Abstract
The progression to alcohol dependence unfolds across multiple stages, including the decision to initiate use, the development of regular patterns of use, and (for some individuals) the subsequent development of problems associated with alcohol use. Using data from two population-based, longitudinal twin studies, FinnTwin16 (FT16) and FinnTwin12 (FT12), we applied multiple stage genetic models (Heath et al., Twin Res. 5 (2002) 113) to better understand the extent to which genetic and environmental influences impact the initiation of alcohol use, frequency of use in adolescence and young adulthood, and alcohol problems in young adulthood. Shared environmental factors played a large role in initiation, and a more moderate role on frequency of use, and it was largely the same influences acting across these stages of use. However, there was no significant evidence of shared environmental influences on alcohol problems in early adulthood. Problems were largely influenced by genetic factors that overlapped with genetic influences on frequency of use. Unique environmental factors were largely specific to each stage, with some overlap between alcohol problems and frequency of use at age 25.
Keywords: alcohol use, initiation, multiple stage genetic models, problems
INTRODUCTION
The World Health Organization (WHO) estimates that alcohol use disorders affect 76.3 million people worldwide (WHO, 2004). Results from the National Comorbidity Study indicate that over 14% of adults in the United States have a lifetime history of alcohol dependence, making it one of the most prevalent adult psychiatric disorders (Kessler et al., 1994). A number of studies have demonstrated that alcohol dependence is under a significant degree of genetic influence, with heritability estimates in the range of 50–70% (Cloninger et al., 1981; Heath et al., 1997; Kaprio, et al., 1987; McGue, 1993, 1999; Prescott et al., 1994b). The frequency and amount of alcohol used by adults is also influenced by genetic factors, accounting for 40–56% of the variance, and these genetic influences remain fairly consistent across adulthood (Carmelli et al., 1990; Kaprio et al., 1987, 1992; Prescott et al., 1994a). Carmelli et al. (1993) estimated that 82–86% of the variance in longitudinal stability of alcohol consumption among a group of WWII veterans was accounted for by genetic factors.
The majority of individuals begin experimenting with alcohol in adolescence. Researchers from the Monitoring the Future study estimated that 80% of American adolescents have used alcohol by the end of 12th grade (Johnston et al., 2001). In data from 1412 Virginia twin pairs, 60% of 16 year-old boys and 48% of girls reported having used alcohol (Maes et al., 1999). Accordingly, the transition from adolescence to young adulthood is a particularly critical period in the development of patterns of substance use, as it is during this period that most individuals initiate alcohol use and transition from initial experimentation to more established, potentially problematic, patterns of use (Schulenberg et al., 1997). Several studies have found that adolescents who begin to use alcohol very early, compared to their peers, are at a greater risk for the later development of alcohol abuse or dependence (Chou and Pickering, 1992; Clapper et al., 1995; Grant and Dawson, 1997; Kandel et al., 1992; Pitkanen et al., 2005; Riala et al.,2004). Results from the National Longitudinal Alcohol Epidemiological Survey (NLAES; Hingson et al., 2000) demonstrated that those who report drinking before the age of 14 vs. those who remained abstinent until age 21 were three times more likely to also report frequent high-density drinking and seven times more likely to drink to intoxication on a weekly basis. These early initiators were also significantly more likely to have placed themselves in a dangerous or injury-prone situation after drinking, suggesting this may be an area to target to decrease adolescent risk behavior.
Twin studies have found that the initiation of alcohol use is largely influenced by environmental risk factors (Heath and Martin, 1988; Heath et al., 1991; Kaprio et al., 1987; Koopmans and Boomsma, 1996; Rose et al., 2001b; Stallings et al., 1999). For example, Rose and colleagues (2001b) found that the shared environment accounted for 76% of the variance in whether or not 14-year-old twin boys and girls had initiated alcohol use, while additive genetic effects were nonexistent for boys and accounted for only 18% of the variance in initiation for girls. In a review of 18 studies examining initiation of substance use, Hopfer et al., (2003) found that alcohol initiation is under a significant degree of shared environmental influence, accounting for 55–80% of the variance in initiation. Once initiation has occurred, genetic factors explain a large amount of the variation in frequency of alcohol use (34–72%) especially as adolescents get older (Heath et al., 1991; Hopfer et al., 2003; Koopsmans and Boomsma, 1996; Maes et al., 1999; Rose et al., 2001b; Viken et al., 1999). Additionally, the amount of time taken to transition from first alcohol use to regular use is largely influenced by genetic factors (Stallings et al., 1999).
Thus, while the substance abuse literature has addressed multiple aspects of use and misuse, most of this literature has accumulated from separate analyses conducted across multiple datasets. In this paper, we apply multiple-stage genetic models to progressive stages of alcohol use and misuse in two longitudinal samples of twins. These multiple-stage models allow one to more accurately assess the importance of genetic and environmental risk factors on patterns of use and misuse by making allowance for partial overlap with risk factors for initiation. In addition, we have further defined initiation, by characterizing individuals as to whether they initiated alcohol use in early or late adolescence. Previous research has typically classified adolescents into two groups, those who have not initiated use and those who have. Heath et al. (2002) demonstrated that, when using a binary initiation variable (e.g., never initiated, initiation), “serious bias may arise for estimates of correlations between genetic (or environmental) effects on Initiation versus Outcome” (p. 121). Accordingly, newer multiple-stage models suggest that initiation should be defined using a multiple-category trait as opposed to a binary construct (Heath et al., 2002). By exploring multiple stages of alcohol use within a multivariate model, we can examine the extent to which risk factors overlap between various stages of alcohol use and misuse. It is possible that some risk factors influence multiple aspects of alcohol use and abuse, while other genetic and environmental factors influence frequency of use and/or the development of problems but have no impact on whether or not an adolescent chooses to try alcohol in the first place (Heath et al., 2002). In order to study such common vs. specific influences on patterns of use, one must account for risk factors impacting the decision to initiate use.
We applied these multiple stage genetic models to data from two population-based, longitudinal twin-family studies, FinnTwin16 (FT16) and Finntwin12 (FT12). The use of parallel assessment procedures and the overlap in ages in these two independent studies of Finnish twins permitted us to examine the replicability of results across samples. We first fit two-stage models to the initiation of alcohol use and frequency of use at age 17 or younger in the FT16 and FT12 datasets. Subsequently, we expanded this model in the FT16 dataset, to incorporate data on drinking frequency and drinking problems in young adulthood. Accordingly, our study allowed us to examine (1) the degree to which genetic and environmental risk factors impact initiation of alcohol use, taking into account both whether and when the adolescent initiated, (2) the degree to which genetic and environmental influences impact drinking frequency in late adolescence/early adulthood, a time by which most adolescents have moved to more experienced and established patterns of use (Rose et al., 2001a, b), (3) the degree to which genetic and environmental influences impact drinking problems, as measured by the Rutgers Alcohol Problem Index (RAPI; White and Labouvie, 1989) at age 25, and (4) the degree to which genetic and environmental factors overlap between these various stages in the progression of substance use.
METHODS
Samples
FinnTwin16 (FT16) and FinnTwin12 (FT12) are two independent, population-based studies, each consisting of five consecutive birth cohorts of Finnish twins. All twins were identified through Finland’s Central Population Registry, permitting exhaustive and unbiased ascertainment. Zygosity was determined in both twin studies using a well-validated questionnaire completed by both co-twins at the baseline, as described elsewhere (Kaprio et al., 1995). Because these twins were younger in FT12, classification was supplemented by parental response to items developed for zygosity classification of twin children (Goldsmith, 1991). FT16 consists of twins born 1975–1979 (Rose et al., 1999). In 1991, the first cohort of twins was mailed baseline questionnaires within 2 months of their 16th birthday. Most responded within a few weeks. This procedure was repeated with the four subsequent cohorts, with the last cohort receiving the baseline questionnaire during 1995. Baseline questionnaires contained items on health habits, including items assessing the initiation and use of alcohol. The staggered administration, rapid completion, and quick return of the baseline questionnaire yielded age-standardized baseline data. The five birth cohorts contained 3065 families of twins in which both twins were living and residing in Finland at the age of 16. With mail and telephone prompts, 5561 of the 6130 twins in these families (90.7%) returned the baseline questionnaire.
All respondent twins were sent follow-up questionnaires when they turned 17 and 18.5 years of age. Retention rates were approximately 90% across the first three waves of data collection for both male and female twins. Starting in the autumn of 2000, a fourth wave of data collection was conducted over a 30-month period when the twins were between the ages of 22 and 27. The last of these questionnaires were returned in 2003. Of those who participated at baseline, we could reach in 2000–2002 a total of 5594 participants (2689 men, 2905 women), and questionnaires were returned by 4929 (2239 men, 2690 women), yielding a response rate of 88.1% (83% for men, 93% for women). The average age for the respondent twins was 24.4 years, but, for ease of presentation, the age of the fourth assessment wave is referred to as 25 throughout this paper. Follow-up questionnaires included the same items assessing the initiation, use, and abuse of alcohol, as well as items assessing problematic behavior related to alcohol use or abuse in the age 18 and 25 follow-ups. For twin analyses, data were available for 1712 same-sex twin pairs of confirmed zygosity: 789 pairs of twin brothers, classified into 342 monozygotic (MZ) and 447 dizygotic (DZ) pairs, and 923 pairs of twin sisters, 496 MZ and 427 DZ female twin pairs.
FT12 consists of 2724 Finnish families with twins born in 1983 through 1987 (Kaprio et al., 2002; Rose, et al., 2001a). The assessment procedure for FT12 was similar to that of FT16 described above, except that baseline assessment was initiated earlier, when the twins were ages 11–12. The first cohort of twins, born in 1983, was sent baseline questionnaires near the end of 1994. Most of these twins returned the questionnaire very early in 1995, the year in which these twins reached the age of 12. This procedure was repeated for subsequent birth cohorts. Mean age at return of the baseline questionnaire was 11.45 years, but, for ease of presentation, baseline age is referred to as age 12. Of the 2548 families with twins that were sent the baseline questionnaire, 2216 (87%) of these families returned the baseline questionnaire and gave consent to participate in this longitudinal study. Follow-up questionnaires were sent out to all twins whose families agreed to participate at baseline within 2 months of the twins’ 14th birthday. Retention rates exceeded 90% at this follow-up. A second follow-up questionnaire, at age 171/2 years, was initiated in autumn of 2000 and is to be completed in the spring of 2005. Results presented here with FT12 data represent 90% of the expected final sample. So far, 3890 questionnaires have been returned out of 4212 mailed, a response rate of 92.4% for those already participating in earlier questionnaires. For twin analyses, data were available for 1297 same-sex twin pairs of confirmed zygosity: 633 pairs of twin brothers, 284 MZ and 349 DZ pairs, and 664 pairs of twin sisters, 347 MZ and 317 DZ female twin pairs.
Alcohol Initiation and Use Measures
In FT16, age of initiation and frequency of use were measured with two items. The first item, assessed at the age of 16 and at all follow-ups, asked how often the twins “drink alcohol at all” and was followed by nine response options: (1) daily; (2) couple of times a week; (3) once a week; (4) a couple times a month; (5) about once a month; (6) about once every 2 months; (7) 3–4 times a year; (8) once a year or less; or (9) I don’t drink at all. The second item, asked only at age 16, asked the age at which the individual first drank a glass of beer, wine, long drink (a drink of beer alcohol content with a soft-drink flavor), and/or hard liquor. In FT12, twins were first asked about substance use at the age 14 follow-up assessment where they were asked how often they “drink alcohol at all” followed by four response options: (1) once a week or more; (2) about 1–2 times a month; (3) less often than once a month; and (4) never; I don’t drink at all. This drinking item was collapsed to four levels from the nine-item measure used in FT16 because of the expected lower frequency of alcohol use at age 14. At age 17 frequency of use was assessed using the same nine level question as in FT16.
Using these items, we created a three-level age of initiation variable for use in genetic models that was parallel across the two datasets. As Heath and his colleagues (2002) suggested, we began by assuming a bivariate probit model for factors that affect alcohol initiation where initiation was defined as having three categories: never initiated, late adolescent initiation, and early adolescent initiation. Those twins who self-reported never drinking alcohol at age 17 or younger were given a zero on the initiation variable, those who reported initiating alcohol use at ages 15, 16, or 17, were coded as 1 and considered “late adolescent initiators”, and those who initiated at age 14 or younger were coded as 2 and considered “early adolescent initiators.” The outcome measure for these bivariate models was the nine-level frequency of use question asked when the twins were 17 in both FT16 and FT12. Those twins who reported never initiating alcohol use cannot be characterized on the frequency of use dimension and were given a missing value for this outcome measure. Only a very small number of twins reported daily drinking (0.17% in FT16 and 0.13% in FT12), so this level was collapsed with “drinking a couple of times per week”, such that the highest category represents drinking more than once per week. Thus, the resulting frequency of use variable ranged from zero (once a year or less) to six (a few times per week or daily), which preserved much of the variance in use and maximized statistical power.
In addition to the alcohol use items described above, the age 25 questionnaire in FT16 contained 22 items from the Rutgers Alcohol Problem Index (RAPI; White and Labouvie, 1989), a scale with good reliability that was designed to assess problematic drinking. The RAPI contains items assessing dependence, withdrawal, blackouts, neglect of responsibilities in several domains, shame and/or embarrassment to self or others, and inappropriate behaviors such as fighting. Individuals indicated how often each consequence of alcohol use had happened in the past twelve months using the following four response options: (1) never, (2) rarely, (3) sometimes, or (4) quite often. For those twins who answered at least 18 of the 22 items, we calculated a RAPI severity score by taking the average response (1–4) across the number of items answered. This method, as opposed to summing across all 22 items, permitted us to retain participants who completed the majority of the items but who may have neglected to answer a few of them had a minimal amount of missing data. Because the initiation and frequency of use dimensions were on ordinal scales, RAPI scores were collapsed for model fitting analyses into five levels using the SAS System’s univariate quintiles procedure, where the first level contains those individuals lowest on problem drinking and the fifth level contains those highest on problem drinking (SAS, 2002–2003). RAPI scores were highly skewed, reflecting the large proportion of individuals reporting no alcohol problems, so the first drinking problem level contained the largest proportion of individuals. For analyses incorporating the age 25 problem drinking outcome variable, initiation was categorized using four levels, extended from the three level initiation variable to include information obtained at age 25. The four levels were: never initiated by age 25, very late initiation (between the ages of 18 and 25), late adolescent initiation (at the ages of 15, 16, or 17), and early initiation (by the age of 14). The frequency of use at age 25 variable was created in the same manner as described above for use at age 17.
Model Fitting Analyses
We applied multiple-stage Cholesky models to the data, as described in Heath et al., 2002. These models are similar to classic multivariate models, however, the outcome, frequency of alcohol use in this case, has structural missing data for those twins with liability values of zero (never used alcohol) on the initiation dimension. Like a standard multivariate model, variance is partitioned into additive genetic (A), common environmental (C), and unique environmental (E) components. Additionally, multiple-stage models permit us to estimate the degree to which shared A, C, and E factors contribute to the covariance between different stages of use. The multiple-stage Cholesky models are also similar to the Causal–Common–Contingent (CCC) model presented in Kendler et al. (1999). Unlike the multiple-stage Cholesky models, however, the CCC model utilizes a single regression coefficient (pathway) to estimate the covariance between different stages and does not allow for the partitioning of the cross-stage covariation into A, C, and E components. We first fit two-stage Cholesky models to the initiation of alcohol use (as defined above) and frequency of use at age 17, separately to data from FT16 and FT12 (see Fig. 1). Subsequently, we expanded the bivariate model to a trivariate model in the FT16 dataset, studying an expanded initiation dimension (as detailed above), frequency of use at age 25, and incorporating a third dimension of drinking problems at age 25 (see Fig. 2).
Fig. 1.

Full Bivariate Cholesky Model of Alcohol Initiation and Frequency of Alcohol Use measured at age 14 and 17.
Fig. 2.
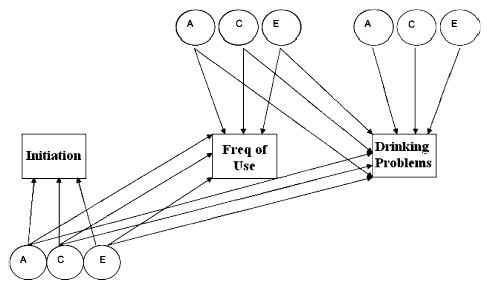
Full Trivariate Cholesky Model of Alcohol initiation, Frequency of Alcohol Use, and Drinking Problems Measured at Ages 14, 17, and 25.
All modeling was conducted using the raw ordinal data option in Mx (Neale et al., 1999). The significance of each of the parameters in the model can be tested by dropping a parameter and evaluating the change in −2 log likelihood between the initial model and the nested submodel. This difference is evaluated using a chi square distribution. A significant change in fit between the models (p <0 05) for the difference in degrees of freedom indicates that dropping the parameter caused a significant decrease in fit of the model, indicating that parameter significantly contributes to the outcome trait and should be retained in the model.
RESULTS
Prevalence of Alcohol Initiation, Use, and Problematic Drinking
The frequency distributions of male and female MZ and DZ twins on alcohol initiation and frequency of use are reported in Table I for FT16 and FT12. Of the 3424 same-sex twins in FT16, 14% reported never drinking at age 17 or younger, 30% reported first drinking at age 15, 16, or 17, and 56% reported early drinking at the age of 14 or younger. Of the 2594 same-sex twins in FT12, 11% reported never drinking at age 17 or younger, 51% reported first drinking at age 15, 16, or 17, and 38% reported early drinking at the age of 14 or younger. Overall, 86% of twins in FT16 and 89% of twins in FT12 self-reported the initiation of alcohol use at the age of 17 or younger, but as the proportions of early drinkers above show, more twins initiated drinking early in FT16 than FT12.
Table I.
Percentage of Twins Self-Reporting the Initiation of Alcohol Use by Age Groups and the Frequency of Use among those Twins who have Initiated Split by Sex and Zygosity
|
FinnTwin16 (N = 1712)
|
FinnTwin12 (N = 1297)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Girls
|
Boys
|
Girls
|
Boys
|
|||||
| MZ | DZ | MZ | DZ | MZ | DZ | MZ | DZ | |
| Initiation of use | ||||||||
| Never | 13% | 12% | 17% | 14% | 14% | 8% | 12% | 9% |
| Age 15–17 | 31% | 28% | 27% | 31% | 50% | 53% | 50% | 52% |
| At age 14 or before | 56% | 60% | 56% | 55% | 36% | 39% | 38% | 39% |
| Frequency of use | ||||||||
| Once a year or less | 9% | 7% | 8% | 7% | 6% | 6% | 5% | 4% |
| 3–4 times a year | 16% | 4% | 13% | 15% | 11% | 13% | 7% | 11% |
| Once every 2 months | 16% | 17% | 11% | 14% | 11% | 11% | 9% | 9% |
| Once a month | 20% | 15% | 16% | 14% | 21% | 15% | 12% | 15% |
| A couple times a month | 26% | 31% | 28% | 27% | 31% | 32% | 37% | 30% |
| Once a week | 10% | 12% | 16% | 16% | 13% | 16% | 20% | 20% |
| Couple of times a week or dailya | 4% | 5% | 8% | 7% | 7% | 6% | 10% | 10% |
“Couple of times per week” and “daily” categories were collapsed because of the small number of twins indicating such regular alcohol use; MZ = Monozygotic; DZ = Dizygotic.
Similar proportions of boys and girls initiated alcohol use at 17 or younger or at 14 or younger in both FT16 and FT12. Although the number of twins who reported initiating alcohol use did not vary substantially by gender, boys reported more frequent alcohol use at age 17 than girls in FT16 (t=2.86, p=0.004, d=0.22) and FT12 (t=2.15, p=0.03, d=0.11). In both samples this difference is most evident at the extreme with more boys, 24% in FT16 and 30% in FT12, than girls, 15% and 21%, reporting regular drinking, once per week or more. To assess whether those who initiated alcohol use early were more likely to report more frequent alcohol use at age 17, we submitted sex (as a covariate) and the three-level initiation variable into a multiple regression procedure predicting the six-level frequency of use variable. After accounting for sex, early initiators in both samples reported significantly higher frequency of alcohol use at age 17 than those who reported initiation after the age of 14 (t=13.69, p<0.0001, pr2=0.12 for FT16; and t=11.63, p<0.0001, pr2=0.07 for FT12).
The frequency distributions of the 3344 FT16 male and female twins who reported on alcohol initiation, frequency of use, and problematic drinking at the age of 25 are presented in Table II. Approximately 2/3 of the twins who had not initiated alcohol use at the age of 17 or younger reported the initiation of drinking by 25 years of age. The proportion of twins drinking a couple of times a week or daily (17% of females, 32% of males) also increased dramatically, particularly for males, while those drinking once a year or less dropped dramatically for both males (1%) and females (3%). The largest proportion of female (43%) and male (46%) twins were placed in the first level of their respective problem drinking distributions based on their responses to RAPI items, which suggests that most twins reported very low levels of alcohol problems. Fourteen percent of females and 15% of males reported problematic drinking in the highest, fifth level. The average RAPI severity score for women in this highest level was 1.69 (possible range of 1–4) and the average score for men was 1.93. On average, females in the highest quintile were reporting the presence 10.39 (SD = 3.32) drinking problems out of a possible 22, and males, on average, reported 13.18 (SD = 3.43) drinking problems. Thus, both females and males in the highest quintile reported experiencing a substantial number of problems associated with alcohol use at age 25.
Table II.
Percentage of Twins in FT16 Self-Reporting the Frequency of Use and Quintile Level of Problematic Drinking at Age 25 Among those Twins who Split by Sex and Zygosity
| Females (n=908)
|
Males (n=762)
|
|||
|---|---|---|---|---|
| MZ | DZ | MZ | DZ | |
| Initiation of use | ||||
| Never | 4% | 5% | 4% | 4% |
| Age 18–25 | 9% | 8% | 13% | 9% |
| Age 15–17 | 32% | 27% | 27% | 31% |
| Age 14 or before | 55% | 59% | 56% | 56% |
| Frequency of use | ||||
| Once a year or less | 2% | 3% | 1% | 1% |
| 3–4 times a year | 6% | 7% | 4% | 4% |
| Once every 2 months | 8% | 7% | 3% | 4% |
| Once a month | 8% | 13% | 6% | 8% |
| A couple times a month | 29% | 25% | 24% | 23% |
| Once a week | 30% | 28% | 31% | 31% |
| Couple of times a week or dailya | 17% | 16% | 32% | 31% |
| Problem drinkingb | ||||
| First level | 42% | 44% | 51% | 41% |
| Second level | 9% | 9% | 10% | 12% |
| Third level | 22% | 19% | 15% | 18% |
| Fourth level | 14% | 14% | 11% | 13% |
| Fifth level | 14% | 14% | 13% | 16% |
“Couple of times per week” and “daily” categories were collapsed because of the small number of twins indicating such regular alcohol use.
Problem Drinking percentages based on the number of twins at each quintile of problematic drinking using scores from the Rutgers Alcohol Problem index (RAPI), with those in the first quintile having the lowest RAPI scores.
MZ = Monozygotic; DZ = Dizygotic.
Bivariate Model Fitting
An example of a two-stage (bivariate Cholesky) model is shown in Fig. 1. Table III presents goodness-of-fit statistics for the two-stage modeling procedure applied to FT16 and FT12 data, with the best-fitting model in bold (model 9g). For both samples, constraining the thresholds to be equal across males and females caused a significant decrease in fit for FT16 (model 2) and FT12 (model 5), reflecting the small differences in frequency of use distributions mentioned above. Constraining standardized estimates of genetic (A), common environmental (C) and unique environmental (E) influences on both traits to be equal across sex did not cause a significant reduction in fit of the model in either FT16 (model 3) or FT12 (model 6) data. We next fit a model jointly analyzing FT16 and FT12 data to test whether the estimates could be constrained equal across samples. As model 9 in Table III shows, constraining A, C, and E estimates on initiation and frequency of use to be equal across samples did not cause a significant decrease in fit of the model. However, constraining thresholds to be equal across samples did cause a significant decrease in fit of the model (model 8). Thus, for all subsequent models, threshold estimates were allowed to vary across sex and sample, while A, C, and E standardized estimates were constrained equal across sex and sample. Dropping genetic effects on the initiation of alcohol use resulted in a significant decrease in fit (model 9a). Similarly, dropping the genetic influence on the frequency of use variable in the combined samples model (model 9b) also resulted in a significantly poorer model fit. Setting the influences of shared environment to zero for the initiation dimension (model 9c) resulted in a significant decrease in model fit. Finally, a substantially poorer fit resulted from dropping the C effects on the frequency of alcohol use (model 9d).
Table III.
Bivariate model fitting results for alcohol initiation and frequency of use at age 17 in FT16 and FT12
| Fit statistics
|
||||
|---|---|---|---|---|
| −2lnL | df | Δχ2 | p | |
| FinnTwin16 model fitting results | ||||
| 1. Baseline model with sex specific effects | 15113.12 | 6227 | ||
| 2. Thresholds constrained across sex | 15158.65 | 6235 | 45.53 | <0001 |
| 3. A, C, E constrained across sex | 15126.31 | 6236 | 13.19 | 0.15 |
| FinnTwin12 model fitting results | ||||
| 4. Baseline model with sex specific effects | 11298.04 | 4683 | ||
| 5. Thresholds constrained across sex | 11323.95 | 4691 | 25.91 | 0.001 |
| 6. A, C, E constrained across sex | 11309.87 | 4692 | 11.83 | 0.223 |
| Combined FT16 and FT12 model fitting results | ||||
| 7. Baseline model sample specific effects | 26436.18 | 10924 | ||
| 8. Thresholds constrained across samples | 26854.18 | 10924 | 418.00 | <0001 |
| 9. A, C, E pathways constrained across samples | 26442.80 | 10930 | 6.62 | 0.357 |
| (a) Dropping A on initiation | 26512.96 | 10931 | 70.16 | <0001 |
| (b) Dropping A on freq. of use | 26518.53 | 10931 | 75.73 | <0001 |
| (c) Dropping C on initiation | 26603.09 | 10931 | 160.29 | <0001 |
| (d) Dropping C on freq. of use | 26535.74 | 10931 | 92.94 | <0001 |
| (e) Dropping shared A | 26456.58 | 10931 | 13.78 | 0.0002 |
| (f) Dropping shared C | 26523.59 | 10931 | 80.79 | <0001 |
| (g) Dropping shared E | 26442.89 | 10931 | 0.09 | 0.76 |
| (h) Same A factors | 26465.77 | 10931 | 22.97 | <0001 |
| (i) Same C factors | 26448.94 | 10931 | 6.14 | 0.01 |
−2lnL = twice the negative log likelihood of the data; A = additive genetic influences; C = common environmental influences; E = unique environmental influences; best fitting model in bold.
Next, we tested for the presence of common A, C, and E factors influencing both the age of alcohol initiation and frequency of use. Dropping the common A estimate (model 9e) and, in a separate model, the common C estimate (model 9f) both resulted in significant reductions in fit, Dropping the common E estimate (model 9g), however, resulted in a very small, nonsignificant change in the chi-square fit statistic. To test whether the genetic variance in initiation completely overlapped with the genetic variance in frequency of use, a model with the specific genetic influences on frequency of use set to zero (which results in a correlation of 1.0 among genetic factors) was fit to the data (model 9h). Setting the genetic correlation to 1.0 resulted in a significant decrease in fit, indicating that while there were significant shared genetic influences on initiation and frequency of use, they were not completely overlapping. Fitting a model with the shared environmental correlation set to 1.0 (model 9i) also resulted in significantly poorer model fit, suggesting that the common environmental influences did not completely overlap. Consequently, the bivariate genetic model that best fit the combined FT16 and FT12 alcohol initiation and frequency of use data was one in which A, C, and E estimates, but not thresholds, were constrained equal across gender with no shared unique environmental influences on the two stages of alcohol use (model 9g).
Standardized estimates of A, C, and E (and 95% Confidence Intervals; CI) for the initiation and frequency of use variables from the full model with A, C, and E constrained across gender are presented in Table IV for the combined model as well as for the models fit separately to FT16 and FT12 data. We presented estimates from the full models in order to show the broader CIs yielded by the full model. Based on results from the full combined model, shared environmental influences accounted for the largest proportion of variance in the age of alcohol initiation, explaining 59% of the variance. Additive genetic factors accounted for 29% of the variance in age of initiation, while unique environmental factors accounted for 12%. For frequency of use at age 17, shared environmental factors accounted for 34%, while the proportion of variance explained by additive genetic factors was 39%. Unique environmental influences on the frequency of alcohol use accounted for 27% of the variance in the combined model.
Table IV.
Standardized Estimates (and 95% CIs) for A, C, and E Influences on Drinking Initiation and Frequency of Alcohol Use at Age 17 for the Full Models Constrained Across Gender in the Combined Model and for FinnTwin16 and FinnTwin12 Separately
| Variable | A | C | E |
|---|---|---|---|
| Combined | |||
| Alcohol initiation | 0.29 (0.22–0.37) | 0.59 (0.51–0.66) | 0.12 (0.10–0.14) |
| Frequency of use | 0.39 (0.30–0.49) | 0.34 (0.25–0.42) | 0.27 (0.24–0.30) |
| FinnTwin16 | |||
| Alcohol initiation | 0.34 (0.24–0.45) | 0.55 (0.45–0.64) | 0.11 (0.09–0.13) |
| Frequency of use | 0.44 (0.32–0.57) | 0.31 (0.19–0.42) | 0.25 (0.22–0.29) |
| FinnTwin12 | |||
| Alcohol initiation | 0.23 (0.12–0.35) | 0.64 (0.52–0.74) | 0.13 (0.11–0.17) |
| Frequency of use | 0.32 (0.17–0.47) | 0.38 (0.24–0.51) | 0.30 (0.26–0.36) |
A = additive genetic influences; C = common environmental influences; E = unique environmental influences.
The correlations (and 95% CIs) between A, C, and E influences on the age of alcohol use initiation and frequency of alcohol use for the full combined model constrained across gender and FT16 and FT12 data are presented in Table V. Based on the combined model fitting results, the estimated genetic correlation between initiation and frequency of use dimensions was 0.51 (0.21–0.81), indicating that genetic factors impacting the decision to initiate drinking accounted for 26% of the variance in frequency of use once initiation has occurred. Results also suggested a large overlap of shared environmental influences impacting both initiation and frequency of alcohol use; a correlation of 0.81 (0.62–1.00) was found across both stages. This correlation suggested that the shared environmental influences on initiation accounted for approximately 66% of the variance in the frequency of alcohol use. The correlation between unique environmental influences across stages was nonsignificant and estimated at 0.02 (0.00–0.19), which suggested that there was almost no overlap of unique environmental factors impacting the age of initiation and the frequency of alcohol use.
Table V.
Correlations (and 95% CIs) among the Standardized A, C, and E Parameter Estimates for the Alcohol Initiation and Frequency of Use Variables for the Full Model Constrained Across Gender and FinnTwin16 and FinnTwin12 Samples
| Combined model | |
|---|---|
| rA | 0.51 (0.21–0.81) |
| rC | 0.81 (0.62–1.00) |
| rE | 0.02 (0.00–0.19) |
A = additive genetic influences; C = common environmental influences; E = unique environmental influences.
Trivariate Model Fitting
Table VI presents goodness-of-fit statistics for the three-stage (trivariate) Cholesky model (see Fig. 2) applied to FT16 data through the age of 25, with the best-fitting model in bold (model 17 for females and 31 for males). Constraining the thresholds to be equal across males and females caused a significant decrease in fit (model 2), reflecting the sizable differences in frequency of regular use at age 25. Constraining standardized A, C, and E estimates on the three stages to be equal across sex also caused a significant reduction in fit of the model (model 3). For all subsequent models, thresholds and A, C, and E estimates at all three stages were allowed to vary across sex.
Table VI.
Trivariate model fitting results for alcohol initiation (Init), frequency of use at age 25 (Freq), and alcohol problems at age 25 (Prob) in FT16
| Fit statistics
|
||||
|---|---|---|---|---|
| Model | −2lnL | df | Δχ2 | p |
| FinnTwin16 model fitting results | ||||
| 1. Baseline model with sex specific effects | 25891.92 | 10133 | ||
| 2. Thresholds constrained across sex | 26010.81 | 10146 | 118.90 | <0001 |
| 3. A, C, E constrained across sex | 25915.75 | 10145 | 23.84 | 0.02 |
| Submodel fitting results for females | ||||
| 4. Dropping A pathway to initiation | 25947.52 | 10134 | 55.60 | <0001 |
| 5. Dropping A pathways from Init to Freq/Prob | 25893.12 | 10135 | 1.20 | 0.55 |
| 6. Dropping A pathways from Freq to Prob | 25898.05 | 10134 | 6.14 | 0.01 |
| 7. Dropping all A pathways from Freq and Prob | 25909.10 | 10136 | 17.18 | 0.0006 |
| 8. Dropping all common A pathways | 25899.48 | 10136 | 7.57 | 0.056 |
| 9. Dropping C pathways from Init to Freq/Prob | 25898.86 | 10135 | 6.94 | 0.03 |
| 10. Dropping C pathways from Freq to Prob | 25911.64 | 10134 | 19.73 | <0001 |
| 11. Dropping all C pathways from Freq and Prob | 25901.92 | 10136 | 10.01 | 0.02 |
| 12. Dropping all common C pathways | 25898.86 | 10136 | 6.95 | 0.07 |
| 13. Dropping E pathways from Init to Freq/Prob | 25894.31 | 10135 | 2.40 | 0.30 |
| 14. Dropping E pathways from Freq to Prob | 25959.26 | 10134 | 67.34 | <0001 |
| 15. Dropping all E pathways from Freq and Prob | 34557.61 | 10136 | 8665.70 | <0001 |
| 16. Dropping all common E pathways | 25967.28 | 10136 | 75.36 | <0001 |
| 17. Dropping A and E pathways from Init to Freq/Prob | 25895.63 | 10137 | 3.72 | 0.45 |
| Submodel fitting results for males | ||||
| 18. Dropping A pathway to Initiation | 25947.52 | 10134 | 8.27 | 0.004 |
| 19. Dropping A pathways from Init to Freq/Prob | 25893.76 | 10135 | 1.85 | 0.40 |
| 20. Dropping A pathways from Freq to Prob | 25896.98 | 10134 | 5.06 | 0.02 |
| 21. Dropping all A pathways from Freq and Prob | 25903.05 | 10136 | 11.14 | 0.01 |
| 22. Dropping all common A pathways | 25906.08 | 10136 | 14.16 | 0.003 |
| 23. Dropping C pathways from Init to Freq/Prob | 25901.12 | 10135 | 9.21 | 0.01 |
| 24. Dropping C pathways from Freq to Prob | 25892.07 | 10134 | 0.16 | 0.69 |
| 25. Dropping all C pathways from Freq and Prob | 25892.31 | 10136 | 0.40 | 0.94 |
| 26. Dropping all common C pathways | 25901.08 | 10136 | 9.17 | 0.03 |
| 27. Dropping E pathways from Init to Freq/Prob | 25896.34 | 10135 | 4.43 | 0.11 |
| 28. Dropping E pathways from Freq to Prob | 25906.60 | 10134 | 14.69 | 0.0001 |
| 29. Dropping all E pathways from Freq and Prob | 25236.21 | 10136 | 655.71 | <0001 |
| 30. Dropping all common E pathways | 25909.27 | 10136 | 17.35 | 0.0006 |
| 31. Dropping A and E pathways from Init to Freq/Prob and all C pathways from Freq and Prob | 25900.16 | 10140 | 8.25 | 0.31 |
−2lnL = twice the negative log likelihood of the data; A = additive genetic influences; C = common environmental influences; E = unique environmental influences; best fitting model in bold.
For both sexes, dropping the genetic pathway on initiation did result in a significant reduction in model fit (models 4 and 18). Dropping the common genetic pathways between initiation and frequency of use and problem drinking did not result in a significant decrease in fit (models 5 and 19), indicating that the significant genetic influences on initiation did not overlap with genetic influences on frequency of use at age 25 or with alcohol problems at age 25, for either sex. However, dropping the common genetic pathway between frequency of use and problematic drinking did significantly reduce model fit (models 6 and 20), indicating that there were common genes influencing frequency of use at age 25 and alcohol problems at 25. Specifying a model that only allowed for one set of shared genetic factors influencing alcohol initiation, use, and problems caused a significant decrease in fit (models 7 and 21), as would be expected based on the results reported above. Finally, a model in which the genetic influences at each stage were independent of other stages (all common genetic pathways set to zero) resulted in a poorer model fit (models 8 and 22). In summary, these results suggest that, for both females and males, genetic factors influencing initiation of alcohol use do not significantly overlap with genetic influences on frequency of use and problem drinking at the age of 25; however, there is significant overlap between the genetic factors influencing frequency of use and alcohol problems at age 25.
The same series of submodels described above for genetic pathways were also applied to common and unique environmental pathways. For both sexes, dropping common C pathways between initiation and frequency of use and problem drinking resulted in a significant decrease in fit (model 9 for females and model 23 for males). Dropping the common C pathway between frequency of use and problem drinking significantly decreased the fit of the model to data for females (model 10) but not for males (model 24). Similarly, stipulating a model that only allowed for one set of shared environmental factors influencing alcohol initiation, use, and problems caused a significant decrease in fit for females (model 11) but not for males (model 25). Lastly, a model fit to the data in which the C influences at each stage were independent of other stages resulted in a moderately significant poorer model fit for females (model 12) and a significantly poorer model fit for males (model 26). These results suggest that, for females, there is a substantial amount of common and specific shared environmental influences across all stages of alcohol use, while, for males, the same shared environmental factors appear to be influencing initiation, frequency of use, and problem drinking at the age of 25. However, we note that for males, the magnitude of influence of shared environmental factors on frequency of use and problematic drinking is rather small. For females, there is considerably greater influence of common environment on frequency of use at age 25, although problematic drinking shows little evidence of common environment, similar to in males.
For unique environmental influences, only the common E pathways from initiation to frequency of use and problem drinking could be dropped without a significant decrease in model fit for both sexes (models 13 and 27). Dropping the common E pathway from use to problem drinking (models 14 and 28), allowing for only one set of E factors influencing all three stages (models 15 and 29), and dropping all common E pathways (models 16 and 30) all caused significant decreases in model fit. In summary, for both females and males, unique environments influencing initiation of alcohol use do not significantly overlap with unique environmental influences on frequency of use and problem drinking at the age of 25; however, there is significant overlap between the unique environmental factors influencing frequency of use and alcohol problems.
Consequently, the trivariate genetic model that best fit the alcohol initiation, frequency of use, and alcohol problem data in FT16 was one in which thresholds and A, C, and E estimates varied across gender. For females, a model in which common A and E pathways between initiation and both frequency of use and alcohol problems were dropped fit the data best (model 17). The best-fitting model for males was one in which common A and E pathways between initiation and both frequency of use and alcohol problems were dropped as well as all C pathways from the frequency of use and problem drinking stages (model 31).
Standardized estimates of A, C, and E (and 95% Confidence Intervals; CI) from the full model for FT16 age 25 initiation, frequency of use, and problem drinking variables are presented in Table VII. As with the bivariate models, C influences accounted for the largest proportion of variance in the age of alcohol initiation, explaining 47% of the variance among females and 61% of the variance among males. Additive genetic factors accounted for 44% and 22% of the variance in age of initiation for females and males, respectively, while E factors accounted for 9% and 17%, respectively. E factors had the largest influence on the frequency of alcohol use at age 25 among women, accounting for 50% of the variance. Additive genetic influences accounted for 19% of the variance in frequency of use, which is substantially less than the proportion of variance accounted for by genetic factors on initiation. The influence of C factors also decreased at this stage, accounting for 31% of the variance in use at age 25 among women. Contrary to the small genetic influences on use for women, A factors accounted for 48% of the variance in frequency of use among men, while C accounted for only 8%. The unique environment accounted for 44% of the variance in use for men. As expected, genetic factors accounted for the largest proportion of variance in problem drinking for both women and men, explaining 47% and 55%, respectively. Unique environmental factors explained another 38% and 36% of the variance in problem drinking, while C accounted for only 15% of the variance for women and 8% of the variance for men. The CIs for the C estimates of problem drinking overlapped zero in both females and males, suggesting that they are nonsignificant.
Table VII.
Standardized Estimates (and 95% CIs) for A, C, and E Influences on Drinking Initiation, Frequency of Alcohol Use, and Problem Drinking for the Full Model in FinnTwin16-Age 25
| Variable | A | C | E |
|---|---|---|---|
| Females | |||
| Alcohol initiation | 0.44 (0.31–0.60) | 0.47 (0.32–0.60) | 0.09 (0.06–0.11) |
| Frequency of use | 0.19 (0.01–0.42) | 0.31 (0.11–0.47) | 0.50 (0.43–0.58) |
| Problem drinking | 0.47 (0.25–0.65) | 0.15 (0.00–0.34) | 0.38 (0.32–0.45) |
| Males | |||
| Alcohol initiation | 0.22 (0.06–0.39) | 0.61 (0.45–0.74) | 0.17 (0.13–0.23) |
| Frequency of use | 0.48 (0.29–0.60) | 0.08 (0.01–0.23) | 0.44 (0.36–0.53) |
| Problem drinking | 0.55 (0.32–0.69) | 0.08 (0.00–0.28) | 0.36 (0.29–0.44) |
A = additive genetic influences; C = common environmental influences; E = unique environmental influences.
Table VIII presents the correlations (and 95% CIs) between A, C, and E influences on the three stages of alcohol use for the full model. The estimated genetic correlation between initiation and frequency of use dimensions was 0.23 (0.00–0.89 for females; 0.00–0.74 for males) for both sexes. The wide confidence interval includes zero, and these correlations were not significant. The genetic correlations between initiation and problem drinking were also nonsignificant: 0.15 (−0.17–0.47) for females and 0.29 (−0.18–0.84) for males. The genetic correlations between frequency of use and problem drinking were much higher, with estimates of 0.78 (0.29–1.00) for females and 0.63 (0.39–0.96) for males, suggesting that 61% and 40%, respectively, of the genetic variance in problem drinking behavior could be accounted for by genetic factors important in frequency of use.
Table VIII.
Correlations (and 95% CIs) among the Standardized A, C, and E Parameter Estimates for the Alcohol Initiation (Init), Frequency of Use (Freq), and Alcohol Problem (Prob) Variables for the Full Models in FinnTwin16-age 25
| Females | Males | |
|---|---|---|
| rA | ||
| Init–Freq | 0.23 (0.00–0.89) | 0.23 (0.00–0.74) |
| Init–Prob | 0.15 (−0.17–0.47) | 0.29 (−0.18–0.84) |
| Freq–Prob | 0.78 (0.29–1.00) | 0.63 (0.39–0.96) |
| rC | ||
| Init–Freq | 0.44 (0.08–0.86) | 1.00 (0.43–1.00) |
| Init–Prob | 0.55 (0.23–1.00) | 0.61 (−1.00–1.00) |
| Freq–Prob | 0.23 (−0.78–0.92) | 0.59 (−0.62–1.00) |
| rE | ||
| Init–Freq | 0.06 (0.00–0.23) | 0.16 (0.00–0.23) |
| Init–Prob | 0.08 (−0.02–0.25) | 0.01 (−0.18–0.22) |
| Freq–Prob | 0.46 (0.35–0.55) | 0.30 (0.15–0.44) |
A = additive genetic influences; C = common environmental influences; E = unique environmental influences.
Overall, the correlations between shared environmental factors across stages were larger than those found for genetic factors. The estimated C correlations between initiation and frequency of use were 0.44 (0.08–0.86) and 1.00 (0.43–1.00) for females and males, respectively (approximately 19% and 100% of the C variance in use could be accounted for by C factors at initiation). For initiation and problem drinking, the correlations were 0.55 (0.23–1.00) for females and 0.61 (−1.00–1.00) for males, suggesting a moderate overlap of 30% of the C variance in these two stages for women. The frequency of use and problem drinking dimensions correlated 0.23 (−0.78–0.92) for females and 0.59 (−0.62–1.00) for males, respectively, which are both nonsignificant. The very large confidence intervals suggesting nonsignificant findings around the correlations between C influences on these stages reflect the very small relative contributions that shared environments made at these stages. The estimated E correlations between initiation and frequency of use, 0.06 (0.00–0.23) for females and 0.16 (0.00–0.37) for males, and the correlation between E factors on initiation and problem drinking, 0.08 (−0.02–0.25) and 0.01 (−0.18–0.22), were small and nonsignificant. The correlations between E influences on frequency of use and problem drinking behavior were estimated at 0.46 (0.35–0.55) for females and 0.30 (0.15–0.44) for males, indicating that E factors important for frequency of use accounted for 21% and 9%, respectively, of the variance in problem drinking.
DISCUSSION
In the present study, we applied multiple-stage genetic models to progressive stages of alcohol use and misuse in two longitudinal samples of twins. Utilizing multiple-stage modeling procedures permitted more accurate assessments of the importance of genetic and environmental risk factors on patterns of use and misuse by making allowance for partial overlap with risk factors for initiation, and by taking into account censoring on the frequency/problem dimensions for individuals who had not yet initiated. Furthermore, we expanded on the previous literature addressing substance use initiation by characterizing individuals as to whether they initiated alcohol use in early or late adolescence. Consistent with previous epidemiological findings (Johnston et al., 2001; Maes, et al., 1999), reported initiation of alcohol use at the age of 17 or younger was high among Finnish twins (86–89%). These numbers are somewhat higher than the estimated 80% of American adolescents who report drinking alcohol (Johnston et al., 2001), which may reflect small cultural differences in attitudes about alcohol use, as well as the availability of alcohol in these two countries. Substantially more twins in FT16 indicated drinking at the age of 14 or younger compared to twin reports of drinking in FT12. The difference in the proportion of twins reporting early initiation is likely due to differences in how early initiation was computed for each study. In FT16, twins were asked at the age of 16 to retrospectively report when they began drinking alcohol, and anyone who reported age 14 or younger was coded as early. In FT12, reports of current drinking behavior were made by the twins at age 14. A second possible explanation for the difference is that drinking patterns, cultural expectations, and the availability of alcohol may have been different across these two time-periods.
In both samples, the age at which twins initiated alcohol use significantly predicted how often the twins reported drinking at age 17 as well as the level of drinking problems the FT16 twins reported, even after accounting for the sex of the twins. For example, 8% and 15% of early initiators in FT16 and FT12, respectively, reported daily or weekly drinking, while only 2% and 5% of late initiators reported daily or weekly drinking. Of those 25 year olds who initiated at age 14 or before, 20% reported drinking problems on the RAPI that placed them in the highest quintile of drinking problems, while 7% of those who initiated after the age of 17 reported drinking problems on the RAPI that placed them in the highest quintile. These results replicated previous findings that earlier alcohol use initiation is associated with heavier and more problematic drinking in later adolescence and young adulthood (Chou and Pickering, 1992; Clapper et al., 1995; Grant and Dawson, 1997; Hingson et al., 2000; Kandel et al., 1992; Pitkanen et al., 2005; Riala et al., 2004).
Bivariate model findings reported here were consistent with reports of large shared environmental influences and small additive genetic factors influencing the decision to initiate use (Heath and Martin, 1988; Heath et al., 1991; Kaprio et al., 1987; Koopmans and Boomsma, 1996; Rose et al., 2001b; Stallings et al., 1999). Across the two Finnish twin samples, the shared environmental estimate on initiation was on the low end of the range of those reported by Hopfer and colleagues (2003), who concluded in their review that shared environmental influences accounted for 55–80% of the variance in alcohol initiation. The somewhat lower estimate of shared environmental influences, and the corresponding higher estimate of genetic influences, in our samples may reflect the incorporation of information about the timing of initiation. Age at first drink has been shown in previous research to be under a degree of genetic influence (Prescott and Kendler, 1999). When initiation was expanded to incorporate those who began drinking after the age of 17 in the FT16 trivariate models, additive genetic and the shared environmental estimates for females were nearly equal, while estimates for males were largely unchanged from the bivariate model. The parameter estimates for genetic influences on the age of alcohol initiation in the FT16 bivariate model that permitted estimates to vary across gender were also higher for females (A = 0.37) than males (A = 0.28). These results suggest that genetic factors may play a more important role in the age of initiation for women.
Results presented here also confirmed the consistent finding that genetic factors increased in importance for drinking behavior once initiation had occurred, while the influence of shared environmental factors dropped appreciably across stages. Unique environmental factors were also more important in impacting frequency of use than the initiation of alcohol use. For males, drinking frequency at age 25 was under similar genetic influence as drinking frequency at age 17. However, genetic factors accounted for a relatively small amount of the variation in drinking frequency at age 25 for females, while unique environmental influences played the largest role in drinking frequency. This finding suggests that there may be unique processes influencing women’s drinking patterns in their early to mid-20s. On the other hand, estimates of unique environmental influences do include error, so it is possible that this difference for women was a result of measurement error. We are not aware of other studies that have reported this effect, and the finding warrants further exploration in future studies. With the exception of small genetic influence on drinking frequency for women in the trivariate modeling results, estimates of genetic and environmental influences on the frequency of alcohol use were all within the range of previous studies (Heath and Martin, 1988; Heath et al., 1991; Hopfer, et al., 2003; Kaprio, et al., 1987; Maes et al., 1999; Rose, et al., 2001b; Stallings et al., 1999).
Our finding of sizeable genetic influences on drinking problems were consistent with previous research that has found large genetic influences on alcohol dependence in adulthood in the range of 50370% (Cloninger et al, 1981; Heath et al., 1997; Kaprio, et al., 1987; McGue, 1993; Prescott et al., 1994b). It is important to note that the analysis of problem drinking is more complex than initiation or frequency of use in our sample and should be interpreted cautiously, as many drinkers who may eventually develop problems may not have yet manifested them in their early to mid-20s.
In addition to examining the genetic and environmental influences on initiation and frequency of use, the multiple stage genetic modeling procedures used here permitted an assessment of the extent to which common (vs. specific) genetic and environmental factors account for the various stages of alcohol use. Results for the model fitting to the combined FT16 and FT12 data suggested that some of the genetic and much of the common environmental influences that are important for the initiation of alcohol use also impact the frequency of alcohol use once initiated. Thus, although common environmental influences played a more important role in impacting initiation, it was largely the same shared environmental influences that impacted frequency of use once initiated. Estimates of common unique environmental influences across stages of use were nonsignificant and near zero.
Finally, we extended these multiple stage models to examine common and/or specific factors important in the transition from regular use to problem drinking in young adulthood using FT16 data. Genetic factors influencing initiation did not overlap substantially with genetic influences on frequency of use and problem drinking in the twins’ 20s. However, the genetic factors influencing frequency of use did overlap substantially with those impacting alcohol problems. This overlap is consistent with results found by Prescott and Kendler (1999), who found an association between genetic factors influencing early drinking and alcohol dependence. The shared environmental factors important at initiation were largely the same factors influencing frequency of use; however, the importance of common environment was considerably reduced for drinking frequency at age 25, and nonsignificant for alcohol problems. Unique environmental factors, which included measurement error, influencing frequency of use did account for some of the variance in alcohol problems.
Several methodological limitations to the multiple stage genetic modeling procedures used in the current study should be noted. Multiple-stage models assume that the initiation and outcome variables have a bivariate normal liability distribution. When this assumption is met and the initiation variable has at least three levels, two of which are characterized in individuals who have data on the outcome dimension, the bivariate genetic model is fully identified with twin data (Heath et al., 2002). Our multiple-level initiation dimension used in these analyses did not meet the assumption of normality. This suggests that our initiation dimension did not represent a single liability, which was likely caused by the incorporation of information about the timing of initiation into the initiation variable. This probably led to the lower estimates of common environmental influences/higher estimates of genetic influence on initiation, as compared to previous studies, and may have also contributed to an inflated estimate of shared genetic influences between initiation and frequency of use at age 17. Another concern is that multiple-stage genetic models are fairly complex and require large sample sizes to accurately estimate parameters and correlations among factors, especially those that are small. The difficulty in estimating some of the small common pathways in the present study may have been due to limited power.
One interesting extension of these models will be to incorporate information about mediators and moderators of genetic and environmental influences on the stages of alcohol use and abuse within the context of multiple stage genetic models (Heath et al., 2002). Several other factors, including age (Koopmans et al., 1997), socioregional factors (Rose et al., 2001a), parental alcohol use (Koopmans and Boomsma, 1996), religiousness (Koopmans et al., 1999), sibling drinking behaviors (McGue et al., 1996), and parental monitoring (Rose et al., 2001b) have all been reported to moderate influences on substance use behavior of adolescents. In future work, we plan to assess the impact of these specific environmental variables on each stage of alcohol use, as well as any impact they may have across stages. Another interesting direction for future research will be to apply these multiple stage genetic modeling procedures to adolescent cigarette smoking and/or drug initiation and use, as several studies have found that adolescents who experiment with one substance are more likely to try others, and, as the use of alcohol increases, so does the use of tobacco products (Maes et al., 1999; Collins, 1990–1991). Finally, the large genetic influences on drinking problems as well as the substantial overlap between genetic factors influencing frequency of alcohol use and alcohol problems suggests that these may be important areas to target for gene identification studies in addition to phenotypes based on substance use disorder diagnoses or dependence.
In conclusion, multiple-stage genetic modeling results from two independent Finnish twin samples suggested the presence strong shared environmental influences, and correspondingly small additive genetic influences, on the decision/timing of alcohol initiation. For the most part, the same shared environmental factors important for initiation also impacted the frequency of alcohol use in later adolescence. However, shared environmental influences were less important for frequency of use, while the influence of additive genetic factors and unique environmental factors were more influential contributors to the frequency of alcohol use. Genetic factors important at initiation overlapped to a small degree with the genetic factors influencing the frequency of use, but we found no overlap of unique environmental factors across stages of use. Genetic factors played the largest role in problematic drinking at age 25 in both men and women, whereas common environmental influences were nonsignificant in both sexes. For both sexes, genetic factors influencing alcohol problems overlapped substantially with those influencing frequency of use at age 25, and shared environmental influences on initiation overlapped moderately with the relatively small shared environmental influences on frequency of use at 25. Unique environmental factors were not very important at initiation and did not overlap much with the moderately important unique environmental factors influencing frequency of use and problem drinking at 25, which did show some overlap with each other. The application of multiple stage genetic models to longitudinal data provided a novel approach to estimating, within the same individuals across time, the impact of genetic and environmental factors at each stage of alcohol use while taking into account those factors important at earlier stages.
Acknowledgments
The Finnish Twin studies have been supported by the National Institute of Alcoholism and Alcohol Abuse (grants AA-12502, AA-00145, AA-08315, and AA-09203 to RJR), the Academy of Finland (grants # 100499 and 44069 to JK) the Finnish Centre of Excellence Programme (to LP) and by grants from the Yrjö Jahnsson Foundation (to JK).
Footnotes
Edited by Michael Stallings
References
- Carmelli D, Heath AC, Robinette D. Genetic analysis of drinking behavior in World War II veteran twins. Genetic Epidemiol. 1993;10:201–213. doi: 10.1002/gepi.1370100306. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Robinette D, Fabsitz R. Heritability of substance use in the NAS-NRC Twin Registry. Acta Genetica Medica Gemellol. 1990;39:91–98. doi: 10.1017/s0001566000005602. [DOI] [PubMed] [Google Scholar]
- Chou SP, Pickering RP. Early onset of drinking as a risk factor for lifetime alcohol-related problems. Brit J Addict. 1992;87:1199–1204. doi: 10.1111/j.1360-0443.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Clapper RL, Buka SL, Goldfield EC, Lipsitt LL, Tsuang MT. Adolescent problem behaviors as predictors of adult alcohol diagnoses. Intl J Addict. 1995;30:507–523. doi: 10.3109/10826089509048741. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: Cross-fostering analysis of adopted men. Arch Gen Psych. 1981;38:861–867. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Collins AC. Genetic influences on tobacco use: A review of human and animal studies. Intl J Addict. 1990–91;25(1A):35–55. doi: 10.3109/10826089009067004. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH. A zygosity questionnaire for young twins: a research note. Behav Genet. 1991;21:257–269. doi: 10.1007/BF01065819. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiological Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden AF, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG. Teenage alcohol use in the Australian twin register: Genetic and social determinants of starting to drink. Alcohol Clin Exp Res. 1988;12:735–741. doi: 10.1111/j.1530-0277.1988.tb01337.x. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PAF. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Res. 2002;5:113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- Heath AC, Meyer J, Jardine R, Martin NG. The inheritance of alcohol consumption patterns in a general population twin sample: II. Determinants of consumption frequency and quantity consumed. J Stud Alcohol. 1991;52:425–433. doi: 10.15288/jsa.1991.52.425. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Jamanka A, Howland J. Age of drinking onset and unintentional injury involvement after drinking. J Amer Med Assoc. 2000;284:1527–1533. doi: 10.1001/jama.284.12.1527. [DOI] [PubMed] [Google Scholar]
- Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. J Acad Child Adolescent Psych. 2003;42:710–719. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- Johnston, L. D., O’Malley, P. M., and Bachman, J. G. (2001). Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2000 Bethesda, MD: National Institute on Drug Abuse.
- Kandel D, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J Stud Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Koskenvuo M, Langinvainio H, Romanov K, Sarna S, Rose RJ. Genetic influences on use and abuse of alcohol: A study of 5638 adult Finnish twin brothers. Alcohol Clin Exp Res. 1987;11:349–356. doi: 10.1111/j.1530-0277.1987.tb01324.x. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Res. 2002;5:366–371. doi: 10.1375/136905202320906101. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Rimpela A, Winter T, Viken RJ, Rimpela M, Rose RJ. Common genetic influences on BMI and age at menarche. Human Biol. 1995;67:739–753. [PubMed] [Google Scholar]
- Kaprio J, Viken R, Koskenvuo M, Romanov K, Rose RJ. Consistency and change in patterns of social drinking: A 6-year follow-up of the Finnish twin cohort. Alcohol Clin Exp Res. 1992;16:234–240. doi: 10.1111/j.1530-0277.1992.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Kendler, Neale, sullivan, Corey, Gardner, Prescott A population-based twin study in women of smoking initiation and nicotine dependence. Psych Med. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psych. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Boomsma DI. Familial resemblances in alcohol use: genetic and cultural transmission? J Stud Alcohol. 1996;57:19–28. doi: 10.15288/jsa.1996.57.19. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Slutske WS, van Baal GC, Boomsma DI. The influence of religion on alcohol use initiation: evidence for genotype × environment interaction. Behav Genet. 1999;29:445–453. doi: 10.1023/a:1021679005623. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, van Doornen LJ, Boomsma DI. Association between alcohol use and smoking in adolescent and young adult twins: a bivariate genetic analysis. Alcohol Clin Exp Res. 1997;21:537–546. [PubMed] [Google Scholar]
- Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, et al. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: the Virginia twin study of adolescent behavioral development. J Stud Alcohol. 1999;60:293–306. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- McGue, M. (1993). Genes, environment and the etiology of alcoholism. In Development of Alcohol-Related Problems in High-Risk Youth: Establishing Linkages Across Biogenetic and Psychosocial Domains, National Institute on Alcoholism and Alcohol Abuse, Research Monograph, Rockville, MD
- McGue M. The behavioral genetics of alcoholism. Curr Direct Psychol Sci. 1999;8:109–115. [Google Scholar]
- McGue M, Sharma A, Benson P. Parent and sibling influences on adolescent alcohol use and misuse; evidence from a US adoption cohort. J Stud Alcohol. 1996;57:8–18. doi: 10.15288/jsa.1996.57.8. [DOI] [PubMed] [Google Scholar]
- Neale, M., Boker, S. M., Xie, G., and Maes, H. H. (1999). Mx: Statistical Modeling, 5th edn. Department of Psychiatry, Virginia Commonwealth University, Box 900126, Richmond, VA 23298
- Pitkanen T, Lyyra AL, Pulkkinen L. Age of onset of drinking and the use of alcohol in adulthood: a follow-up study from age 8–42 for females and males. Addiction. 2005;100:652–61. doi: 10.1111/j.1360-0443.2005.01053.x. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Hewitt JK, Heath AC, Truett KR, Neale MC, Eaves LJ. Environmental and genetic influences on alcohol use in a volunteer sample of older twins. J Stud Alcohol. 1994a;55:18–33. doi: 10.15288/jsa.1994.55.18. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Hewitt JK, Truett KR, Heath AC, Neale MC, Eaves LJ. Genetic and environmental influences on lifetime alcohol-related problems in a volunteer sample of older twins. J Stud Alcohol. 1994b;55:184–202. doi: 10.15288/jsa.1994.55.184. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: a noncausal association. Alcohol Clin Exp Res. 1999;23:101–107. [PubMed] [Google Scholar]
- Riala K, Hakko H, Isohanni M, Jarvelin MR, Rasanen P. Teenage smoking and substance use as predictors of severe alcohol problems in late adolescence and in young adulthood. J Adoles Health. 2004;35:245–54. doi: 10.1016/j.jadohealth.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: regional residency moderates longitudinal influences on alcohol use. Alcohol Clin Exp Res. 2001a;5:637–643. [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14? A genetic epidemiological study. Alcohol Clin Exp Res. 2001b;25:1594–1604. [PubMed] [Google Scholar]
- Rose RJ, Kaprio J, Winter T, Koskenvuo M, Viken RJ. Familial and socioregional environmental effects on abstinence from alcohol at age sixteen. J Stud Alcohol. 1999;13(Suppl):63–74. doi: 10.15288/jsas.1999.s13.63. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. (2002–2003). SAS Version 91. Cary, NC USA
- Schulenberg, J., Maggs, J. L., and Hurrelmann, K. (1997). Health Risks and Developmental Transitions During Adolescence Cambridge: Cambridge University Press.
- Stallings MC, Hewitt JK, Beresford T, Heath AC, Eaves LJ. A twin study of drinking and smoking onset and latencies from first use to regular use. Behav Genet. 1999;29:409–421. doi: 10.1023/a:1021622820644. [DOI] [PubMed] [Google Scholar]
- The World Health Report 2004 – Global Status Report on Alcohol Geneva, World Health Organization, 2004
- Viken RJ, Kaprio J, Koskenvuo M, Rose RJ. Longitudinal analyses of the determinants of drinking and of drinking to intoxication in adolescent twins. Behav Genet. 1999;29:455–461. doi: 10.1023/a:1021631122461. [DOI] [PubMed] [Google Scholar]
- White HR, Labouvie EW. Toward the assessment of adolescent problem drinking. J Stud Alcohol. 1989;50:30–37. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]


