittemore. 2000. Human cytomegalovirus causes productive infection and neuronal injury in differentiating fetal human central nervous system neuroepithelial precursor cells. J. Hum. Virol. 3:215-228. [PubMed] [Google Scholar]
- 44.Mocarski, E. S., Jr. 2002. Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 10:332-339. [DOI] [PubMed] [Google Scholar]
- 45.Molina-Holgado, E., A. Arevalo-Martin, A. Castrillo, L. Bosca, J. M. Vela, and C. Guaza. 2002. Interleukin-4 and interleukin-10 modulate nuclear factor κB activity and nitric oxide synthase-2 expression in Theiler's virus-infected brain astrocytes. J. Neurochem. 81:1242-1252. [DOI] [PubMed] [Google Scholar]
- 46.Murray, P. D., K. Krivacic, A. Chernosky, T. Wei, R. M. Ransohoff, and M. Rodriguez. 2000. Biphasic and regionally-restricted chemokine expression in the central nervous system in the Theiler's virus model of multiple sclerosis. J. Neurovirol. 6(Suppl. 1):S44-S52. [PubMed] [Google Scholar]
- 47.Nadeau, S., and S. Rivest. 2000. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor kappa B activity in the brain during endotoxemia. J. Neurosci. 20:3456-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neville, L. F., G. Mathiak, and O. Bagasra. 1997. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 8:207-219. [DOI] [PubMed] [Google Scholar]
- 49.Oh, J. W., L. M. Schwiebert, and E. N. Benveniste. 1999. Cytokine regulation of CC and CXC chemokine expression by human astrocytes. J. Neurovirol. 5:82-94. [DOI] [PubMed] [Google Scholar]
- 50.Palma, J. P., and B. S. Kim. 2001. Induction of selected chemokines in glial cells infected with Theiler's virus. J. Neuroimmunol. 117:166-170. [DOI] [PubMed] [Google Scholar]
- 51.Persidsky, Y. 1999. Model systems for studies of leukocyte migration across the blood-brain barrier. J. Neurovirol. 5:579-590. [DOI] [PubMed] [Google Scholar]
- 52.Peterson, P. K., S. Hu, J. Salak-Johnson, T. W. Molitor, and C. C. Chao. 1997. Differential production of and migratory response to beta chemokines by human microglia and astrocytes. J. Infect. Dis. 175:478-481. [DOI] [PubMed] [Google Scholar]
- 53.Poluektova, L., T. Moran, M. Zelivyanskaya, S. Swindells, H. E. Gendelman, and Y. Persidsky. 2001. The regulation of alpha chemokines during HIV-1 infection and leukocyte activation: relevance for HIV-1-associated dementia. J. Neuroimmunol. 120:112-128. [DOI] [PubMed] [Google Scholar]
- 54.Ransohoff, R. M., T. Wei, K. D. Pavelko, J. C. Lee, P. D. Murray, and M. Rodriguez. 2002. Chemokine expression in the central nervous system of mice with a viral disease resembling multiple sclerosis: roles of CD4+ and CD8+ T cells and viral persistence. J. Virol. 76:2217-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reddehase, M. J. 2000. The immunogenicity of human and murine cytomegaloviruses. Curr. Opin. Immunol. 12:390-396. [DOI] [PubMed] [Google Scholar]
- 56.Renard, P., I. Ernest, A. Houbion, M. Art, H. Le Calvez, M. Raes, and J. Remacle. 2001. Development of a sensitive multi-well colorimetric assay for active NFκB. Nucleic Acids Res. 29:E21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sambucetti, L. C., J. M. Cherrington, G. W. Wilkinson, and E. S. Mocarski. 1989. NF-κB activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 8:4251-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sauder, C., W. Hallensleben, A. Pagenstecher, S. Schneckenburger, L. Biro, D. Pertlik, J. Hausmann, M. Suter, and P. Staeheli. 2000. Chemokine gene expression in astrocytes of Borna disease virus-infected rats and mice in the absence of inflammation. J. Virol. 74:9267-9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sawada, M., A. Suzumura, H. Hosoya, T. Marunouchi, and T. Nagatsu. 1999. Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J. Neurochem. 72:1466-1471. [DOI] [PubMed] [Google Scholar]
- 60.Schneider-Schaulies, J., U. Liebert, R. Dorries, and V. Meullen. 1997. Establishment and control of viral infections of the nervous system, p. 576-610. In R. W. Keane and W. F. Hickey (ed.), Immunology of the nervous system. Oxford University Press, New York, N.Y.
- 61.Schottelius, A. J., M. W. Mayo, R. B. Sartor, and A. S. Baldwin, Jr. 1999. Interleukin-10 signaling blocks inhibitor of κB kinase activity and nuclear factor κB DNA binding. J. Biol. Chem. 274:31868-31874. [DOI] [PubMed] [Google Scholar]
- 62.Shi, Y., B. S. Kornovski, R. Savani, and E. A. Turley. 1993. A rapid, multiwell colorimetric assay for chemotaxis. J. Immunol. Methods 164:149-154. [DOI] [PubMed] [Google Scholar]
- 63.Spaete, R. R., and E. S. Mocarski. 1987. Insertion and deletion mutagenesis of the human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 84:7213-7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spencer, J. V., K. M. Lockridge, P. A. Barry, G. Lin, M. Tsang, M. E. Penfold, and T. J. Schall. 2002. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J. Virol. 76:1285-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tebo, J. M., H. S. Kim, J. Gao, D. A. Armstrong, and T. A. Hamilton. 1998. Interleukin-10 suppresses IP-10 gene transcription by inhibiting the production of class I interferon. Blood 92:4742-4749. [PubMed] [Google Scholar]
- 66.Vanguri, P., and J. M. Farber. 1994. IFN and virus-inducible expression of an immediate early gene, crg-2/IP-10, and a delayed gene, I-Aα, in astrocytes and microglia. J. Immunol. 152:1411-1418. [PubMed] [Google Scholar]
- 67.Vitkovic, L., S. Maeda, and E. Sternberg. 2001. Anti-inflammatory cytokines: expression and action in the brain. Neuroimmunomodulation 9:295-312. [DOI] [PubMed] [Google Scholar]
- 68.Wang, P., P. Wu, M. I. Siegel, R. W. Egan, and M. M. Billah. 1995. Interleukin (IL)-10 inhibits nuclear factor kappa B (NFκB) activation in human monocytes: IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J. Biol. Chem. 270:9558-9563. [DOI] [PubMed] [Google Scholar]
- 69.Wesselingh, S. L., B. Levine, R. J. Fox, S. Choi, and D. E. Griffin. 1994. Intracerebral cytokine mRNA expression during fatal and nonfatal alphavirus encephalitis suggests a predominant type 2 T cell response. J. Immunol. 152:1289-1297. [PubMed] [Google Scholar]
- 70.Yurochko, A. D., and E. S. Huang. 1999. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J. Immunol. 162:4806-4816. [PubMed] [Google Scholar]
- 71.Zhou, Y., G. Lin, M. J. Baarsch, R. W. Scamurra, and M. P. Murtaugh. 1994. Interleukin-4 suppresses inflammatory cytokine gene transcription in porcine macrophages. J. Leukoc. Biol. 56:507-513. [DOI] [PubMed] [Google Scholar]