Abstract
Depo-provera, a long-acting progestational formulation, is widely used to facilitate infection of sexually transmitted diseases in animal models. We have previously reported that hormone treatments change susceptibility and immune responses to genital tract infections. In this study we compared the changes in susceptibility of mice to genital herpes simplex virus type 2 (HSV-2) after Depo-provera or a saline suspension of progesterone (P-sal). We found that following Depo-provera-treatment, mice had prolonged diestrus that lasted more than 4 weeks. This coincided with a 100-fold increase in susceptibility to genital HSV-2 compared to that of untreated mice. Mice given P-sal were in diestrous stage for 4 to 6 days before returning to irregular reproductive cycles. When these mice were infected at diestrus they showed a 10-fold increase in susceptibility compared to that of normal, untreated mice. P-sal-treated mice infected at estrus were susceptible to HSV-2, depending on the infectious dose. Normal, untreated mice in estrus were not susceptible to HSV-2, even at a high infectious dose of 107 PFU. In addition to alterations in susceptibility, Depo-provera treatment had inhibitory effects on immune responses to HSV-2. Mice immunized with HSV-2 protein (gB) and treated with Depo-provera showed significant lowering of local HSV-2-specific immunoglobulin G (IgG) and IgA in their vaginal washes. Mice immunized with an attenuated strain of HSV-2 2 weeks after Depo-provera treatment failed to develop protection when challenged intravaginally with wild-type HSV-2. In contrast, mice given progesterone and immunized at diestrus or estrus were completely protected from intravaginal challenge. These studies show that Depo-provera treatment changes susceptibility and local immune responses to genital HSV-2 infection. Animal models and vaccine strategies for sexually transmitted diseases need to consider the effect of hormone treatments on susceptibility and immune responses.
Herpes simplex virus type 2 (HSV-2) is a sexually transmitted agent that attaches, penetrates, and undergoes infectious cycles of replication in the epithelium of the genital tract. Statistics show that approximately one in four sexually active adults has genital herpes (9). Despite significant progress in understanding the virus and the disease caused by it, presently there are no effective vaccines against HSV-2 infection. One of the reasons why this goal has remained elusive is that it is difficult to establish correlates of protection against genital infection. An effective vaccine has to elicit a protective immune response locally, in the mucosal compartment of the genital tract, where immune responses are regulated by factors such as sex hormones (3).
Studies done by us and others have clearly shown that both susceptibility and immune responses in the female genital tract are regulated by sex hormones (2, 8, 19). In a rat model it was previously shown that the stage of the estrous cycle and treatment with sex hormones, specifically estradiol and progesterone, influence both the inductive and effector arm of the immune system in the genital tract (20). Antigen presentation, immunoglobulin A (IgA) and IgG levels, IgA transport, and presence of immune cells are all under hormonal regulation (5-7, 22). More recently these studies have been extended to cover the reproductive tract of women, demonstrating that similar regulation of immune responses exists in women during the menstrual cycle (18, 23).
In other studies it was shown that susceptibility and immune responses to other sexually transmitted diseases (STDs), such as genital Chlamydia infection, are profoundly affected by sex hormones (8). Hormonally treated rats exhibited remarkable changes in their susceptibility and immune responses depending on the hormone treatment they received. Progesterone-treated rats became heavily infected following genital exposure to Chlamydia organisms and had severe inflammation, while estradiol-treated rats remained uninfected and showed no signs of inflammation. Thus, the hormonal environment at the time of genital infection may play a significant role in determining both susceptibility and immune responses.
A mouse model of HSV-2 has been used by a number of groups over the past few years to study vaginal infection and immune responses (4, 13-15). In all studies that used this model, mice were pretreated with Depo-provera (Depo; dihydroxyprogesterone acetate) prior to infection. In the absence of Depo treatment, susceptibility to vaginal HSV-2 infection is dependent on the stage of the estrous cycle (2). Pretreatment with Depo, while facilitating vaginal infection, may have unknown effects on susceptibility and immune responses. The aim of the present study was to investigate these issues. The susceptibility of mice in diestrous stage induced by Depo versus that by a saline suspension of progesterone (P-sal) that does not have the prolonged effects of Depo was compared. Since mice in estrous stage have been shown to be resistant to HSV-2 infection, mice were also infected at estrus, following P-sal treatment, to examine any changes in susceptibility. In addition to analyzing changes in susceptibility, we also examined if treatment with Depo following immunization with either an attenuated HSV-2 virus or HSV-2 antigen (glycoprotein B [gB]) led to alterations in immune responses and protection against HSV-2.
MATERIALS AND METHODS
Animals and hormone treatments.
Inbred 8- to 10-week-old C57Bl/6 mice purchased from Charles River Canada (Constant, Quebec, Canada) were used in these studies. Mouse colonies were maintained on a 12-h dark:12-h light cycle. Mice were injected subcutaneously with Depo (Upjohn, Don Mills, Ontario, Canada) or progesterone (Calbiochem, La Jolla, Calif.). Progesterone suspension was made in saline by glass-glass homogenization.
Infection of animals.
Mice were anesthetized by injectable anesthetic (ketamine-xylazine [0.75 ml: 0.25 ml]) given intraperitoneally, swabbed intravaginally with a cotton applicator, placed on their backs, and infected intravaginally with 10 μl of wild-type HSV-2 strain 333 or an attenuated strain of HSV-2, TK-HSV-2. Mice were kept on their backs under the influence of anesthesia for 45 min to 1 h to allow the inoculum to infect.
Vaginal smears and lavage collection.
Vaginal lavage for reproductive cycle staging and plaque assays was collected by pipetting 30 μl of phosphate-buffered saline (PBS) in and out of the vagina several times. For vaginal smears the fluid was smeared on glass slides and was examined by light microscopy to determine the stage of the estrous cycle as described previously (21). The following classification was used for identifying the stage of the cycle: estrus, >90% cornified epithelial cells; diestrus, >75% polymorphonuclear cells; diestrus-estrus, 50% epithelial, 50% polymorphonuclear cells. For plaque assays the vaginal wash was followed by swabbing with a cotton tip applicator. Both the wash and applicator were combined with 0.97 ml of PBS and were frozen at −70°C. The dilution of each vaginal wash supernatant was considered to be 10−2.
Viral replication and pathology in the reproductive tract.
Genital pathology following infection with HSV-2 was monitored daily and was scored on a 5-point scale: 0, no infection; 1, slight redness of external vagina; 2, swelling and redness of external vagina; 3, severe swelling and redness of both vagina and surrounding tissue and hair loss in genital area; 4, genital ulceration with severe redness, swelling, and hair loss of genital and surrounding tissue; 5, severe genital ulceration extending to surrounding tissue. Animals were sacrificed after stage 4.
Vaginal washes or tissue homogenates were analyzed for viral titers by plaque assays. Vero cells were grown in α-modified Eagle’s medium (GIBCO Laboratories, Burlington, Canada) supplemented with 10% fetal calf serum (FCS; GIBCO, Burlington, Canada), 1% penicillin-streptomycin, and l-glutamine (GIBCO). For plaque assays, Vero cells were grown to confluence in 24-well plates. Samples were diluted and were added to monolayers in duplicate. Infected monolayers were incubated at 37°C for 1 h and were rocked every 15 min for viral absorption. Infected monolayers were overlaid with α-modified Eagle’s medium supplemented with 0.05% human immune serum globulin (Canadian Blood Services). Infection was allowed to occur for 48 h at 37°C. Monolayers were then fixed and stained with crystal violet, and viral plaques were counted under a light microscope. Positive controls were run with every assay with previously titered laboratory stocks of HSV-2 (strain 333). The number of PFU per milliliter was calculated by taking the plaque count mean for every sample and taking into account the dilution factors.
ELISA for anti-HSV-2 gB IgG and IgA.
HSV-2 gB-specific antibody titers were determined by an enzyme-linked immunosorbent assay (ELISA) modified from a protocol described previously (2). Briefly, Maxisorp 96-well plates (Invitrogen, Burlington, Ontario) were coated overnight with 2.5 μg of recombinant gB protein (Chiron Inc., Emeryville, Calif.)/ml in PBS at 4°C. Plates were blocked with 2% bovine serum albumin for 2 h at room temperature and were loaded with 100 μl of twofold serial dilutions of samples or controls. Incubation was carried out at room temperature for 1 to 2 h. Plates were washed and reacted for 1 h with one of the following biotinylated antibodies: goat anti-mouse IgG or goat anti-mouse IgA at 1:1,000 dilution (Pharmingen, Mississauga, Ontario, Canada). Plates were developed with extravidin-peroxidase (1:2,000 dilution) and tetramethyl benzidine. Endpoint titers were determined and expressed as geometric mean titers ± standard errors of the means. Background values were obtained by using vaginal lavages from nonimmunized mice. Two times the background optical density value was the cutoff for determining positive values.
Intranasal gB immunization.
Mice were immunized with 10 μg of recombinant gB of HSV-2 (Chiron, Emeryville, Calif.)/mouse plus CpG oligodeoxynucleotides (ODN) (10 μg/mouse) in a volume of 15 μl given intranasally as described before (1). Briefly, mice were anesthetized and held inverted with their noses down until droplets of solution applied to their external nares were completely inhaled.
Statistical analysis.
All experiments were repeated at least two times with six animals in each group. Where applicable, data was analyzed by using unpaired two-tailed t test, and significance was defined as a P value of <0.05.
RESULTS
Effect of Depo versus progesterone on reproductive cyclicity.
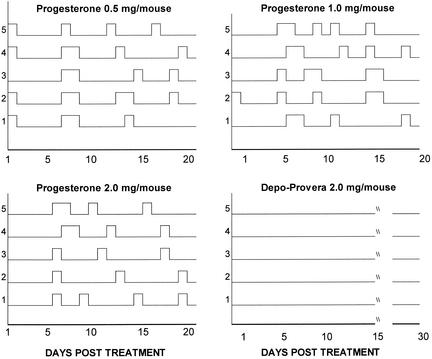
Groups of mice were given either Depo (2 mg/mouse) or P-sal at a dose of 2, 1, or 0.5 mg/mouse in the same volume as that of Depo. Vaginal smears of each group were examined daily prior to and following each hormone treatment. As shown in Fig. 1, mice given Depo went into diestrous phase following hormone treatment and continued to stay in diestrus until day 30, when daily smears were terminated, without any sign of reproductive cyclicity. Vaginal smears of mice given progesterone in all three doses showed diestrous stage within 24 h of administration of the hormone, but the majority of them started cycling irregularly around day 5 or 6 posttreatment. By 2 weeks following hormone treatment, most of the mice returned to the regular 4- to 5-day estrous cycles seen with normal, untreated mice. Estrous stage was seen in these mice every 3 to 4 days, indicating that they were ovulating regularly. Subsequent repeat experiments confirmed these results, and the mice always started cycling between 4 to 6 days following progesterone treatment, whereas Depo-treated mice took 5 to 6 weeks to start irregular cycles.
FIG. 1.
Vaginal smears from hormone-treated mice. Four groups of mice were treated with either Depo (2 mg/mouse) or progesterone (2, 1, or 0.5 mg/mouse) subcutaneously. Vaginal smears were taken daily, and the stage of the cycle was determined as described in Materials and Methods. Graphs show the cycles of each mouse followed for 20 (progesterone groups) or 30 days posttreatment (Depo group). Estrus is shown by peaks on the Y axis, whereas diestrus is denoted by the baseline.
Dose of progesterone does not have an effect on susceptibility to genital HSV-2 infection.
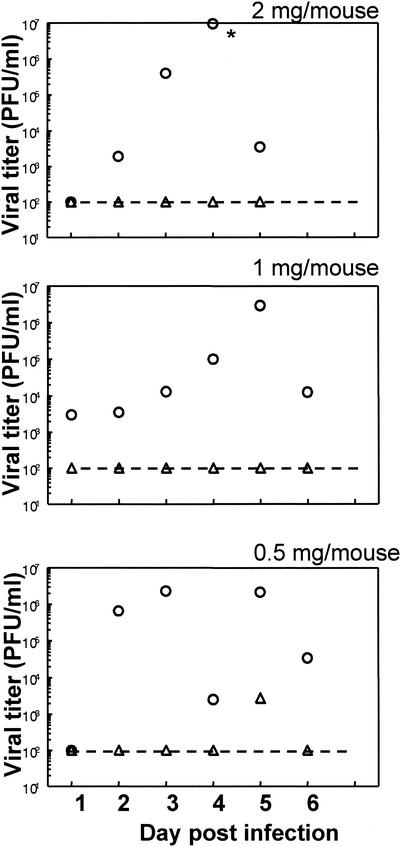
Since most studies use Depo, a long-acting progestational preparation, to render mice susceptible to genital HSV-2 infection, we investigated whether P-sal would also make the mice susceptible in similar or smaller doses. Three groups of mice were administered 2, 1, or 0.5 mg of progesterone suspension subcutaneously. Reproductive cyclicity was monitored in these animals by vaginal smears. Four to 6 days later, when the mice started cycling irregularly, mice were sorted into either diestrous phase or estrous phase and were inoculated with 105 PFU of HSV-2 strain 333. Vaginal pathology was followed for 6 days, and vaginal washes were collected daily to monitor viral titers. External pathology scores showed that mice inoculated in estrus in all three dose groups of progesterone treatment showed no or minimal signs of vaginal pathology. On the other hand, mice inoculated in diestrus phase in all dose groups started showing vaginal pathology within 48 h postinfection, and by day 6 all of them scored 4 or 5 on the scale and had to be sacrificed. Viral titers showed good correlation with the pathology (Fig. 2). No viral shedding could be detected in the vaginal washes of mice inoculated in estrus stage in all three groups. In contrast, all mice inoculated at diestrus had viral titers in the vaginal washes by day 2 until they were sacrificed on day 6. These results indicate that, following progesterone treatment, mice that were in diestrous stage were susceptible to genital HSV-2 infection. Despite progesterone treatment, mice that started cycling and were in estrous phase were not susceptible at this dose of viral challenge. Also, in all the doses used in these studies progesterone made mice in diestrous stage susceptible to genital HSV-2 infection, and an increase in the dose of hormone did not change susceptibility.
FIG. 2.
Viral titers from mice following progesterone treatment. Three groups of mice (n = 6) were given subcutaneous progesterone doses of 2, 1, or 0.5 mg/mouse. Vaginal smears were taken daily as described in Materials and Methods. Mice were inoculated intravaginally with 105 PFU of HSV-2 (strain 333) at estrus (▵) or diestrus (○). Vaginal washes were collected daily, and viral plaque assays were done as described in Materials and Methods. Plaques were counted and viral titers were expressed as PFU/milliliter. Geometric mean values are shown for each group. An asterisk indicates a value of >108 PFU, the upper detection limit of the assay. The dashed lines show the lower detection limit of the assay. Results are representative of three independent experiments with similar results.
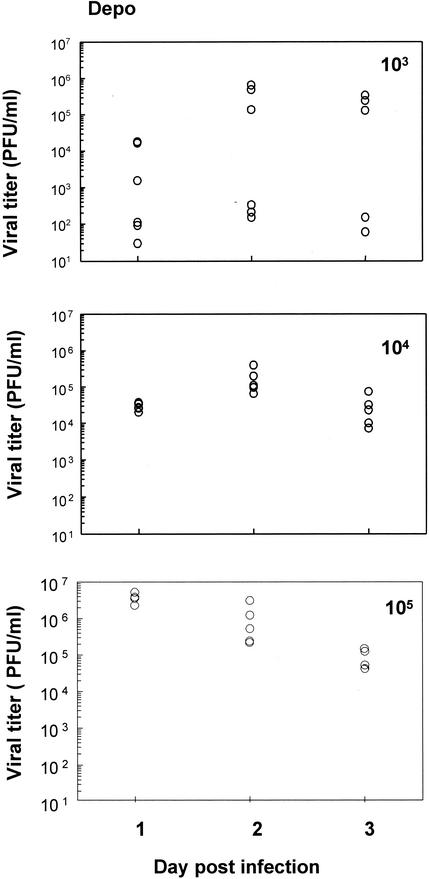
Effect of different doses of virus on susceptibility following Depo treatment.
In order to investigate how Depo treatment affects susceptibility to genital HSV-2 infection, mice were inoculated intravaginally with different doses of HSV-2 strain 333 following Depo treatment. Figure 3 shows the viral titers in vaginal washes collected for 3 days postinfection from mice inoculated with different infectious doses, from 103 to 105 PFU. Only 50% of mice inoculated with 103 PFU showed significant shedding of virus which correlated with pathology and survival. One-hundred percent of the mice were heavily infected at doses of 104 and 105 PFU, as seen with viral titers on all three days postinfection. All of these mice succumbed to the infection.
FIG. 3.
Viral titers of mice following Depo treatment. Three groups of mice (n = 5 to 6 mice in each group) were given Depo (2 mg/mouse) subcutaneously and were intravaginally inoculated 5 days later with different HSV-2 doses (103 to 105 PFU). Vaginal washes were collected daily, and viral plaque assays were done as described in Materials and Methods. Plaques were counted and viral titers were expressed as PFU/milliliter. Each symbol represents a single animal. Experiments were repeated twice with comparable results.
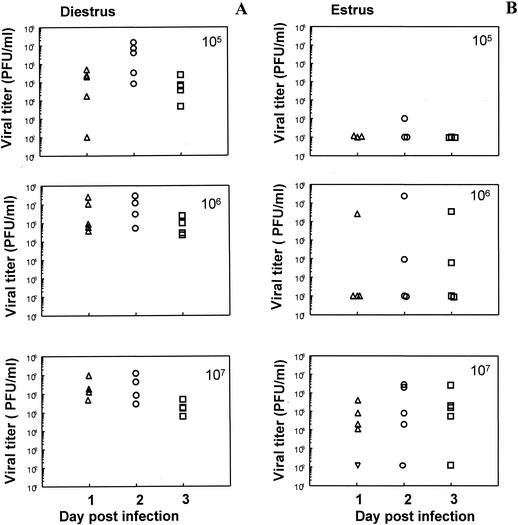
Effect of different doses of virus on susceptibility following progesterone priming.
Mice were given P-sal (1 mg/mouse) subcutaneously. Five days later P-sal-treated mice were sorted out according to their vaginal smears as either diestrus or estrus and were inoculated intravaginally with HSV-2 strain 333. Three different inoculation doses (105, 106, or 107 PFU) were used in each group, and viral titers were measured in vaginal washes for 3 days postinfection. The results are shown in Fig. 4. Mice treated with progesterone and then inoculated at diestrus had viral shedding on all three days at all three infectious doses. At the lowest infectious dose (105 PFU) the viral shedding increased by a log from day 1 to day 2 (Fig. 4A). All of these mice eventually succumbed to the infection. Viral shedding kinetics of mice treated with progesterone and inoculated at estrous stage showed that infection of mice is dose dependent. None of the mice had any significant viral shedding in vaginal washes when inoculated with 105 PFU. Only two out of five mice had any significant viral titers at a dose of 106 PFU. However, at an inoculum dose of 107 PFU, four out of five mice had significant viral shedding on all three days (Fig. 4B).
FIG. 4.
Viral titers of mice infected with different doses of HSV-2 (strain 333) following P-sal treatment. (A) Mice treated with P-sal (1 mg/mouse) and inoculated intravaginally at diestrus with different infectious doses. (B) Mice treated with P-sal and inoculated intravaginally at estrus with different infectious doses. Vaginal washes were collected daily, and viral plaque assays were done as described in Materials and Methods. Plaques were counted, and viral titers were expressed as PFU/milliliter. Each symbol represents a single animal (n = 5 to 6 mice in each group). Results shown are representative of two separate experiments.
Effect of different doses of virus on susceptibility in normal mice at different stages of the reproductive cycle.
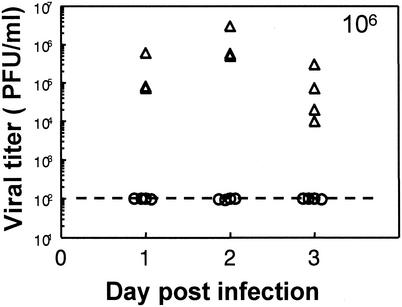
To answer the question of whether susceptibility to genital infection had been altered by progesterone or Depo treatment, susceptibility to HSV-2 in mice at different stages of the estrous cycle was examined. Normal mice at the estrous or diestrous stage of the cycle were inoculated intravaginally with HSV-2 (strain 333), and viral titers of vaginal washes were determined for 3 days postinfection. Mice were inoculated at estrous stage with 105, 106, or 107 PFU. HSV-2 (strain 333) had no shedding of virus in their vaginal smears. No signs of external pathology were observed even after 21 days. Diestrus mice inoculated with 104 and 105 PFU had no viral shedding in the vaginal washes (data not shown). However, at the dose of 106 PFU significant viral shedding was observed in the vaginal washes, accompanied by external pathology. The results are shown in Fig. 5. These results show that without any progesterone treatment, mice are not susceptible to genital infection with HSV-2 at estrus and require much higher infectious doses at diestrus than do mice treated with Depo or progesterone.
FIG. 5.
Viral titers of normal, untreated mice following infection with HSV-2. Vaginal smears were taken daily as described in Materials and Methods. Mice were inoculated intravaginally with 106 PFU of HSV-2 (strain 333) at estrus (○) or diestrus (▵). Vaginal washes were collected daily, and viral plaque assays were done as described in Materials and Methods. Plaques were counted, and viral titers were expressed as PFU/milliliter. Each symbol represents a single animal (n = 5 to 6 mice in each group). The dashed line shows the lower detection limit of the assay. Results are representative of two separate experiments.
Effect of hormone treatment on immunization with TK-HSV-2 and subsequent protection against wild-type virus challenge.
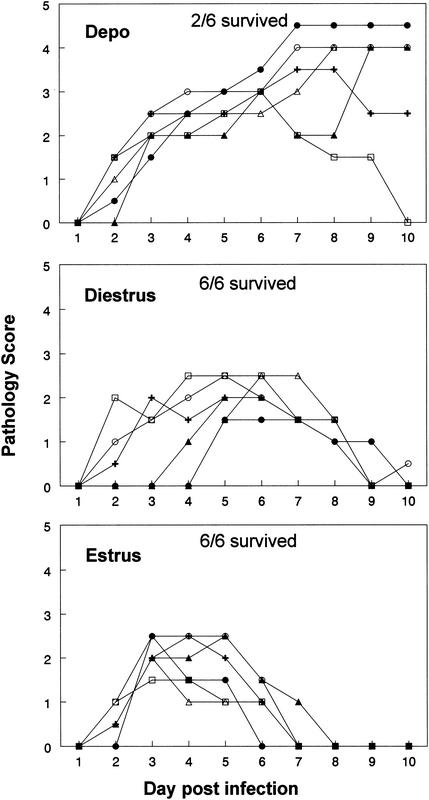
Data from the above experiments clearly indicated that Depo and P-sal treatment increased susceptibility to genital HSV-2. In previous studies we have seen that hormone treatment also affects immune responses (19). Therefore, the effects of hormonal treatment on efficacy of vaccination against HSV-2 were examined. P-sal-treated mice were sorted into diestrus or estrus 5 days later and were immunized intravaginally with TK-HSV-2. Depo-treated mice were immunized with the same dose of HSV-2 15 days after single treatment with Depo. Three weeks postimmunization all three groups were challenged intravaginally with HSV-2 strain 333 at diestrous stage, which is when they are susceptible. Four out of six Depo-treated mice failed to show protection against the challenge (Fig. 6). Severe pathology and lesions were observed in these animals 3 to 4 days after challenge, and within 10 days four out of six animals had to be sacrificed. Mice in the two groups that had been immunized at diestrus or estrus and challenged at diestrus showed complete protection. Both groups showed some pathology for a few days and then made a complete recovery. While the mice immunized at diestrus took 9 to 10 days to return to normal, the recovery time was relatively faster for mice immunized at estrus (6 to 8 days) (Fig. 6).
FIG. 6.
Pathology of mice immunized with TK-HSV-2 and challenged with wild-type HSV-2. One group of mice was given Depo (2 mg/mouse) subcutaneously and was immunized intravaginally with 105 PFU of TK-HSV-2 15 days later. A second group of mice was given progesterone (1 mg/mouse) subcutaneously. Vaginal smears were examined, and mice were sorted according to the stage of the cycle (diestrus or estrus) and were immunized intravaginally with 105 PFU of TK-HSV-2. All mice in all three groups (n = 5 to 6 mice per group) were challenged intravaginally 15 days postimmunization with 106 PFU of HSV-2 (strain 333) at diestrus. Pathology and survival were scored for all animals, as explained in Materials and Methods. The experiment was repeated two times with comparable results.
Effect of Depo on local antibody levels in the genital tract postimmunization.
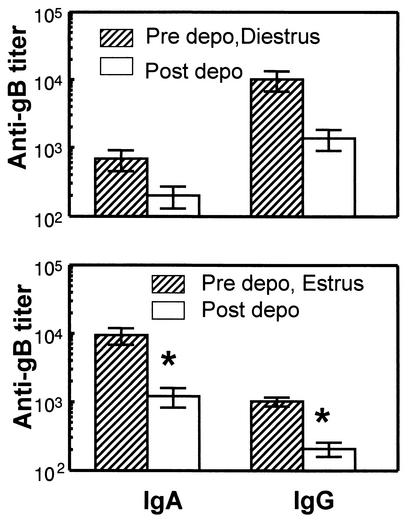
The experiments described here showed that susceptibility increased and protection decreased significantly following Depo treatment. Previous studies have shown that local antibody levels play an important role in protection against genital HSV-2 infection (2). Therefore, the effects of Depo on local antibody levels in the genital tract were examined. In order to do this, a previously described model of intranasal immunization was used (1). Mice were immunized intranasally with recombinant gB of HSV-2 in combination with CpG ODN as adjuvant. Two weeks later mice were boosted by using the same regime. Three weeks after the booster, mice were treated with Depo, and vaginal washes were collected before and four days after hormone treatment. Antibody measurement in vaginal washes showed that both IgG and IgA levels decreased in all mice after Depo treatment (Fig. 7). The most significant effects in both IgA and IgG were observed in mice that were in estrus prior to Depo treatment, where the decrease was eightfold and fivefold, respectively. Mice that were in diestrus also had similar decreases in antibody levels post-Depo treatment, although the decrease did not reach significance.
FIG. 7.
Mice were immunized intranasally with gB and CpG oligonucleotides, boosted 2 weeks later, and treated with Depo 3 weeks later. gB-specific IgG and IgA titers were measured prior to and 4 days after Depo treatment. Results were sorted out on the basis of the stage of the animal prior to Depo treatment. n = 6 to 10 mice per group. An asterisk indicates a P value of <0.05 compared to data for mice prior to Depo inoculation. Data shown are representative of two separate experiments.
DISCUSSION
The results from this study show that not only did Depo induce a prolonged diestrus stage in mice, allowing them to be easily infected with HSV-2, but there was also a change in the susceptibility of the treated mice. Susceptibility of Depo-treated mice increased by 100-fold compared to that of normal untreated mice in diestrus, and susceptibility of Depo-treated mice increased by 10-fold compared to that of mice treated with P-sal at a similar dose. Treatment with P-sal, which does not have the slow-release effect of Depo, also induced diestrus for 4 to 6 days in the treated animals. Compared to that of normal, untreated mice in diestrus, the P-sal-treated mice had 10-fold-increased susceptibility. The results also show that though some of the mice started cycling 4 to 6 days following progesterone treatment and showed estrus stage by vaginal cytology, these mice had increased susceptibility as well and were susceptible to genital HSV-2 infection at high inoculum doses (106 and 107 PFU). Normal, untreated mice in estrus were not susceptible to HSV-2 infection, even at very high infectious doses (107 PFU).
That hormone treatment enhances genital tract infection by agents such as HSV-2 has been known for a while (15). Most studies, in fact, use Depo to induce the diestrous state in mice to get consistent infection. However, the possibility of changed susceptibility due to the hormone treatment has not been considered by most investigators. To the best of our knowledge, this is the first time that susceptibility changes following different progesterone treatments have been documented and compared. The results clearly show that, depending on the hormone treatment, susceptibility of mice to genital herpes infection can vary significantly. Compared to normal, untreated mice, treatment with progesterone increases susceptibility to HSV-2 significantly both at diestrus and estrus. Depo-treated mice are the most susceptible and remain so for prolonged periods of time. Studies with Depo-treated mice have to take into account the effect on their results of altered susceptibility due to hormone treatment.
The mechanism by which progesterone increases susceptibility in mice is not clear. Animals in estrus, when the epithelial lining in the vagina is several layers thick, normally have been shown to be resistant to genital HSV-2 infection (2, 17). In this study, untreated animals in estrus also were resistant to inoculation dose as high as 107 PFU. Under the influence of progesterone the epithelial lining in the vagina is thinned out, making it possible for microbial organisms to cross the protective barrier and establish infection. The high titers of viral shedding observed in Depo- and progesterone-treated mice support this possibility. What is unclear is why the mice administered the long-lasting formulation of progesterone, Depo, were more susceptible than those administered similar doses of P-sal at the same time point (days 4 to 6). On the other hand, it is interesting that the thickness of the epithelial lining may not be the only factor in modulating susceptibility. This is indicated by results where progesterone-treated animals in estrus were also susceptible at high infectious doses of HSV-2. It is possible that other factors, such as receptors for HSV-2 which would play a critical role in regulating susceptibility, may be differentially expressed under various hormone treatments. This may contribute to changes in susceptibility. Previous studies have also shown that local IgA levels in the genital tract are affected profoundly by progesterone (22). IgA plays a critical role in defending the genital tract against viral infections (10). Downregulation of IgA levels by Depo could also enhance infection by HSV-2. We are presently examining these possibilities.
The results from TK-HSV-2 immunization experiments underline the importance of taking into consideration the hormonal state in designing vaccines against STDs. Vaccination was successful or unsuccessful in our studies depending on the hormonal state at the time of initial exposure to the attenuated virus vaccine. Mice immunized 15 days post-Depo treatment showed the least protection following subsequent challenge with wild-type HSV-2. Mice immunized at diestrus or estrus following progesterone treatment showed complete protection against subsequent challenges. These results indicate that adequate immune responses were induced when mice were immunized at estrus or diestrus. The length of exposure to Depo treatment appears to be quite critical in determining whether the mice are protected from subsequent challenges with wild-type virus. In other studies we have seen that mice that were immunized within 4 to 5 days following Depo treatment did not show lack of subsequent protection compared to mice that were immunized 15 days after Depo treatment (A. Gillgrass, A. A. Ashkar, K. L. Rosenthal, and C. Kaushic, submitted for publication). This explains why other studies, where mice were immunized 3 to 5 days following Depo treatment, found that TK-HSV-2 immunization protects mice from subsequent challenges (13-14).
Our data from the present study indicate that alterations in immune responses under different hormonal conditions may affect the ability to raise an adequate immune response that protects the genital tract from viral infections. In the intranasal gB immunization experiments, it was clear that local antibody levels to gB in the genital tract were significantly lowered following Depo treatment. Local antibody responses following mucosal immunization may be critical to subsequent protection against HSV-2 exposure in the genital tract (1). In related studies we have observed that long-term exposure to Depo leads to a decreased antibody response in the genital tract following immunization with TK-HSV-2, and this correlates with a lack of protection against subsequent challenges (A. Gillgrass et al., submitted).
We and others have also demonstrated that immune responses in the genital tract are regulated by sex hormones (20). Studies show that following progesterone treatment and at diestrus (when progesterone is the predominant hormone), immune responses, such as IgA and IgG levels in the uterine fluid, IgA transport, and immune cell trafficking in the uterus, are all suppressed (20). Similar results have recently been reported for the reproductive tract of women (18). Antigen-independent CD3+ T-lymphocyte cytolytic activity was found to be high in the proliferative phase under the influence of estradiol and was absent in the secretory phase of menstrual cycle when progesterone levels are high.
The results from this study also raise questions regarding the change in susceptibility of women to STDs. Studies have already indicated that intake of oral contraceptives influences susceptibility to candidiasis, HSV-2, human immunodeficiency virus type 1, and chlamydial infections in women (11). A recent study also shows that estrogen may protect against vaginal transmission of simian immunodeficiency virus in a rhesus macaque model, while earlier studies have shown that subcutaneous progesterone implants made monkeys more susceptible to simian immunodeficiency virus vaginal transmission (12, 16). Depo treatment is a popular form of contraception. The results from these studies and clinical data on oral contraceptives make it very likely that there are changes in susceptibility to STDs in women who use Depo as a method of birth control (11). This is of concern, since many women on hormonal birth control methods, such as Depo, do not use barrier methods, which would provide some degree of protection against transmission of STDs. Further studies are needed to completely understand the implication of the present study for women, since our experiments were done with inbred mice and the conclusions may not be directly applicable in humans.
In summary, the results of this study document for the first time that pretreatment with different formulations of progesterone not only induces diestrous state in mice but also significantly increases their susceptibility to genital HSV-2 infection. In addition, the study also indicates that Depo may suppress immune responses following immunization. These studies emphasize the need to take into consideration hormonal influences in designing vaccination strategies to preventing STDs.
Acknowledgments
This work was supported by grants from CIHR Canada (K.L.R. and C.K.) and the Bickell Foundation (C.K.).
We thank Jen Newton, Amy Patrick, and Amy Gillgrass for technical help. We also thank Denis Snider and Dario DiLuca for critical reading of the manuscript.
REFERENCES
- 1.Gallichan, W. S., T. Gurasachi, and K. L. Rosenthal. 2001. Intranasal immunization with CpG oligonucleotides as an adjuvant dramatically increases IgA and protection against HSV-2 in the genital tract. J. Immunol. 166:3451-3457. [DOI] [PubMed] [Google Scholar]
- 2.Gallichan, W. S., and K. L. Rosenthal. 1996. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology 224:487-497. [DOI] [PubMed] [Google Scholar]
- 3.Gallichan, W. S., and K. L. Rosenthal. 1996. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J. Exp. Med. 184:1879-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallichan, W. S., and K. L. Rosenthal. 1998. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J. Infect. Dis. 177:1155-1161. [DOI] [PubMed] [Google Scholar]
- 5.Kaushic, C., E. Frauendorf, R. M. Rossoll, J. M. Richardson, and C. R. Wira. 1998. Influence of estrous cycle on the presence and distribution of immune cells in the rat reproductive tract. Am. J. Reprod. Immunol. 39:209-216. [DOI] [PubMed] [Google Scholar]
- 6.Kaushic, C., E. Frauendorf, and C. R. Wira. 1997. Polymeric immunoglobulin A receptor in the rodent reproductive tract: influence of estradiol in the vagina and differential expression of messenger ribonucleic acid expression in rodent uteri. Biol. Reprod. 57:958-966. [DOI] [PubMed] [Google Scholar]
- 7.Kaushic, C., J. M. Richardson, and C. R. Wira. 1995. Regulation of polymeric immunoglobulin A receptor messenger ribonucleic acid expression in rodent uteri: effect of sex hormones. Endocrinology 136:2836-2844. [DOI] [PubMed] [Google Scholar]
- 8.Kaushic, C., F. Zhou, A. D. Murdin, and C. R. Wira. 2000. Effect of estradiol and progesterone on susceptibility and immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infec. Immun. 68:4207-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuklin, N. A., M. Daheshia, S. Chun, and B. T. Rouse. 1998. Role of mucosal immunity in herpes simplex virus infection. J. Immunol. 160:5998-6003. [PubMed] [Google Scholar]
- 10.Lamm, M. E., J. G. Nedrud, C. S. Kaetzel, and M. B. Mazanec. 1995. IgA and mucosal defense. APMIS 103:241-246. [DOI] [PubMed] [Google Scholar]
- 11.Martin, H. L., Jr., P. M. Nyange, B. A. Richardson, L. Lavreys, K. Mandaliya, D. J. Jackson, J. O. Ndinya-Achola, and J. Kreiss. 1998. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J. Infect. Dis. 178:1053-1059. [DOI] [PubMed] [Google Scholar]
- 12.Marx, P. A., A. I. Spira, A. Gettie, et al. 1996. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 2:1084-1089. [DOI] [PubMed] [Google Scholar]
- 13.McDermott, M. R., B. J. Smiley, L. J. Brias, et al. 1984. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated stain of herpes simplex virus. J. Virol. 51:247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milligan, G. N., and D. I. Bernstein. 1995. Analysis of herpes specific T-cells in the murine female genital tract following genital infection with herpes virus type 2. Virology 212:481-489. [DOI] [PubMed] [Google Scholar]
- 15.Parr, M. B., L. Kepple, M. R. McDermott, M. D. Drew, J. J. Bozzola, and E. L. Parr. 1994. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab. Investig. 70:369-380. [PubMed] [Google Scholar]
- 16.Smith, S. M., G. B. Baskin, and P. A. Marx. 2000. Estrogen protects against vaginal transmission of simian immunodeficiency virus. J. Infect. Dis. 182:708-715. [DOI] [PubMed] [Google Scholar]
- 17.Teepe, A. G., L. B. Allen, R. J. Wordinger, and E. F. Harris. 1990. Effect of the estrous cycle on susceptibility of female mice to intravaginal inoculation of herpes simplex virus type 2 (HSV-2). Antivir. Res. 14:227-235. [DOI] [PubMed] [Google Scholar]
- 18.White, H. D., K. M. Crassi, A. L. Givan, J. E. Stern, J. L. Gonzales, V. A. Memoli, W. R. Green, and C. R. Wira. 1997. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage and menstrual cycle and menopause. J. Immunol. 158:3017-3027. [PubMed] [Google Scholar]
- 19.Wira, C. R., and C. Kaushic. 1996. Mucosal immunity in the female reproductive tract: effect of sex hormones on immune recognition and responses, p. 375-386. In H. Kiyono, P. L. Ogra, and J. R. McGhee (ed.), Mucosal vaccines: new trends in immunization. Academic Press, New York, N.Y.
- 20.Wira, C. R., C. Kaushic, and J. Richardson. 1999. Role of sex hormones and cytokines in regulating mucosal immune system in the female reproductive tract, p. 1449-1461. In by P. L. Ogra, M. E. Lamm, J. Bienenstock, J. Mestecky, W. Strober, and J. R. McGhee (ed.), Mucosal immunology. Academic Press, New York, N.Y.
- 21.Wira, C. R., and R. M. Rossoll. 1995. Antigen presenting cells in the female reproductive tract: influence of estrous cycle on antigen presentation by uterine epithelial and stromal cells. Endocrinology 136:4526-4534. [DOI] [PubMed] [Google Scholar]
- 22.Wira, C. R., and C. P. Sandoe. 1977. Sex steroid hormone regulation of immunoglobulin G (IgG) and A (IgA) in rat uterine secretions. Nature 268:534-536. [DOI] [PubMed] [Google Scholar]
- 23.Yeaman, G. R., P. M. Guyre, M. W. Fanger, J. E. Collins, et al. 1997. Unique CD8+ T cell rich lymphoid aggregate in human uterine endometrium. J. Leukoc. Biol. 61:427-435. [PubMed] [Google Scholar]