Abstract
Wounding chickpea (Cicer arietinum) internodes or cotyledons resulted in an increase in the steady-state level of copper amine oxidase (CuAO) expression both locally and systemically. Dissection of the molecular mechanisms controlling CuAO expression indicated that jasmonic acid worked as a potent inducer of the basal and wound-inducible CuAO expression, whereas salicylic acid and abscisic acid caused a strong reduction of the wound-induced CuAO expression, without having any effect on the basal levels. Epicotyl treatment with the CuAO mechanism-based inhibitor 2-bromoethylamine decreased hydrogen peroxide (H2O2) levels in all the internodes, as evidenced in vivo by 3,3′-diaminobenzidine oxidation. Moreover, inhibitor pretreatment of wounded epicotyls resulted in a lower accumulation of H2O2 both at the wound site and in distal organs. In vivo CuAO inhibition by 2-bromoethylamine after inoculation of resistant chickpea cv Sultano with Ascochyta rabiei resulted in the development of extended necrotic lesions, with extensive cell damage occurring in sclerenchyma and cortical parenchyma tissues. These results, besides stressing the fine-tuning by key signaling molecules in wound-induced CuAO regulation, demonstrate that local and systemic CuAO induction is essential for H2O2 production in response to wounding and indicate the relevance of these enzymes in protection against pathogens.
Plant defense responses are accomplished by the deployment of a complex array of events that are differentially modulated depending on the incoming stress (Maleck and Dietrich, 1999). Wounding different plant organs or interaction with pathogens induce local and systemic accumulation of defense-related proteins (Hammond-Kosack and Jones, 1996; Ryals et al., 1996; Ryan, 2000). The study of signaling events inducing local and systemic responses led to the discovery of systemin, jasmonates, ethylene, salicylic acid (SA), and abscisic acid (ABA) as signal molecules (Peña-Cortés et al., 1989; Farmer and Ryan, 1990; Pearce et al., 1991; Xu et al., 1994; O'Donnell et al., 1996; Schweizer et al., 1998; van Loon et al., 1998; Knoester et al., 1999).
The existence of multiple defense strategies and complex signaling networks leads to an enhanced defense capacity of the plants. The signal transduction pathways of wounding and pathogen attack may be common, different, or exclusive, depending on the biological system, but likewise the establishment of defense mechanisms requires the presence or accumulation of hydrogen peroxide (H2O2; Sutherland, 1991; Mehdy, 1994; Hammond-Kosack et al., 1996). In particular, H2O2 behaves as a direct cytotoxic compound against pathogens and as a second messenger in the activation of defense genes (Lamb and Dixon, 1997). Moreover, this compound is involved in systemic acquired resistance and acts synergistically with NO in the induction of hypersensitive cell death (Delledonne et al., 1998). As a cosubstrate of the peroxidases, H2O2 has been implicated in the oxidative cross-linking of apoplastic structural proteins as well as in lignin and suberin polymerization. These events strengthen the plant cell wall after mechanical damage or pathogen challenge and make it less susceptible to the action of microbial lytic enzymes (Mehdy, 1994; Hammond-Kosack et al., 1996). Given its limited lifetime and its toxicity potential, H2O2 must be generated in situ and its level must be finely regulated. In this context, proteins involved in the regulation of H2O2 levels in the extracellular matrix probably play a crucial role. In the apoplast, the accumulation of H2O2 may result by the activity of a plasma membrane NAD(P)H oxidases (Doke, 1995; Lamb and Dixon, 1997), cell wall oxalate oxidases (Lane, 1994), peroxidases (Bolwell et al., 1995), and FAD and copper-containing amine oxidase (Allan and Fluhr, 1997; Rea et al., 1998; Laurenzi et al., 1999).
Copper amine oxidase (CuAO; EC 1.4.3.6) catalyzes the oxidative deamination of various biological active amines with the production of the corresponding aminoaldehydes, H2O2, and NH3 (Smith, 1985). The production of H2O2 raised upon amine degradation has been correlated with oxidative burst, cell death, as well as peroxidase-mediated lignification, suberization, and cell wall polymer cross-linking occurring during ontogenesis and defense responses (Allan and Fluhr, 1997; Møller and McPherson, 1998; Rea et al., 1998; Wisniewski et al., 2000). CuAO is the most abundant soluble protein detected in the extracellular fluids from Fabaceae, in particular, pea (Pisum sativum), lentil (Lens culinaris), and chickpea (Cicer arietinum) seedlings (Federico and Angelini, 1991). CuAO and peroxidase activities are spatially correlated and induced during wound healing (Angelini et al., 1990; Scalet et al., 1991). Rapid accumulation of CuAO mRNA was observed upon wounding and it has been correlated with the repair process occurring after injury (Rea et al., 1998). Moreover, CuAO activity is higher, and increases to a greater extent upon infection, in chickpea cultivars resistant to the fungus Ascochyta rabiei (Pass.) Lab., compared with the susceptible ones (Angelini et al., 1993). In this host-pathogen interaction resistance is not related to a hypersensitive response. The reaction of resistant cultivars includes necroses as disease symptoms, although of reduced extent as compared with necroses observed on susceptible cultivars (Porta-Puglia et al., 1996). It has been reported that haloamines behave as selective, suicide inhibitors of CuAO. In particular, 2-bromoethylamine inactivates the enzyme irreversibly with a Ki = 10−6 m (Medda et al., 1997; Yu et al., 2001). The high specificity of 2-bromoethylamine inhibition of plant CuAO is explained by the analysis of the reaction mechanism (Medda et al., 1997). 2-Bromoetylamine actually leads to the irreversible inhibition of CuAO through a mechanism-based inactivation of the enzyme cofactor tri-hydroxy-Phe chinone. Inhibition is because of the aldehyde oxidation product rather than the short chain amine itself. In fact, the aldehyde oxidation product forms a stable six-membered ring causing irreversible inhibition of the enzyme (Medda et al., 1997).
In this paper, we report a detailed study on the regulatory mechanisms and the molecular signals leading to the modulation of chickpea CuAO after wounding and we demonstrate the relevance of these enzymes as an H2O2-delivering system in wound response, as well as in the protection against fungal invasion, also exploiting in vivo 2-bromoethylamine inhibition of CuAO.
RESULTS
CuAO Is Locally and Systemically Induced in Response to Wounding
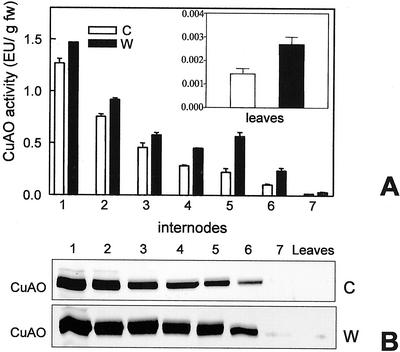
It is now well established that some classes of defense-related proteins accumulate only at the site of injury in response to wounding, whereas others accumulate both locally and systemically (Bowles, 1990). It has been demonstrated recently that in chickpea seedlings, CuAO expression is increased locally (Rea et al., 1998). To assess if CuAO is also associated with metabolic changes leading to systemic protection, we have undertaken a detailed study on the wound-induced expression of this enzyme. The fifth internode of 10-d-old seedlings was longitudinally injured with a blade and all the internodes as well as the apical leaves were tested for CuAO accumulation, through enzymatic activity determination and immunoblotting analysis, 24 h after damage. Wounding induced an increase in the level of CuAO activity not only at the site of injury, but also throughout the whole stem and leaves (Fig. 1A). In the internodes and the leaves above the lesion, the increase in CuAO activity level was similar to that observed in the wounded internode (2.5-fold), whereas in the internodes below the lesion only a small increase was observed (1.2-fold). Western-blot analysis revealed a close correlation between CuAO enzymatic activity and protein level (Fig. 1B).
Figure 1.
Local and systemic induction of CuAO in response to internode wounding. A, CuAO activity assayed in internodes and leaves of control (C) and wounded (W) plants 24 h after wounding the fifth internode. B, Western-blot analyses performed on internodes of control and wounded plants, respectively. 1 through 7, Internodes numbered from the base toward the seedling apex. Bars represent sd from three independent experiments.
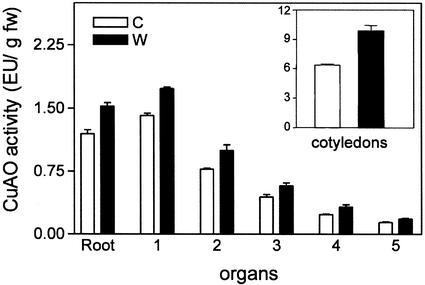
To test whether the systemic induction of CuAO expression occurs also by wounding other organs, cotyledons of 10-d-old seedlings were wounded with several cuts and CuAO activity evaluated 24 h later both locally and at distal organs (roots, internodes, and apical leaves). As shown in Figure 2, wounding of cotyledons also determined a systemic increase of CuAO activity in all the organs tested.
Figure 2.
Local and systemic induction of CuAO in response to cotyledon wounding. CuAO activity determined in cotyledons, roots, and internodes 24 h after wounding of cotyledons. 1 through 5, Internodes numbered from the base toward the seedling apex. C, Control; W, wounded. Bars represent sd from three independent experiments.
It has been demonstrated previously that CuAO expression is locally induced 3 h after wounding (Rea et al., 1998). To assess whether the systemic response is also a rapid process, we analyzed the time course of CuAO accumulation in wounded and distal internodes. The results reported in Figure 3 demonstrate that CuAO expression is induced as early as 3 h after damage in all the analyzed organs, indicating a rapid systemic response.
Figure 3.
Timing of local and systemic induction of CuAO in response to wounding. The fourth internode of chickpea seedlings was longitudinally injured with a blade and CuAO activity determined in the third, fourth, and fifth internodes at the indicated times. C, Control; W, wounded. Bars represent sd from four independent experiments.
Jasmonic Acid (JA) Is a Potent Inducer of CuAO Expression
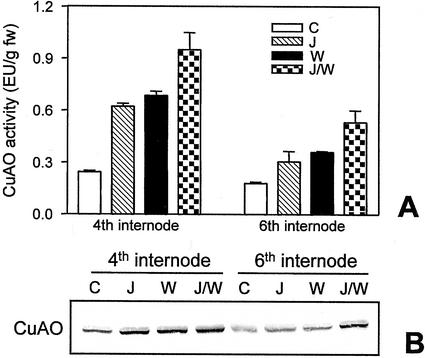
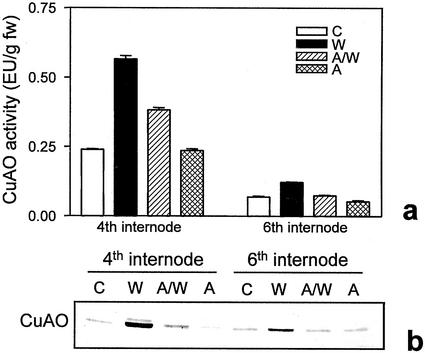
It has been shown that wounding triggers an increase in the endogenous levels of the plant growth regulator JA that is required to activate several wound-responsive genes both locally and systemically (Creelman and Mullet, 1997; Wasternack and Parthier, 1997). To investigate whether JA is part of the signal transduction pathway leading to wound-induced CuAO expression, we analyzed the effect of JA on 10-d-old excised chickpea epicotyls. The exogenous application of this compound caused a pronounced increase (2.5-fold) of CuAO activity and protein level in unwounded epicotyls and potentiated the wound-induced CuAO expression in the same organs (Fig. 4, A and B). These regulatory mechanisms were also observed in the upper adjacent internode of wounded plants suggesting that this hormone may mediate local and systemic induction of CuAO in response to wounding.
Figure 4.
JA effect on CuAO expression. A, CuAO activity determined in the fourth and sixth internodes of excised chickpea control epicotyls (C), or treated with JA (J), or wounded at the fourth internode (W), or wounded 8 h after the onset of JA-treatments (J/W). Analyses were carried out 24 h after epicotyl detachment. Bars represent sd from three independent experiments. B, Western-blot analyses performed on the same samples analyzed for CuAO activity.
SA Blocks Wound- and JA-Induced CuAO Expression
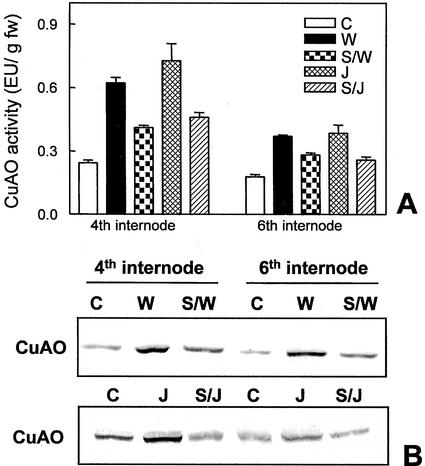
Because it has been reported that SA can act as a negative regulator for several wound-induced genes (Doherty et al., 1988; Peña-Cortés et al., 1993; Doares et al., 1995; Harms et al., 1998), we studied the role of SA on CuAO expression. Chickpea epicotyls excised from the cotyledons were supplied with 1 mm SA and then analyzed for CuAO accumulation in response to wounding. Results from enzyme activity determination and western-blot analyses indicated that although SA did not affect the steady-state level of CuAO expression (data not shown), it led to a 50% reduction of the wound-induced CuAO accumulation (Fig. 5, A and B). Furthermore, SA also repressed the systemically induced CuAO expression in upper internodes (Fig. 5, A and B), indicating a common feature in both local and systemic regulation of the wound-inducible CuAO expression. Our experiments demonstrated also that SA strongly inhibited the JA-induced increases of CuAO activity (Fig. 5A) and protein levels (Fig. 5B) in wounded and distal unwounded internodes. Control experiments revealed that activity of purified chickpea CuAO was not affected by incubation with 1 mm SA or 50 μm JA (data not shown), excluding a direct effect of these compounds on CuAO activity.
Figure 5.
Effect of SA on the wound- or JA-induced CuAO accumulation. A, CuAO activity determined in the fourth and sixth internodes of excised chickpea control epicotyls (C), or wounded at the fourth internode (W), or supplied with JA (J), or pretreated with SA and then wounded (S/W), or pretreated with SA and then supplied with JA (S/J). Analyses were performed 24 h after epicotyl detachment. Values presented are the mean of five independent experiments. B, Western-blot analyses performed on the same samples analyzed for CuAO activity. Experiments were performed three times giving highly reproducible results.
ABA Negatively Regulates Wound-Induced CuAO Expression
ABA is an essential mediator in triggering the plant responses to adverse environmental stimuli such as water stress, high salinity, and low temperature (Giraudet et al., 1994; Shinozaki and Yamaguchi-Shinozaki, 1996). Recently, it has been included among the primary components of the systemic wound-signaling cascade leading to proteinase inhibitors accumulation (Herde et al., 1996; Peña-Cortés et al., 1996; Wasternack and Parthier, 1997), even if its role in the wound response is still debated (Birkenmeier and Ryan, 1998). We have assessed the involvement of ABA in CuAO regulation in healthy and wounded plants. Figure 6 shows that in seedlings continuously supplied with ABA, CuAO activity and protein levels are unaffected compared with control plants supplied with buffer alone. In contrast, treating plants with ABA before wounding caused a strong reduction of the wound-induced enzyme activity and protein level. Similarly to what was observed for SA, ABA also repressed the systemically induced CuAO expression in distal internodes (Fig. 6).
Figure 6.
ABA effect on CuAO expression. a, CuAO activity determined in the fourth and sixth internodes of excised chickpea control epicotyls (C), or treated with ABA (A), or wounded at the fourth internode (W), or pretreated with ABA, and then wounded (A/W). Analyses were carried out 24 h after epicotyl detachment. Bars represent sd calculated from values obtained in four independent experiments. b, Western-blot analyses were performed on the same samples analyzed for CuAO activity.
Chickpea CuAO mRNA Accumulation after Hormonal Treatment and Wounding
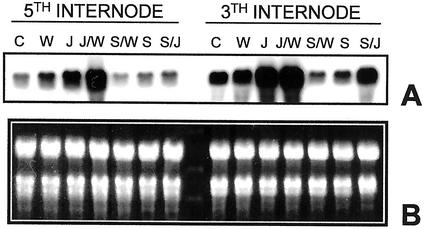
To gain a further insight into the molecular mechanism controlling CuAO expression after mechanical injury, we measured the CuAO transcript accumulation by northern-blot analysis on plants treated with SA and/or JA and then wounded. Figure 7 shows that in response to wounding, the steady-state level of CuAO mRNA increased locally and systemically compared with unwounded plants. This increase was higher at the site of injury in respect to that observed in the adjacent organs, similar to what has been observed at the protein level. As far as the JA effect is concerned, densitometric analyses of the hybridization signals indicated that CuAO transcript levels increased 5 times in plants treated with the hormone and 10 times in JA-treated/-wounded plants. Northern-blot analysis has also demonstrated that SA treatment inhibited the wound- and JA-induced CuAO transcript accumulation in both wounded and distal internodes. A similar CuAO regulation was observed in ABA-treated plants (data not shown). As a whole the above results suggest, although they do not prove, that wounding, JA, and SA may regulate CuAO expression acting at the transcriptional level, without excluding the possibility of additional posttranscriptional and posttranslational regulation mechanisms.
Figure 7.
Effect of salicylic and JA on local and systemic CuAO mRNA accumulation. A, Northern blot hybridized to chickpea 32P-labeled CuAO cDNA and carried out on total RNA extracted from the third and fifth internodes of control epicotyls (C), or treated with JA (J), or SA (S), or wounded at the third internode (W), or pretreated with SA and then wounded (S/W), or pretreated with SA and then supplied with JA (S/J). B, The fluorescence intensity of ethidium bromide stained rRNA evidenced equal loading for each sample. Experiments were performed three times yielding highly reproducible results.
A Mechanism-Based CuAO Inhibitor Reduces H2O2 Accumulation in Chickpea Epicotyls
2-Bromoethylamine is a specific and irreversible CuAO inhibitor (Medda et al., 1997). This compound was effective in totally inhibiting CuAO activity in all internodes, when supplied in excised epicotyls (data not shown). The CuAO inhibitor was used to evaluate the relevance of this enzyme in H2O2 production during physiological conditions and the wound response. To detect changes in H2O2 levels, we used 3,3′-diaminobenzidine (DAB), which is oxidized by endogenous peroxidases in the presence of H2O2-generating deep brown polymers (Thordal-Christensen et al., 1997). The accumulation of the DAB oxidation product in chickpea epicotyls, in the presence or absence of 2-bromoethylamine, is shown in Figure 8. Our experiments demonstrated lower levels of brown DAB polymers accumulated in the inhibitor-treated plants (Fig. 8B, clearly visible in internodes IV–VI) compared with untreated control plants (Fig. 8A). Treatment of plants with 2-bromoethylamine before wounding caused a decrease of wound-induced H2O2 production (Fig. 8D, internodes IV and V, versus Fig. 8C). Cross inhibition study revealed that 2-bromoethylamine at concentrations up to 8 × 10−3 m did not affect peroxidase, catalase, superoxide dismutase, and oxalate oxidase activity either in pure enzymes or crude homogenates obtained from inhibitor-treated plants (percent activity inhibition was less than 5% compared with the untreated plants or non pre-incubated pure enzyme).
Figure 8.
Effect of CuAO inhibition on H2O2 levels in healthy and wounded chickpea epicotyls. Analyses were carried out by allowing uptake of DAB by control epicotyls (A), or treated with 2-bromoethylamine (B), or wounded (C), or pretreated with inhibitor and then wounded (D). Arrows indicate the wounded fourth internodes.
CuAO Inhibition by 2-Bromoethylamine Decreases Defense Capacity of a Chickpea Cultivar Resistant to A. rabiei
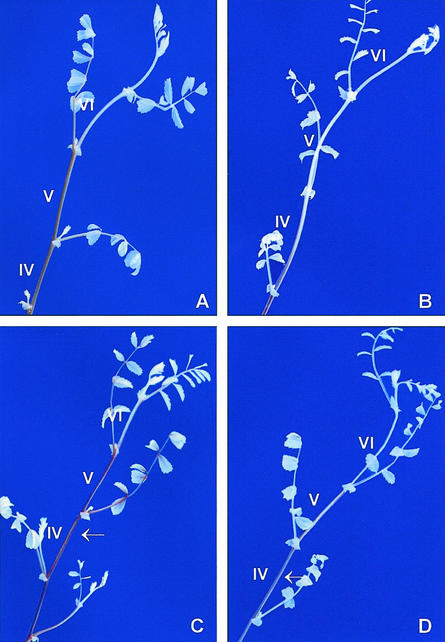
The necrotrophic fungus A. rabiei is the major pathogen of chickpea causing blight on all the aboveground parts of the plants. On stems, the symptoms of disease appear as expanding necrotic areas and, depending on pathotype aggressiveness and cultivar susceptibility, lesions elongate to varying extents, often girdling the stem. Severe attacks of the pathogen can result in stem breakage and, as a consequence, in heavy yield losses (Akem, 1999). During the chickpea/A. rabiei interaction, CuAO activity is induced in parallel with polyamine levels and peroxidase activity (Angelini et al., 1993). These results led us to hypothesize the involvement of CuAO in chickpea defense responses against A. rabiei. Thus, we studied the effect of in vivo CuAO inhibition by 2-bromoethylamine on symptom development of the resistant chickpea cv Sultano, inoculated with the isolate ER33 of A. rabiei. As soon as necrosis appeared, epicotyls were treated with the CuAO inhibitor and were analyzed 7 d later (see “Materials and Methods”). Chickpea cv Sultano plants challenged by the fungus exhibited localized and restricted necroses with average disease rating of 1.2 ± 0.42 (sd; Fig. 9A). In contrast, the infected and inhibitor-treated plants (Fig. 9B) showed higher disease rating, averaging 3.7 ± 0.48 (sd), together with a dramatic modification on necroses morphology. In the latter, in fact, as a consequence of CuAO inhibition, lesions extended lengthwise along the internode, even if they never girdled the organ.
Figure 9.
Effect of CuAO inhibition on the defense capacity of the chickpea cv Sultano resistant to A. rabiei. Photographs A and B depict internodes of inoculated chickpea epicotyls treated or not treated with 2-bromoethylamine, respectively. Magnification 16×. Photographs C through F show berberine-aniline blue staining of transverse sections from chickpea epicotyls. C, Control internode; D, inhibitor-treated internode; E, inoculated internode; F, inoculated and inhibitor-treated internode. Sections from infected internodes (E and F) were cut at the center of the superficial area of the necrosis. Magnification 116×, bar = 70 μm.
The effects of haloamine treatment on healthy and infected tissues were also analyzed at the microscopic level by lignin-suberin staining with berberine-aniline blue (Fig. 9, C–F). It is interesting to note that in plants treated with 2-bromoethylamine (Fig. 9, D and F), the number of lignified xylem cells and derivatives of vascular cambium in contiguity with xylem cells (white-bluish fluorescence) is lower compared with those of the untreated plants (Fig. 9, C and E). The number of fluorescent cells is actually 7.65 ± 0.57 (sd) and 7.30 ± 0.75 (sd) in noninfected and infected plants, respectively, whereas it is 3.85 ± 0.28 (sd) and 3.6 ± 0.38 (sd) in inhibitor-treated noninfected and infected plants, respectively. In infected plants treated with 2-bromoethylamine, the fungus penetrated the cuticle and dramatically spread, causing extensive damage to the cortical parenchyma and sclerenchyma (Fig. 9F). In contrast, in infected plants not treated with the inhibitor, the fungus invaded a small number of epidermal and cortical parenchyma cells, causing only limited damage (Fig. 9E).
No CuAO activity was detected in the A. rabiei mycelium or in liquid culture medium for the fungus growth (A. Porta-Puglia, personal communication). Moreover, control experiments performed on A. rabiei growth revealed that 2-bromoethylamine does not modify morphology, growing rate, and development of the mycelial mass forming pycnidia.
DISCUSSION
Infection by opportunistic microorganisms can arise from mechanical wounding caused by environmental stresses. As a consequence, plants respond to physical injury activating genes involved in the repair process of the lesions, as well as in the enhancement of resistance to parasites (Bowles, 1990). Most of these inducible responses occur in a complex temporal pattern around the wound site and systemically throughout the whole plant.
The involvement of CuAO in both wound healing and pathogen defenses (Scalet et al., 1991; Angelini et al., 1993) prompted us to examine its specific role in these processes. In particular, wounding of chickpea organs resulted in the local and systemic induction of CuAO expression with the highest induction levels at the wound site. The kinetics of CuAO accumulation was the same in damaged as well as in distal internodes, starting 3 h after injury and increasing thereafter in a time-dependent way (Rea et al., 1998; this paper). The occurrence of local and systemic responses suggests the possible relevance of these enzymes in defense. Determination of CuAO activity at shorter times did not evidence significant increases. However, the high basal CuAO activity levels found in chickpea plants may represent a constitutive defense at the onset of wounding or pathogen attack. The inducible increases boost the defense response and may be related to the specific incoming stress (suberin synthesis and wall fortification during wound healing; production of H2O2 to kill the pathogen and for suberizing barrier to confine it). There is a positive correlation between basal CuAO activity levels as well as the extent of enzyme activity increase upon infection and degree of resistance of chickpea to A. rabiei (Angelini et al., 1993).
The mechanisms by which plants regulate inducible defenses are not yet completely understood. Several molecules are involved in different transduction pathways and, among them, SA and JA play important roles. Many reports support the hypothesis that interaction between pathways, rather than linear signaling cascades, fine-tunes these complex defense responses, providing great regulatory potential (Maleck and Dietrich, 1999; van Wees et al., 2000). The best evidence for cross talk between induced defenses is observed in the systemic acquired resistance and wound response pathways, which require SA and JA as local and systemic effectors. SA has been shown to be a negative regulator for defense gene induction by JA in several plant species (Doherty et al., 1988; Peña-Cortés et al., 1993; Doares et al., 1995; Penninckx et al., 1996; Bowling et al., 1997; Harms et al., 1998; van Wees et al., 1999). Similarly, JA action has been reported to inhibit salicylate-dependent pathways (Niki et al., 1998). Conversely, JA and ethylene have been shown to stimulate SA effects in Arabidopsis plants (Lawton et al., 1994; Xu et al., 1994).
In an attempt to dissect the molecular mechanisms controlling CuAO expression upon mechanical injury, we have tested several candidates that are known to mediate the wound and pathogen signaling in plants. Experiments focusing on SA and JA interactions indicated the existence of an inverse relationship between these compounds. Exogenously applied JA induced CuAO accumulation in healthy as well as wounded plants, mimicking the effect of mechanical injury. In contrast, SA was unable to significantly modulate the constitutive expression of CuAO, but it strongly suppressed the wound- and JA-induced increases of the enzyme expression. These effects were observed in wounded as well as in distal unwounded internodes, indicating a common feature in both local and systemic CuAO activation. Because JA was exogenously applied in these experiments, SA could act as a negative effector in the signaling pathway leading to CuAO induction, proceeding on steps that are downstream from the JA biosynthesis. These regulatory mechanisms closely resemble those observed for proteinase inhibitors of solanaceous species that help to protect plants against herbivores (Ryan, 1990; Peña-Cortés et al., 1993; Doares et al., 1995).
The phytohormone ABA has been included among the components of the systemic signaling cascade leading to wound-induced gene activation, even though its specific role is still debated (Peña-Cortés et al., 1989, 1993, 1996; Herde et al., 1996; Birkenmeier and Ryan, 1998). Our studies indicate that ABA plays a negative role in the wound-induced expression of CuAO in chickpea seedlings. This phenomenon remains to be elucidated for CuAO as well as for other wound-induced metabolic pathways similarly regulated. ABA, in fact, inhibits the defense-induced transcriptional activation of Phe ammonia lyase, the glucan-induced isoflavone response, and glyceollin accumulation in soybean (Glycine max; Ward et al., 1989; Graham and Graham, 1996).
H2O2 produced during the wound response as well as in plant-pathogen interactions is also involved in the modification of the plant cell wall occurring after pathogen challenge (Mehdy, 1994; Hammond-Kosack et al., 1996). We have demonstrated that 2-bromoethylamine, a CuAO inhibitor, strongly reduces H2O2 accumulation generated in physiological conditions as well as in response to wounding. The latter phenomenon was observed both locally and in distal organs. Hence, in these species CuAO could play a role in the balance of reactive oxygen species produced in the extracellular matrix. Furthermore, these H2O2-producing enzymes have also a role in defense against A. rabiei. By using 2-bromoethy-lamine, we have demonstrated that CuAO inhibition can modify the defense capacity of the chickpea cv Sultano, resistant to the fungus. A possible explanation is that CuAO inhibition may result in a low H2O2 production in the apoplast, leading to a reduced mechanical resistance and suberization of cell walls. This phenomenon may allow higher fungal spread and diffusion of solanapyrone toxins (Alam et al., 1989). At the macroscopic level, in fact, treatment with the inhibitor caused the formation of more extended necrotic lesions, compared with the untreated ones. Histology revealed that germ tubes of conidia penetrate the stem tissues producing extended mycelial mass in both treated and untreated plants. These events caused extensive damage to cortical parenchyma cells (Höhl et al., 1990; this paper). It is interesting that infection symptoms were more extended in 2-bromoethylamine treated plants that, in addition, displayed damaged sclerenchyma tissue and a lower number of lignified xylem tracheary elements.
These results, beside stressing that wound-induced CuAO regulation is under the fine-tuning by the key signaling molecules JA, SA, and ABA, demonstrate that local and systemic CuAO induction are essential for H2O2 production during ontogenesis as well as in response to wounding and suggest that a similar phenomenon can be operating in defense strategies against pathogens.
MATERIALS AND METHODS
Plant Materials
Chickpea (Cicer arietinum L. cv Sultano) seeds were soaked overnight in aerated water and grown in soil in a greenhouse under natural light conditions for 10 d at 25°C. After treatments, organs were harvested, frozen in liquid N2, and stored at −80°C until use.
Wounding and Treatments
Ten-day-old chickpea seedlings were longitudinally wounded with a blade at the fourth or fifth internode or on the cotyledons, as indicated in the specific experiments. Wounded as well as distal organs were collected in parallel with those from control plants, at the indicated times.
CuAO modulation by JA, SA, and ABA was studied using excised epicotyls supplied through their cut surface with 15 mm NaPi, pH 6.5 (NaPi buffer), or 50 μm JA, or 1 mm SA, or 100 μm ABA for 24 h. Analyses were also performed on a set of epicotyls wounded immediately after excision, or 8 h after the onset of a pretreatment with NaPi buffer containing 50 μm JA, or 1 mm SA, or 100 μm ABA. After injury, plants were continuously supplied with the same solutions for 16 h. The effect of SA on the JA-inducible CuAO expression was determined pretreating excised epicotyls for 8 h with NaPi buffer containing 1 mm SA, before supplying them for 16 h with the same solution containing or not containing 50 μm JA.
Infection Experiments
The fourth internodes of 10-d-old chickpea seedlings were inoculated using a paintbrush with 0.4% (w/v) agar-water containing 1.5 × 106 spores mL−1 of an Ascochyta rabiei (Pass.) Lab. isolate characterized by an intermediate level of virulence (Porta-Puglia et al., 1996) and maintained at the Istituto Sperimentale per la Patologia Vegetale collection (Rome) as isolate ER33 (A. Porta-Puglia, personal communication). After inoculation, seedlings were transferred into a growth cabinet at 20°C in which humidity was constantly maintained near saturation. Inoculated internodes were checked daily for the appearance of symptoms. Epicotyls with necroses comparable in extension were detached from the cotyledons and treated for 7 d with NaPi buffer containing or not containing the CuAO inhibitor 2-bromoethylamine, at a 5 mm final concentration. Seven days after treatment, both inhibitor-treated and untreated internodes were evaluated for symptoms. A 0 to 5 scale, based on the visual estimation of necrotic internode surface, was used for disease rating (0, no symptoms; 1, superficial lesions covering up to 10% of the internode surface; 2, superficial lesions covering more than 10% and up to 25% of the internode surface; 3, superficial lesions covering more than 25% and up to 50% of the internode surface; 4, superficial lesions covering more than 50% and up to 75% of the internode surface; and 5, superficial lesions covering more than 75% of the internode surface).
Northern-Blot Analyses
Total RNA was isolated using the Trizol Reagent (Gibco-BRL, Cleveland) and following the manufacturer's instruction. Blotting and hybridization procedures were performed as reported by Sambrook et al. (1989). Twenty micrograms of total RNA were loaded for each sample. Membranes were hybridized by using 50 ng of [32P]ATP-labeled chickpea cDNA (pCSAO, Rea et al., 1998), using high-stringency conditions.
Enzyme Assays and Western-Blot Analyses
The extraction of soluble proteins from different chickpea organs was performed by homogenization (1:2 [w/v]) in 0.2 m NaPi, pH 7.0. CuAO activity was assayed as previously reported (Angelini et al., 1996). Enzymatic unit is defined as the enzyme amount catalyzing the oxidation of one μmol of substrate × min−1. The inhibitory effect of 2-bromoethylamine was checked on pure chickpea CuAO, horseradish peroxidase, bovine liver and Aspergillus niger catalase, horseradish superoxide dismutase, barley oxalate oxidase (all purchased from Sigma [St. Louis] except chickpea CuAO that was previously purified by conventional chromatographic procedures) or on enzyme activities determined on inhibitor-treated plant homogenates according to assays described by Angelini et al. (1996), Egley et al. (1983), Beers and Sizer (1952), Mishra and Fridovich (1972), and Lane et al. (1993), respectively). Total soluble proteins were quantified according to Bradford (1976) using bovine serum albumin as a standard reference. Western-blot analyses were performed on 20 μg of total soluble proteins of each extract. After electroblotting, nylon membranes were tested for equal loading by staining with Ponceau S (Sigma; 0.1% [w/v] Ponceau S in 5% [v/v] acetic acid; data not shown). A 1,000-fold diluted lentil (Lens culinaris)-CuAO rabbit polyclonal antibody (Federico et al., 1985) and a 5,000-fold diluted peroxidase-conjugated goat anti-rabbit IgG (Sigma) were employed to detect CuAO protein accumulation using 4-chloro-1-naphtol (Sigma) and H2O2 as substrates, according to the manufacturer's instruction. Experiments were performed independently at least five times, yielding reproducible results. Single representative experiments are shown in the figures.
In Vivo Detection of H2O2
H2O2 was directly visualized in detached chickpea epicotyls by using 1 mg mL−1 DAB water solution, pH 3.8, as described by Thordal-Christensen et al. (1997). Excised epicotyls were pretreated either with NaPi buffer containing 5 mm 2-bromoethylamine or buffer alone for 12 h and subsequently wounded on the fourth internode and supplied for 8 h with DAB solution in parallel with unwounded control plants. After treatments, all the plants were soaked 15 min in 95% (v/v) boiling ethanol to remove pigments, transferred to fresh ethanol, and photographed.
Histochemical Methods
Ten-day-old chickpea cv Sultano seedlings were inoculated with A. rabiei on the fourth internodes as described above. Epicotyls were excised 10 d postinoculation and supplied with NaPi buffer containing or not containing 5 mm 2-bromoethylamine for 7 d. The fourth internodes were then cut in 4- to 5-mm segments, oriented in 4% (w/v) agar, and cut to a thickness of 100 μm with a vibratome. Lignin and suberin depositions were detected by the berberine-aniline blue fluorescent staining procedure according to Brundrett et al., (1988). The experiments were repeated three times with very similar results.
ACKNOWLEDGMENTS
We wish to thank Paraskevi Tavladoraki (University “Roma Tre”) and Angelo Porta-Puglia (Istituto Sperimentale per la Patologia Vegetale) for critical reading of the manuscript. We also gratefully acknowledge Renato D'Ovidio (University of Tuscia, Viterbo, Italy) for many helpful suggestions and technical assistance.
Footnotes
This work was supported by the Italian Ministry for University and Scientific Research and by the European Commission (project Faba Bean Resistance and Yield in Mediterranean Area, contract no. 18–CT 98–0300, as part of O.M.'s doctorate thesis).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010646.
LITERATURE CITED
- Akem C. Ascochyta blight of chickpea: present status and future priorities. Int J Pest Manag. 1999;45:131–137. [Google Scholar]
- Alam SS, Bilton JM, Slavin AMZ, Williams DJ, Sheppard RN, Strange RN. Chickpea blight: production of the phytotoxins solanapyrones A and C by Ascochyta rabiei. Phytochemistry. 1989;28:2627–2630. [Google Scholar]
- Allan AC, Fluhr R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell. 1997;9:1559–1572. doi: 10.1105/tpc.9.9.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini R, Manes F, Federico R. Spatial and functional correlation between diamine oxidase and peroxidase activities and their dependence upon de-etiolation and wounding in chickpea stem. Planta. 1990;182:89–96. doi: 10.1007/BF00239989. [DOI] [PubMed] [Google Scholar]
- Angelini R, Bragaloni M, Federico R, Infantino A, Porta-Puglia A. Involvement of polyamines, diamine oxidase and peroxidase in resistance of chick-pea to Ascochyta rabiei. J Plant Physiol. 1993;142:704–709. [Google Scholar]
- Angelini R, Rea G, Federico R, D'Ovidio R. Spatial distribution and temporal accumulation of mRNA encoding diamine oxidase during lentil (Lens culinaris Medicus) seedling development. Plant Sci. 1996;119:103–110. [Google Scholar]
- Beers RF, Sizer IW. Colorimetric method for estimation of catalase. J Biol Chem. 1952;195:133–139. [PubMed] [Google Scholar]
- Birkenmeier GF, Ryan CA. Wound signaling in tomato plants: evidence that ABA is not a primary signal for defense gene activation. Plant Physiol. 1998;117:687–693. doi: 10.1104/pp.117.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Butt VS, Davies DR, Zimmerlin A. The origin of the oxidative burst in plant. Free Radic Res. 1995;23:517–532. doi: 10.3109/10715769509065273. [DOI] [PubMed] [Google Scholar]
- Bowles DJ. Defense related proteins in higher plants. Annu Rev Biochem. 1990;28:113–138. [Google Scholar]
- Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X. The cpr5 mutant of arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell. 1997;9:1573–1584. doi: 10.1105/tpc.9.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brundrett MC, Enstone DE, Peterson CA. A berberine-aniline blue fluorescent staining procedure for suberin, lignin and callose in plant tissue. Protoplasma. 1988;146:133–142. [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonate in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–387. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- Doares SH, Narvaez-Vasquez J, Conconi A, Ryan CA. Salicylic acid inhibits synthesis of proteinase inhibitor in tomato leaves induced by systemin and jasmonic acid. Plant Physiol. 1995;108:1741–1746. doi: 10.1104/pp.108.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty HM, Selvendram RR, Bowles DJ. The wound response of tomato plants can be inhibited by aspirin and related hydroxy-benzoic acid. Physiol Mol Plant Pathol. 1988;33:377–384. [Google Scholar]
- Doke N. NADPH-dependent O2− generation in membrane fraction isolated from wounded potato tubers inoculated with Phytophtora infestans. Physiol Plant Pathol. 1995;27:311–322. [Google Scholar]
- Egley GH, Paul RN, Vaughn KC, Duke SO. Role of peroxidase in the development of water impermeable seed coats in Sida spinosa L. Planta. 1983;157:224–232. doi: 10.1007/BF00405186. [DOI] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci USA. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico R, Angelini R. Polyamine catabolism in plant. In: Slocum RD, Flores HE, editors. Biochemistry and Physiology of Polyamines in Plants. Boca Raton, FL: CRC Press; 1991. pp. 41–56. [Google Scholar]
- Federico R, Di Lisi F, Angelini R. Purification of diamine oxidase from Lens culinaris by affinity chromatography. Plant Sci. 1985;38:9–12. [Google Scholar]
- Giraudet J, Parcy F, Bertauche N, Gosti F, Leung J, Morris PC, Bouvier Durand M, Vartanian N. Current advances in abscisic acid action and signaling. Plant Mol Biol. 1994;26:1557–1577. doi: 10.1007/BF00016490. [DOI] [PubMed] [Google Scholar]
- Graham TL, Graham MY. Signaling in soybean phenylpropanoid responses: dissection of primary, secondary and conditioning effects of light, wounding and elicitor treatments. Plant Physiol. 1996;110:1123–1133. doi: 10.1104/pp.110.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JDG. Resistance gene-dependent plant defense responses. Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms K, Ramirez I, Peña-Cortés H. Inhibition of wound-induced accumulation of allene oxide synthase transcripts in flax leaves by aspirin and salicylic acid. Plant Physiol. 1998;118:1057–1065. doi: 10.1104/pp.118.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herde O, Atzorn R, Fisahn J, Wasternack C, Willmitzer L, Peña-Cortés H. Localized wounding by heat initiates the accumulation of proteinase inhibitor II in abscisic acid-deficient plant by triggering jasmonic acid biosynthesis. Plant Physiol. 1996;112:853–860. doi: 10.1104/pp.112.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhl B, Pfautsch M, Barz W. Histology of disease development in resistant d susceptible cultivars of chickpea (Cicer arietinum L.) inoculated with spores of Ascochyta rabiei. J Phytopathol. 1990;129:31–45. [Google Scholar]
- Knoester M, Pieterse CMJ, Bol JF, van Loon LC. Systemic resistance in Arabidopsis induced by rhizobacteria requires ethylene-dependent signaling at the site of application. Mol Plant-Microbe Interact. 1999;12:720–727. doi: 10.1094/MPMI.1999.12.8.720. [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Lane BG. Oxalate, germin, and the extracellular matrix of higher plants. FASEB J. 1994;8:294–301. doi: 10.1096/fasebj.8.3.8143935. [DOI] [PubMed] [Google Scholar]
- Lane BG, Dunwell JM, Ray JA, Schmitt MR, Cuming AC. Germin, a protein marker of early plant development, is an oxalate oxidase. J Biol Chem. 1993;268:12239–12242. [PubMed] [Google Scholar]
- Laurenzi M, Rea G, Federico R, Tavladoraki P, Angelini R. De-etiolation causes a phytochrome-mediated increase of polyamine oxidase expression in outer tissues of the maize mesocotyl: a role in the photomodulation of growth and cell wall differentiation. Planta. 1999;208:146–154. [Google Scholar]
- Lawton KA, Potter SL, Uknes S, Ryals J. Systemic acquired resistance signal transduction in Arabidopsis is ethylene independent. Plant Cell. 1994;6:581–588. doi: 10.1105/tpc.6.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleck K, Dietrich RA. Defense on multiple fronts: How do plants cope with diverse enemies? Trends Plant Sci. 1999;4:215–219. doi: 10.1016/s1360-1385(99)01415-6. [DOI] [PubMed] [Google Scholar]
- Medda R, Padiglia A, Pedersen JZ, Agrò AF, Rotilio G, Floris G. Inhibition of copper amine oxidase by haloamines: a killer product mechanism. Biochemistry. 1997;36:2595–2602. doi: 10.1021/bi961410w. [DOI] [PubMed] [Google Scholar]
- Mehdy MC. Active oxygen species in plant defense against pathogen. Plant Physiol. 1994;105:467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Møller SG, McPherson MJ. Developmental expression and biochemical analyses of the Arabidopsis atao1 gene encoding an H2O2-generating diamine oxidase. Plant J. 1998;13:781–791. doi: 10.1046/j.1365-313x.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- Niki T, Mitsuhara I, Seo S, Ohtsubo N, Ohashi Y. Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tobacco leaves. Plant Cell Physiol. 1998;108:1741–1746. [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyse HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- Pearce G, Strydom D, Johnson S, Ryan CA. A polypeptide from tomato induces wound-inducible proteinase inhibitor proteins. Science. 1991;253:895–898. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- Peña-Cortés H, Prat S, Atzorn R, Wasternack C, Willmitzer L. Abscisic acid-deficient plants do not accumulate proteinase inhibitor II following systemin treatment. Planta. 1996;198:447–451. [Google Scholar]
- Peña-Cortés H, Albrecht T, Prat S, Weller EW, Willmitzer L. Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta. 1993;191:123–128. [Google Scholar]
- Peña-Cortés H, Sanchèz-Serrano JJ, Mertens R, Willmitzer L, Prat S. Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci USA. 1989;86:9851–9855. doi: 10.1073/pnas.86.24.9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IAMA, Eggermont K, Terras FRG, Thomma BPHJ, De Sambianx GW, Buchala A, Metraux JP, Manners JM, Broekaert WF. Pathogen-induced systemic activation of a plant defensin gene in arabidopsis follows a salicylic acid-independent pathway. Plant Cell. 1996;8:2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta-Puglia A, Crinò P, Mosconi C. Variability in virulence to chickpea of an Italian population of Ascochyta rabiei. Plant Dis. 1996;80:30–41. [Google Scholar]
- Rea G, Laurenzi M, Tranquilli E, D'Ovidio R, Federico R, Angelini R. Developmentally and wound regulated expression of the gene encoding a cell wall copper amino oxidase in chickpea seedlings. FEBS Lett. 1998;437:177–182. doi: 10.1016/s0014-5793(98)01219-8. [DOI] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MJ, Molina A, Steiner HY, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CA. Protease inhibitors in plants: genes for improving defenses against pathogens. Annu Rev Phytopathol. 1990;93:1134–1139. [Google Scholar]
- Ryan CA. The systemin signaling pathway: differential activation of plant defensive genes. Biochim Biophys Acta. 2000;1477:112–121. doi: 10.1016/s0167-4838(99)00269-1. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Scalet M, Federico R, Angelini R. Time courses of diamine oxidase and peroxidase activities and polyamine changes after mechanical injury of chickpea seedlings. J Plant Physiol. 1991;137:571–575. [Google Scholar]
- Schweizer P, Buchala A, Dudler R, Métraux JP. Induced systemic resistance in wounded rice plants. Plant J. 1998;14:475–481. [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Molecular responses to drought and cold stress. Curr Opin Biotechnol. 1996;7:161–167. doi: 10.1016/s0958-1669(96)80007-3. [DOI] [PubMed] [Google Scholar]
- Smith TA. The di- and polyamine oxidase of higher plants. Biochem Soc Trans. 1985;13:319–322. doi: 10.1042/bst0130319. [DOI] [PubMed] [Google Scholar]
- Sutherland MW. The generation of oxygen radicals during host plant responses to infection. Physiol Mol Plant Pathol. 1991;39:79–93. [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei YD, Collinge DB. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- van Loon LC, Bakker PAHM, Pieterse CMJ. Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol. 1998;36:453–483. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- van Wees SCM, de Swart EAM, van Pelt JA, van Loon LC, Pieterse CMJ. Enhancement of induced disease resistance by simultaneous activation of salicylate- and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2000;97:8711–8716. doi: 10.1073/pnas.130425197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wees SCM, Luijendijk M, Smoorenburg I, van Loon LC, Pieterse CMJ. Rhizobacteria-mediated induced systemic resistance (ISR) in Arabidopsis is not associated with a direct effect on expression of known defense-related genes but stimulates the expression of the jasmonate-inducible genes Atvsp upon challenge. Plant Mol Biol. 1999;41:537–549. doi: 10.1023/a:1006319216982. [DOI] [PubMed] [Google Scholar]
- Ward EWB, Cahill DM, Bhattacharyya MK. Abscisic acid suppression of phenylalanine ammonia-lyase activity and messenger RNA and resistance of soybeans to Phytophtora megasperma f. sp. glycinea. Plant Physiol. 1989;91:23–27. doi: 10.1104/pp.91.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Parthier B. Jasmonate-signaled plant gene expression. Trends Plant Sci. 1997;2:1360–1385. [Google Scholar]
- Wisniewski JP, Rathbun EA, Knox JP, Brewin NJ. Involvement of diamine oxidase and peroxidase in insolubilization of the extracellular matrix: implications for pea nodule initiation by Rhizobium leguminosarum. Mol Plant-Microbe Interact. 2000;13:413–420. doi: 10.1094/MPMI.2000.13.4.413. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chang PFL, Liu D, Narasimhan ML, Raghothana KG, Hasegawa PM, Bressan RA. Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell. 1994;6:1077–1085. doi: 10.1105/tpc.6.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PH, Davis BA, Deng Y. 2-bromoethylamine as a potent selective suicide inhibitor for semicarbazide-sensitive amine oxidase. Biochem Pharmacol. 2001;61:741–748. doi: 10.1016/s0006-2952(01)00524-x. [DOI] [PubMed] [Google Scholar]