Abstract
Small noncoding RNAs, including small interfering RNAs (siRNAs) and micro RNAs (miRNAs) of ∼21 nucleotides (nt) in length, have emerged as potent regulators of gene expression at both transcriptional and post-transcriptional levels in diverse organisms. Here we report the identification of a novel class of small RNAs in the mouse male germline termed piwi-interacting RNAs (piRNAs). piRNAs are ∼30 nt in length. They are expressed during spermatogenesis, mostly in spermatids. piRNAs are associated with MIWI, a spermatogenesis-specific PIWI subfamily member of the Argonaute protein family, and depend on MIWI for their biogenesis and/or stability. Furthermore, a subpopulation of piRNAs are associated with polysomes, suggesting their potential role in translational regulation.
Keywords: piRNA, gsRNA, spermatogenesis, spermatid, mouse, MIWI
The importance of small, noncoding RNAs as regulators of transcription, RNA stability, and translation is becoming increasingly evident (Storz et al. 2005; Plasterk 2006). Two major classes of endogenous small RNAs, the small interfering RNAs (siRNAs) and microRNAs (miRNAs), have been characterized in a number of eukaryotes (Valencia-Sanchez et al. 2006). siRNAs and miRNAs are small, ∼21-nucleotide (nt) RNAs that elicit their function through post-transcriptional silencing of cognate mRNAs by targeted destruction and translational repression, respectively. Biochemical analysis suggests that these two species are functionally interchangeable based on the level of complementarity to target mRNAs (Doench et al. 2003). These two classes of small RNAs are products of the cleavage of double-stranded RNA (dsRNA) precursors by the ribonuclease III Dicer enzymes. However, they differ in their origins: siRNAs arise from long dsRNAs, whereas miRNAs are processed from hairpin precursors.
Recently, additional classes of small RNAs have been discovered. In Trypanosoma brucei and Drosophila melanogaster, repeat-associated siRNAs (rasiRNAs) are slightly longer (24–26 nt) than siRNAs and miRNAs, and are derived from bidirectional transcription of repetitive elements in the genome (Djikeng et al. 2001; Aravin et al. 2003). rasiRNAs are highly abundant in the fly testis, where they presumably act to protect the genome from transposable element mobilization and/or to establish chromatin structure (Aravin et al. 2001). Zebrafish (Danio rerio) also contains large numbers of rasiRNAs (Chen et al. 2005). In Arabidopsis thaliana, both rasiRNAs and trans-acting siRNAs represent distinct classes of small RNAs (Lippman et al. 2004; Vazquez et al. 2004). In mammals, some miRNAs and their precursors share sequence homology with repetitive elements and are thought to target complementary sequences contained within the 3′ untranslated regions (UTRs) of multiple mRNAs (Smalheiser and Torvik 2005). Finally, Tetrahymena thermophila contains at least two distinct classes of small RNAs, one functioning in antisense regulation of mRNAs while the other, called scan (scn) RNAs, is involved in the elimination of particular DNA sequences in the newly formed macronucleus after conjugation (Mochizuki and Gorovsky 2004; Lee and Collins 2006).
Although hundreds of small RNAs have been identified from mammalian somatic tissues, relatively little is known about small RNAs in germ cells. To date, only 43 miRNAs have been cloned from the mouse testis (Lagos-Quintana et al. 2002; Yu et al. 2005). One such miRNA, miR-122a, targets a reporter mRNA containing sequences from the 3′-UTR of the Transition Protein 2 (Tnp2) mRNA. Tnp2 is a small basic protein that initially replaces the histones during the condensation of haploid chromosomes in spermatids as they undergo morphogenetic changes to become sperm, a process known as spermiogenesis. Because transcription ceases as chromosomes condense, all genes involved in spermiogenesis, such as Tnp2, must be transcribed in early spermatids, but translationally repressed for up to 7 d, until later stages of spermiogenesis (for review, see Kleene 2003).
Here, we report the identification and preliminary characterization of a novel class of small RNAs present in spermatogenic cells of the mouse. These small RNAs are termed piRNAs (piwi-interacting RNAs) because they are ∼30 nt in length, longer than the known small RNAs. piRNAs map to intergenic, RNA-coding, and repetitive sites in the mouse genome. piRNAs accumulate significantly during spermiogenesis and associate with MIWI, a PIWI family protein that is required for piRNA accumulation. A subpopulation of these piRNAs associate with polysomes, suggesting a potential role in translational regulation.
Results and Discussion
A novel small RNA species in mouse testis that depends on MIWI for expression
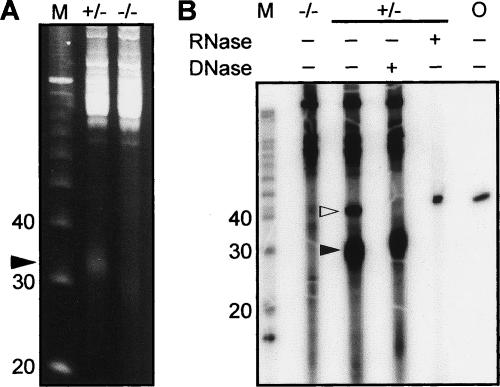
When we separated total testicular RNA by polyacrylamide gel eletrophoresis (PAGE) to resolve small RNAs, we identified an abundant species of RNA migrating at 31–35 nt relative to a DNA ladder (Fig. 1A). Because DNA migrates 10% faster than RNA in our denaturing PAGE, these small RNAs are 28–32 nt in length (see also Fig. 1B with RNA ladder), distinct from the canonical miRNAs and siRNAs that are ∼21 nt or rasiRNAs that are 24–26 nt. This novel species is much more abundant than canonical small RNAs because only the novel species, but not known small RNAs, can be visualized by ethidium-bromide staining and 32P-end labeling (Fig. 1A,B). To ensure that this 28–32-nt species is indeed comprised of RNA, we performed RNase and DNase digestion of 32P-end-labled total RNA samples, adding a 45-nt single-stranded DNA oligomer to the RNA samples as an internal control. The RNase did not have any contaminating DNase activity as the DNA control was unaffected, yet effectively degraded the entire RNA sample, including the 28–32 nt species (Fig. 1B). In contrast, the DNase treatment eliminated the 45-nt internal DNA control, but did not affect the 28–32-nt species, confirming that they are RNA (Fig. 1B).
Figure 1.
PAGE analysis of a novel small RNA species. (A) A 15% PAGE gel containing equal amounts of total RNA from 24-dpp miwi+/− (+/−) and miwi−/− (−/−) testes and visualized with ethidium bromide. A closed arrowhead points to the novel small RNA species. (B) A 15% PAGE gel containing equal amounts of 32P-end-labled total RNA from adult mili+/− (+/−) and mili−/− (−/−) testes, with or without treatment by RNase or DNase. (M) 10-nt DNA (A) or RNA (B) molecular-weight ladder; (O) 45-nt DNA oligomer only. (Closed arrowhead) The novel small RNA species; (open arrowhead) DNA oligomer.
Because of the key role of the PIWI/Argonuate protein MIWI in regulating spermatogenesis, we examined whether the expression of the novel RNA species is dependent on MIWI. MIWI is specifically expressed in spermatocytes and round spermatids starting at the pachytene stage of meiosis (Deng and Lin 2002). MIWI is required for initiating spermiogenesis—a process that transforms round spermatids into mature sperm (Deng and Lin 2002). Spermatogenesis in miwi-null testes is arrested uniformly at the onset of spermiogenesis at 24 d postpartum (dpp). We observed that the expression of the novel species of RNA is significantly reduced in 24 dpp miwi−/− testes (Fig. 1A). These results suggest that these novel RNAs are mostly, if not exclusively, expressed in spermatocytes and spermatids and that their expression is dependent on MIWI function.
Cloning and sequence characterization of the novel small RNAs
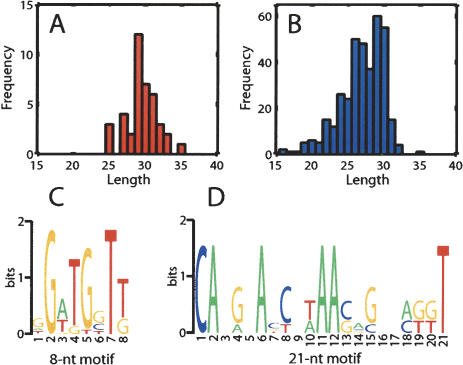
To further characterize these novel RNAs, we cloned them using the method of Lau et al. (2001). This method takes advantage of the characteristic 5′-phosphate and 3′-hydroxyl moieties present on siRNAs and miRNAs, allowing effective selection against typical breakdown products that contain 5′-hydroxyl and 3′-phosphate groups. As a result, 40 independent novel RNA species were cloned (Supplementary Table 1). The cloned sequences were subjected to BLAT searches against the current mouse genomic databases, which reveal that 35 cloned small RNAs each has at least one corresponding sequence in the genome. The length of these small RNAs ranges from 25 to 35 nt, with the majority between 29 and 31 nt (Fig. 2A), confirming our original observations (Fig. 1). Interestingly, two of the 40 RNAs have also been identified by the H. Imai laboratory in their cloning of 367 germline-specific small RNAs from the mouse (gsRNAs, GeneBank accession nos. AB250971–AB251337, herein called the Imai set). Given that the length distributions of these two independent sets of clones are very similar (Fig. 2A,B) and that they are both from the mouse germline, they likely represent two subsets of the same novel class of gsRNAs. Because the expression of these novel RNAs requires a piwi subfamily gene (miwi), yet there is no definitive evidence at present that these small RNAs are all germline specific (see below), these small RNAs are referred to as piwi-interacting RNAs (piRNAs). The small overlap between these two sets of small RNAs suggests that the total number of piRNAs is much larger than these two sets combined.
Figure 2.
Sequence analyses of piRNAs. (A,B) Length distributions of piRNAs from this study (A) and from the Imai set (B). (C,D) Sequence logos showing conserved motifs among 40 cloned piRNAs (T is used to replace U).
Although there is little sequence similarity among the 40 piRNAs we cloned, there is an 8-nt motif shared by 17 sequences and a 21-nt motif shared by five sequences (Fig. 2C,D). Interestingly, eight piRNAs from the Imai set contain the 8-nt motif, but none contains the 21-nt motif. This suggests that our set partially overlaps with the Imai set, but is distinct from the Imai set. This further testifies to the complexity of the piRNA population and the incomplete coverage of the cloning efforts.
It has been reported that Dicer products, including miRNAs, siRNAs, and rasiRNAs have a strong preference for pyrimidine residues, especially uridine, at their 5′-most position (Aravin et al. 2003). piRNAs overall show a very strong sequence bias toward U (77.6%) (Supplementary Table 2), suggesting that they may also be generated by Dicer-like cleavage. Among them, 82.8% of piRNAs from the Imai set contain 5′U, yet only 30% of piRNAs from our set contain 5′U. The chance for this to be a statistical fluctuation from the Imai set is <0.0001 (see Materials and Methods). This further suggests that our set is distinct from the Imai set.
Among Dicer-generated small RNAs, miRNAs are known to be generated from precursors that have unique fold-back hairpin secondary structure (Bartel 2004). To test whether piRNAs share similar structures, we randomly selected eight piRNAs, added 50 bases of flanking genomic sequence from both ends of each piRNA coding sequence, and predicted their secondary structures. None of the eight structures resembles those of miRNA precursors, nor do these structures resemble each other. Therefore, it remains unclear whether piRNAs are derived from precursors with unique secondary structures.
The distribution of piRNAs in the genome
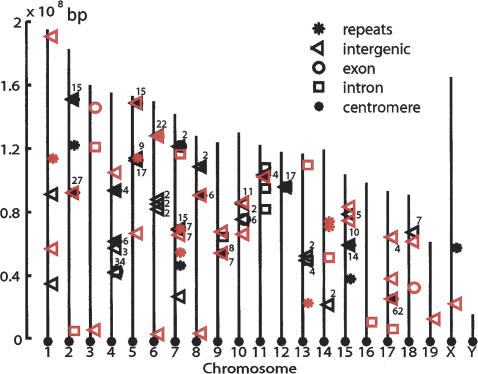
To further characterize the piRNAs, we determined the corresponding genomic region of our piRNAs and the Imai set, totaling 407 piRNAs. We first conducted BLAT searches against the mouse genome to map these RNAs (Material and Methods; Fig. 3; Supplementary Table 2). Of the 407 piRNAs, 75.4% were successfully annotated. The remaining 24.6% (37) sequences did not match to any genomic sequences. Several striking features are revealed through the following analysis:
Figure 3.
Distribution of piRNA-encoding sequences in the genome. The chromosomes were drawn in scale and aligned by their centromere positions. piRNAs from this study and from the Imai set are indicated in red and black, respectively. The number of piRNAs in a particular cluster is indicated by the number next to the cluster.
First, piRNA-coding sequences display highly uneven distribution among chromosomes (Fig. 3; Supplementary Table 2). For example, Chromosome (Chr) 17, representing only 3.1% of the genome, encodes 17.6% of the piRNAs. Other piRNAs are enriched on Chr 5 (11.6%), Chr 4 (10.7%), and Chr 2 (10.2%), but are underrepresented on Chr 1, Chr 3, Chr 16, Chr 19, and Chr X. For example, the X chromosome, representing 5.5% of the genome, contains only two piRNA sequences (0.4%), 14-fold lower than the expected value for random distribution. The Y chromosome is devoid of known piRNAs.
Second, piRNA-coding sequences show a strong tendency to cluster (Fig. 3; Supplementary Table 3). Eighty-nine percent of piRNAs form 35 clusters containing two to 62 piRNAs, with an average of 10.3 piRNAs per cluster. Each cluster spans 0.4–73.5 kb (median 21.8 kb). The largest cluster is located on Chr17 and contains 62 piRNAs in a 73.5-kb region. Among the 35 clusters, five may be created by intrachromosomal segmental duplications, with one cluster on Chr 4 containing 17 duplicated 152-base-pair (bp) sequences defined by two piRNAs. However, the other 30 clusters do not appear to be caused by duplications. In either case, this highly clustered organization raises the possibility that the piRNAs within each cluster are coordinately expressed and might share related functions.
Third, piRNAs are diversely distributed among exonic, intronic, intergenic, and repeat sequences (Supplementary Table 4). The majority of piRNAs (90%) are found within intergenic regions, 9.3% within introns of known genes, and 1.3% in exons. The exonic piRNAs can be distinguished from the degradation products of mRNAs by Northern blot analysis,, where piRNAs are detected as discrete bands of ∼30 nt, rather than the size of the corresponding mRNA (data not shown). This distribution is not significantly different from the genome-wide averages of 68.1% intergenic, 29.9% intronic, and 2.1% exonic sequences. Interestingly, 17% of piRNAs map to repetitive sequences, noticeably lower than the presence of such sequences in 42.3% of the genome. Overall, the relatively even distribution of piRNAs among different types of genomic sequences is in contrast to the high bias of miRNAs toward exonic sequences or rasiRNAs toward repeat sequences. The distribution of piRNAs among all types of genomic sequences suggests their diverse roles in regulating gene expression.
piRNAs are only detectable in developing spermatids
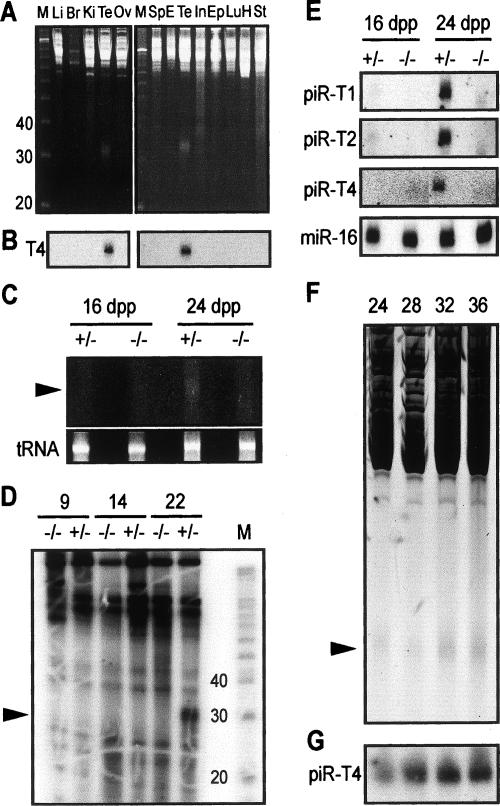
To examine whether piRNAs also exist in other tissues, we examined total RNA from many mouse tissues by ethidium-bromide staining of denaturing PAGE gels (Fig. 4A), and by Northern blot analysis of an individual piRNA, piRNA-T4 (Fig. 4B). The piRNAs were only detected in testes, suggesting that piRNAs are only abundantly present in testes. Notably, piRNAs are not detected in the caudal epididymus, suggesting that they are not loaded into mature sperm. Furthermore, since piRNAs are drastically reduced in miwi−/− testes, they are expected to be mostly, if not exclusively, present in spermatocytes and early spermatids where MIWI is expressed. To test this prediction, we fractionated total testicular RNA isolated from different timepoints of testicular development by PAGE to detect total piRNAs by ethidium-bromide staining (Fig. 4C,F) and 32P-end labeling (Fig. 4D) or individual piRNAs by Northern blot analysis (Fig. 4E,G). By any of these methods, piRNAs are not detectable in miwi+/− and miwi−/− testes at 16 dpp (Fig. 4C,E), 2 d after the initiation of MIWI expression, when only spermatogonia and primary spermatocytes are present (Bellve et al. 1977). piRNAs are detectable at 22 dpp (Fig. 4D), when step 1 round spermatids begin to form, and are more abundant at 24 dpp, when steps 1–3 round spermatids have formed in both genotypes (Fig. 4C,E). piRNAs persist at this level in the wild-type testes through 36 dpp (sexual maturity), as indicated by both ethidium-bromide staining of total piRNAs (Fig. 4F) or Northern blot analysis of piRNA-T4 (Fig. 4G). This expression profile indicates that piRNAs are predominantly produced post-meiotically in early round spermatids in a MIWI-dependent fashion. This accumulation corresponds to a major phase of translation (Hecht 1998; Braun 2000; Kimmins and Sassone-Corsi 2005), suggesting that some piRNAs might be involved in translational regulation. This speculation is consistent with the likely role of MIWI in translational regulation (S.T. Grivna, B. Pyhtila, and H. Lin, in prep.).
Figure 4.
Tissue and spermatogenic stage specificity of piRNA expression. (A) PAGE analysis of total RNA extracts from liver (Li), brain (Br), kidney (Ki), testis (Te), ovary (Ov), spleen (Sp), embryonic stem cells (E), intestine (In), caudal epididymus (Ep), lung (Lu), heart (H), and stomach (St), showing that piRNAs are only detected in the testis by ethidium-bromide staining. (B) Northern blot analysis of RNA from A, showing that piRNA-T4 is only detectable in the testis. (C) PAGE analysis with ethidium-bromide staining showing that piRNAs are only detected in the miwi+/− testis at 24 dpp. (D) PAGE analysis with 32P-end-labeled total RNA indicating that piRNAs are first detectable at 22 dpp in mili+/− testes, but are absent in 22-dpp mili−/− testes. (E) Northern blot analysis of RNA from C, showing that piRNA-T1, piRNA-T2, and piRNA-T4 are detectable in 24 dpp, but not 16-dpp, miwi+/− testes. (F) Negative image of PAGE analysis with ethidium-bromide-stained PAGE gel showing that piRNAs are present in 24, 28, 32, and 36 dpp testes. (G) Northern blot analysis of total RNA from F, showing that piRNA-T4 is increasing in abundance from 24–32 dpp.
piRNAs associate with polysomes
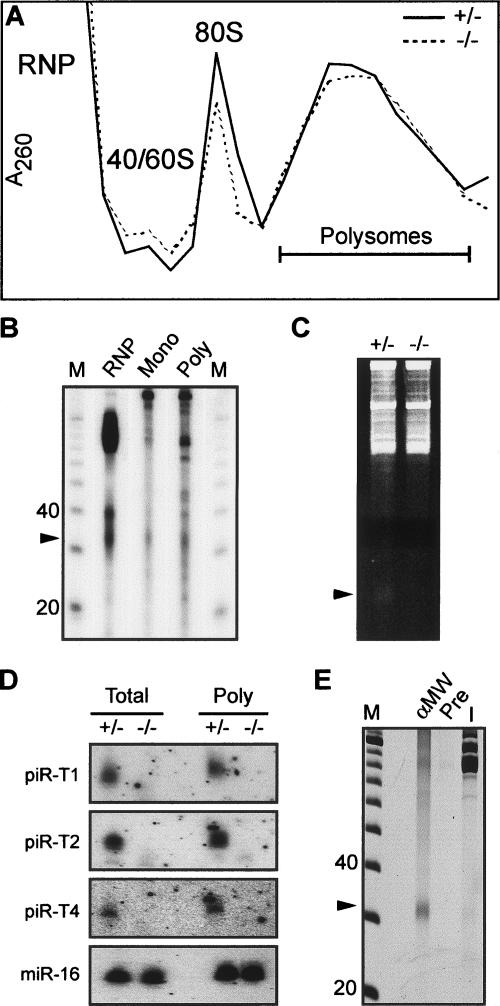
To test the potential role of piRNAs in translational regulation, we examined whether piRNAs associate with polysomes. We fractionated testicular extracts from 24-dpp and adult miwi+/− and miwi−/− mice on continuous sucrose gradients (see Materials and Methods; 24 dpp shown in Fig. 5A). RNA was then isolated from pooled free RNP, monosomal, and polysomal fractions from adult miwi+/− mice, end-labled with 32P, and separated by denaturing PAGE (Fig. 5B). piRNAs are present in RNP, monosomal, and polysomal fractions at a relative abundance of 38%, 28%, and 34%, respectively. The presence of piRNAs in the polysome fraction was abundant enough to be detectable by ethidium-bromide staining (Fig. 5C). When the polysome-cosedimenting RNA was used for Northern blot analysis of individual piRNAs, we observed that all three tested piRNAs cosediment with polysomes (Fig. 5D). As expected, piRNAs are absent from the polysomal fractions of miwi−/− testes.
Figure 5.
piRNAs are associated with MIWI and polysomes. (A) A260 profile of 24 dpp miwi+/− (+/−) and miwi−/− (−/−) testicular extracts separated by sucrose density gradient fractionation with RNP, 40S, 60S, monosomal, and polysomal fractions indicated. (B) PAGE analysis of RNA from pooled RNP, monosome, and polysome fractions of the adult miwi+/− testicular extracts end-labeled with 32P. piRNAs, indicated by the arrowhead, were quantified with the NIH Image J software. (C) PAGE analysis of RNA from pooled polysome fractions in A stained with ethidium bromide. piRNAs are indicated by the arrowhead. (D) Northern blots of total RNA and polysome-associated RNA from C probed with three individual piRNAs. miRNA miR-16 was used as a loading control. (E) Negative image of ethidium-bromide-stained PAGE gel containing RNA coimmunoprecipitated with MIWI antibody (αMW) or its preimmune sera (Pre), and 10 μg of testicular RNA input (I). (M) 10-nt DNA ladder.
Since MIWI is required for piRNA expression, we examined whether piRNAs are associated with MIWI. MIWI was immunoprecipitated from 24-dpp miwi+/− testicular extract by anti-MIWI antibody (Deng and Lin 2002). The coprecipitated RNA was size fractionated by PAGE and visualized by ethidium-bromide staining. piRNAs specifically coimmunoprecipite with MIWI (Fig. 5E), indicating their association. Since MIWI is implicated in translational regulation (S.T. Grivna, B. Pyhtila, and H. Lin, in prep.), the association of piRNAs with MIWI suggests a possible role for piRNAs in MIWI-mediated translational regulation.
Whereas some piRNAs might have a role in translational regulation, others encoded by repetitive sequences might function like rasiRNAs to protect the haploid genome from the rearrangement of transposable elements. The biogenesis of piRNAs also opens a new challenge. The conventional siRNAs and miRNAs are generally 21–23 nt in length as determined by the physical distance between the PAZ and RNaseIII domains of Dicer molecules (Macrae et al. 2006). How these 28–35 nt piRNAs are generated remains elusive. Further investigation of these questions should allow us to discover a new dimension of small RNA biology.
Materials and methods
Total RNA isolation and separation
Total and polysomal RNA was isolated using Trizol (Invitrogen) according to the manufacturer’s protocol. Concentrations were determined by UV spectrometry and gel quantification.
Cloning of testicular piRNAs
Small RNAs of 29–34 nt from the testis were purified from 500 μg of total testicular RNA by excision of corresponding gel slices from ethidium-bromide-stained 15% denaturing polyacrymide gels. Small RNAs were recovered from the excised gel slices and cloned according to the protocol of Lau et al. (2001). A total of 46 clones were isolated. Sequence analysis revealed that five are cloning artifacts, and 41 are genuine clones that represent 40 independent sequences.
PAGE analysis of piRNAs
For total RNA analysis by ethidium-bromide staining, 40 μg of total RNA from 16- and 24-dpp miwi+/− and miwi−/− testes was used. For total RNA analysis by 32P-end labeling, 1 μg of total RNA samples isolated from 9, 14, and 22 dpp, and adult (∼3-mo-old) mili+/− and mili−/− testes were 5′-end-labeled with 32P by T4 polynucleotide kinase (New England Biolabs) following dephosphorylation with calf intestinal phosphatase (New England Biolabs). One nanogram of 5′-end-labeled DNA 45-mer was added to each sample as indicated in Figure 1B. Labeled RNA and DNA was purified using a Sephadex G25 spin column (Roche). Nuclease digestion, where indicated, was performed with 100 μg of RNase A (Sigma), and 7.5 U RQ1 DNase (Promega) for 30 min at 37°C. For polysomal RNA analysis, one adult testis equivalent (160-mg testis) of polysomal RNA was used with 200 μM cycloheximide. Forty micrograms of total miwi+/− testicular RNA was used for ethidium-bromide staining of RNA at 24, 28, 32, and 36 dpp. Ten nucleotide DNA markers and 10 nt RNA markers were purchased from Invitrogen and Ambion, respectively. Typhoon 9400 PhosphorImager was used for radioactive imaging analysis.
Northern blot analysis of piRNAs
For Northern blot analysis, RNA was separated by 6 M Urea/15% PAGE and transferred to Hybond-N+ membrane (Amersham Biosciences). Forty micrograms of total testicular RNA from 16- and 24-dpp miwi+/− and miwi−/− mice were used for total RNA analysis. For polysomal analysis, one-tenth of one testis equivalent of 24-dpp miwi+/− total testicular RNA and one testis equivalent of polysomal RNA were used. Hybridizations were performed at 42°C in hybridization buffer (1× SSC, 7% SDS, 20 mM Na2HPO4 at pH 7.2, 1× Denhardt’s solution, 0.1 mg/mL salmon sperm DNA). Blots were probed with radiolabeled DNA oligos (IDT DNA) complementary to mature piRNA or miRNA sequences. Probes were labeled with [32P-γ]ATP and T4 PNK (NEB). Blots were washed twice in 1× SSC and 0.1% SDS at 42°C for 10 min and analyzed by PhosphorImager.
RNA isolation from polysomes
Two testes from 24-d-old miwi+/− and miwi−/− mice were subjected to continuous sucrose density gradient fractionation as in S.T. Grivna, B. Pyhtila, and H. Lin (in prep.). Fractions containing polysomes were combined and total RNA was isolated as above.
Coimmunoprecipitation of piRNAs
MIWI and its associated RNA was immunoprecipitated from 2 mg of post-nuclear extract from 24-dpp miwi+/− testes by a rabbit polyclonal antibody R133 against amino acid residues 239–524 of MIWI (Deng and Lin 2002). The extract was combined with an equal volume of NT2 buffer (50 mM Tris at pH 7.4, 150 mM NaCl, 1 mM MgCl2, 0.05% NP-40) and incubated with Protein A-Sepharose beads coated with either anti-MIWI or preimmune serum. To recover coprecipitated RNA, 200 μL of TE/1% SDS was added to the precipitate and heated to 95°C for 3 min, followed by phenol/chloroform extraction and isopropanol precipitation. Precipitated RNA was separated by 15% denaturing PAGE and visualized with ethidium bromide.
piRNA sequence analyses
Motif finding was carried out by the MEME program (http://meme.sdsc.edu/meme/meme.html). piDNA sequence logos were drawn by WebLogo (http://weblogo.berkeley.edu/logo.cgi). piRNA (plus 50 bp upstream and 50 bp downstream) secondary structure predictions were carried out with RNAfold (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi). To annotate piRNAs, BLAT searches were conducted to map them onto individual chromosomes (http://genome.ucsc.edu/cgi-bin/hgBlat). For individual piRNAs with multiple hits, only the highest scoring hits with a sequence identity of >96% were kept. Exonic, intronic, intergenic, and repeat annotations were retrieved based on mouse genome assembly 34, by a computer program developed in-house.
The 77.6% frequency of 5′U in piRNAs refers to the percentage of sequences that have a 5′U in a combined data set of the Imai Set (367 sequences) and our set (40 sequences). The frequency of 5′U in our set is 30%, in contrast to the Imai Set (82.8%). To determine whether it is likely that our set is a subset of the Imai set, we randomly subsampled 40 sequences from the Imai’s 367 sequences to form 10,000 sets of 40 sequences and calculated the number of sequences that have 5′Us. Among these random sets, there are 22–40 sequences per set that have a 5′U (mean = 33.15 and standard deviation = 2.25). Therefore, the probability of observing 30% 5′U-containing piRNAs from our set as a fluctuation of the Imai set is <0.0001. This result suggests that our sequences likely represent a distinct population of smaller RNAs than the Imai’s piRNAs.
Acknowledgments
S.T.G. is supported in part by a NIH predoctoral training grant (T32 CA59365). This work was funded by NIH (HD42012) to H.L.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.1434406
References
- Aravin A.A., Naumova N.M., Tulin A.V., Vagin V.V., Rozovsky Y.M., Gvozdev V.A. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr. Biol. 2001;11:1017–1027. doi: 10.1016/s0960-9822(01)00299-8. [DOI] [PubMed] [Google Scholar]
- Aravin A.A., Lagos-Quintana M., Yalcin A., Zavolan M., Marks D., Snyder B., Gaasterland T., Meyer J., Tuschl T. The small RNA profile during Drosophila melanogaster development. Dev. Cell. 2003;5:337–350. doi: 10.1016/s1534-5807(03)00228-4. [DOI] [PubMed] [Google Scholar]
- Bartel D.P. microRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bellve A.R., Cavicchia J.C., Millette C.F., O’Brian D.A., Bhatnagar Y.M., Dym M. Spermatogenic cells of the prepubertal mouse: Isolation and morphological characterization. J. Cell Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R.E. Temporal control of protein synthesis during spermatogenesis. Int. J. Androl. 2000;23(Suppl. 2):92–94. doi: 10.1046/j.1365-2605.2000.00027.x. [DOI] [PubMed] [Google Scholar]
- Chen P.Y., Manninga H., Slanchev K., Chien M., Russo J.J., Ju J., Sheridan R., John B., Marks D.S., Gaidatzis D., et al. The developmental miRNA profiles of zebrafish as determined by small RNA cloning. Genes & Dev. 2005;19:1288–1293. doi: 10.1101/gad.1310605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev. Cell. 2002;2:819–830. doi: 10.1016/s1534-5807(02)00165-x. [DOI] [PubMed] [Google Scholar]
- Djikeng A., Shi H., Tschudi C., Ullu E. RNA interference in Trypanosoma brucei: Cloning of small interfering RNAs provides evidence for retroposon-derived 24-26-nucleotide RNAs. RNA. 2001;7:1522–1530. [PMC free article] [PubMed] [Google Scholar]
- Doench J.G., Petersen C.P., Sharp P.A. siRNAs can function as miRNAs. Genes & Dev. 2003;17:438–442. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht N.B. Molecular mechanisms of male germ cell differentiation. Bioessays. 1998;20:555–561. doi: 10.1002/(SICI)1521-1878(199807)20:7<555::AID-BIES6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kimmins S., Sassone-Corsi P. Chromatin remodelling and epigenetic features of germ cells. Nature. 2005;434:583–589. doi: 10.1038/nature03368. [DOI] [PubMed] [Google Scholar]
- Kleene K.C. Patterns, mechanisms, and functions of translation regulation in mammalian spermatogenic cells. Cytogenet. Genome Res. 2003;103:217–224. doi: 10.1159/000076807. [DOI] [PubMed] [Google Scholar]
- Lagos-Quintana M., Rauhut R., Yalcin A., Meyer J., Lendeckel W., Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- Lau N.C., Lim L.P., Weinstein E.G., Bartel D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- Lee S.R., Collins K. Two classes of endogenous small RNAs in Tetrahymena thermophila. Genes & Dev. 2006;20:28–33. doi: 10.1101/gad.1377006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman Z., Gendrel A.V., Black M., Vaughn M.W., Dedhia N., McCombie W.R., Lavine K., Mittal V., May B., Kasschau K.D., et al. Role of transposable elements in heterochromatin and epigenetic control. Nature. 2004;430:471–476. doi: 10.1038/nature02651. [DOI] [PubMed] [Google Scholar]
- Macrae I.J., Zhou K., Li F., Repic A., Brooks A.N., Cande W.Z., Adams P.D., Doudna J.A. Structural basis for double-stranded RNA processing by Dicer. Science. 2006;311:195–198. doi: 10.1126/science.1121638. [DOI] [PubMed] [Google Scholar]
- Mochizuki K., Gorovsky M.A. Conjugation-specific small RNAs in Tetrahymena have predicted properties of scan (scn) RNAs involved in genome rearrangement. Genes & Dev. 2004;18:2068–2073. doi: 10.1101/gad.1219904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasterk R.H.A. Micro RNAs in animal development. Cell. 2006;124:877–881. doi: 10.1016/j.cell.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Smalheiser N.R., Torvik V.I. Mammalian microRNAs derived from genomic repeats. Trends Genet. 2005;21:322–326. doi: 10.1016/j.tig.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Storz G., Altuvia S., Wassarman K.M. An abundance of RNA regulators. Annu. Rev. Biochem. 2005;74:199–217. doi: 10.1146/annurev.biochem.74.082803.133136. [DOI] [PubMed] [Google Scholar]
- Valencia-Sanchez M.A., Liu J., Hannon G.J., Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes & Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- Vazquez F., Vaucheret H., Rajagopalan R., Lepers C., Gasciolli V., Mallory A.C., Hilbert J.L., Bartel D.P., Crete P. Endogenous trans-acting siRNAs regulate the accumulation of Arabidopsis mRNAs. Mol. Cell. 2004;16:69–79. doi: 10.1016/j.molcel.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Yu Z., Raabe T., Hecht N.B. MicroRNA Mirn122a reduces expression of the posttranscriptionally regulated germ cell transition protein 2 (Tnp2) messenger RNA (mRNA) by mRNA cleavage. Biol. Reprod. 2005;73:427–433. doi: 10.1095/biolreprod.105.040998. [DOI] [PubMed] [Google Scholar]