Abstract
Objective
To report the authors’ experience with hepatic vein reconstruction and plasty in living donor liver transplantation for adult patients.
Summary Background Data
A right liver graft without the middle hepatic vein (MHV) trunk (modified right liver graft) can cause severe congestion of the right paramedian sector. However, the need for MHV reconstruction has not been fully recognized.
Methods
From June 2000 to December 2001, 30 adult patients received a modified right liver graft. Major MHV tributaries were preserved and reconstructed under the authors’ criteria. Plasty of recipient hepatic veins for a wide outflow orifice was performed when necessitated. The regeneration of paramedian and lateral sectors of the grafts was examined by computed tomography 1 and 3 months after the operation.
Results
MHV tributaries were reconstructed in 18 grafts. Plasty of recipient hepatic veins was performed in 15 patients. All patients survived the operation. The regeneration of paramedian and lateral sectors was equivalent.
Conclusions
A modified right liver graft can provide satisfactory surgical results if hepatic vein reconstruction and plasty are performed using the present techniques.
Living donor liver transplantation (LDLT) was originally developed as a solution for the organ shortage with pediatric recipients 1,2 and has recently been extended to adult recipients. An extended right liver graft, 3 which includes the trunk of the middle hepatic vein (MHV), was devised to alleviate the problem of graft size disparity. However, this graft increases the extent of the donor operation and might raise an important ethical issue in LDLT. 4
A right liver graft without the MHV trunk (modified right liver graft) is now commonly used but can cause severe congestion of the right paramedian sector (corresponding to Couinaud segments 5 and 8 5). Such congestion can lead to severe graft dysfunction and septic complications 6 because hepatic venous outflow of the right paramedian sector is drained mostly into the MHV. 7
MHV drainage into the recipient’s venous system can be reconstructed using vein grafts. This provides a functioning liver mass comparable to an extended right liver graft. We previously proposed reconstruction criteria and have performed LDLT using the graft under these criteria. 8 A wide outflow orifice seems to be another crucial issue. We present here our surgical indications, techniques, and results for hepatic vein reconstruction in modified right liver graft.
METHODS
Patients
From June 2000 to December 2001, 30 modified right liver grafts were implanted in adult patients (20 men, 10 women). The indications for LDLT included hepatitis C virus/cirrhosis in eight, primary biliary cirrhosis in six, hepatitis B virus/cirrhosis associated with hepatocellular carcinoma in six, hepatitis C virus/cirrhosis associated with hepatocellular carcinoma in five, fulminant hepatic failure in four, and primary sclerosing cholangitis in one. The recipients were 18 to 61 years old (mean 45) and weighed 47 to 70 kg (mean 61). The total bilirubin and albumin levels ranged from 2.6 to 27.8 mg/dL (mean 8.7) and 2.1 to 3.1 g/dL (mean 2.8), respectively.
The donors were 14 men and 16 women. They ranged in age from 20 to 60 years (mean 37) and weighed 43 to 75 kg (mean 57). They consisted of 13 children, 8 siblings, 6 spouses, 2 parents, and 1 nephew. All cases were approved by the ethics committee of University of Tokyo.
Preoperative Assessment of Donor Liver
Right liver volume was estimated by computed tomography (CT). Candidates in whom the right liver represented more than 70% of the whole liver were rejected as prospective donors. An estimated graft volume to recipient standard liver volume 9 ratio of 40% was the lower limit for right liver transplantation.
The number and diameter of thick MHV tributaries draining the right paramedian sector were evaluated on CT. The tributaries were classified as V8, which drained the cranial part of the portal trunk of the right paramedian sector, and V5, which drained the corresponding caudal part. The other details regarding selection criteria and evaluation have been described elsewhere. 10
Intraoperative Evaluation of Hepatic Venous Congestion
A J-shaped incision was made and the abdominal cavity was entered. Hepatectomy started with a careful hilar dissection. Intraoperative ultrasound was then performed to confirm the hepatic vein anatomy and to verify the transection plane. The major short hepatic veins (inferior or middle right hepatic veins [IRHV or MRHV]), V5 or V8, if present and greater than 5 mm, were isolated and preserved. Parenchymal transection was performed using a combination of the clamp fracture technique and a Cavitron Ultrasonic Surgical Aspirator (CUSA System 200; Valleylab Inc., Boulder, CO). All sizable vascular and biliary structures were divided between ligatures.
Hepatic venous congestion in the right paramedian sector was investigated intraoperatively after parenchyma transection. First, liver surface discoloration in the right paramedian sector was observed after 5 minutes of simultaneous clamping of MHV tributaries and the right hepatic artery. Next, intraoperative Doppler ultrasonography (SSD-2000 or SSD-5500; Aloka, Tokyo, Japan) was performed after declamping of only the hepatic artery. If the portal flow of the paramedian sector was hepatofugal, the area was confirmed to be congested.
If the congested area was dominant by the clamping test or ultrasonography, we proceeded with bench reconstruction of MHV tributaries. The need for MRHV or IRHV reconstruction was determined by the same criteria. The other details of donor hepatectomy have been described elsewhere. 11
Bench Surgery
The harvested liver graft was flushed with 1 L University of Wisconsin solution through a cannula inserted into the right portal vein.
Autogenous vein grafts for interposition were harvested from the recipients, donors or cadavers. Anastomosis between V5 or V8 and autogenous vein grafts was carried out using a continuous 6-0 prolene suture. When the recipient’s RHV orifice was smaller than that of the graft, the proximal end of the vein grafts were sutured to the anterior wall of the graft RHV (Fig. 1).
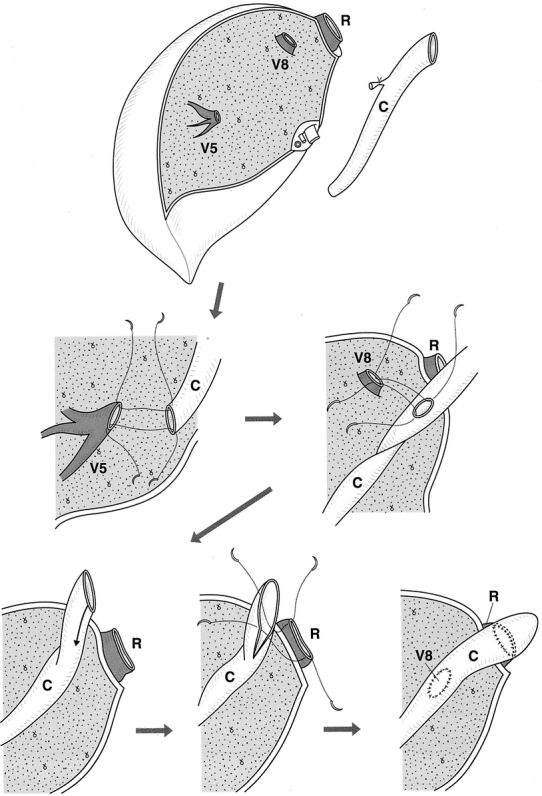
Figure 1. Anastomosis between stump of middle hepatic vein tributaries and vein grafts on the bench when the orifice of the graft right hepatic vein was smaller than that of the recipient. C, vein graft; R, right hepatic vein; V5, V8, stump of middle hepatic vein tributaries.
Recipient Operation
When the size of the RHV orifice was comparable to that of the graft and MHV reconstruction was not indicated, the left hepatic vein (LHV) and MHV were closed with a running suture. A V-shaped venous patch was added to the anterior wall of the recipient RHV, which was anastomosed with graft RHV. If MHV reconstruction was necessary, the stumps of the LHV and MHV of the recipients were preserved longer and served as anastomotic sites with graft MHV tributaries (Fig. 2).
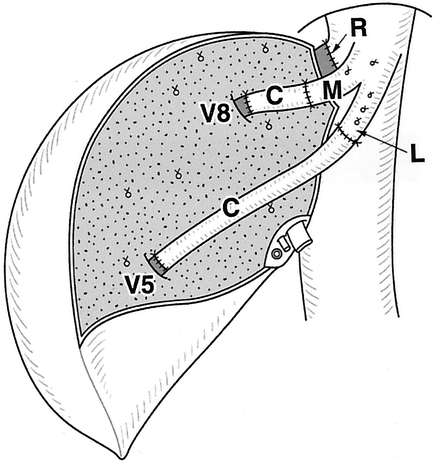
Figure 2. Hepatic vein reconstruction when the orifice of the graft right hepatic vein was comparable with that of the recipient. C, vein graft; R, right hepatic vein; M, middle hepatic vein; L, left hepatic vein; V5, V8, stump of middle hepatic vein tributaries.
When the recipient’s RHV orifice was smaller than that of the graft, a wide orifice was created by plasty of three hepatic veins, the LHV and MHV or MHV and RHV (Fig. 3). After plasty, the approximation was checked with another clamp placed at the distal end. The anastomosis was made with a continuous everted mattress or over-and-over suture using a 6-0 monofilament material. Graft MHV tributaries were anastomosed with a common orifice when necessary.
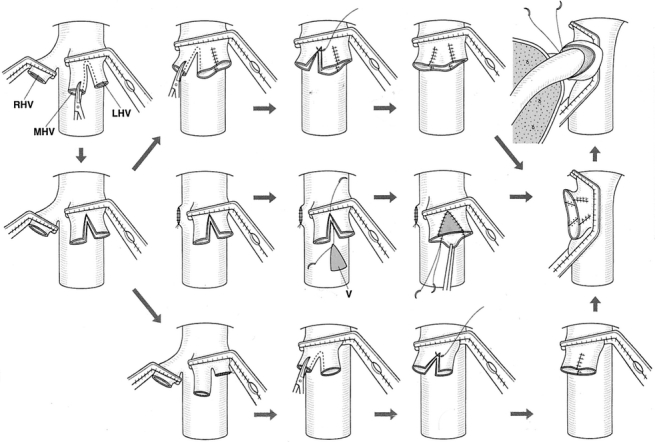
Figure 3. Recipient venoplasty of triple hepatic veins. V, venous patch; LHV, left hepatic vein; MHV, middle hepatic vein; RHV, right hepatic vein.
If the IRHV or MRHV was preserved in the graft, it was anastomosed to the side of the recipient’s retrohepatic vena cava in an end-to-side fashion. On reconstruction of the inflow, adequate hepatic venous drainage was confirmed by a complex or triphasic Doppler waveform. Inflow vascular or bile duct anastomosis followed hepatic vein reconstruction; the details have been described previously. 4
Postoperative Evaluation
Vascular flow in the graft or interposition vein patency was checked by Doppler ultrasound every day until the postoperative day 14 and once a week thereafter until hospital discharge. Enhanced CT was performed 1 and 3 months after LDLT. The volumes of the paramedian and lateral sectors were calculated as described elsewhere. 9 Aspartate aminotransferase (AST) and total bilirubin levels were measured every day after LDLT for 4 weeks.
RESULTS
Venous Reconstruction and Patency
In 15 patients, the RHV was anastomosed to that of the graft (see Fig. 2). In the other 15 patients, plasty of hepatic veins was performed: a triple venoplasty in 7, LHV and MHV in 5, and MHV and RHV in 3.
A total of 28 MHV tributaries were reconstructed in 18 grafts. The veins consisted of cryopreserved iliac veins (n = 17), the left portal branch of the recipient (n = 8), the saphenous vein of the donor (n = 2), and cryopreserved superior vena cava (n = 1). No MHV tributaries were reconstructed in 12 grafts because of a negligible area of congestion in 7 and a lack of dominant tributaries in 2. Major short hepatic veins were found in 17 grafts, and 15 of these were reconstructed.
No complications were recognized in RHV reconstruction. No evidence of congestion in the right paramedian sector was recognized in the patients using Doppler ultrasound.
Laboratory Data
Levels of AST peaked on postoperative day 1 and then decreased gradually (Fig. 4). The total bilirubin level decreased rapidly after LDLT, and none of the patients had significant cholestasis.
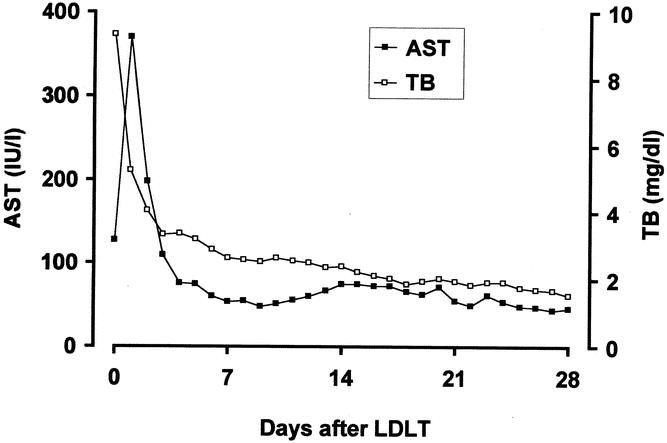
Figure 4. The changes in aspartate aminotransferase (AST, closed square) and total bilirubin (TB, open square) levels for 4 weeks after transplantation.
Morbidity and Mortality
The right liver grafts weighed 488 to 780 g (mean 622), which corresponded to 40% to 67% (mean 52%) of the standard liver volume of the recipients. All the patients survived the operation. In one patient, hepatofugal flow of the graft was recognized on postoperative day 5 and was corrected by shunt ligation. 12 Other complications included acute rejection in 10 and bile duct leakage of the anastomosis that necessitated surgical revision in 2. CT examination revealed that regeneration of the paramedian and lateral sectors was compatible after LDLT (Fig. 5).
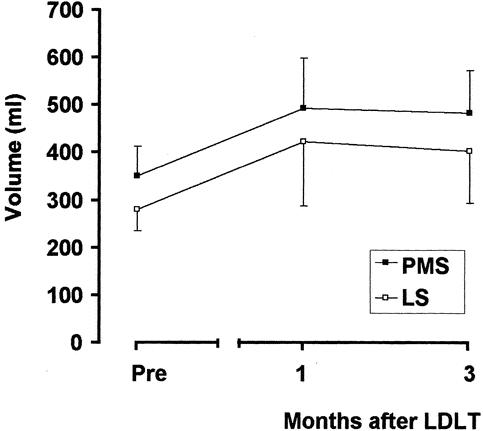
Figure 5. Regeneration of the paramedian (PMS, closed square) and lateral (LS, open square) sectors 1 and 3 months after transplantation.
One patient died 8 months after LDLT due to the recurrence of hepatocellular carcinoma. The other patients achieved long-term survival with a median follow-up of 22 months. Neither mortality nor life-threatening postoperative complications were observed in the donors.
DISCUSSION
It has been previously reported that ligation of the major hepatic veins does not pose any risk for patients undergoing hepatectomy, since collateral circulation develops via the sinusoids and short hepatic veins and occasionally via the portal veins after ligation of the major hepatic veins. 13 In the initial reports regarding LDLT using a modified right liver graft, the MHV tributaries were not reconstructed. 14–17 Colledan et al. 18 reported a new in situ split liver technique for liver grafts from cadavers and did not address this problem.
An important concern was raised by Lee et al., 19 who emphasized the need for MHV drainage when using a modified right liver graft. They noted that the graft could cause severe congestion of the right paramedian sector because hepatic venous outflow of the right paramedian sector is drained mostly into the MHV. Such congestion can cause severe graft dysfunction and septic complications. This concern might be supported by the findings of Nakamura et al., who reported that the RHV drains the right lateral sector and a small part of the paramedian sector, particularly segment 8. 20 Venous drainage through the sinusoids appeared to be insufficient to relieve congestion after hepatic vein ligation in rats.
After this impressive proposal, some transplant teams seem to have recognized the value of reconstructing the MHV tributaries. Cattral et al. 21 reported a case of reconstruction using the recipient’s left portal branch. Ghobrial et al. 22 found a venous variant type of small RHV and large MHV branch and proposed that MHV reconstruction should be performed in such cases.
It has remained unclear whether all modified right liver grafts require MHV drainage. Lee et al. 19 emphasized aggressive reconstruction of MHV under any circumstances. However, most of the initial cases 14–16 using a modified right liver graft seemed to achieve successful results. Sano et al. 8 proposed clear criteria for MHV reconstruction, and MHV tributaries were reconstructed under these criteria in our series. Reconstruction was performed in 18 of 30 grafts, and all of the grafts showed uneventful functional recovery.
It has been reported that a single wide outflow orifice is important in left liver transplantation. 23–25 Matching the size of the donor RHV to that of the recipient is another requirement. 22 A triple recipient venoplasty was performed in seven cases; this useful when the orifice of the recipient RHV is small compared to that of graft.
The use of a venous patch or a long anterior wall of the reconstructed hepatic vein may raise some objections. We were concerned about stretching the anastomosis of the hepatic veins, which might cause outflow obstruction. Using the present technique, the reconstructed RHV is thick and long enough, allowing the graft to sit in a dorsal space of the abdominal cavity without tension. Marcos et al. 17 performed simple end-to-end anastomosis between the donor and recipient RHV. Ghobrial et al. 22 commented that a long reconstructed vein was predisposed to kinking after reperfusion. The appropriate length of the reconstructed RHV remains controversial.
In summary, using the criteria we proposed previously, 8 MHV tributaries were reconstructed in 18 of 30 modified right liver grafts. All grafts achieved prompt functional recovery with equivalent regeneration of the paramedian and lateral sectors. These satisfactory results support the feasibility of the MHV reconstruction criteria and our surgical technique.
Footnotes
Supported by a Grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Public Trust Fund for the Promotion of Surgery, Welfide Medical Research Foundation, Mitsui Life Social Welfare Foundation, and a Grant-in-aid for Research on Human Genome, Tissue Engineering, Food Biotechnology, Health Sciences Research Grants, Ministry of Health, Labor and Welfare of Japan
Correspondence: Yasuhiko Sugawara, MD, Artificial Organ and Transplantation Division, Department of Surgery, Graduate School of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
E-mail: yasusuga-tky@umin.ac.jp
Accepted for publication May 16, 2002.
References
- 1.Raia S, Nery JR, Mies S. Liver transplantation from live donors. Lancet 1989; 2: 497. [DOI] [PubMed] [Google Scholar]
- 2.Strong RW, Lynch SV, Ong TH, et al. Successful liver transplantation from a living donor to her son. N Engl J Med 1990; 322: 1505–1507. [DOI] [PubMed] [Google Scholar]
- 3.Lo CM, Fan ST, Liu CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg 1997; 226: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugawara Y, Makuuchi M. Technical advances in living-related liver transplantation. J Hepatobiliary Pancreat Surg 1999; 6: 245–253. [DOI] [PubMed] [Google Scholar]
- 5.Couinaud C. Schema general de la distribution intra-hepatique. In: Couinaud C, ed. Le foie. Etudes anatomiques et chirurgicales. Paris: Massaon & Cie; 1957: 9–12.
- 6.Lee SG, Park KM, Hwang S, et al. Adult-to-adult living donor liver transplantation at Asian Medical Center, Seoul, Korea. Transplant Proc 1999; 31: 456–458. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura S, Tsuzuki T. Surgical anatomy of the hepatic veins and the inferior vena cava. Surg Gynecol Obstet 1981; 152: 43–50. [PubMed] [Google Scholar]
- 8.Sano K, Makuuchi M, Miki K, et al. Evaluation of hepatic venous congestion: proposed indication criteria for hepatic vein reconstruction. Ann Surg 2002; 236: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urata K, Kawasaki S, Matsunami H, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 1995; 21: 1317–1321. [PubMed] [Google Scholar]
- 10.Sugawara Y, Makuuchi M, Takayama T, et al. Living-related liver transplantation for primary biliary cirrhosis. Transplantation 2001; 72: 1087–1091. [DOI] [PubMed] [Google Scholar]
- 11.Makuuchi M, Kawasaki S, Noguchi T, et al. Donor hepatectomy for living related partial liver transplantation. Surgery 1993; 113: 395–402. [PubMed] [Google Scholar]
- 12.Cescon M, Sugawara Y, Kaneko J, et al. Restoration of portal vein flow by splenorenal shunt ligation and splenectomy after living-related liver transplantation. Hepato-Gastroenterology 2001; 48: 1453–1454. [PubMed] [Google Scholar]
- 13.Ou QJ, Hermann RE. Hepatic vein ligation and preservation of liver segments in major hepatic resections. Arch Surg 1987; 122: 1198–1200. [DOI] [PubMed] [Google Scholar]
- 14.Yamaoka Y, Washida M, Honda K, et al. Liver transplanation using a right lobe graft from a living related donor. Transplantation 1994; 57: 1127–1230. [PubMed] [Google Scholar]
- 15.Wachs M, Bak T, Karrer F, et al. Adult living donor liver transplantation using a right hepatic lobe. Transplantation 1998; 66: 1313–1316. [DOI] [PubMed] [Google Scholar]
- 16.Marcos A, Fisher RA, Ham JM, et al. Right lobe living donor liver transplantation. Transplantation 1999; 68: 798–803. [DOI] [PubMed] [Google Scholar]
- 17.Marcos A, Ham JM, Fisher RA, et al. Surgical management of anatomical variations of the right lobe in living donor liver transplantation. Ann Surg 2000; 231: 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colledan M, Andorno E, Valente U, et al. A new splitting technique for liver grafts. Lancet 1999; 53: 1763. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Park K, Hwang S, et al. Congestion of right liver graft in living donor liver transplantation. Transplantation 2001; 71: 812–814. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura S, Sakaguchi S, Hachiya T, et al. Significance of hepatic vein reconstruction in hepatectomy. Surgery 1993; 114: 59–64. [PubMed] [Google Scholar]
- 21.Cattral MS, Greig PD, Muradali D, et al. Reconstruction of middle hepatic vein of a living-donor right lobe liver graft with recipient left portal vein. Transplantation 2001; 71: 1864–1866. [DOI] [PubMed] [Google Scholar]
- 22.Ghobrial RM, Hsieh CB, Lerner S, et al. Technical challenges of hepatic venous outflow reconstruction in right lobe adult living donor liver transplantation. Liver Transplant 2001; 7: 551–555. [DOI] [PubMed] [Google Scholar]
- 23.Matsunami H, Makuuchi M, Kawasaki S, et al. Venous reconstruction using three recipient hepatic veins in living related liver transplantation. Transplantation 1995; 59: 917–919. [PubMed] [Google Scholar]
- 24.Takayama T, Makuuchi M, Kawasaki S, et al. Outflow Y-reconstruction for living related partial hepatic transplantation. J Am Coll Surg 1994; 179: 226–229. [PubMed] [Google Scholar]
- 25.de Villa VH, Chen CL, Chen YS, et al. Outflow tract reconstruction in living donor liver transplantation. Transplantation 2000; 70: 1604–1608. [DOI] [PubMed] [Google Scholar]


