Abstract
Adenovirus dodecahedron is a virus-like particle composed of only two viral proteins of human adenovirus serotype 3 that are responsible for virus attachment and internalization. We show here that this dodecameric particle, devoid of genetic information, efficiently penetrates human cells and can deliver large multimeric proteins such as immunoglobulins.
Human adenoviruses (Ads) are nonenveloped viruses responsible for respiratory, ocular, and enteric infections. Their icosahedral capsid, containing the 36-kpb double-stranded DNA genome, is composed of three major proteins: the hexon, the penton (Pt) base, and the fiber. At the 12 vertices of the capsid, the protruding fiber is noncovalently attached to the Pt base, thus forming the Pt complex. It has been reported that the fiber interacts with a high affinity with a primary receptor and that the subsequent interaction of the Pt base RGD motif with cellular integrins triggers endocytosis (17). A 42-kDa protein, called the coxsackievirus and Ad receptor (CAR), is recognized by at least one serotype of each of the six subgroups of Ad except for the serotypes belonging to subgroup B (i.e., Ad3) (1, 12). Even though that Ad capsid is composed of at least 11 proteins, it has been shown that Pt alone can penetrate into human cell lines, thus making of this complex a potential vector for DNA delivery (2, 5, 7, 8, 16). Remarkably, Ad3 but not Ad5 Pt expressed in the baculovirus system led to the formation of symmetric complexes of 12 Pts called dodecahedron (Dd). We have previously shown that the non-CAR-binding Ad3-Dd can be used as a gene transfer vector (2), and we show here that this virus-like particle is also able to transduce large multimeric proteins into human cells.
Dd internalization.
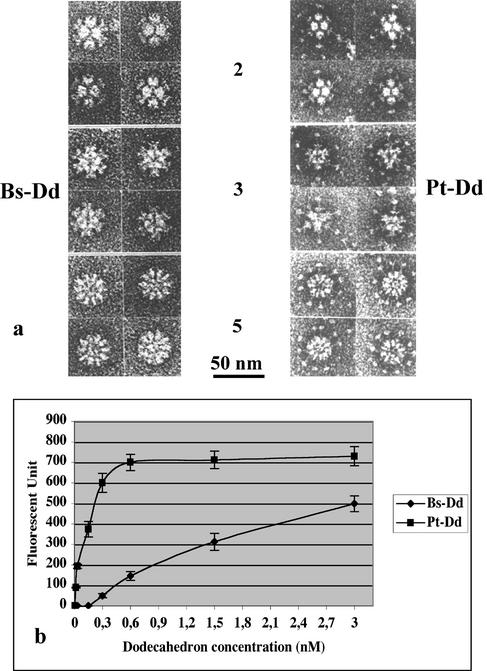
Coexpression of Ad3 Pt base and fiber proteins in the baculovirus system led to the formation of a symmetric dodecameric particle (2, 14), Pt-Dd (Fig. 1a). This complex results from the interaction between the pentameric base proteins, as attested by the Dd formation upon expression of base proteins alone (Bs-Dd; Fig. 1a). The respective roles of the fiber and the Pt base protein in Ad3 Dd entry into HeLa cells was assessed by immunofluorescence. HeLa cells grown in a 96-multiwell plate (Falcon) at 2 × 104 cells per well were incubated in 100 μl of phosphate-buffered saline (PBS) with a range of Dd concentrations. After 1 h at 37°C, cells were permeabilized, and the amount of Dd present in the cells was estimated by using a rabbit polyclonal serum recognizing the Ad3 Pt base, followed by incubation with a goat anti-rabbit secondary fluorescein isothiocyanate (FITC)-labeled antibody (Santa Cruz). After PBS washes, fluorescence quantification was performed by using a plate reader fluorimeter (485 and 535 nm; Wallac). The antibody background was subtracted, and the mean of three measurements is shown in Fig. 1b. The Bs-Dd was capable of entering human cells, suggesting that a direct interaction between the RGD motifs of the Pt base and the cellular integrins or between the Pt base and another cellular component (5) is sufficient for cell entry. However, the presence of fiber in the Pt-Dd dramatically increased the level of Dd entry, suggesting a more efficient two-step endocytosis mediated by this protein.
FIG. 1.
Dd structure and function. (a) Negative-stain micrographs of purified Bs-Dd and Pt-Dd. Views along the two-, three-, and five-fold axes of Dds are shown. (b) Fluorescence quantification of Dd entry into HeLa cells after 1 h of incubation at 37°C. Incoming Bs-Dd or Pt-Dd was quantified by indirect immunofluorescence (at 485 and 535 nm) in permeabilized cells with an antibody directed against the Ad3 Pt base protein and an FITC-conjugated secondary antibody.
MAb production and characterization.
Monoclonal antibodies (MAbs) have been produced against ion-exchange chromatography-purified Bs-Dd according to the classical protocol (6). Two retained hybridomas were analyzed by Western blot and enzyme-linked immunosorbent assay (ELISA). The 5C12 hybridoma recognized both Bs-Dd and Pt-Dd only in their native forms (as determined by ELISA), indicating that the recognized epitopes are exposed on the Dd surface and that the presence of the fiber does not interfere with MAbs binding to the base protein. In contrast, the 5F5 clone, which was strongly reactive toward the sodium dodecyl sulfate-denatured base protein (detection of 0.1 ng of denaturated base in a Western blot), recognized only weakly the native Dd by ELISA.
Electron microscopy study of Bs-Dd-5C12 complex.
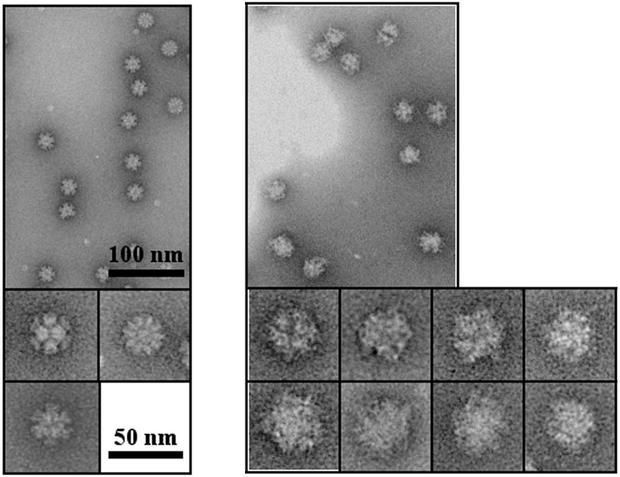
MAb-Dd complexes were obtained by incubation of purified Dds with purified MAbs. After sucrose gradient (15 to 40%) density centrifugation (4 h at 200,000 × g in a TLS55 rotor [Beckman]), 5C12 MAb was found at the bottom of the tube, together with Dd, whereas the 5F5 MAb that is unable to strongly bind native Dd remained in the upper part of the gradient (data not shown). An aliquot of purified Bs-Dd-5C12 complex was applied to the carbon on mica and negatively stained with 1% silicotungstate (pH 7.0) for electron microscopy (Fig. 2). Micrographs were obtained under low-dose conditions by using a JEOL 1200 EX II microscope at 100 kV and a nominal magnification of ×40,000. The fuzzy outline and the increased size of Bs-Dd-5C12 versus Bs-Dd alone suggest that the particle is decorated by immunoglobulin G.
FIG. 2.
Negative-stain electron microscopy of Bs-Dd (left panel) and Bs-Dd-5C12 complex (right panel). Proteins were purified by using a 15 to 40% sucrose density gradient, and a portion of the fractions corresponding to Bs-Dd or Bs-Dd-5C12 complexes was analyzed.
MAb entry into HeLa cells with Dd as a vector.
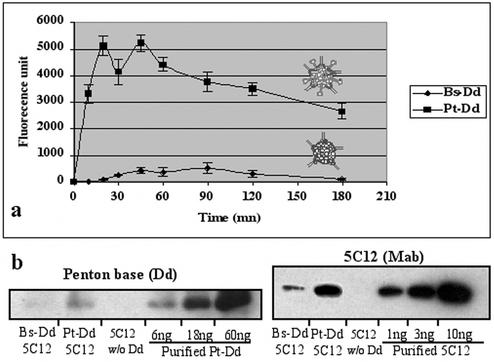
In order to find out whether MAbs can be delivered to the cell by Dd, Dd-MAb complexes (0.6 nM with respect to Dd) were incubated for different times with living HeLa cells in a 96-multiwell plate (Falcon) at 2 × 104 cells per well. After methanol permeabilization, the level of MAb entry was determined by using a fluorescent secondary antibody directed against the mouse monoclonal antibody. After PBS washes, fluorescence quantification was performed by using a plate reader fluorimeter (485 and 535 nm; Wallac). The antibody background was subtracted, and the mean of three measurements is shown in Fig. 3a. Bs-Dd permitted a limited transduction of 5C12 MAbs with a maximum level obtained at 90 min, whereas Pt-Dd permitted a very efficient transduction of 5C12 MAbs, with a maximum reached after 20 min only. These results show that the fiber in Pt-Dd improved considerably the protein transduction efficiency and also reduced the time needed by the Pt-Dd-MAb complex to bind and enter the cells.
FIG. 3.
5C12 entry into HeLa cells. (a) Kinetic experiment. Bs-Dd-5C12 or Pt-Dd-5C12 complexes (0.6 nM) were incubated with HeLa cells for different times at 37°C. Transduced 5C12 antibody was quantified in permeabilized cells by using a secondary anti-mouse FITC-labeled antibody. Background signal raised by 5C12 antibody alone was subtracted. (b) Dd and MAbs quantification. Lysis extract of HeLa cells incubated for 1 h at 37°C with a 0.6 nM concentration of Pt-Dd-5C12, Bs-Dd-5C12, or 5C12 without Dd was analyzed by Western blot with either anti-Ad3 base serum and anti-rabbit HRP-labeled antibody (recognition of internalized Dd) or anti-mouse HRP-labeled antibody (recognition of transduced 5C12 MAb). Estimation of the amount of protein was performed by comparison to a range of purified Dd or 5C12.
The approximate amount of MAbs and Pt-Dd present inside the HeLa cell was determined by Western blot analysis. HeLa cells grown in a 96-multiwell plate (Falcon) at 2 × 104 cells per well were transduced for 1 h at 37°C with either 0.6 nM concentrations of Bs-Dd-5C12 or Pt-Dd-5C12 complexes or with 5C12 alone. After washes with PBS, cells were lysed in 100 μl of cell lysis buffer (Promega), and 10-μl aliquots were boiled and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, together with known amounts of either purified Pt-Dd or 5C12 MAb. After transfer to a polyvinylidene difluoride membrane, the blot was treated with either anti-mouse horseradish peroxidase (HRP) antibody (visualization of transduced 5C12) or anti-Pt base antibody and anti-rabbit HRP antibody (visualization of internalized Dd). Estimation of Dd and 5C12 present inside the cell was performed by band scanning (Molecular Analyst; Bio-Rad), and the results were scaled to the number of cells per well (Fig. 3b). About 6 ng of Pt-Dd and 3 ng of 5C12 MAbs were found in 2 × 104 HeLa cells, representing an average of 5 × 104 Pt-Dd and 5.7 × 105 MAbs per cell. This result indicates an average ratio of about 12 MAbs per Dd, suggesting a stoichiometric process of MAb attachment to Dd. This experiment shows an efficient and stable interaction of 5C12 MAb with Dd, as well as very efficient cell transduction of attached MAbs. As expected, the amount of Bs-Dd was much lower than that of Pt-Dd. Since we determined by ELISA that equivalent amounts of 5C12 MAb were bound to Bs-Dd and Pt-Dd (data not shown), the 10-fold-smaller amount of antibody transduced by Bs-Dd is consistent with the lower penetration efficiency of this particle (Fig. 3).
MAb localization upon transduction.
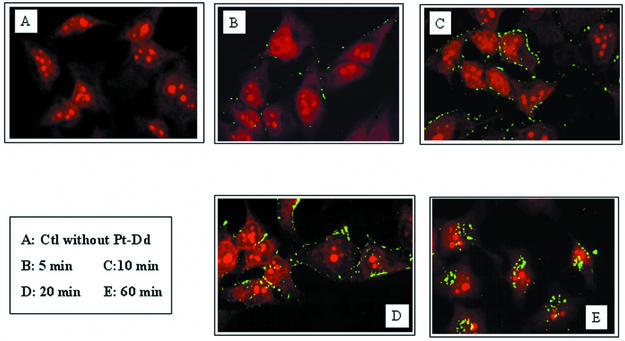
MAb localization in transduced cells was monitored by confocal microscopy. About 105 HeLa cells were incubated for different periods of time with a 0.6 nM concentration of the Pt-Dd-5C12 complex. After a given period of entry, cells were washed and permeabilized and 5C12 MAb was detected by using an FITC-labeled secondary antibody (Fig. 4). Laser scanning confocal microscopy was performed on an MRC600 (Bio-Rad). Incubation times as short as 5 min were sufficient to detect some Pt-Dd-5C12 complexes bound to the plasma membrane and, after 10 min of incubation, a green signal surrounding the cells was observed. Consistent with the kinetic experiment (Fig. 3a), complex internalization began after 20 min (Fig. 4D). After 1 h of incubation, MAbs were detected close to the nucleus, whereas a control experiment performed with 5C12 antibody without Dd gave no signal, thus attesting that MAbs delivery into the cells is mediated by the Pt-Dd (Fig. 4E and A, respectively). Moreover, incubation of Dd with the 5F5 MAb (which was unable to recognize native Dd) on a HeLa cell produced no signal as in Fig. 4A, thus confirming that protein delivery occurs only when the MAb is attached to Dd. A similar experiment of confocal microscopy done with Bs-Dd-5C12 complexes confirmed the weaker and delayed transduction of 5C12 in HeLa cells observed in Fig. 3a (data not shown).
FIG. 4.
MAb localization upon transduction into HeLa cells as determined by confocal microscopy. Pt-Dd-5C12 complexes (0.6 nM) were incubated for different times at 37°C. Cells were washed, and transduced MAbs were detected by using a secondary FITC-labeled mouse antibody (green signal). Cells nuclei were counterstained with propidium iodide (red signal).
Activity of the transduced protein.
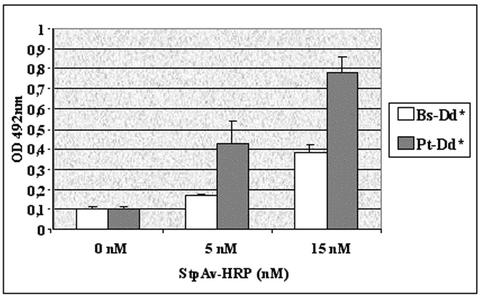
It has been reported that the cell entry of subgroup B Ads (i.e., Ad3 and Ad7) is different from that of the subgroup C Ads (i.e., Ad2 and Ad5). The major difference lies in the ability of subgroup C Ads to escape endosomes, whereas subgroup B Ads accumulate in late endosomes and to a small extent in lysosomes prior to their cytosol escape (9, 10). It was therefore important to assess whether the activity of the transduced protein is retained during the trafficking process. For this, we developed an enzyme delivery system. Purified Pt-Dd and Bs-Dd were biotinylated with 1 mM Sulfo-NHS-LC-LC-biotin (Pierce). After removal of the unbound biotin, a 0.6 nM concentration of biotinylated Bs-Dd or Pt-Dd was incubated for 30 min in PBS with a 0 to 15 nM concentration of streptavidin conjugated to HRP (SptAv-HRP; Jackson Laboratories). HeLa cells grown in a 96-multiwell plate (2 × 104 cells per well) were then incubated with the complexes and, after 1 h at 37°C, the cells were lysed in 100 μl of cell lysis buffer (Promega). The enzyme activity was determined by photometry at 492 nm after the addition of 100 μl of the colorimetric peroxidase substrate o-phenylenediamine (3 mg/ml; Sigma). Nonbiotinylated Dd incubated with 0 to 15 nM of StpAv-HRP was used for background substraction. The experiment was performed in triplicate, and the results are expressed as the mean of the values (Fig. 5). This experiment shows an exogenous peroxidase activity in cells incubated with biotinylated Dd-StpAv-HRP, thus demonstrating that enzyme was delivered by biotinylated Dd and that at least a part of the transduced proteins remained active 1 h after transduction. During biotinylation of Pt-Dd, fiber protein is also biotinylated which could, in part, neutralize its function, and it can possibly explain the two- to threefold difference in transduction efficiency of Pt-Dd and Bs-Dd in this experiment compared to the 10-fold difference observed with MAb delivery (Fig. 3).
FIG. 5.
Biological activity of the transduced protein. Complexes made of either biotinylated Dd (Bs-Dd* or Pt-Dd*), both at 0.6 nM, and streptavidin-HRP (0 to 15 nM) were incubated with HeLa cells for 1 h at 37°C. Cells were lysed, and peroxidase activity was determined at 492 nm by using the colorimetric peroxidase substrate o-phenylenediamine.
MAb delivery into human cells by Dd shows that this particle can act as a protein transduction vector. Protein transduction technology represents an alternative to gene transfer technology when no gene insertion into genome is required (3, 11, 15). This technology may be appropriate for restricted applications; it is also potentially a basis for an entirely new form of therapy and may allow us to overcome some of the technical hurdles of gene therapy. Protein transduction could be useful for the efficient antigen loading of dendritric cells for a range of vaccination purposes, including anti-tumor immune therapy. Interestingly, both Dd and the Ad virion contain 12 Pts and efficiently enter human cells. However, contrary to the complete Ad capsid, the Dds are compact particles devoid of any viral DNA. Consequently, Ad Dd might be a safe alternative vector to use instead of the whole Ad. In order to deliver proteins other than MAbs into the cells by Dd, a strategy aiming to fuse the protein of interest to a single-chain antibody derived from 5C12 can be considered. Moreover, it has been recently observed that the two PPxY motifs present in the Ad Pt base sequence interacted with WW domains of several proteins (4). As reported for polyomavirus-like particle, in which the WW domain has been inserted in order to target PPxY motif (13), we can hypothesize that the fusion of therapeutic protein to WW domains might permit its attachment to the Dd, thus allowing its transduction into the target cell.
Acknowledgments
We thank R. Ruigrok for discussions and advice with the electron microscopy study and R. H. Wade for help with the manuscript.
This work was supported in part by grant EMERGENCE 2001-Région Rhône Alpes.
REFERENCES
- 1.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Protein structure and isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 2.Fender, P., R. W. Ruigrok, E. Gout, S. Buffet, and J. Chroboczek. 1997. Adenovirus dodecahedron, a new vector for human gene transfer. Nat. Biotechnol. 15:52-56. [DOI] [PubMed] [Google Scholar]
- 3.Ford, K. G., B. E. Souberbielle, D. Darling, and F. Farzaneh. 2001. Protein transduction: an alternative to genetic intervention? Gene Ther. 8:1-4. [DOI] [PubMed] [Google Scholar]
- 4.Galinier, R., E. Gout, H. Lortat-Jacob, J. Wood, and Chroboczek, J. 2002. Adenovirus protein involved in virus internalization recruits ubiquitin-protein ligases. Biochemistry 41:14299-14305. [DOI] [PubMed] [Google Scholar]
- 5.Hong, S. S., B. Gay, L. Karayan, M. C. Dabauvalle, and P. Boulanger. 1999. Cellular uptake and nuclear delivery of recombinant adenovirus penton base. Virology 262:163-177. [DOI] [PubMed] [Google Scholar]
- 6.Kohler, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495-497. [DOI] [PubMed] [Google Scholar]
- 7.Medina-Kauwe, L. K., N. Kasahara, and L. Kedes. 2001. 3PO, a novel nonviral gene delivery system using engineered Ad5 penton proteins. Gene Ther. 8:795-803. [DOI] [PubMed] [Google Scholar]
- 8.Medina-Kauwe, L. K., M. Maguire, N. Kasahara, and L. Kedes. 2001. Nonviral gene delivery to human breast cancer cells by targeted Ad5 penton proteins. Gene Ther. 8:1753-1761. [DOI] [PubMed] [Google Scholar]
- 9.Miyazawa, N., P. L. Leopold, N. R. Hackett, B. Ferris, S. Worgall, E. Falck-Pedersen, and R. G. Crystal. 1999. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J. Virol. 73:6056-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyazawa, N., R. G. Crystal, and P. L. Leopold. 2001. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J. Virol. 75:1387-1400. [DOI] [PMC free article] [PubMed]
- 11.Morris, M. C., J. Depollier, J. Mery, F. Heitz, and G. Divita. 2001. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 19:1173-1176. [DOI] [PubMed] [Google Scholar]
- 12.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt, U., R. Rudolph, and G. Böhm. 2001. Binding of external ligands onto an engineered virus capsid. Protein Eng. 14:769-774. [DOI] [PubMed] [Google Scholar]
- 14.Schoehn, G., P. Fender, J. Chroboczek, and E. A. Hewat. 1996. Adenovirus 3 penton dodecahedron exhibits structural changes of the base on fibre binding. EMBO J. 15:6841-6846. [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz, J. J., and S. Zhang. 2000. Peptide-mediated cellular delivery. Curr. Opin. Mol. Ther. 2:162-167. [PubMed] [Google Scholar]
- 16.Smith, C. C., M. Kulka, and L. Aurelian. 2000. Modified adenovirus penton base protein (UTARVE) as a non-replicating vector for delivery of antisense oligonucleotides with antiviral and/or antineoplastic activity. Int. J. Oncol. 17:841-850. [DOI] [PubMed] [Google Scholar]
- 17.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvβ3 and βvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]