Abstract
Objective
To identify patient characteristics associated with the development of local recurrence and the effect of local recurrence on subsequent morbidity and mortality in patients with intermediate- to high-grade extremity soft tissue sarcomas.
Summary Background Data
Numerous studies on extremity soft tissue sarcomas have consistently shown that presentation with locally recurrent disease is associated with the development of subsequent local recurrences and that large tumor size and high histologic grade are significant factors associated with decreased survival. However, the effect of local recurrence on patient survival remains unclear.
Methods
From 1975 to 1997, 753 patients with intermediate- to high-grade extremity soft tissue sarcomas were treated at UCLA. Treatment outcomes and patient characteristics were analyzed to identify factors associated with both local recurrence and survival.
Results
Patients with locally recurrent disease were at a significantly increased risk of developing a subsequent local recurrence. Local recurrence was a morbid event requiring amputation in 38% of the cases. The development of a local recurrence was the most significant factor associated with decreased survival. Once a patient developed a local recurrence, he or she was about three times more likely to die of disease compared to similar patients who had not developed a local recurrence.
Conclusions
Local recurrence in patients with intermediate- to high-grade extremity soft tissue sarcomas is associated with the development of subsequent local recurrences, a morbid event decreasing functional outcomes and the most significant factor associated with decreased survival. Although 85% to 90% of patients with high-grade extremity soft tissue sarcomas are treatable with a limb salvage approach, patients who develop a local recurrence need aggressive treatment and should be considered for trials of adjuvant systemic therapy.
Limb-sparing surgery as a treatment for patients with extremity soft tissue sarcomas is an approach that has evolved over the years. The initial concept was that it was possible to attain survival rates with wide surgical excisions that were comparable to amputative surgery. Numerous studies have confirmed this, employing either a radical surgical excision or a more limited wide surgical excision with adjuvant therapy. 1–4
The influence of local recurrence on the survival of patients with extremity soft tissue sarcomas is controversial. The results of two prospective randomized trials have provided the basis for suggesting that local recurrence is not associated with decreased survival. Rosenberg et al. 3 at the National Cancer Institute (NCI) randomized patients to undergo either amputation or limb-sparing surgery combined with postoperative radiation. Despite no local recurrences in the amputation group and a 20% local recurrence rate in the limb salvage group, no survival differences were noted. Brennan et al. at Memorial Sloan-Kettering Cancer Center (MSKCC) randomized patients to undergo surgical resection with adjuvant brachytherapy or surgical resection alone. Although the results showed that adjuvant brachytherapy reduced local recurrence rates in patients with high-grade sarcomas, the overall survival of the two groups was not different. 5–7 In addition to these studies, multiple retrospective analyses have arrived at conclusions covering the entire spectrum from no association with decreased survival, 8–10 to an indeterminate association with decreased survival, 11,12 and finally to being associated with decreased survival. 13,14 Thus, the influence of local recurrence on overall survival remains unclear.
Our hypothesis is that local recurrence is not only a morbid event reducing functional outcomes but that it is also a significant prognostic factor associated with decreased survival. To study this we evaluated the factors that were associated with development of a local recurrence and the effect of local recurrence on subsequent morbidity and mortality in patients with intermediate- to high-grade extremity soft tissue sarcomas.
METHODS
From 1975 to 1997 1,290 patients with soft tissue sarcomas were treated at UCLA. Fifty-six were located in the head and neck region, 287 in the retroperitoneum or abdominal cavity, 92 in the trunk or chest wall, and 855 in the extremity. Of the 855 patients with extremity sarcomas, 102 with low-grade tumors were excluded from this analysis. The remaining 753 patients with intermediate- to high-grade histologies and no evidence of metastatic disease represent the study population.
All 753 patients underwent surgery at UCLA. Surgery was performed by one of two surgeons over the duration of this study. Seven hundred four patients underwent limb-sparing surgery and 49 patients required primary amputation due to tumor location, size, neurovascular involvement, or a combination of these factors. Four hundred ninety-eight patients were treated in a preoperative, protocol fashion with neoadjuvant therapy. Neoadjuvant therapy consisted of radiation using various regimens of either intravenous or intra-arterial Adriamycin, combinations of Adriamycin/platinum, and most recently Adriamycin, platinum, and ifosfamide. The neoadjuvant protocols used were performed in a nonrandomized manner and reflect the evolution of treatment at UCLA during the past 20 years (Fig. 1). The remaining 255 patients did not receive neoadjuvant therapy. These patients were not included in the protocols for various reasons including superficial tumors, epithelioid sarcoma, or prior radiation.
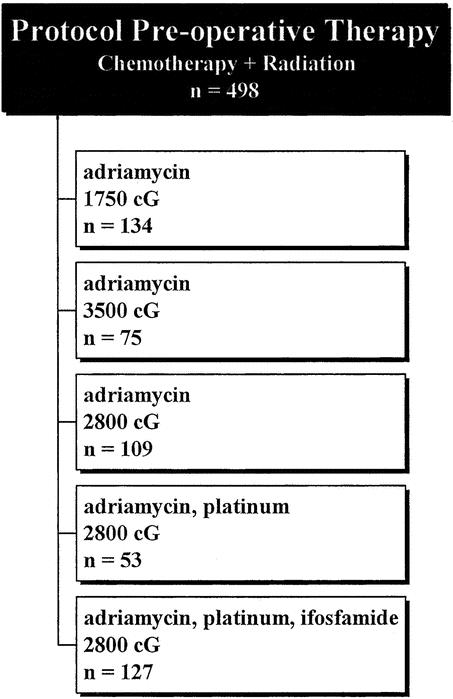
Figure 1. Protocol pre-operative therapy.
The following definitions were used. A tumor was considered primary (group I) if it was previously untreated or only a biopsy (incisional or excisional) had been performed at the time of presentation. Locally recurrent disease (group II) was defined as tumor recurrence at the site of previous treatment. Tumor size was defined as maximum diameter at pathologic analysis. Tumors were grouped into three size ranges: less than 5 cm, 5 to 10 cm, and more than 10 cm. Upper extremity was defined as a tumor at or distal to the shoulder. Upper extremity proximal refers to tumors within the shoulder region, including the axilla. Upper extremity medial refers to tumors in the region from the shoulder to the forearm. Upper extremity distal refers to tumors at or distal to the forearm. Lower extremity was defined as a tumor at or distal to the groin or gluteal area. Lower extremity proximal refers to tumors within the groin or gluteal region. Lower extremity medial refers to tumors located in the region from the groin to the calf. Lower extremity distal refers to tumors at or distal to the calf. Tumor grade was classified as high, intermediate, or low based on established criteria, including degree of differentiation, nuclear pleomorphism, and number of mitoses per high-power field. 15
Postoperative follow-up included physical examination, chest radiograph, and computed tomography (CT) of the primary site at 6-month intervals for the first 2 years and yearly thereafter. End points for evaluation included local recurrence, date of death, or date of last follow-up. The interval to recurrence was measured from the date of surgery at UCLA to the date of recurrence. Survival duration was measured from the date of surgery at UCLA to the date of last follow-up or death. Follow-up in this group of patients was 100%.
Statistical Analysis
Statistical analysis was performed using the STATA software package. Actuarial survival curves were determined using the Kaplan-Meier method and were compared using the log-rank test. Univariate analysis was performed using the log-rank test for grouped variables and the Cox proportional hazards model for continuous variables. Multivariate analysis was performed using the Cox proportional hazards model. Use of the Cox proportional hazards model requires certain assumptions. Validation of the proportional hazards assumption (that is, that the relative risk of failure between subgroups in the model does not change over time) was made through a graphical examination of log minus log plots of the various Kaplan-Meier survival curves versus the log of time. The resulting approximately parallel straight lines indicated that the proportional hazards assumptions for the dichotomous variables examined were not unreasonable. More importantly, validation of the overall final model was made graphically by comparing the Kaplan-Meier survival curves for various subgroups to the predicted survival curves based on the Cox model. Since no gross departure was observed in any of the subgroups with adequate sample sizes, the final model was accepted.
Factors related to local recurrence were investigated using competing risks survivorship analysis, with patient death and local recurrence as the two competing risks. 16,17 This analysis is different from the usual cause-specific analysis, where patients who die before having a local recurrence are censored at the time of their death. The cause-specific analysis is estimating the probability of local recurrence in the presence of death, but this analysis is strictly valid only if the risk of death and local recurrence are independent. In this study the competing risk analysis was preformed using a program written by Dr. Bob Gray. 16
The relationship between local recurrence and the risk of death was investigated using local recurrence as a time-dependent variable. 13,18 In this analysis, patients are switched from the nonrecurrent group to the recurrent group at the time of local recurrence. The effect of local recurrence on the risk of death is illustrated by comparing all patients who recurred locally within the first year to patients who were alive and had not recurred locally at year 1. The time-dependent analysis can be viewed as a means of comparing these groups, from the times of local recurrence to death, over the time period of the study.
RESULTS
Patient and Tumor Characteristics
Of the 753 patients evaluated, 607 (81%) were primary (group I) and 146 (19%) were locally recurrent (group II) at presentation to UCLA. The mean age for the group I and group II patients was 47 years and 50 years, respectively. Gender distribution was similar, with 56% (n = 338) male and 44% (n = 269) female for the group I patients and 51% (n = 74) male and 49% (n = 72) female for the group II patients.
The two most common anatomic sites of the primary tumor were the lower extremity medial (60% [n = 367] for group I, 44% [n = 64] for group II) and upper extremity medial (15% [n = 89] for group I and 21% [n = 30] for group II). Forty-six percent (n = 280) of the group I tumors and 58% (n = 85) of the group II tumors were in the 5-to-10-cm range. Of the group I tumors, 72% (n = 436) were high grade and 28% (n = 171) were intermediate grade. Of the group II tumors, 53% (n = 78) were high grade and 47% (n = 68) were intermediate grade. The two most common histologies were liposarcoma (26% [n = 156] for group I, 24% [n = 35] for group II) and malignant fibrous histiocytoma (25% [n = 153] for group I, 21% [n = 31] for group II) (Table 1).
Table 1
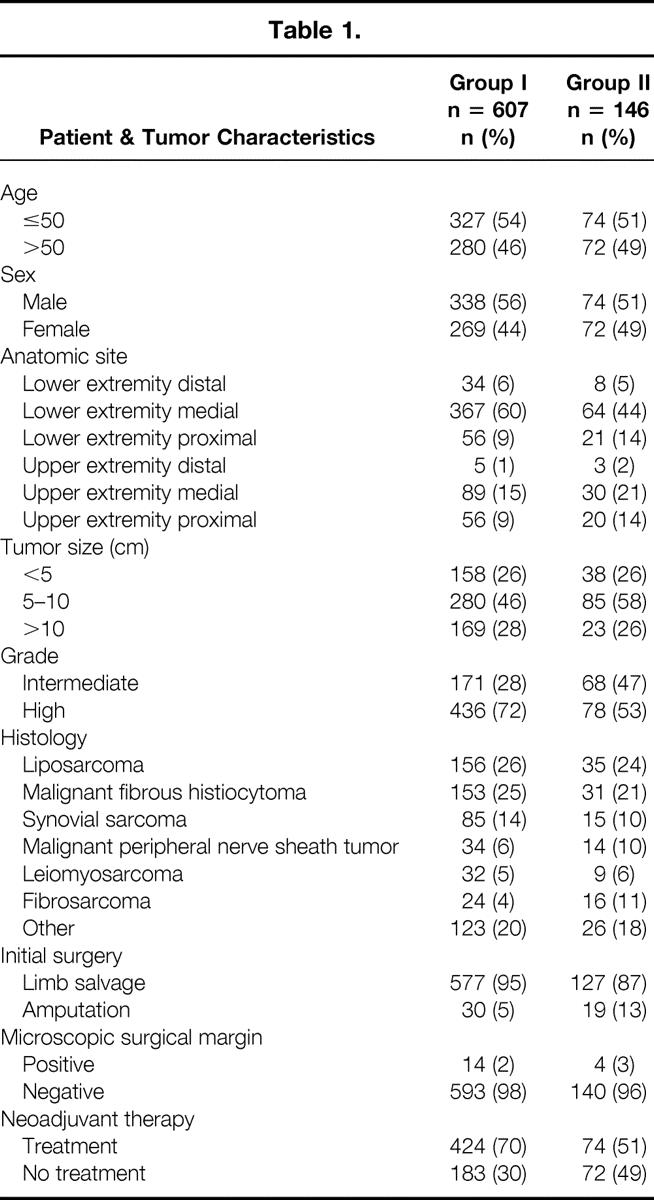
The mean interval between diagnosis and initial treatment at UCLA for the group I patients was 2 months (median 1, range 1–39). The mean interval between initial treatment and treatment of locally recurrent disease at UCLA for the group II patients was 30 months (median 13, range 6–192). Seventy percent (n = 424) of the group I and 51% (n = 74) of the group II patients received neoadjuvant therapy.
Treatment Outcomes
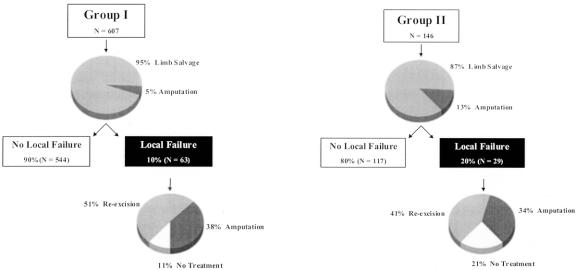
Ninety-five percent (n = 577) of the 607 group I patients underwent limb salvage surgery as the initial surgery at UCLA, with the remaining 5% (n = 30) requiring amputation. Ninety percent (n = 544) of these group I patients remained free of disease, with 10% (n = 63) having developed a local recurrence. Of these 63 group I patients with locally recurrent disease, 24 (38%) required amputation to control the recurrent tumor, 32 (51%) had a local re-excision, and 7 (11%) had no further treatment due to the concurrent development of metastatic disease (Fig. 2).
Figure 2. Treatment outcomes.
By comparison, 87% (n = 127) of the 146 group II patients underwent limb salvage surgery as the initial surgery at UCLA, with the remaining 13% (n = 19) requiring amputation. Eighty percent (n = 117) of these group II patients remained free of disease, with 20% (n = 29) having developed a subsequent local recurrence. Of these 29 group II patients with additional local recurrences, 11 (34%) required amputation to control their local failure, 12 (41%) had local re-excision, and 6 (21%) had no further therapy due to the concurrent development of metastatic disease (see Fig. 2).
Eighteen (2%) patients (14 [2%] of the group I patients, 4 [3%] of the group II patients) had microscopically positive surgical margins following surgery at UCLA (see Table 1). These patients were treated immediately by amputation (n = 3), additional local excision (n = 5), or additional radiation therapy (n = 8). Two of these patients had no further therapy. Five of the 15 (33%) nonamputated patients with positive resection margins developed a subsequent local recurrence despite treatment with re-excision or adjuvant radiation therapy.
Local Recurrence
With a mean follow-up of 98 months for surviving patients (median = 88 months), the local recurrence rate for all 753 patients was 12 ± 1.2% at 5 years and 14 ± 1.3% at 10 years. Of the 92 patients who developed a local recurrence, 25% recurred by 8 months, 50% by 16 months, and 75% by 36 months. However, 13% developed a local recurrence greater than 5 years following initial treatment at UCLA.
The 5- and 10-year local recurrence rates for group I patients were 10 ± 1.2% and 12 ± 1.4%, respectively. The 5- and 10-year local recurrence rates for group II patients were 19 ± 3.4% and 22% ± 3.6%, respectively (Fig. 3). The time range to local failure for group I patients was from 2 to 151 months, with a mean of 28 months (median 17 months). The time range to local failure for group II patients was 1 to 113 months, with a mean of 26 months (median 13 months).
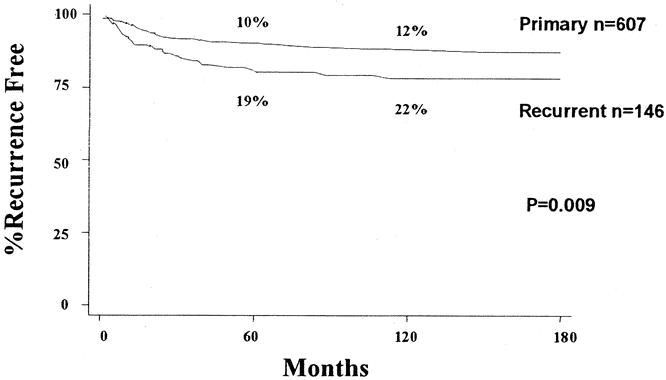
Figure 3. Local recurrence: Group I vs. Group II.
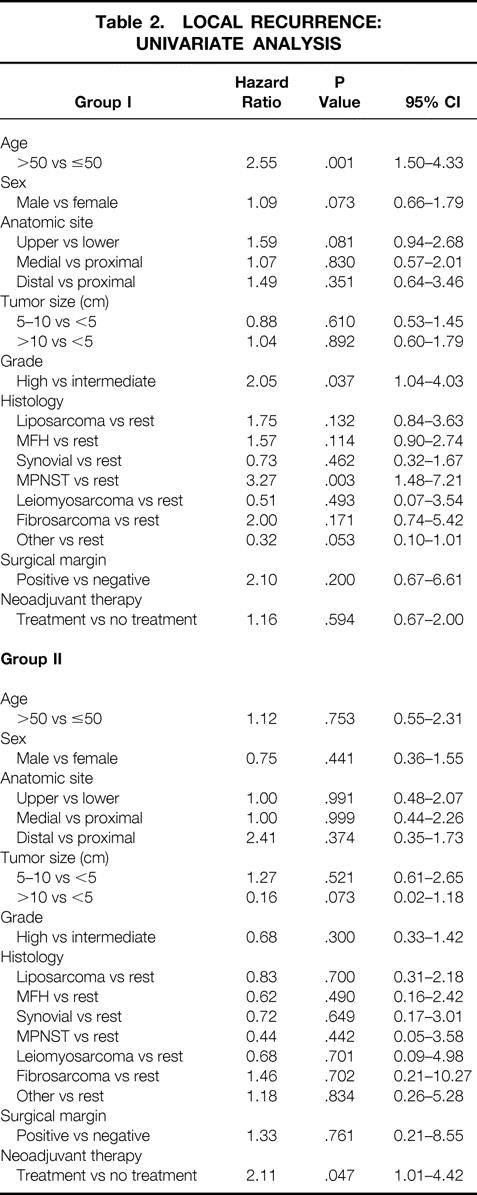
A univariate analysis of patient characteristics related to local recurrence for group I patients showed that age older than 50 years, high histologic grade, and malignant peripheral nerve sheath tumor were associated with an increased risk of developing a local recurrence. However, in group II patients, failure to receive neoadjuvant therapy was the only factor associated with an increased risk of developing a local recurrence (Table 2).
Table 2. LOCAL RECURRENCE: UNIVARIATE ANALYSIS
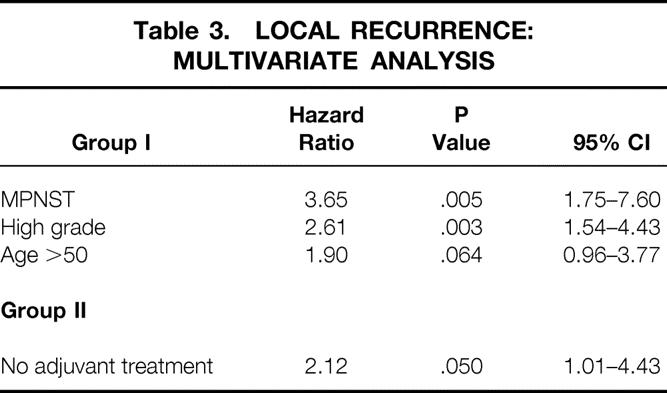
A multivariate analysis of patient characteristics related to local recurrence for group I patients showed that (in order of hazard ratio) malignant peripheral nerve sheath tumor, high histologic grade, and age older than 50 years were all independently associated with an increased risk of developing a local recurrence. As with the univariate analysis, failure to receive neoadjuvant therapy was the only factor associated with an increased risk of developing a local recurrence for the group II patients (Table 3).
Table 3. LOCAL RECURRENCE: MULTIVARIATE ANALYSIS
Survival
With a mean follow-up of 98 months for surviving patients (median 88 months), the overall survival for all 753 patients was 70 ± 1.8% at 5 years and 60 ± 2.1% at 10 years. The 5- and 10-year survival rates for group I patients were 71 ± 2.0% and 62 ± 2.3%, respectively. The 5- and 10-year survival rates for group II patients were only 67 ± 4.1% and 52 ± 4.6%, respectively (Fig. 4).
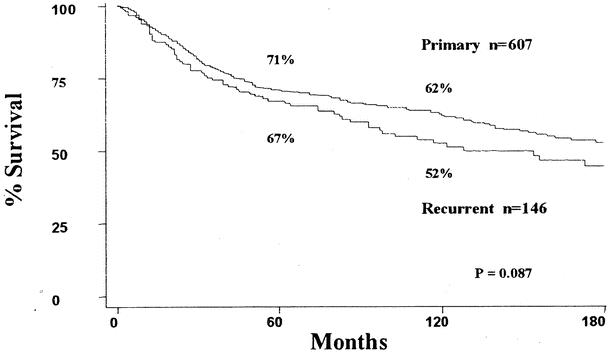
Figure 4. Survival: Group I vs. Group II.
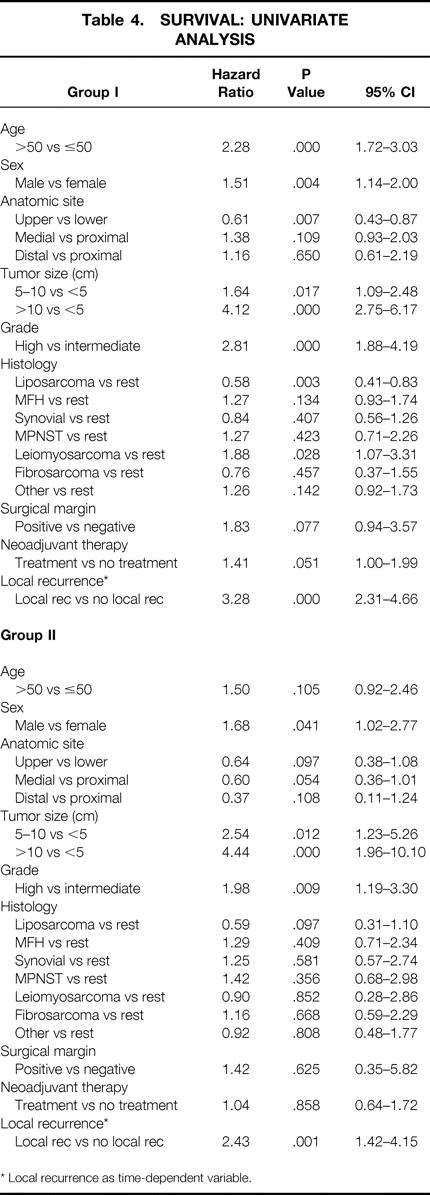
A univariate analysis of patient characteristics related to survival for group I patients showed that age older than 50 years, male gender, lower extremity location, size, high histologic grade, non-liposarcoma or leiomyosarcoma histology, and local recurrence were associated with decreased survival. For the group II patients, male gender, size, high histologic grade, and local recurrence were associated with decreased survival (Table 4).
Table 4. SURVIVAL: UNIVARIATE ANALYSIS
* Local recurrence as time-dependent variable.
A time-dependent multivariate analysis of patient characteristics related to survival for group I patients showed that (in order of hazard ratio) local recurrence, high histologic grade, leiomyosarcoma histology, size, age older than 50 years, and lower extremity location were all independently associated with decreased survival. For the group II patients (in order of hazard ratio), local recurrence, high histologic grade, size, and male gender were all independently associated with decreased survival (Table 5).
Table 5. SURVIVAL: TIME-DEPENDENT MULTIVARIATE ANALYSIS
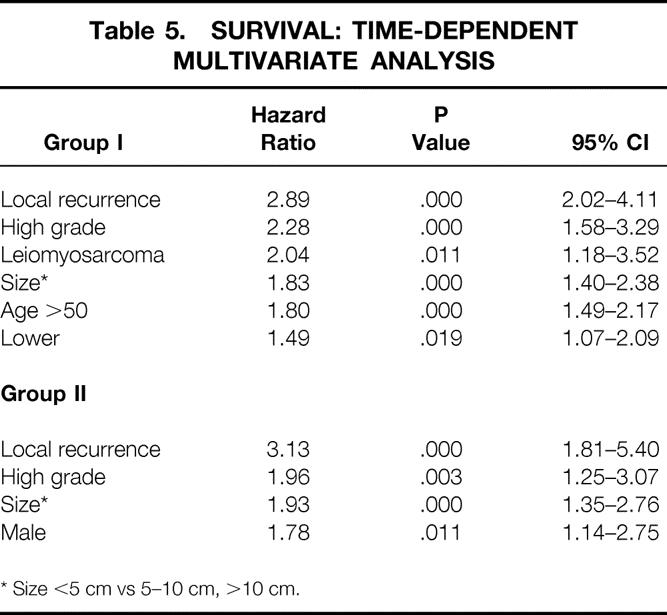
* Size <5 cm vs 5–10 cm, >10 cm.
To illustrate the effect of local recurrence on survival, we compared (among group I patients surviving at least 1 year) group I patients who recurred locally within the first year to group I patients who did not recur locally within the first year. The survival rates for the group I patients who had not recurred locally within the first year were 77 ± 1.9% at 5 years and 67 ± 2.4% at 10 years. By comparison, the survival rate for group I patients who recurred locally within the first year was only 29 ± 9.6% at 5 years (Fig. 5).
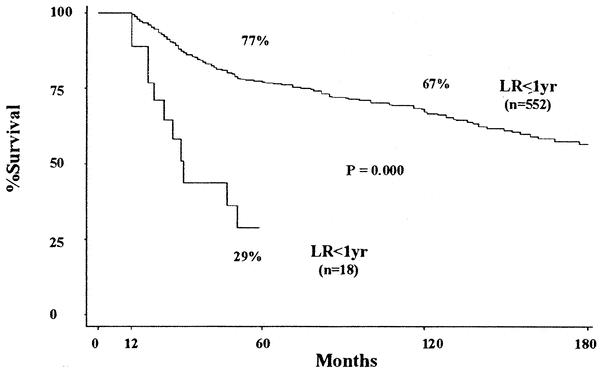
Figure 5. Survival Group I: local recurrence with in first year (LR < 1yr) versus no local recurrence with in first year (no LR < 1yr).
DISCUSSION
A number of studies have evaluated the factors influencing both local recurrence and overall survival in patients with extremity soft tissue sarcomas. 8–14,19–23 From these studies several questions have emerged. One such question pivotal to the treatment of this disease is what effect local recurrence has on overall survival. In an attempt to answer this, we evaluated the factors associated with developing a local recurrence and the effect of local recurrence on subsequent morbidity and mortality in 753 patients with intermediate- to high-grade extremity soft tissue sarcomas. In addition to analyzing all 753 patients together, we believed it was important to analyze the patients who presented to UCLA with primary disease (group I) separately from the patients who presented with locally recurrent disease (group II). Despite being very similar in patient and tumor characteristics, the treatment outcomes and prognostic factors associated with both local recurrence and survival were noticeably different for group I and group II patients.
The morbidity of developing a local recurrence is readily apparent. Of the 753 patients, only 7% (n = 49) required amputation on initial treatment at UCLA. This increased to 38% (n = 35) in the treatment of the 92 patients who developed a subsequent local recurrence. This is further evident by comparing the patients who presented with primary disease (group I) with those who presented with locally recurrent disease (group II). Only 5% of the group I patients required amputation on initial treatment at UCLA, compared to 13% of the group II patients. Following the development of subsequent local recurrence, 21% of the group II could not be treated further due to the concurrent development of metastatic disease, compared to only 11% of the group I patients. Although the amputation rate was similar among the patients who developed a local failure, 51% of the group I patients were amenable to local re-excision, compared to only 41% of the group II patients (see Fig. 2). Thus, patients who presented with locally recurrent disease had a significantly higher local recurrence rate and worse treatment outcomes both initially and following subsequent local failure.
With a mean follow-up of over 8 years for surviving patients, the overall local recurrence rate for all 753 patients was 12% at 5 years and 14% at 10 years. Although the majority of patients recurred locally within 36 months of treatment, there was a subset of patients that recurred late. In fact, we found that 13% developed a local recurrence at greater than 5 years from initial treatment at UCLA. The 5- and 10-year local recurrence rates for the patients who presented to UCLA with primary disease (group I, 10% and 12%, respectively) were significantly better than for patients who presented with locally recurrent disease (group II, 19% and 22%, respectively) (see Fig. 4). In fact, having a prior local recurrence was found to be associated with development of a subsequent local recurrence, and the group II patients were about two times more likely to develop a local recurrence compared to the group I patients.
Patient characteristics associated with developing a local recurrence were analyzed in a univariate and more importantly in a multivariate manner. For the patients who presented with primary disease (group I), malignant peripheral nerve sheath tumor, high histologic grade, and age more than 50 years (in order of hazard ratio) were all found to be independently associated with an increased risk of developing a local recurrence. Interestingly, not receiving neoadjuvant therapy was the only factor found to be associated with an increased risk of developing a local recurrence for the patients who presented with locally recurrent disease (group II). Although we found this to be a noteworthy observation, this study was not designed to look at the impact of any of the specific adjuvant therapies, and thus no treatment-related conclusions can be made. 24,25 However, clearly the group II patients are different, and the factors associated with an increased risk of local recurrence for patients with primary disease did not apply to patients presenting with locally recurrent disease.
Only 18 of the 753 (2%) patients had a microscopically positive surgical margin. Sixteen of these patients had additional local treatment with re-excision (n = 5), radiation therapy (n = 8), or amputation (n = 3). Two of these patients had no further therapy. Despite the immediate additional therapy, 33% (5 of 15) of the nonamputated patients with a positive surgical margin developed a local recurrence. This factor, however, was not found to be significantly associated with developing a local recurrence. We believe that microscopically positive surgical margins are associated with an increased risk of local recurrence, and if more patients in this study were left with microscopically positive margins and/or less aggressively treated, then this variable may have reached statistical significance.
The influence of local recurrence on mortality in patients with extremity soft tissue sarcomas has been a source of debate since the development of limb salvage therapy. The inference that local recurrence is not associated with overall survival is primarily based on two prospective randomized studies performed on this disease. Rosenberg et al. at the NCI randomized 43 patients with extremity soft tissue sarcomas to undergo either amputation or limb-sparing surgery combined with postoperative radiation. 3 Despite no local recurrences in the amputation group and a 20% local failure rate in the limb salvage group, no survival differences were noted. Brennan et al. at MSKCC randomized 126 patients with soft tissue sarcomas to undergo surgical resection with either adjuvant brachytherapy or no further therapy. Despite a local recurrence rate of 18% for the patients who received brachytherapy versus 33% in the group who received no further therapy, there was not a significant impact on disease-specific survival. 5–7 Thus, the differences in local recurrence rates in either study were not associated with a difference in overall survival. In addition to these studies, multiple retrospective analyses have arrived at conflicting conclusions regarding the influence of local recurrence on survival. 8–14 In reviewing the literature it is clear that there is no consensus on the association of local recurrence with overall survival.
With a mean follow-up of over 8 years for surviving patients, the overall survival rate for all patients was 70% at 5 years and 60% at 10 years. The 5- and 10-year survival rates for the patients who presented to UCLA with primary disease (group I, 71% and 62%, respectively) were better than for patients who presented with locally recurrent disease (group II, 67% and 52%, respectively) (see Fig. 4).
Patient characteristics associated with survival were analyzed in a univariate and a more appropriately in a time-dependent multivariate fashion. This analysis showed that local recurrence was the most significant factor independently associated with decreased survival. Group I patients who developed a local recurrence were 2.89 times more likely to die from their disease than patients who did not develop a local recurrence. Group II patients who developed a subsequent local recurrence were 3.13 times more likely to die from their disease than patients who did not develop a local recurrence. High histologic grade and size were also found to be significantly associated with decreased survival for both group I and group II patients.
To graphically illustrate the association between local recurrence and decreased survival, we compared group I patients who recurred locally within the first year to group I patients who did not recur locally within the first year. The 5- and 10-year survival rates for group I patients who did not recur locally within the first year were 77% and 67%, respectively. By comparison, the survival rate for group I patients who recurred locally within the first year was only 29% at 5 years. Similar differences were also observed when different time points beyond 1 year were chosen. Essentially, the time-dependent analysis can be viewed as a means of comparing the surviving patients among these groups (local recurrence vs. no local recurrence) from the time of local recurrence to death at each time point over the entire period of the study.
The question remains as to why there is such a divergence of opinion in the literature concerning the effect of local recurrence on survival. We believe this is due to several factors. First, the majority of studies on extremity soft tissue sarcomas tend to have a relatively small number of cases with a broad diversity in both patient and tumor characteristics. 23 This is of particular significance if there is a large mix of high- and low-grade tumors and/or primary and recurrent tumors. Each has a distinct biologic behavior and may affect the overall interpretation of the outcome. This is clearly exemplified by the difference between the group I and group II patients in this study. Second, the vast majority of these studies focus on early recurrence and mortality, with few data on long-term follow-up and survival. 23 This is an important consideration when patients recur late, as in this study, where 13% of the patients had local recurrences at greater than 5 years following treatment at UCLA.
Finally, local recurrence is a time-dependent variable and is more appropriately analyzed in a time-dependent fashion. The distinction between a regular multivariate analysis and a time-dependent multivariate analysis is described by Stotter et al. and is exemplified by the following example. 13 Consider a patient who develops a local recurrence 8 years following treatment and subsequently dies 2 years later. In the non-time-dependent analysis, this patient is in the local recurrence group yet is a 10-year survivor. In the time-dependent analysis, this patient is at high risk of early death (within 2 years) after developing the local recurrence. The time-dependent analysis thus more appropriately emphasizes the increased risk of death following a local recurrence.
The adverse effect of local recurrence on survival may explain why the difference in survival between the group I patients and the group II patients was not more significant. The mean interval between initial treatment and treatment of recurrent disease at UCLA for group II patients was 30 months. In addition, 40% of the patients with primary disease (group I) that locally recurred die within 2 years of their local recurrence. Thus, we believe there is a selection process occurring whereby the high-risk patients within group II have likely died before arrival at UCLA. As such, the overall survival rate of the group II patients, although less than the group I patients, would likely be worse.
While this study clearly shows that local recurrence is strongly associated with decreased survival, no conclusions can be drawn regarding a causal relationship between local recurrence and survival. In addition, this study was not designed to look at the efficacy of different treatment methods. However, we believe that patients who either develop a local recurrence or present with locally recurrent disease should be strongly considered for adjuvant systemic therapy. 24–27
From the analysis of these 753 patients with intermediate- to high-grade extremity soft tissue sarcomas, we believe several issues become clear. Patients with locally recurrent disease are at a significantly increased risk of developing a subsequent local recurrence. Patient follow-up with either computed tomography or magnetic resonance imaging of the surgical site needs to be performed for at least 5 years. In addition, local recurrence is extremely morbid, with 38% of the patients who developed a local recurrence requiring amputation.
The development of a local recurrence was the most significant factor associated with decreased survival, regardless of whether the patient presented with primary or locally recurrent disease. Once a patient developed a local recurrence, he or she was about three times more likely to die from disease compared to a similar patient who had not developed a local recurrence. Although 85% to 90% of the patients with high-grade extremity soft tissue sarcomas are treatable with a limb salvage approach, patients who develop a local recurrence need aggressive treatment and should be considered for trials of adjuvant systemic therapy.
Footnotes
Correspondence: Frederick R. Eilber, MD, Division of Surgical Oncology, 54-140 CHS, UCLA Medical Center, 10833 Le Conte Avenue, Los Angeles, CA 90095-1782.
E-mail: eilberf@mskcc.org
Accepted for publication June 5, 2002.
References
- 1.Morton DL, Eilber FR, Townsend CM, et al. Limb salvage from a multidisciplinary treatment approach for skeletal and soft tissue sarcomas of the extremity. Ann Surg 1976; 184: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eilber FR, Mirra JJ, Grant TT, et al. Is amputation necessary for sarcomas? A seven-year experience with limb salvage. Ann Surg 1980; 192: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcoma of the extremities: Prospective randomized evaluation of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982; 196: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williard WC, Hajdu SI, Casper ES, et al. Comparison of amputation with limb-sparing operations for adult soft tissue sarcoma of the extremity. Ann Surg 1992; 215: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan MF, Hilaris B, Shiu MH, et al. Local recurrence in adult soft tissue sarcoma. A randomized trial of brachytherapy. Arch Srug 1987; 122: 1289–1293. [DOI] [PubMed] [Google Scholar]
- 6.Harrison LB, Franzese F, Gaynor JJ, et al. Long term results of a prospective trial of adjuvant brachytherapy in the management of completely resected soft tissue sarcomas of the extremity and superficial trunk. Int J Radiat Oncol Biol Phys 1993; 27: 259–265. [DOI] [PubMed] [Google Scholar]
- 7.Pisters PWT, Harrison LB, Leung DHT, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996; 14: 859–868. [DOI] [PubMed] [Google Scholar]
- 8.Brennan MF, Casper ES, Harrison LB, et al. The role of multimodality therapy in soft tissue sarcoma. Ann Surg 1991; 214: 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potter DA, Kinsella T, Glatstein E, et al. High-grade soft tissue sarcomas of the extremities. Cancer 1986; 58: 190–205. [DOI] [PubMed] [Google Scholar]
- 10.Gustafson P, Rooser B, Rydholm A. Is local recurrence of minor importance for metastese in soft tissue sarcoma? Cancer 1991; 67: 2083–2086. [DOI] [PubMed] [Google Scholar]
- 11.Singer S, Corson JM, Gonin R, et al. Prognostic factors predictive of survival and local recurrence for extremity soft tissue sarcoma. Ann Surg 1994; 219: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karakousis CP, Proimakis C, Rao U, et al. Local recurrence and survival in soft-tissue sarcomas. Ann Surg Oncol 1995; 3: 255–260. [DOI] [PubMed] [Google Scholar]
- 13.Stotter AT, Ahern RP, Fisher C, et al. The influence of local recurrence of extremity soft tissue sarcoma on metastases and survival. Cancer 1990; 65: 1119–1129. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JJ, Leung D, Heslin M, et al. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol 1997; 15: 646–652. [DOI] [PubMed] [Google Scholar]
- 15.Hajdu SI, Shiu MH, Brennan MF. The role of the pathologist in the management of soft tissue sarcomas. World J Surg 1988; 12: 326–331. [DOI] [PubMed] [Google Scholar]
- 16.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16 ( 3): 1141–1154. [Google Scholar]
- 17.Tai B, Machin D, White I, et al. Competing risk analysis of patients with osteosarcoma: a comparison of four different approaches. Stat Med 2001; 20 ( 5): 661–684. [DOI] [PubMed] [Google Scholar]
- 18.Kalbfleisch EL, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley & Sones 1980; 122–126.
- 19.Huth JF, Eilber FR. Patterns of metastatic spread following resection of extremity soft tissue sarcomas and strategies for treatment. Semin Surg Oncol 1988; 4: 20–26. [DOI] [PubMed] [Google Scholar]
- 20.Giuliano AE, Eilber FR, Morton DL. The management of locally recurrent soft-tissue sarcoma. Ann Surg 1982; 196: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pisters PWT, Leung DHY, Woodruff J, et al. Analysis of prognostice factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996; 14: 1679–1689. [DOI] [PubMed] [Google Scholar]
- 22.Coindre J, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma: a study of 546 patients from the french federation of cancer centers sarcoma group. J Clin Oncol 1996; 14: 869–877. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JJ, Leung D, Casper ES, et al. Multifactorial analysis of long-term follow-up (more than 5 years) of primary extremity sarcoma. Arch Surg 1999; 134: 190–194. [DOI] [PubMed] [Google Scholar]
- 24.Eilber FC, Rosen G, Eckardt J, et al. Treatment induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high grade extremity soft tissue sarcomas. J Clin Oncol 2001; 19: 3203–3209. [DOI] [PubMed] [Google Scholar]
- 25.Pisters PW. Chemoradiation treatment strategies for localized sarcoma: conventional and investigational approaches. Semin Surg Oncol 1999; 17: 66–71. [DOI] [PubMed] [Google Scholar]
- 26.Patel SR, Benjamin RS. New chemotherapeutic strategies for soft tissue sarcomas. Semin Surg Oncol 1999; 17: 47–51. [DOI] [PubMed] [Google Scholar]
- 27.Benjamin RS. Evidence for using adjuvant chemotherapy as standard treatment of soft tissue sarcomas. Semin Radiat Oncol 1999; 9: 349–351. [DOI] [PubMed] [Google Scholar]