Abstract
Galactolipid biosynthesis in plants is highly complex. It involves multiple pathways giving rise to different molecular species. To assess the contribution of different routes of galactolipid synthesis and the role of molecular species for growth and photosynthesis, we initiated a genetic approach of analyzing double mutants of the digalactosyldiacylglycerol (DGDG) synthase mutant dgd1 with the acyltransferase mutant, act1, and the two desaturase mutants, fad2 and fad3. The double mutants showed different degrees of growth retardation: act1,dgd1 was most severely affected and growth of fad2,dgd1 was slightly reduced, whereas fad3,dgd1 plants were very similar to dgd1. In act1,dgd1, lipid and chlorophyll content were reduced and photosynthetic capacity was affected. Molecular analysis of galactolipid content, fatty acid composition, and positional distribution suggested that the growth deficiency is not caused by changes in galactolipid composition per se. Chloroplasts of dgd1 were capable of synthesizing monogalactosyldiacylglycerol, DGDG, and tri- and tetragalactosyldiacylglycerol. Therefore, the reduced growth of act1,dgd1 and fad2,dgd1 cannot be explained by the absence of DGDG synthase activity from chloroplasts. Molecular analysis of DGDG accumulating in the mutants during phosphate deprivation suggested that similarly to the residual DGDG of dgd1, this additional lipid is synthesized in association with chloroplast membranes through a pathway independent of the mutations, act1, dgd1, fad2, and fad3. Our data imply that the severe growth defect of act1,dgd1 is caused by a reduced metabolic flux of chloroplast lipid synthesis through the eukaryotic and prokaryotic pathway as well as by the reduction of photosynthetic capacity caused by the destabilization of photosynthetic complexes.
The two galactolipids, monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG), are the major lipids of photosynthetic membranes (Browse and Somerville, 1991; Joyard et al., 1998). Their biosynthesis is complex. Two parallel pathways for the production of diacylglycerol, the precursor for galactolipid biosynthesis, are present in the plastid and the endoplasmic reticulum (ER) of plants such as Arabidopsis (Heinz and Roughan, 1983; Browse et al., 1986). The prokaryotic pathway is restricted to the plastid, whereas the eukaryotic pathway involves reactions at the plastid and the ER. This requires bidirectional transport of lipid moieties between the ER and the plastid with the actual mechanisms remaining obscure. Thylakoid lipids derived from each of the two pathways can be distinguished by their fatty acid composition such that certain molecular species are diagnostic for the responsible pathway (Heinz and Roughan, 1983). Their specific occurrence and high abundance in chloroplasts implies that galactolipids are important for the integrity of the phototsynthetic complexes in the thylakoids. However, not much is known about the role of the individual molecular species of galactolipids for the functionality of light absorption and electron transfer during photosynthesis or about their localization in different suborganellar membranes.
Mutants of Arabidopsis deficient in different aspects of lipid biosynthesis are available (Browse and Somerville, 1994; Vijayan et al., 1998) and can be used to study the genetic interaction between different loci encoding enzymes of thylakoid lipid biosynthesis. In the dgd1 mutant, the biosynthesis of DGDG is impaired, resulting in a reduction in DGDG content by 90% (Dörmann et al., 1995). Because the molecular analysis of the dgd1 mutant allele suggested complete inactivation of the DGD1 gene (Dörmann et al., 1999), another reaction must be responsible for the biosynthesis of the residual DGDG in the dgd1 mutant. Clear evidence for a DGD1-independent pathway of DGDG synthesis came from the analysis of Arabidopsis plants raised under phosphate-limiting conditions (Härtel et al., 2000). The fact that DGDG synthesis was increased under phosphate deficiency in the dgd1 mutant strongly suggested the existence of a second, DGD1-independent pathway. Membrane fractionation experiments indicated that under phosphate starvation, DGDG also accumulated in extraplastidic membranes.
The act1 mutant of Arabidopsis is deficient in the plastidic acyl-ACP:glycerol-3-phosphate acyltransferase leading to the inactivation of the prokaryotic pathway (Kunst et al., 1988). We recently constructed an Arabidopsis act1,dgd1 double mutant as part of our effort to understand the function of the DGD1 gene (Dörmann et al., 1999). The severe growth phenotype of the act1,dgd1 double mutant was suggested to be caused by a strong reduction in overall membrane biosynthesis.
The aim of this study was: (a) to analyze the contribution of the different pathways to the overall metabolic flux of galactolipid biosynthesis under normal growth conditions, (b) to characterize the contribution of the different pathways to the synthesis of galactolipids in plants raised under phosphate-limiting growth conditions, and (c) to unravel the function of the molecular species of galactolipids derived from the different pathways for plant growth and photosynthesis. For this purpose, we initiated a series of experiments including further studies to address the possible causes for the severe growth phenotype observed for act1,dgd1. Changes of chloroplast lipid biosynthesis might lead to a decreased photosynthetic capacity of the double mutant as compared with dgd1. Therefore, we measured total lipids and photosynthetic pigments, analyzed chloroplast ultrastructure, and recorded chlorophyll fluorescence for act1,dgd1 plants. Additional double mutant lines (fad2,dgd1 and fad3,dgd1) were constructed to address the question of whether metabolic flux through different lipid biosynthesis pathways, the amounts of the lipids, or fatty acid composition of the residual DGDG are changed in mutant lines homozygous for dgd1. FAD2 and FAD3 encode 18:1 and 18:2 desaturases, respectively, which are both localized at the ER (Miquel and Browse, 1992; Browse et al., 1993). In the fad2 mutant, thylakoid lipids derived from the eukaryotic pathway as well as extraplastidic lipids are affected and show an altered fatty acid composition (Miquel and Browse, 1992), whereas in the fad3 mutant, only extraplastidic lipids but none of the thyalkoid lipids are altered (Browse et al., 1993). Furthermore, chloroplasts isolated from wild type and dgd1 were compared for their capability of galactolipid synthesis to find out if the residual DGDG found in dgd1 homozygous lines might still be synthesized in the chloroplast. Finally, we compared the fatty acid composition and positional distribution of the residual amount of DGDG of the double mutants with that of plants raised under phosphate-limiting conditions to address the question of whether these functionally important pools of DGDG are synthesized by related pathways.
RESULTS
Double Mutants of dgd1 with Mutants in the Prokaryotic (act1) or Eukaryotic Pathway (fad2,fad3) Are to Different Extents Affected in Growth
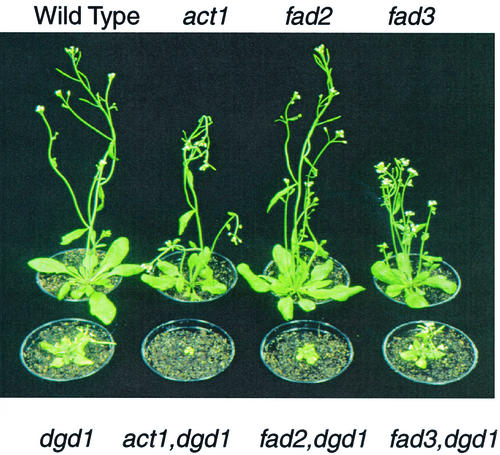
The act1,dgd1 double mutant carrying a block in the plastid acyl-ACP:glycerol-3-phosphate acyltransferase and in the DGDG synthase DGD1 was severely affected in growth (Dörmann et al., 1999; Fig. 1). The act1,dgd1 plants could be propagated on Suc-supplemented medium, but were virtually unable to survive on soil (Fig. 1), suggesting that this mutant is incapable of photoautotrophic growth. To further dissect residual DGDG synthesis in dgd1, we generated double mutants between dgd1 and the mutants fad2 and fad3 affected in the activity of ER-localized desaturases. The fad2,dgd1 mutant was impaired in growth as compared with dgd1 and produced only very few seeds. The plants grew better on Suc-supplemented medium, and only a small fraction survived on soil (Fig. 1). In contrast to fad2,dgd1, the fad3,dgd1 mutant was very similar in growth to the dgd1 single mutant, could be maintained on soil, and was fertile (Fig. 1).
Figure 1.
Visible phenotype of dgd1 and double mutants. Six-week-old plants raised on soil are shown for Arabidopsis wild type, act1, fad2, and fad3 (top, from left to right) as well as dgd1 and the double mutants act1,dgd1, fad2,dgd1, and fad3,dgd1 (bottom, from left to right).
The Amount of Total Lipids and Photosynthetic Membranes Is Drastically Reduced in act1,dgd1
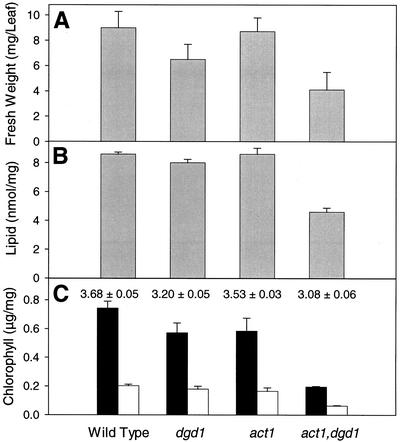
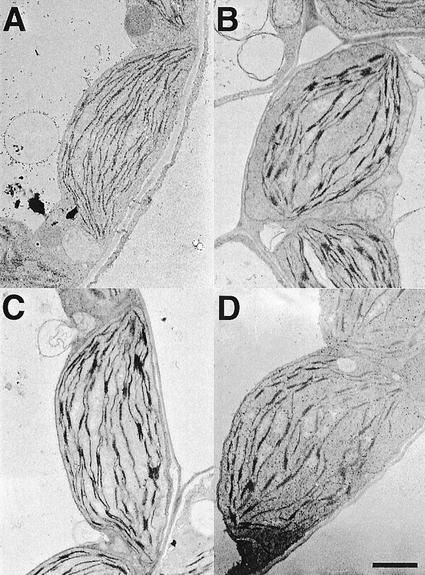
To estimate the capacity of overall lipid biosynthesis of the most severely affected line, act1,dgd1, the total amount of fatty acids per gram leaf fresh weight was determined (Fig. 2). Total fatty acids were slightly reduced in dgd1 as compared with wild type and act1, but were reduced to about 50% of wild-type amounts in act1,dgd1. Also taking into consideration the reduced fresh weight per leaf in the double mutant (Fig. 2), one can conclude that overall lipid biosynthesis is severely affected far beyond what is observed for dgd1. We quantified photosynthetic pigments in wild type, dgd1, act1, and act1,dgd1 (Fig. 2) to estimate the effect of the reduction in overall lipid biosynthesis on the amount of photosynthetic membranes. Total chlorophyll, which was reduced in dgd1 and in act1, was even further decreased in the double mutant to approximately 25% of wild type. The chlorophyll a/b ratio was also decreased in act1,dgd1, but was similar to dgd1. A decrease of total chlorophyll content without changes in the chlorophyll a/b ratio in act1,dgd1 indicates that the ratio of light-harvesting antenna to the reaction center/core complexes remains constant; therefore, all pigment-protein complexes, including photosystem (PS) I and II, are decreased in parallel. To study the reduction of photosynthetic membranes in act1,dgd1 at the level of chloroplasts, light and electron microscopic analysis was done with leaf thin sections of all four lines. Chloroplast numbers per cell cross section were reduced in the act1,dgd1 mutant to approximately 50% of wild type (7.6 ± 0.4, 8.3 ± 0.5, 7.3 ± 0.3, and 3.9 ± 0.4 for WT, dgd1, act1, and act1,dgd1, respectively; n = 10 cell cross sections). As observed for dgd1 (Dörmann et al., 1995), thylakoid membranes of the act1,dgd1 mutant were curved and stroma areas were increased (Fig. 3). Apparently, the combinations of two blocks in lipid biosynthesis of act1 (prokaryotic pathway) and dgd1 (eukaryotic pathway) drastically affect overall lipid biosynthesis and as a result the total amount of photosynthetic membranes is reduced in the act1,dgd1 double mutant.
Figure 2.
Amounts of lipid and photosynthetic pigments of the act1,dgd1 double mutant. A, Fresh weight per rosette leaf. B, Lipid measured as nanomoles fatty acid per milligram leaf fresh weight. C, Chlorophyll a (black bars) and chlorophyll b (white bars) in leaves in micrograms per milligram fresh weight The numbers indicate the chlorophyll a to b ratio in each line. All values represent averages ± se of four measurements. Plants were grown on solidified Murashige and Skoog medium supplemented with Suc.
Figure 3.
Ultrastructure of chloroplasts of the act1,dgd1 double mutant. Representative chloroplasts are shown for four-week-old plants of Arabidopsis wild type (A), dgd1 (B), and act1 (C) single mutants and the act1,dgd1 double mutant (D). Bar = 1 μm. Plants were grown on solidified Murashige and Skoog medium supplemented with Suc.
The Photosynthetic Capacity of the Residual PSs Is Compromised in act1,dgd1 as Compared with dgd1
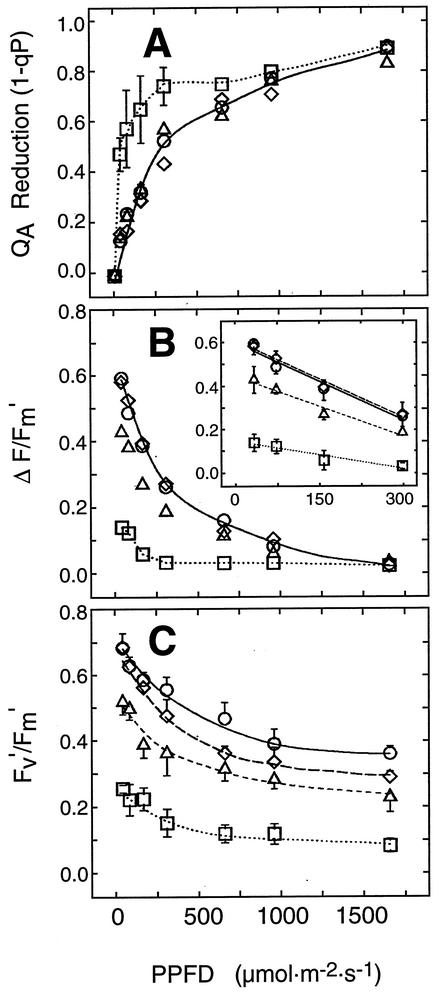
The reduction in lipid biosynthesis and the drastic impairment of photosynthetic membranes observed for act1,dgd1 prompted us to investigate the photosynthetic competence of this mutant in greater detail. Pulse amplitude-modulated chlorophyll fluorescence analysis is a sensitive, noninvasive method that gives information about changes in electron-transport reactions within thylakoids and the overall photosynthetic capability of leaves under in vivo conditions (for review, see Krause and Weis, 1991; Horton et al., 1996). Figure 4A shows light response curves for the fluorescence parameter 1-qP, an estimate for the reduction state of the primary electron acceptor of PSII (QA). The QA reduction increases more strongly with increase in the photosynthetic photon flux density (PPFD) in leaves of act1,dgd1 than in the wild type and the other mutant plants, and is almost saturated at 75 μmol photons m−2 s−1, the PPFD employed for plant growth in this study. The quantum yield of linear electron flux through the photosynthetic electron transport chain (ΔF/Fm′) is strongly reduced in act1,dgd1 (Fig. 4B). As previously shown for plants grown on soil, ΔF/Fm′ is also reduced in dgd1 (Dörmann et al., 1995; Härtel et al., 1998), whereas virtually no differences are observed between act1 and wild type. The intrinsic efficiency of open PSII reaction centers in the light-adapted state (Fv′/Fm′) was drastically decreased in act1,dgd1 as compared with wild type, dgd1, and act1 (Fig. 4C), which indicates a reduced photochemical efficiency of PSII. Taken together, in vivo chlorophyll fluorescence data indicate a severe impact on the utilization of light energy by the residual photosynthetic complexes of act1,dgd1.
Figure 4.
Photosynthtetic capability in act1,dgd1. Chlorophyll fluorescence of Arabidopsis wild type (circle), act1 (diamond), dgd1 (triangle), and act1,dgd1 (square) was measured in whole leaves exposed to different light intensities. The parameters 1-qP (A), ΔF/Fm′ (B), and Fv′/Fm′ (C) were obtained from fluorescence measurements. The inset in B shows ΔF/Fm′ on a different scale. Values represent the means ± se of five measurements.
The Total Amount and Molecular Species Distribution of Galactolipids Are Similar in the Double Mutants and dgd1
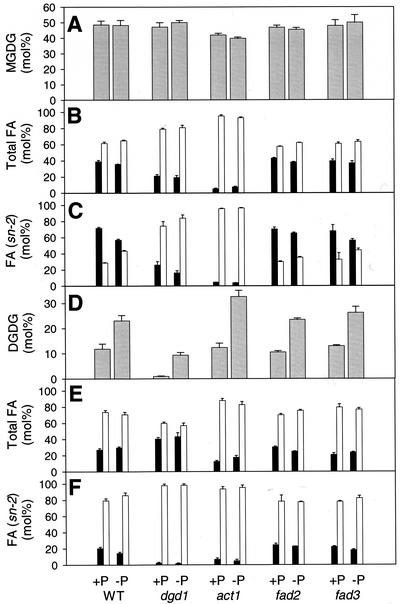
The growth defects observed for act1,dgd1 and fad2,dgd1 might be caused by changes in the total amounts of DGDG, alterations of its fatty acid composition, or distribution at the sn-1 and sn-2 positions of the glycerol backbone. Therefore, molecular species composition of act1,dgd1, fad2,dgd1, fad3,dgd1, and dgd1 was analyzed in greater detail. The relative amount of DGDG in all four lines was very similar (2.0%–2.8%; Table I). Therefore, the additional growth defect of act1,dgd1 and fad2,dgd1 cannot be explained by a further reduction in the total amount of DGDG. Fatty acid composition of the residual DGDG in act1,dgd1 was very similar to that of dgd1 (Table I; Dörmann et al., 1999), excluding the possibility that the severe growth retardation of this line is because of changes in the DGDG fatty acid composition. The fad2,dgd1 mutant showed a shift in fatty acid composition, i.e. an increase of 18:1 in DGDG with a further decrease in 18:3 when compared with either parent (Table I). However, because the shift of 18:3 to 18:1 caused by the fad2 mutation can already be observed in the fad2 single mutant that shows normal growth, it is unlikely that this small change might affect growth of fad2,dgd1 plants.
Table I.
DGDG lipid content and fatty acid composition of DGDG in fad2,dgd1, fad3,dgd1, and act1,dgd1
| Lipid | Wild Type | dgd1 | fad2 | fad2,dgd1 | fad3 | fad3,dgd1 | act1 | act1,dgd1 |
|---|---|---|---|---|---|---|---|---|
| DGDG | 16.5 | 2.0 | 15.4 | 2.8 | 17.0 | 2.8 | 15.9 | 2.1 |
| 16:0 | 10.1 | 21.3 | 13.3 | 24.1 | 10.2 | 21.9 | 7.2 | 23.0 |
| 16:1 | 1.1 | 3.8 | 1.8 | 6.1 | 1.3 | 3.7 | 1.1 | 4.7 |
| 16:2 | 0.6 | 2.8 | 1.4 | 2.7 | 1.0 | 2.6 | 0.3 | 1.9 |
| 16:3 | 2.0 | 2.0 | 3.3 | 2.6 | 1.8 | 2.0 | n.d. | n.d. |
| 18:0 | 0.9 | 4.2 | 1.2 | 3.7 | 1.1 | 2.6 | 1.1 | 6.7 |
| 18:1 | 1.7 | 10.9 | 8.0 | 19.5 | 3.1 | 6.7 | 2.2 | 10.1 |
| 18:2 | 3.2 | 7.3 | 5.1 | 5.3 | 6.2 | 8.4 | 5.2 | 9.0 |
| 18:3 | 80.4 | 47.7 | 65.9 | 36.2 | 75.4 | 52.0 | 83.0 | 44.6 |
Values are given as mol % and represent averages of three measurements. se was less than 2% for all measurements. Plants were grown on soil except act1 and act1,dgd1, which were grown on solidified Murashige and Skoog medium supplemented with Suc. n.d., Not detected.
The DGDG fatty acid composition in the double mutants act1,dgd1 and fad2,dgd1 was not very different from dgd1 (Table I), still these two lines showed a stronger growth retardation than the dgd1 single mutant. We considered the possibility that the molecular species composition of the galactolipids produced by the prokaryotic or eukaryotic pathways might be altered by the respective mutations and thus add to the growth phenotype. Because the two double mutants act1,dgd1 and fad2,dgd1 were not fertile and had to be maintained on Suc-supplemented medium, it was difficult to obtain sufficient amounts of tissue for positional analysis of galactolipids. Therefore, we analyzed the positional fatty acid distribution in the respective parental lines dgd1, act1, and fad2 (Fig. 5). The amount of C16 fatty acids in the sn-2 position of DGDG of dgd1 and act1 is extremely low (Fig. 5F), which confirms previous studies showing that in these two mutants, only very little DGDG is made via the prokaryotic pathway (Kunst et al., 1988; Härtel et al., 2000). Therefore, in DGDG of act1,dgd1, C16 fatty acids supposedly are also excluded from the sn-2 position, which in turn suggests that similarly to the dgd1 single mutant, the high amount of C16 fatty acids in DGDG of act1,dgd1 (Table I) must be localized at sn-1. The total amounts of C16 and C18 fatty acids in DGDG (Fig. 5E) as well as the positional distribution at the sn-2 position (Fig. 5F) are very similar for wild type and fad2. In dgd1, an increase in total C16 of DGDG was observed that must be located at the sn-1 position, because C16 fatty acids are largely excluded from sn-2 (compare with Härtel et al., 2000). Because the positional distribution of fatty acids in DGDG of wild type and fad2 is very similar, this mutation apparently has no large effect on the fatty acids at sn-2, and therefore positional distribution in DGDG of fad2,dgd1 should be very similar to that of dgd1. For these reasons, the growth differences observed between dgd1, act1,dgd1, and fad2,dgd1 cannot be explained by alterations in total amount of DGDG, its fatty acid composition, or positional distribution.
Figure 5.
Fatty acid composition and positional distribution in the galactolipids of act1, fad2, and fad3 grown on phosphate-supplied and -deficient medium. Plants were raised on Murashige and Skoog medium for 12 to 14 d and further propagated for an additional 10 d on phosphate-containing or -deficient medium (Estelle and Somerville, 1987). A, Amount of MGDG; B, total fatty acids in MGDG; C, fatty acids in lyso-MGDG (sn-2 position); D, amount of DGDG; E, total fatty acids in DGDG; F, fatty acids in lyso-DGDG (sn-2 position). Black bars, C16 fatty acids; white bars, C18 fatty acids. Values represent mean and se of three experiments.
DGDG in dgd1 Homozygous Lines Is Synthesized at the Chloroplast
Härtel et al. (2000) demonstrated that the additional amount of DGDG synthesized during phosphate deprivation was at least in part localized in extraplastidic membranes. Therefore, we wondered whether the residual DGDG in dgd1 was actually produced in the chloroplast, because the integrity of the photosynthetic apparatus might be affected if it were associated with extraplastidic membranes (Härtel et al., 2000, 2001). This question could be addressed in part by analyzing the fatty acid composition of the fad3,dgd1 double mutant. No changes in the fatty acid composition of leaf DGDG were found in fad3,dgd1 as compared with dgd1. In particular, the amount of 18:3 in DGDG of fad3,dgd1 and dgd1 was very similar (52.0 and 47.7 mol %, respectively; Table I). The fad3 mutation preferentially affects extraplastidic lipids (Browse et al., 1993). We detected large amounts of 18:3 in DGDG of fad3,dgd1 that must be derived from the plastid FAD7 desaturase, because the FAD3 enzyme localized at the ER is not active in this line. These findings suggest that DGDG in dgd1 is accessible to chloroplast desaturases.
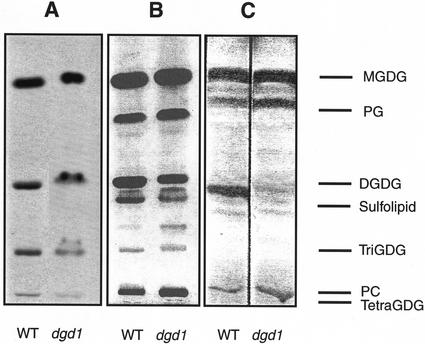
We performed galactolipid synthesis assays with isolated chloroplasts as a direct way to determine whether dgd1 chloroplasts harbor the enzyme(s) of a second DGDG synthesis pathway. In a previous study (Dörmann et al., 1995), assays with chloroplasts directly isolated from homogenized leaves resulted in low DGDG synthase activity. In this study, we isolated chloroplasts from protoplasts, which resulted in plastid preparations with much higher DGDG synthase activity. We observed very similar incorporation of radiolabel from UDP-[14C]Gal into the galactolipids MGDG, DGDG, and oligogalactolipids comigrating with trigalactosyldiacylglycerol (TriGDG) and tetragalactosyldiacylglycerol (TetraGDG) for wild type and dgd1 (Fig. 6A). The chloroplasts isolated from wild-type and dgd1 protoplasts contained high amounts of DGDG and oligogalactolipids that could easily be detected by staining with iodine (Fig. 6B) and the sugar-specific reagent α-naphthol (not shown). However, in chloroplasts isolated directly from fresh leaves, only low amounts of DGDG were found in dgd1 and oligogalactolipids were absent (Fig. 6C). Apparently, during protoplastation, a galactosyltransferase activity was induced in wild-type and dgd1 chloroplasts that synthesized DGDG and the oligogalactolipids TriGDG and TetraGDG. This clearly demonstrates that a second DGDG synthase activity is present in dgd1 chloroplasts.
Figure 6.
DGDG synthesis in chloroplasts of Arabidopsis wild type and dgd1 mutant. A, Chloroplasts of Arabidopsis wild type and dgd1 mutant were isolated from protoplasts and incubated with MGDG and UDP-[14C]Gal. After extraction, lipids were separated by thin-layer chromatography and radiolabeled lipids visualized by autoradiography. B, Chloroplasts of wild type and dgd1 were isolated from protoplasts, lipids extracted, and stained with iodine. C, Chloroplasts of wild type and dgd1 were isolated by homogenization of leaves (Price et al., 1994), lipids extracted, and stained with iodine. Galactolipids and sulfolipid were identified by staining with α-naphthol (not shown).
Additional DGDG Synthesized during Phosphate Deprivation Has Similar Characteristics as the Residual DGDG of dgd1 Homozygous Lines
During phosphate limitation, DGDG was found to accumulate in membranes of wild-type and dgd1 mutant leaves (Härtel et al., 2000). This DGD1-independent pathway of DGDG biosynthesis was induced by phosphate deprivation, but might also be active to a minor extent under phosphate-sufficient conditions and thus be involved in the synthesis of the residual amount of DGDG in dgd1. We measured total amounts, fatty acid composition, and positional distribution of galactolipids in wild type and different mutant lines raised under normal and phosphate-limiting conditions to investigate the relationship between DGDG synthesized during phosphate deprivation and the residual amount of DGDG in dgd1. As shown in Figure 5 (A–C), the total amount of MGDG and its fatty acid composition and positional distribution was not drastically changed in any of the mutants, dgd1, act1, fad2, or fad3, during growth on phosphate-deficient medium. The amount of DGDG increased to a similar extent in all lines when propagated on phosphate-deficient medium (Fig. 6D). Therefore, the synthesis of this extra amount of DGDG was not affected by any of these mutations. The fatty acid composition of DGDG was not drastically altered during phosphate deprivation (Fig. 6E). The amount of C16 fatty acids in the sn-2 position of DGDG slightly decreased under phosphate deficiency in all lines analyzed (Fig. 6F; compare with Härtel et al., 2000), indicating a redirection of fatty acids in DGDG derived from the prokaryotic to the eukaryotic pathway. During phosphate deficiency, DGDG increased in leaves of the fad3,dgd1 double mutant from 1.1 to 9.5 mol % (Table II), whereas the amount of 18:3 in DGDG remained unchanged (53.2 and 53.4 mol %, respectively). As already pointed out for the residual DGDG lipid in fad3,dgd1, the high amount of 18:3 in DGDG isolated from this line after induction by phosphate deprivation is derived from desaturation by the chloroplast FAD7 enzyme. Therefore, DGDG biosynthesis induced by phosphate deprivation involves desaturases associated with the chloroplast.
Table II.
Changes in DGDG lipid content and fatty acid composition of DGDG in fad3,dgd1 double mutants after phosphate deprivation
| Lipid | WT
|
dgd1
|
fad3
|
fad3,dgd1
|
||||
|---|---|---|---|---|---|---|---|---|
| + | – | + | – | + | – | + | – | |
| DGDG | 11.8 | 23.0 | 0.9 | 9.3 | 12.9 | 26.2 | 1.1 | 9.5 |
| 16:0 | 15.4 | 21.8 | 26.0 | 29.8 | 15.3 | 16.0 | 22.7 | 22.5 |
| 16:1 | 2.1 | 1.6 | 4.2 | 2.3 | 2.2 | 1.4 | 2.4 | 0.7 |
| 16:2 | 0.9 | n.d. | 1.6 | 0.3 | 0.7 | 0.2 | 3.1 | n.d. |
| 16:3 | 2.6 | 1.9 | 3.4 | 1.1 | 3.4 | 2.5 | 1.9 | 1.8 |
| 18:0 | 1.2 | 2.5 | 3.7 | 5.3 | 4.5 | 2.4 | 2.1 | 3.1 |
| 18:1 | 2.0 | 2.9 | 6.4 | 4.9 | 3.2 | 2.3 | 5.4 | 2.3 |
| 18:2 | 4.9 | 10.8 | 9.2 | 12.5 | 7.1 | 12.2 | 9.2 | 15.6 |
| 18:3 | 70.9 | 58.6 | 45.2 | 44.0 | 63.7 | 63.0 | 53.2 | 53.4 |
Values are given as mol % and represent averages of three measurements. se error was less than 2% for all measurements. Plants were grown on solidified medium supplemented with Suc containing 1 mm (+) or no phosphate (−). n.d., Not detected.
DISCUSSION
The act1,dgd1 double mutant has one of the most extreme phenotypes of all known Arabidopsis lipid mutants. In a previous study (Dörmann et al., 1999), the drastically impaired growth of act1,dgd1 led us to hypothesize that an overall reduction in membrane lipid synthesis might be one of the reasons for the severe phenotype and suggested that DGD1 plays a critical role in chloroplast lipid production. This idea was based on the proposed reaction mechanism of the DGDG synthase, which releases one diacylglycerol molecule per molecule of DGDG synthesized (van Besouw and Wintermans, 1978; Heemskerk et al., 1990). Diacylglycerol produced in this reaction could be used for the biosynthesis of other eukaryotic lipids in the plastid (Williams and Khan, 1996; Dörmann et al., 1999) and may be limiting in all plants homozygous for dgd1. In the present study, we observed a strong reduction in the amounts of total fatty acids and of photosynthetic pigments as well as a reduction in the number of chloroplasts per cross section in act1,dgd1. This clearly points toward a reduction in overall thylakoid membrane lipid biosynthesis as one of the causes for the drastic growth retardation of act1,dgd1. The strong phenotype caused by the combination of blocks in two parallel pathways of lipid biosynthesis emphasizes the importance of the prokaryotic and eukaryotic pathways for overall chloroplast lipid biosynthesis in Arabidopsis.
Two additional double mutants of dgd1 and the ER-localized deasturases fad2 and fad3 were generated during this study. Whereas fad2,dgd1 plants were affected in growth, fad3,dgd1 plants were very similar to dgd1. As part of the eukaryotic pathway of lipid synthesis, diacylglycerol moieties, which are enriched in 18:2, are transported from the ER back to the chloroplast (Miquel and Browse, 1992). Because fad2 is critical for synthesis of 18:2 at the ER, a block in fad2, but not in fad3, would be expected to affect the metabolic flux through the eukaryotic pathway. The finding that the fad2,dgd1 double mutant, but not the fad2 single mutant, showed a reduction as compared with the respective parental lines (dgd1 and wild type, respectively), suggests that the fad2 mutation affects lipid synthesis to a higher extent, when the flux through the eukaryotic pathway in the dgd1 mutant background is already compromised. Therefore, the combinations of two mutations in the eukaryotic pathway, dgd1 and fad2, results in strong reduction of growth caused by additive effects of two blocks in a linear pathway of lipid synthesis.
Figure 4 demonstrates that the utilization of light energy by the residual PSs of the act1,dgd1 double mutant is impaired as compared with the parental mutants act1 and dgd1 (Kunst et al., 1989; Härtel et al., 1997). One possibility for this might be the specific association of the galactolipids with different complexes of photosynthesis. It has been shown that the lumen-exposed water oxidation complex is affected in dgd1 (Reifarth et al., 1997). Consistent with this, the oxygen evolution rate in in vitro preparations of PS II was dependent on the amount of DGDG added (Gounaris et al., 1983). Furthermore, DGDG was found to be bound to the light-harvesting complex II, and one MGDG molecule was recently discovered in the crystal structure of PS I (Nuβberger et al., 1993; Jordan et al., 2001). Whereas in dgd1, the prokaryotic lipid pathway is still active (as indicated by the molecular species composition of MGDG; Fig. 5C), the additional block in act1 in the double mutant may result in a specific loss of prokaryotic-type MGDG and DGDG in the chloroplast, giving rise to a destabilization and dysfunction of complexes of the photosynthetic apparatus which in turn could explain why the act1,dgd1 double mutant was unable to grow photoautotrophically.
To investigate the amount and origin of DGDG synthesized in dgd1, we analyzed lipid and fatty acid composition in double mutants of dgd1 with act1 and the desaturase mutants fad2 and fad3. The fatty acid composition of DGDG in lines with dgd1 mutant background was found to be constant for plants analyzed within one set of experiments but somewhat variable between different sets (e.g. Dörmann et al., 1995, 1999; Härtel et al., 2000; this study). This variability might be caused by slight differences in growth conditions (e.g. propagation on soil or Murashige and Skoog medium) because it was shown that the amount of phosphate in the growth medium has a strong impact on fatty acid composition of DGDG (Härtel et al., 2000). The fact that the total amount of DGDG in the double mutants, the fatty acid composition, and positional distribution was not drastically altered as compared with the dgd1 single mutant suggests that the growth deficiencies of act1,dgd1 and fad2,dgd1 are not caused by alterations in galactolipid composition per se.
The fatty acid composition of the act1,dgd1 and fad2,dgd1 double mutants suggested that a major portion of the residual DGDG in lines with dgd1 genetic background is of eukaryotic origin: In act1,dgd1, we found a very similar fatty acid composition for DGDG as in dgd1, i.e. increased amounts of 16:0 and 18:1 at the expense of 18:3 (Table I). Palmitic acid is particularly enriched at the sn-1 position of DGDG, indicating that the prevalent fraction of DGDG in dgd1 is of eukaryotic structure (Härtel et al., 2001; Fig. 5F). Furthermore, the act1 mutation did not eliminate residual amounts of DGDG in act1,dgd1 as would be expected if the residual DGDG were completely synthesized via the prokaryotic pathway. Because the block in desaturation of 18:1 to 18:2 caused by the fad2 mutation is reflected in the DGDG fatty acid composition in the fad2,dgd1 plants, the residual amount of DGDG still contains molecular species derived from the eukaryotic pathway. Apparently, the block in the ER-localized desaturation in fad2 cannot entirely be bypassed by plastid desaturases (i.e. FAD6).
Chloroplasts directly isolated from leaves of wild type and dgd1 mutant were found to synthesize all galactolipids normally present in plants (MGDG and DGDG; Dörmann et al., 1995). This result again suggests that a second DGDG synthase is localize in chloroplasts. No oligogalactolipids (TriGDG and TetraGDG) were detected in these preparations (Dörmann et al., 1995; Fig. 6C). However, chloroplasts isolated from leaf protoplasts of wild type and dgd1 were capable of producing DGDG, TriGDG, and TetraGDG from radioactive UDP-Gal (Fig. 6A). High amounts of DGDG and oligogalactolipids accumulated in chloroplasts of wild type and dgd1 during protoplastation, diminishing the differences in the amounts of DGDG in these two lines (Fig. 6B). We concluded that during protoplastation, a DGDG synthase activity is induced leading to the formation of DGDG, TriGDG, and TetraGDG. These oligogalactolipids are normally absent from leaves and are only found in low amounts in non-photosynthetic tissues (e.g. Fujino and Miyazawa, 1979). Dorne et al. (1982) and Heemskerk et al. (1986) demonstrated that in isolated chloroplasts, DGDG, TriGDG, and TetraGDG accumulate at the expense of MGDG. The repeated glycosylation of glycolipids that was also described for bacterial genes was referred to as “processive” (Jorasch et al., 1998, 2000). There has been some debate on whether or not the enzyme responsible for producing DGDG, TriGDG, and TetraGDG, the galactolipid:galactolipid galactosyltransferase, represents the main activity for DGDG synthesis in chloroplasts (Heemskerk et al., 1988, 1990), or whether it merely is an “artificial” activity detectable in vitro only (Dorne et al., 1982). The result for the dgd1 mutant clearly shows that the formation of TriGDG and TetraGDG are independent of the main pathway of DGDG synthesis through DGD1.
In addition to DGD1, which was shown to be responsible for the production of the predominant fraction of DGDG in chloroplasts (Dörmann et al., 1995), Härtel et al. (2000) and Härtel and Benning (2000) demonstrated that a second, DGD1-independent pathway is induced in Arabidopsis after phosphate deprivation, giving rise to accumulation of extraplastidic DGDG. Analysis of the fatty acid composition of fad3,dgd1 plants revealed that DGDG produced after phosphate deprivation still contains fatty acids desaturated at the chloroplast. However, in roots of fad3 single mutants, DGDG produced during phosphate deficiency showed a reduction in 18:3 (Härtel et al., 2000). This apparent discrepancy can be explained by the reduced activity of plastid desaturases in roots (Miquel and Browse, 1992). For this reason, the fad3 mutation in roots cannot efficiently be circumvented by the plastid FAD7 desaturase as in leaves. Taken together, fatty acid data obtained for fad3,dgd1 plants grown in the presence or absence of phosphate suggest that DGDG in this line is desaturated at chloroplast membranes, which in turn points to a second DGDG synthase activity localized at the chloroplast membranes. During phosphate deficiency, the additional amount of DGDG may be assembled in the plastid and then transported to the ER depending on lipid demand.
It will be interesting to elucidate how the DGD1-independent DGDG synthase induced by phosphate deprivation (Härtel et al., 2000) is related to the processive galactosyltransferase detected in isolated chloroplasts of dgd1 (this study). In chloroplasts isolated from protoplasts as well as in plants raised under phosphate deficiency, a DGDG synthase activity is induced, resulting in massive production of DGDG (Dorne et al., 1982; Heemskerk et al., 1986; Härtel et al., 2000; this study). The processive galactosyltransferase is localized in the outer chloroplast envelope, where it presumably accepts eukaryotic MGDG as a precursor for DGDG synthesis. Similarly, DGDG produced by the phosphate-dependent DGDG synthase is mostly eukaryotic. Contrary to the processive enzyme, no oligogalactolipids were synthesized under phosphate-limiting conditions. However, this apparent discrepancy might be explained by the fact that DGDG produced during phosphate deprivation can be transported to extraplastidic membranes to substitute for their deficiency in phospholipids (Härtel et al., 2000). Under these conditions, DGDG may not be available for further galactosylation by plastid-localized enzymes. Because of the absence of extraplastidic membranes in the isolated chloroplast system, DGDG lipid cannot be removed from the outer envelope. Furthermore, because of the deficiency of diacylglycerol supplied by the ER, MGDG may become limiting in isolated chloroplasts and the processive galactosyltransferase may use DGDG for further galactosylation. Recently, a second DGDG synthase (DGD2) was described in Arabidopsis that was induced by phosphate deprivation and showed processive galactosylation activity after heterologous expression in Escherichia coli (Kelly and Dörmann, 2002). Therefore, DGD2 represents a candidate enzyme for both the DGD1-independent DGDG synthase induced by phosphate deficiency as well as for the processive galactosyltransferase detectable in dgd1. Further studies will be required to reveal which additional factors are involved in the induction of DGD1-independent galactolipid synthesis in Arabidopsis.
MATERIALS AND METHODS
Plant Growth Conditions and Generation of Double Mutants
Arabidopsis wild type (Arabidopsis, ecotype Columbia-2) and different mutants were grown at light conditions of 60 to 70 μmol photons m−2 s−1 on soil or solidified Murashige and Skoog medium as described below (Murashige and Skoog, 1962). For phosphate deprivation experiments, plants raised on Murashige and Skoog medium for 11 to 14 d were transferred to Arabidopsis medium at one-half strength, where they were grown for an additional 10 d as described (Estelle and Somerville, 1987; Härtel et al., 2000). Double mutants were obtained by crossing dgd1 plants (Dörmann et al., 1995) with act1 (Nottingham Arabidopsis Stock Center, Nottingham University, Loughborough, UK; Kunst et al., 1988), fad2-1 (Lemieux et al., 1990; Miquel and Browse, 1992), or fad3 (Nottingham Arabidopsis Stock Center; Lemieux et al., 1990; Browse et al., 1993). Because all mutations analyzed are recessive, double homozygous plants were searched for in the F2 generation, where they were expected to occur in a ratio of 1:16.
The act1,dgd1 double mutant was obtained as described by Dörmann et al. (1999). The act1,dgd1 plants were grown on solidified 1× Murashige and Skoog medium containing 1% (w/v) Suc, if not otherwise stated. F2 plants derived from a cross of dgd1 and fad2 were screened for double-mutant plants by analyzing lipid and fatty acid patterns. No double homozygous plant was found in 405 plants of the F2 generation, but only one line homozygous for fad2 and heterozygous for dgd1. This segregation pattern indicates close linkage of the two genes with a calculated genetic distance of 1.4 cM, which is in good agreement with the published genetic locations of dgd1 (about 16 cM; Dörmann et al., 1999) and fad2 (12.5 cM; Okuley et al., 1994) on chromosome 3 of Arabidopsis. After germination on Murashige and Skoog medium with 1% (w/v) Suc, plants were transferred to soil. Double mutant plants of fad3,dgd1 were found in an F2 population of a cross between dgd1 and fad3 by screening for the respective lipid and fatty acid patterns. After germination on solidified Murashige and Skoog medium with 1% (w/v) Suc, these plants were transferred to soil.
Upon request, all seed stocks of double mutants described in this publication will be made available for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this paper that would limit their use in noncommercial research purposes.
Analysis of Lipids and Chlorophyll and Chlorophyll Fluorescence
Lipids were extracted from leaves, separated by thin-layer chromatography, and quantified by gas chromatography as described by Dörmann et al. (1999). Chlorophyll was measured photometrically (Lichtenthaler, 1987). Positional analysis of fatty acids was done according to Miquel et al. (1998) and Siebertz and Heinz (1977).
In vivo chlorophyll fluorescence at room temperature was registered as described previously (Härtel et al., 1998). All plants were dark adapted for 1 h before fluorescence measurements. Fluorescence parameters used are as defined (Genty et al., 1989; van Kooten and Snel, 1990). PPFDs were measured with a quantum sensor (LI-189A; LI-COR, Lincoln, NE).
Electron Microscopy
Leaf tissue was cut into 1-mm-wide slices and immersed in a primary fixative of 2.5% (v/v) glutaraldehyde in 0.05 m sodium cacadylate, pH 6.8. The samples were fixed under vacuum until exhausted then further fixed for a total of 2 h at room temperature. After washing in three 20-min changes of 0.05 m cacadylate buffer, pH 6.8, the samples were post fixed in 1% (w/v) aqueous osmium tetroxide for 2 h. This was followed by three washes with deionized water, then a serial dehydration with 25%, 50%, 75%, and 100% (v/v) of acetone in water. The specimens were infiltrated with a series of 33%, 66%, and 100% (w/v) of epoxy resin in acetone (Equiequivalent vinyl cyclohexane dioxide and Quetol 651, with nonenyl succinic anhydride and dimethylaminoethanol). After three changes of pure resin over a 24-h period, the samples were cast into blocks and polymerized at 65°C for 12 h. Sections, 70 to 90 nm thick, were cut with an RMC MT-X ultramicrotome (RMC-Boeckeler Instruments Inc., Tucson, AZ) and mounted on plain 300-μm mesh copper grids. These were stained in aqueous uranyl acetate and lead citrate prior to viewing in a Philips CM-10 Transmission Electron Microscope (Eindhoven, The Netherlands) operating at 100 kV.
Chloroplast Isolation and Galactolipid Biosynthesis Assay
Chloroplasts were isolated from 3-week-old wild-type and dgd1 plants grown in tissue culture using a protoplast method as described (Fitzpatrick and Keegstra, 2001). In brief, leaves were cut from the plants and digested with cellulase and macerozyme in 400 mm sorbitol; 20 mm MES-KOH, pH 5.2; and 0.5 mm CaCl2. After 3 h, the protoplasts were purified by filtration through 200-μm mesh nylon. Chloroplasts were obtained by resuspending in 300 mm sorbitol, 20 mm EDTA, 5 mm EGTA, 10 mm NaHCO3, and 0.1% (w/v) bovine serum albumin by passage through a 10-μm mesh nylon membrane. Intact chloroplasts were obtained by centrifugation through a Percoll gradient as described Bruce et al. (1994). Chloroplasts were directly isolated from fresh leaves without protoplastation according to Price et al. (1994).
Intact chloroplasts of wild type and dgd1 mutant corresponding to 36 μg of total chlorophyll each were incubated in assay buffer (0.3 m sorbitol; 20 mm Tricine-KOH, pH 7.6; 5 mm MgCl2; and 2.5 mm EDTA) in a total reaction volume of 250 μL containing 130 nmol of MGDG (isolated from wild-type leaves), 100 nmol sodium deoxycholate, and 61.5 pmol UDP-[U-14C]Gal (325 mCi/mmol) for 1 h at room temperature. Lipids were extracted and separated by thin-layer chromatography as previously described (Dörmann et al., 1999). Radioactive lipids were visualized by autoradiography.
ACKNOWLEDGMENTS
We thank John Browse (Washington State University, Pullman) for the fad2-1 mutant seeds. The help of Ilse Balbo and Antje Bolze (Max-Planck-Institute) for the generation and analysis of double mutants is especially acknowledged.
Footnotes
This work was supported in part by the U.S. Department of Energy (grant no. DE–FG02–98ER20305 to C.B.) and by the Alexander von Humboldt Foundation (Feodor-Lynen fellowship to P.D.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010780.
LITERATURE CITED
- Browse J, McConn M, James D, Jr, Miquel M. Mutants of Arabidopsis deficient in the synthesis of α-linolenate: biochemical and genetic characterization of the endoplasmic reticulum linoleoyl desaturase. J Biol Chem. 1993;268:16345–16351. [PubMed] [Google Scholar]
- Browse J, Somerville C. Glycerolipid synthesis: biochemistry and regulation. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:467–506. [Google Scholar]
- Browse J, Somerville C. Glycerolipids. In: Meyerowitz E, Somerville C, editors. Arabidopsis. New York: Cold Spring Harbor Press; 1994. pp. 881–912. [Google Scholar]
- Browse J, Warwick N, Somerville CR, Slack CR. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the 16:3 plant Arabiodpsis thaliana. Biochem J. 1986;235:25–31. doi: 10.1042/bj2350025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce BD, Perry S, Froehlich J, Keegstra K. In vitro import of proteins into chloroplasts. In: Gelvin SB, Schilperoort RB, editors. Plant Molecular Biology Manual. Vol. 2. Boston: Kluwer Academic Publishers; 1994. pp. 1–15. [Google Scholar]
- Dörmann P, Balbo I, Benning C. Arabidopsis galactolipid biosynthesis and lipid trafficking mediated by DGD1. Science. 1999;284:2181–2184. doi: 10.1126/science.284.5423.2181. [DOI] [PubMed] [Google Scholar]
- Dörmann P, Hoffmann-Benning S, Balbo I, Benning C. Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. Plant Cell. 1995;7:1801–1810. doi: 10.1105/tpc.7.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorne A-J, Block MA, Joyard J, Douce R. The galactolipid:galactolipid galactosyltransferase is located on the outer membrane of the chloroplast envelope. FEBS Lett. 1982;145:30–34. [Google Scholar]
- Estelle MA, Somerville C. Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol Gen Genet. 1987;206:200–206. [Google Scholar]
- Fitzpatrick LM, Keegstra K. A method for isolating a high yield of Arabidopsis chloroplasts capable of efficient import of precuroso proteins. Plant J. 2001;27:59–65. doi: 10.1046/j.0960-7412.2001.01061.x. [DOI] [PubMed] [Google Scholar]
- Fujino Y, Miyazawa T. Chemical structures of mono-, di-, tri- and tetraglycosyl gycerides in rice bran. Biochim Biophys Acta. 1979;572:442–451. [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Gounaris K, Sundby C, Andersson B, Barber J. Lateral heterogeneity of polar lipids in the thylakoid membranes of spinach chloroplasts. FEBS Lett. 1983;156:170–174. [Google Scholar]
- Härtel H, Benning C. Can digalactosyldiacylglycerol substitute for phosphatidylcholine upon phosphate deprivation in leaves and roots of Arabidopsis? Biochem Soc Trans. 2000;28:729–732. [PubMed] [Google Scholar]
- Härtel H, Dörmann P, Benning C. DGD1-independent biosynthesis of extraplastidic galactolipids following phosphate deprivation in Arabidopsis. Proc Natl Acad Sci USA. 2000;97:10649–10654. doi: 10.1073/pnas.180320497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtel H, Dörmann P, Benning C. A galactolipid pool not associated with the photosynthetic apparatus in phosphate-deprived plants. J Photochem Photobiol B Biol. 2001;61:46–51. doi: 10.1016/s1011-1344(01)00144-0. [DOI] [PubMed] [Google Scholar]
- Härtel H, Lokstein H, Dörmann P, Grimm B, Benning C. Changes in the composition of the photosynthetic apparatus in the galactolipid deficient dgd1 mutant of Arabidopsis thaliana. Plant Physiol. 1997;115:1175–1184. doi: 10.1104/pp.115.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtel H, Lokstein H, Dörmann P, Trethewey NT, Benning C. Photosynthetic light utilization and xanthophyll cycle activity in the galactolipid deficient dgd1 mutant of Arabidopsis thaliana. Plant Physiol Biochem. 1998;36:407–417. [Google Scholar]
- Heemskerk JWM, Bögemann G, Helsper JPFG, Wintermans JFGM. Synthesis of mono- and digalactosyl diacylglycerol in isolated spinach chloroplasts. Plant Physiol. 1988;86:971–977. doi: 10.1104/pp.86.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk JWM, Strorz T, Schmidt RR, Heinz E. Biosynthesis of digalactosyldiacylglycerol in plastids from 16:3 and 18:3 plants. Plant Physiol. 1990;93:1286–1294. doi: 10.1104/pp.93.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk JWM, Wintermans JFGM, Joyard J, Block MA, Dorne A-J, Douce R. Localization of galactolipid:galactolipid galactosyltransferase and acyltransferase in outer envelope membrane of spinach chloroplasts. Biochim Biophys Acta. 1986;877:281–289. [Google Scholar]
- Heinz E, Roughan PG. Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 1983;72:273–279. doi: 10.1104/pp.72.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Jorasch P, Warnecke DC, Lindner B, Zähringer U, Heinz E. Novel processive and nonprocessive glycosyltransferases from Staphylococcus aureus and Arabidopsis thaliana synthesize glycoglycerolipids, glycophospholipids, glycosphingolipids and glycosylsterols. Eur J Biochem. 2000;267:3770–3783. doi: 10.1046/j.1432-1327.2000.01414.x. [DOI] [PubMed] [Google Scholar]
- Jorasch P, Wolter FP, Zähringer Y, Heinz E. A UDP glycosyltransferase from Bacillus subtilis successively transfers up to four glucose residues to 1:2-diacylglycerol: expression of ypfP in Escherichia coli and structural analysis of its reaction products. Mol Microbiol. 1998;29:419–431. doi: 10.1046/j.1365-2958.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N. Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature. 2001;411:909–917. doi: 10.1038/35082000. [DOI] [PubMed] [Google Scholar]
- Joyard J, Teyssier E, Miège C, Berny-Seigneurin D, Maréchal E, Block MA, Dorne A-J, Rolland N, Ajlani G, Douce R. The biochemical machinery of plastid envelope membranes. Plant Physiol. 1998;118:715–723. doi: 10.1104/pp.118.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AA, Dörmann P. DGD2, an Arabidopsis gene encoding a UDP-galactose dependent digalactosyldiacylglycerol synthase is expressed during growth under phosphate limiting conditions. J Biol Chem. 2002;277:1166–1173. doi: 10.1074/jbc.M110066200. [DOI] [PubMed] [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:313–349. [Google Scholar]
- Kunst L, Browse J, Somerville CR. Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc Natl Acad Sci USA. 1988;85:4143–4147. doi: 10.1073/pnas.85.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst L, Browse J, Somerville C. Altered chloroplast structure and function in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Plant Physiol. 1989;90:846–853. doi: 10.1104/pp.90.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux B, Miquel M, Somerville C, Browse J. Mutants of Arabidopsis with alterations in seed lipid fatty acid composition. Theor Appl Genet. 1990;80:234–240. doi: 10.1007/BF00224392. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol. 1987;148:350–382. [Google Scholar]
- Miquel M, Browse J. Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis: biochemical and genetic characterization of a plant oleoyl-phosphatidylcholine desaturase. J Biol Chem. 1992;267:1502–1509. [PubMed] [Google Scholar]
- Miquel M, Cassagne C, Browse J. A new class of Arabidopsis mutants with reduced hexadecatrienoic acid fatty acid levels. Plant Physiol. 1998;117:923–930. doi: 10.1104/pp.117.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Nuβberger S, Dörr K, Wang N, Kühlbrandt W. Lipid-protein interactions in crystals of plant light harvesting complex. J Mol Biol. 1993;234:347–356. doi: 10.1006/jmbi.1993.1591. [DOI] [PubMed] [Google Scholar]
- Okuley J, Lightner J, Feldmann K, Yadav N, Lark E, Browse J. Arabidopsis FAD2 gene encodes the enzyme that is essential for polyunsaturated lipid synthesis. Plant Cell. 1994;6:147–158. doi: 10.1105/tpc.6.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CA, Hadjeb N, Newman L, Reardon EM. Isolation of chloroplasts and chloroplast DNA. In: Gelvin SB, Schilperoort RA, editors. Plant Molecular Biology Manual, Section D4. Dordrecht, The Netherlands: Kluwer Academic Press; 1994. pp. 1–15. [Google Scholar]
- Reifarth F, Christen G, Seeliger AG, Dörmann P, Benning C, Renger G. Modification of the water oxidizing complex in leaves of the dgd1 mutant of Arabidopsis thaliana deficient in the galactolipid digalactosyldiacylglycerol. Biochemistry. 1997;36:11769–11776. doi: 10.1021/bi9709654. [DOI] [PubMed] [Google Scholar]
- Siebertz HP, Heinz E. Labeling experiments on the origin of hexa- and octadecatrienoic acids in galactolipids from leaves. Z Naturforsch. 1977;32:193–205. [Google Scholar]
- van Besouw A, Wintermans JFGM. Galactolipid formation in chloroplast envelopes: I. Evidence for two mechanisms in galactosylation. Biochim Biophys Acta. 1978;529:44–53. doi: 10.1016/0005-2760(78)90102-9. [DOI] [PubMed] [Google Scholar]
- van Kooten O, Snel JFH. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- Vijayan P, Routaboul J-M, Browse J. A Genetic approach to investigating membrane lipid structure and function. In: Siegenthaler PA, Murata N, editors. Lipids in Photosynthesis: Structure, Function and Genetics. Dordrecht, The Netherlands: Kluwer Academic Press; 1998. pp. 263–285. [Google Scholar]
- Williams JP, Khan MU. Lipid biosynthesis in Brassica napus leaves: I. 14C-labeling kinetics of the fatty acids of the major glycerolipids. Plant Physiol Biochem. 1996;34:93–100. [Google Scholar]