Abstract
Objective
To determine if medium-chain triglycerides (MCTs) prevent organ injuries and mortality in rats administered endotoxin and to investigate effects of MCT on the gut.
Summary Background Data
Since dietary MCTs prevent alcohol-induced liver injury by inhibiting activation of Kupffer cells in the enteral feeding model, the authors hypothesized that MCT could prevent deleterious conditions in endotoxemia.
Methods
After a preliminary experiment determined the optimal dose of MCT, rats were given MCT (5 g/kg per day) or the same dose of corn oil by gavage daily for 1 week. Then, lipopolysaccharide (LPS) was administered intravenously and survival was assessed for the next 24 hours. For analysis of mechanisms, rats were killed 9 hours after LPS injection and serum and liver sections were collected. To investigate effects of MCT on the gut, pathologic change, permeability, and microflora were assessed. Kupffer cells isolated by collagenase digestion and differential centrifugation were used for endotoxin receptor CD14 immunoblotting, phagocytic index, and TNF-α production assay.
Results
All rats given corn oil died after LPS administration; however, this mortality was prevented by MCT in a dose-dependent manner. Rats given corn oil showed liver injury after LPS administration. In contrast, MCT prevented this pathologic change nearly completely. MCT blunted CD14 expression on the Kupffer cells and TNF-α production by isolated Kupffer cells; however, there were no differences in phagocytic index between the two groups. The length of the intestinal epithelium was increased in the MCT group compared to the corn oil group. Further, after LPS administration, increases in gut permeability and injury were prevented by MCT. Importantly, MCT also prevented hepatic energy charge and gut injuries in this condition.
Conclusions
Enteral feeding using MCT could be a practical way of protecting the liver and intestine during endotoxemia.
Systemic infection that involves gram-negative bacteria results in the development of endotoxin shock. The Kupffer cell, the resident macrophage in the liver, removes most bacteria and endotoxin and is activated in this process. 1 Activated Kupffer cells release pathophysiologically active substances such as eicosanoids, inflammatory cytokines (i.e., interleukin [IL]-6 and tumor necrosis factor [TNF]-α), and free radicals, all of which may participate in the mechanism of endotoxemia and multiple organ failure. 2,3 Collectively, Kupffer cells play an important role in inflammation and the immune response. 3,4
Inhibition of Kupffer cells by gadolinium chloride (GdCl3), a toxic compound of the Kupffer cell, prevented liver injury and mortality nearly completely in rats given endotoxin 3 by inhibiting superoxide and TNF-α production by activated Kupffer cells. 2,5 Thus, inhibition of Kupffer cells is useful in preventing pathophysiologic changes caused by endotoxin; however, GdCl3 is not suitable for clinical use due to its toxicity. Thus, an appropriate method for inhibiting activation of the Kupffer cell is still lacking.
Nutrition is an important factor in the pathogenesis of acute and chronic liver disease. 6 Further, enteral delivery of nutrients benefits severely injured or critically ill patients more than parenteral nutrition. 7 Indeed, dietary supplement of an essential amino acid glycine prevented liver injury after endotoxin administration in the rat. 8 Further, oral feeding with glutamine prevents lymphocyte and glutathione depletion of Peyer’s patches in endotoxemic mice. 9 It was previously reported that dietary medium-chain triglycerides (MCTs) prevent alcohol-induced liver injury by inhibiting activation of Kupffer cells in the Tsukamoto-French enteral model. 10 Thus, nutritional manipulation could be a useful tool to inhibit activation of the Kupffer cell in endotoxemia. Accordingly, the purpose of this study was to test the hypothesis that supplementation of MCTs would inhibit activation of the Kupffer cell and prevent mortality in rats administered endotoxin. We also investigated effects of MCTs on the hepatocyte and the gut.
METHODS
Animals and Treatment
We used a total of 96 male Sprague-Dawley rats (250 g body weight) in these experiments. The experimental protocol followed the institution’s and the National Research Council’s criteria for the care and use of laboratory animals in research. Further, all animals received humane care in compliance with institutional guidelines.
To determine the optimal dose of MCTs (saturated fat, trioctanoin 8:0, a kind gift from Clinico Co., Ltd.) for the proposed experiment, rats were given MCTs at a dosage of 0, 1, 2.5, and 5 g/kg per day or the same dose of corn oil (polyunsaturated fat) by gavage daily for 1 week. Rats were given food and water ad libitum throughout the study. After 1 week, animals were administered either saline vehicle or lipopolysaccharide (LPS, 10 mg/kg, Escherichia coli serotype 0111:B4; Sigma, St. Louis, MO) via the tail vein, and survival was assessed for the next 24 hours (n = 6 for each point).
For further analysis of the mechanisms in different sets of six rats, a dose of 5 mg/kg MCTs was used, since maximum effects of improvement in survival rates were observed at this dose.
Blood Sampling
Rats (n = 6 in each group) were given MCTs (5 g/kg per day) or the same dose of corn oil for 1 week. Then, animals were administered either LPS (10 mg/kg) or saline vehicle via the tail vein. Blood samples were collected from the aorta 0 or 9 hours later; samples were centrifuged at 1,200 g for 10 minutes at 4°C. Serum was stored at −80°C until assays for alanine aminotransferase (ALT). ALT was measured by standard enzymatic procedures (Nissui Transnase, Nissui Pharmaceutical Co., Japan). 11
Arterial Ketone Body Ratio
To assess hepatic energy levels in experimental animals, the arterial ketone body ratios (AKBR) were measured. While AKBR is an indirect measurement of the end point of interest, it was shown to accurately reflect hepatic mitochondrial energy charge in humans as well as in several animal models. 12,13 Further, AKBR is used clinically to assess hepatic energy charge. 14 Briefly, rats (n = 6 in each group) were given either MCTs (5 mg/kg) or the same dose of corn oil for 1 week. Two milliliters heparinized arterial blood was collected from the aorta 9 hours after LPS administration and centrifuged at 1,200 g for 10 minutes at 4°C. Then, 1 mL plasma was added to ice-cold 6% perchloric acid (1 mL) and mixed immediately. After standing for 10 minutes, the mixture was recentrifuged at 1,200 g for 10 minutes at 4°C. Supernatant was used for measurement of ketone bodies (acetoacetate and β-hydroxybutyrate) by the methods described by Williamson and Mellanby 15,16 using a Ketorex kit (Sanwa Kagaku Kenkyusho, Kyoto, Japan). AKBR was then calculated as follows 17: AKBR = acetoacetate/β-hydroxybutyrate.
Pathologic Evaluation
After 9 hours of LPS administration, sections from the liver and the small intestine near the cecum were fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin to assess inflammation and necrosis. Histologic samples were evaluated in a blinded manner by one of the authors and an outside expert in rodent liver pathology.
Kupffer Cell Isolation and Culture
Rats (n = 6 in each group) given either MCTs (5 mg/kg) or the same dose of corn oil for 1 week, and Kupffer cells were isolated by collagenase digestion and differential centrifugation using Percoll (Pharmacia, Uppsala, Sweden) as described elsewhere, with slight modifications. 18 Briefly, the liver was perfused through the portal vein with Ca2+- and Mg2+-free Hank’s balanced salt solution (HBSS) at 37°C for 5 minutes. Subsequently, perfusion was with HBSS containing 0.025% collagenase IV (Sigma, St. Louis, MO) at 37°C for 5 minutes. After the liver was digested, it was excised and cut into small pieces and placed in collagenase buffer. The suspension was filtered through a nylon gauze, and the filtrate was centrifuged at 450 g for 10 minutes at 4°C. Cell pellets were resuspended in buffer, parenchymal cells were removed by centrifugation at 50 g for 3 minutes, and the nonparenchymal cell fraction was washed twice with buffer. Cells were centrifuged on a density cushion of Percoll at 1,000 g for 15 minutes, and the Kupffer cell fraction was collected and washed with buffer again. The viability of cells determined from trypan blue exclusion exceeded 90%. Cells were seeded onto 25-mm glass coverslips and cultured in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO Laboratories Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 10 mmol/L HEPES, and antibiotics (100 U/mL penicillin G and 100 mg/mL streptomycin sulfate) at 37°C with 5% CO2. Nonadherent cells were removed after 1 hour by replacing the culture medium. All adherent cells phagocytosed latex beads, indicating that they were Kupffer cells. Cells were cultured for 24 hours and used for measurement of TNF-α production and phagocytic index.
Phagocytic Index of Isolated Kupffer Cells
Isolated Kupffer cells (1 × 105 cells per culture dish, n = 6 in each group) were plated in 35-mm culture dishes on cover slips in DMEM supplemented with 10% fetal calf serum and antibiotics. 19 The same concentration of fluorescein isothiocyanate (FITC)-labeled latex beads (1 mm, Polysciences, Warrison, PA) were added and cells were incubated for 5 minutes at 37°C and washed three times with phosphate-buffered saline (PBS), and fluorescence was determined in situ with a Universal Imaging Corp. Image-1/AT image acquisition and analysis system (Chester, PA) incorporating an Axioskop 50 microscope (Carl Zeiss, Thornwood, NY). The same microscopic field was photographed in normal light or with FITC. Further, the number of FITC-labeled beads phagocytosed by cells was counted in five different microscopic fields in each dish to assess function of phagocytosis.
TNF-α Production by Isolated Kupffer Cells
Kupffer cells isolated from rats given corn oil or MCTs were seeded onto 24-well plates and cultured in DMEM supplemented with 10% FBS and antibiotics at 37°C in the presence of 5% CO2. After 24 hours of incubation, cells were incubated with fresh media containing LPS (10 μg/mL) supplemented with 5% rat serum for an additional 4 hours. Media were collected and kept at −80°C until assay. TNF-α in the culture media was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Cosmo Bio Co., Tokyo, Japan), and data were corrected for dilution.
Further, to test the effects of fatty acids on LPS-induced TNF-α production by Kupffer cells, isolated Kupffer cells were incubated in the presence of linoleic acid, arachidonic acid, and MCTs. Various concentrations of linoleic, arachidonic, and octanoic acids (10–1,000 μmol/L) were added to cells and incubated in DMEM supplemented with 10% FBS. After 12 hours, LPS was added to each well and cells were incubated for an additional 4 hours. TNF-α concentrations in the culture media were measured by ELISA.
Kupffer Cell Preparation and Western Blotting for CD14
Kupffer cells were isolated by collagenase digestion and differential centrifugation using Percoll as described elsewhere, with slight modifications. 10 Cells were plated on plastic culture dishes and cultured in RPMI 1640 media supplemented with 25 mmol/L HEPES, 10% FBS, and antibiotics (100 U/mL penicillin G and 100 μg/mL streptomycin sulfate). After 1 hour of incubation, Kupffer cells were scraped with a sterile cell scraper and pelleted by centrifugation at 500 g for 7 minutes. Cell pellets were resuspended in 250 μL suspension buffer with Triton X-100, agitated for 15 minutes at 4°C, and centrifuged at 12,000 g for 10 minutes at 4°C. The supernatant was removed and the pellet was resuspended in suspension buffer. Protein was stored at −20°C for subsequent Western blotting.
Extracted proteins (30 μg) were separated by 10% sodium-dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked with Tris-buffered saline-Tween 20 containing 5% skim milk, probed with mouse antirat ED9 monoclonal antibody (Serotec, Oxford, UK), followed by horseradish peroxidase-conjugated secondary antibody as appropriate. Membranes were incubated with a chemiluminescence substrate (ECL reagent, Amersham Life Science, Buckinghamshire, UK) and exposed to X-OMAT films (Eastman Kodak, Rochester, NY).
Gut Microflora and Gut Permeability
Rats were given either MCTs (5 mg/kg) or the same dose of corn oil daily for 1 week by gavage. After 1 week of treatment, feces were collected to assess the effects of lipids on gut microflora. Feces were swabbed on agar and cultured aerobically at 37°C for 2 days. Fecal bacteria were identified and the total number of bacteria was counted.
Gut permeability was measured in isolated intestinal segments as described previously. 10 Briefly, 8-cm segments of the small intestine were removed, rinsed with ice-cold saline, everted, filled with 1 mL Tris buffer (125 mmol/L NaCl, 10 mmol/L fructose, and 30 mmol/L Tris; pH 7.5), and ligated at both ends. The filled gut segments were incubated in Tris buffer containing 0.4 mg/mL horseradish peroxidase (type VI, 300 units/mg solid, Sigma Chemical Co.). After 45 minutes, gut sacs were removed and blotted lightly to eliminate excess horseradish peroxidase, and the contents (∼750 μL) of each sac were collected carefully using a 1-mL syringe. Horseradish peroxidase activity in the contents of each sac was determined spectrophotometrically from the rate of oxidation of pyrogallol, as described elsewhere. 10
Statistical Analysis
Data are expressed as mean values ± SEM. Analysis of variance or the Student t test was used to determine statistical significance as appropriate. P < .05 was selected before the study as the level of significance.
RESULTS
Effect of Lipid Type on Mortality and ALT Levels After LPS Administration
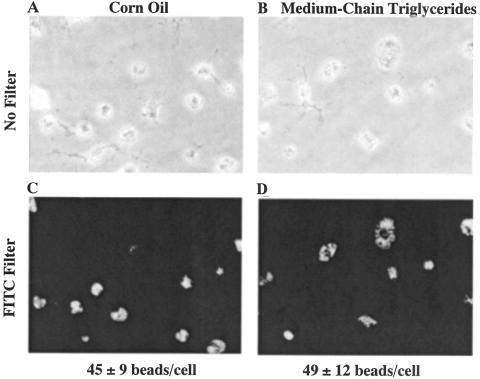
After LPS administration, all control rats died within 24 hours, suggesting that this dose was lethal (data not shown). Rats given corn oil appeared moribund 9 hours after LPS injection and died at all doses studied within 12 hours (Fig. 1). This mortality was prevented by MCTs in a dose-dependent manner.
Figure 1. Effect of LPS and lipid type on mortality after LPS administration. Rats were given either corn oil or medium-chain triglycerides. After 1 week of treatment, LPS (10 mg/mL) was administered via the tail vein and survival rates were monitored for 24 hours. Data represent percentage of survival animal.
ALT levels were about 20 IU/L in untreated rats (Table 1). Further, neither corn oil nor MCTs affected serum ALT levels before LPS administration. After LPS administration, ALT levels were increased to about 1,650 IU/L in the control group. Values were increased to about 1,800 IU/L in rats given corn oil. MCTs blunted this increase by about 90%.
Table 1. EFFECT OF TYPE OF FAT AND LPS ON SERUM ALT LEVELS AND AKBR
Serum ALT levels and AKBR were determined as described in text. Data represent mean ± SEM (n = 6).
AKBR, arterial ketone body ratio.
*P < .01 vs. control rats given saline.
†P < .01 vs. rats given corn oil with saline.
‡P < .01 vs. rats given corn oil with LPS.
§P < .01 vs. control rats with LPS by two-way ANOVA using Bonferroni post hoc test.
Pathologic Evaluation of Liver and Gut
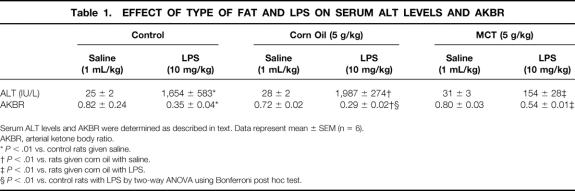
No pathologic changes were observed in liver sections from rats given either corn oil or MCTs for 1 week (Fig. 2). Inflammation, focal necrosis, and hemorrhagic change were observed after LPS administration in the corn-oil group. MCTs prevented these pathologic changes significantly.
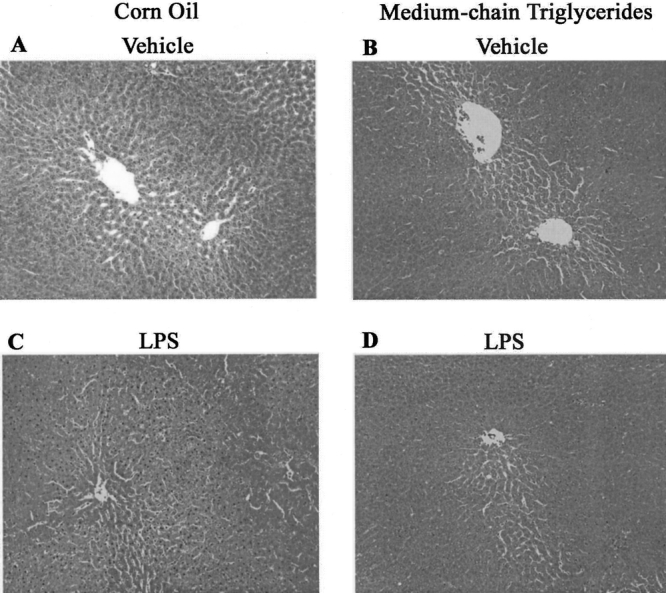
Figure 2. Effect of LPS and lipid type on the liver. Liver sections from rats given saline (A, corn oil; B, medium-chain triglycerides) and LPS (C, corn oil; D, medium-chain triglycerides) are shown. Original magnification, ×200. Representative photomicrographs.
No pathologic changes were observed in gut sections from rats given corn oil (Fig. 3). MCTs increased the length of intestinal epithelial cells and the number of the goblet cell in the small intestine compared to either the control (data not shown) or the corn oil group. After LPS administration, gut sections from the corn oil group showed hemorrhagic change in the submucosal layer and necrosis of epithelial cells; these changes were prevented by MCTs nearly completely.
Figure 3. Effect of LPS and lipid type on the gut. Gut sections from rats given saline (A, corn oil; B, medium-chain triglycerides) and LPS (C, corn oil; D, medium-chain triglycerides) are shown. Original magnification, ×200. Representative photomicrographs.
Effect of Lipid Type and LPS on AKBR
In control rats, AKBR was about 0.8 before LPS administration (see Table 1). Neither corn oil nor MCTs alone affected this level. LPS administration decreased AKBR by about 60% in the control group. AKBR was significantly lower by about 20% in rats given corn oil compared with the control group. MCTs prevented this decrease by about 50%.
Effect of Lipid Type on Gut Microflora and Gut Permeability
Changes in the intestinal bacterial population in response to supplement of these lipids were investigated. Escherichia coli, Enterococcus faecalis, α-Streptococcus, and Bacteroides were predominant bacterial species detected in feces from both groups. The number of total bacteria was decreased significantly in feces from rats given MCTs compared with corn oil (Table 2).
Table 2. EFFECT OF TYPE OF FAT ON THE TOTAL NUMBER OF BACTERIA IN FECES
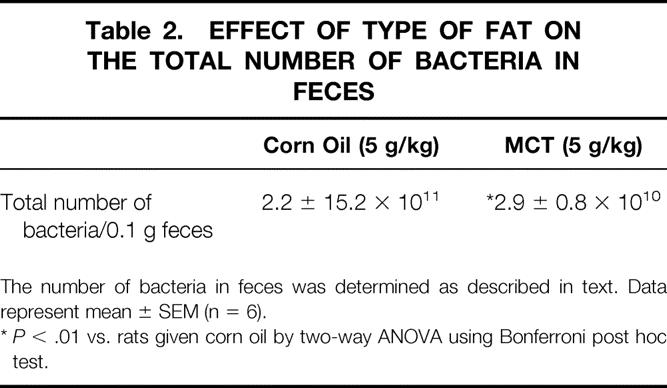
The number of bacteria in feces was determined as described in text. Data represent mean ± SEM (n = 6).
*P < .01 vs. rats given corn oil by two-way ANOVA using Bonferroni post hoc test.
Gut permeability to horseradish peroxidase was about 30 U/L in the corn oil group (Fig. 4). In rats given MCTs, values were decreased significantly compared with the corn oil group. Gut permeability was increased about threefold in the corn oil group after LPS administration. This increase was blunted by about 70% by MCT administration.
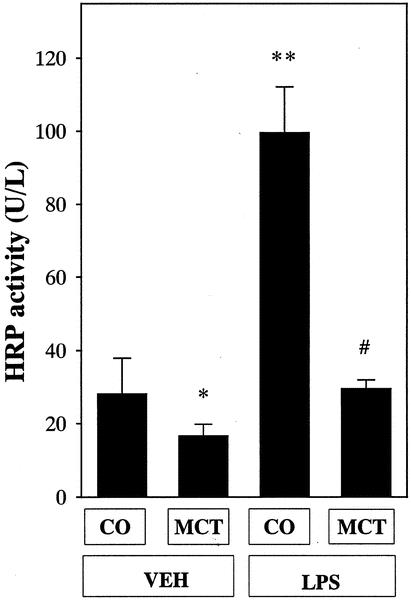
Figure 4. Effect of lipid type on gut permeability. Data represent mean ± SEM (n = 6). CO, corn oil; MCT, medium-chain triglycerides; HRP, horseradish peroxidase. *P < .05 vs. rats given corn oil with vehicle administration; #P < .05 vs. rats given corn oil with LPS administration by ANOVA and Bonferroni post hoc test.
Purity of Kupffer Cells and Phagocytic Index
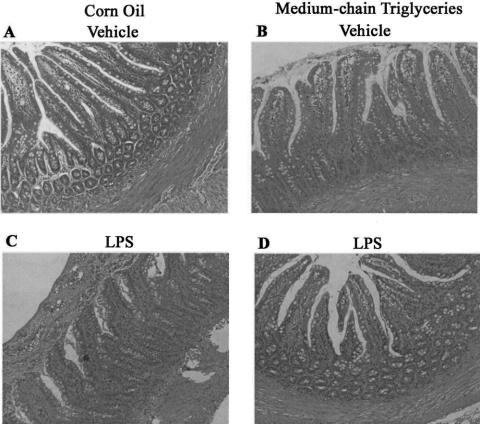
In photographs in normal light, isolated cells from rats given corn oil or MCTs contained large Kupffer cells predominantly, with a few small Kupffer cells (Fig. 5). In photographs in normal light, isolated cells from rats given either corn oil or MCTs were morphologically the same. The purity of cell preparation exceeded 99% in each group, as verified by uptake of FITC-labeled latex beads. Kupffer cells from both groups showed the same extent of phagocytosis of the latex beads, suggesting that MCTs did not affect phagocytosis of the Kupffer cell.
Figure 5. Photomicrographs of isolated hepatic macrophages and phagocytotic ability. Isolated Kupffer cells were incubated with FITC-labeled latex beads as described in text. The same microscopic field was photographed in normal light (upper panel) or with FITC filter (lower). Original magnification, ×200. (A, C) Cells from rats given vehicle. (B, D) Cells from rats given medium-chain triglycerides. Representative photomicrographs.
Effect of Lipid Type on CD14 Expression on the Kupffer Cell
In the control group, expression of the endotoxin receptor CD14 on the Kupffer cell was minimal (Fig. 6). After 1 week of treatment with fatty acids, expression of the endotoxin receptor CD14 on the Kupffer cell was increased about twofold in rats given corn oil compared with control rats. MCTs reduced this expression by about 70%.
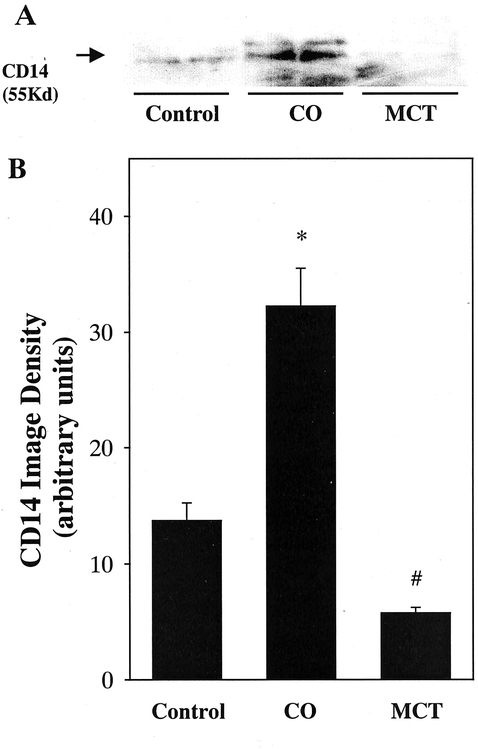
Figure 6. Effect of lipid type on Cd14 expression on the Kupffer cell. Protein extracts (50 μg) from isolated Kupffer cells were analyzed by Western blotting using mouse antirat ED9 antibody. (A) Specific band for Cd14 (55 kilodaltons). (B) Densitometric analysis of CD14 was carried out by image analysis as described in text. CO, corn oil; MCT, medium-chain triglycerides. Data represent mean ± SEM (n = 6). *P < .05 vs. control rats; #P < .05 vs. rats given corn oil by ANOVA and Bonferroni post hoc test.
Effect of Lipid Type on TNF-α Production by Isolated Kupffer Cells
TNF-α production by isolated Kupffer cells was minimal in media with saline administration (Fig. 7). Production was increased markedly by LPS stimulation in the control group. Values were about twofold greater in the corn oil than the control group. These increases were blunted by about 60% by MCT administration.
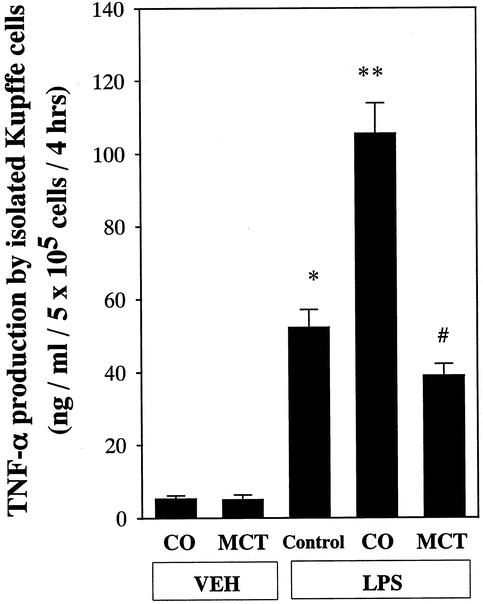
Figure 7. Effect of lipid type on LPS-induced TNF-α production by isolated Kupffer cells. TNF-α was measured in the culture media using an enzyme-linked immunosorbent assay (ELISA) as described in text. Kupffer cells were isolated and cultured in 24-well culture plates. After 24 hours of incubation, changes in TNF-α production at 4 hours after addition of various doses of LPS supplemented with 5% rat serum are plotted. Data represent mean ± SEM (n = 6). *P < .05 vs. control rats without LPS administration; **P < .05 vs. control rats with LPS administration; #P < .05 vs. rats given corn oil with LPS administration by ANOVA and Bonferroni post hoc test.
Effects of Fatty Acids on TNF-α Production by Kupffer Cells In Vitro
To determine if fatty acids can activate Kupffer cells in vitro, cells were isolated from untreated rats and incubated and TNF-α production was measured. TNF-α production was minimal before LPS stimulation. Although LPS increased this production in each group, linoleic acid, the most abundant fatty acid in corn oil, maximally increased TNF-α production in a dose-dependent manner, with a peak at 1 mmol/L (Fig. 8). This increase was significantly blunted by about 45% by MCT administration.
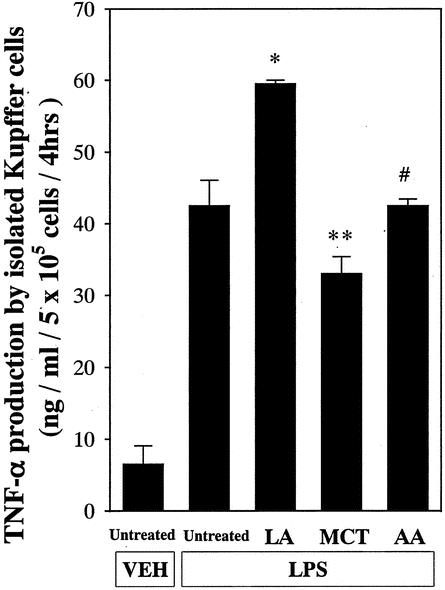
Figure 8. Effect of fatty acid on TNF-α production by the Kupffer cell in vitro. Linoleic acid, medium-chain triglycerides (octanoic acid), and arachidonic acid (LA, MCT, and AA, respectively; 0.01–1 mmol/L) were added to cultured Kupffer cells and TNF-α production was measured as described in text. Data represent mean ± SEM (n = 6). *P < .05 vs. untreated Kupffer cells; **P < .05 vs. cells treated with linoleic acid; #P < .05 vs. cells treated with medium-chain triglycerides by ANOVA and Bonferroni post hoc test.
DISCUSSION
Effect of MCTs on Kupffer Cells in Endotoxemia
The major target of endotoxin is the Kupffer cell. 20 Activated Kupffer cells release mediators, such as cytokines, eicosanoids, and free radicals, that cause liver injury. Activation of Kupffer cells is a critical factor in the pathophysiology of endotoxemia or sepsis. Indeed, elimination of Kupffer cells by GdCl3 prevented liver injury and mortality. 5 Thus, inhibition of the Kupffer cell is useful in preventing pathophysiology caused by endotoxin;3,5 however, an appropriate method is still lacking. Nutrition and dietary factors are important in the pathogenesis of acute and chronic liver disease. 8,21,22 Indeed, saturated fat prevents early alcohol-induced liver injury by inhibiting the Kupffer cell. 10 The dietary essential amino acid glycine also prevented mortality in rats given a lethal dose of endotoxin by inhibition of the Kupffer cell. 8 These results suggested that the Kupffer cell could be inhibited by nutritional manipulation. Therefore, this study was designed to test the hypothesis that supplementation with MCTs would prevent liver injury and mortality caused by a lethal dose of endotoxin. MCTs prevented pathophysiologic changes by inhibiting the activation of the Kupffer cell (see Table 1, Fig. 1). MCTs did not affect the phagocytosis of the Kupffer cell, indicating that the Kupffer cell still removed endotoxin after treatment with MCTs (see Fig. 5). This is important for clinical application, since GdCl3 inhibits both cytokine production and phagocytosis nearly completely.
MCTs prevented LPS-induced liver injury by inhibiting activation of the Kupffer cell (see Fig. 2). Since Kupffer cells are activated by endotoxin via the endotoxin receptor CD14, which is on the plasma membrane on Kupffer cells, it was hypothesized that MCTs could affect CD14 expression on the Kupffer cell. Indeed, MCTs suppressed CD14 mRNA expression in the liver. 23 MCT supplementation also reduced expression of CD14 on the Kupffer cell (see Fig. 6). 23 Thus, we can conclude that MCTs blunt the response of Kupffer cells to endotoxin in part by inhibiting expression of CD14. On the other hand, calcium is essential for the activation of Kupffer cells, 24 which contain voltage-dependent calcium channels. 25 Increases in the intracellular calcium concentration caused by LPS were blunted in isolated Kupffer cells from rats fed MCTs chronically. 10 Further, it was reported that the dietary fatty acids of the omega-3 series are rapidly incorporated into cell membranes and profoundly influence biologic responses. 26 MCTs also blunted intrahepatic free radical formation in rats given enteral alcohol. 10 Alternatively, it was reported that arachidonic acid, a major metabolite of linoleic acid and the most abundant fatty acid in corn oil, activated superoxide production by Kupffer cells in vitro. 27 Linoleic acid also increased TNF-α production by isolated Kupffer cells (see Fig. 8). MCTs blunted these increases significantly. These results suggested that MCTs could influence stability, membrane fluidity, cell mobility, formation of receptors, binding of ligands to their receptors, activation of intracellular signaling pathways either directly or through the formation of eicosanoids, gene expression, and cell differentiation (Fig. 9).
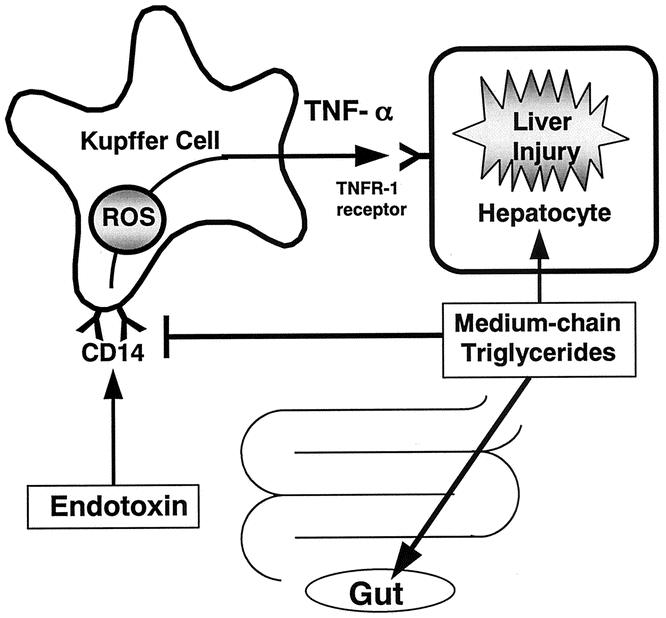
Figure 9. Working hypothesis. Endotoxin activates Kupffer cells to release inflammatory mediators such as free radicals and TNF-α. It is proposed that medium-chain triglycerides prevent liver injury by inhibiting TNF-α production due to endotoxin-mediated activation of Kupffer cells via CD14. This most likely involves effects on the cell membrane structure of Kupffer cells, leading to decreased responsiveness of Kupffer cells to endotoxin. Medium-chain triglycerides also affect the intestinal mucosa. Thus, immunonutrition intended to protect the liver and the gut could be provided in a practical fashion clinically by enteral feeding using medium-chain triglycerides. ROS, reactive oxygen species.
TNF-α stimulates endothelial cells to synthesize adhesion molecules, such as ICAM-1, that induce infiltration of neutrophils in the liver and cause microcirculatory disturbances leading to liver injury. 1 Anti-TNF-α antibody reduced inflammatory cell infiltration and necrosis in the liver. 28 Nanji et al. reported that MCTs decreased TNF-α mRNA expression in the liver in the Tsukamoto-French model. 29 Here, the increase of TNF-α production in isolated Kupffer cells from rats given corn oil was blunted significantly by MCTs (see Fig. 7). MCT administration in vitro also blunted TNF-α production by the Kupffer cell (see Fig. 8). Taken together, MCTs prevented liver injury most likely by reducing TNF-α production from activated Kupffer cells (see Fig. 9).
Advantages of Using MCTs During Endotoxemia
MCTs are absorbed from the duodenum and jejunum without micelle formation, different from long-chain triglycerides, and are hydrolyzed rapidly by lipoprotein lipase in plasma. 30,31 The liberated medium-chain fatty acids reach the liver rapidly and are taken up independently of carnitine by mitochondria and oxidized to CO2 faster than long-chain fatty acids, and serum carnitine levels were decreased in sepsis. 32 On the other hand, mitochondria in the hepatocyte use fatty acids as a substrate to produce ATP by β-oxidation under conditions of depletion of AKBR, such as sepsis. 33–35 Therefore, it was hypothesized that MCTs could prevent hepatic energy charge in endotoxemia. Decreases in AKBR after LPS administration were prevented significantly by MCTs (see Table 1). Collectively, MCTs are useful energy carriers in endotoxemia or sepsis.
Oral feeding with glutamine prevents lymphocyte and glutathione depletion of Peyer’s patches in endotoxemic mice. 9 Enteral feeding also decreases gut apoptosis and permeability during endotoxemia. 36 Thus, enteral feeding could affect the inflammatory immune system in the gut. It has been reported that MCTs prevented intestinal injury in rats fed alcohol enterally. 10 In this study, the length of the intestinal epithelium and the number of goblet cells were increased by MCTs (see Fig. 3). Increases in gut permeability caused by LPS were blunted by MCTs nearly completely (see Fig. 4). The total number of microflora was also reduced significantly by MCTs (see Table 2). Thus, MCTs may prevent intestinal atrophy during parenteral nutrition and prevent intestinal immune system during endotoxemia. Taken together, we can conclude that MCTs have protective effects on the gut. Recently it was reported that there is no evidence to support a difference between enteral and parenteral nutrition in sepsis and nonseptic morbidity and mortality. 7 However, since MCTs have protective effects on the gut in addition to the liver, enteral nutrition could have advantages by selection of MCT components during endotoxemia.
Clinical Implications
We propose that MCTs prevent liver injury by inhibiting TNF-α production due to endotoxin-mediated activation of Kupffer cells via CD14. This most likely involves effects on the cell membrane structure of Kupffer cells, leading to decreased responsiveness of Kupffer cells to endotoxin (see Fig. 9). MCTs are useful hepatic energy substrates in critical illness such as endotoxemia or sepsis. MCTs also affect the intestinal mucosa. Since enteral nutrition prevents remote organ injury and death after a gut ischemic insult and endotoxin challenge, 36,37 immunonutrition intended to protect the liver and intestine could be provided in a practical fashion clinically by enteral feeding using MCTs.
Footnotes
Correspondence: Hiroshi Kono, MD, PhD, First Department of Surgery, Yamanashi Medical University, 1110 Shimokato, Tamaho, Nakakoma, Yamanashi 409-3898, Japan.
E-mail: hkouno@res.yamanashi-med.ac.jp
Accepted for publication May 16, 2002.
References
- 1.Nolan JP. Endotoxin, reticuloendothelial function, and liver injury. Hepatology 1981; 1: 458–465. [DOI] [PubMed] [Google Scholar]
- 2.Kono H, Yamamoto M, Iimuro Y, et al. The role of splenic macrophages in plasma tumor necrosis factor levels in endotoxemia. Eur Surg Res 1997; 29: 176–186. [DOI] [PubMed] [Google Scholar]
- 3.Iimuro Y, Yamamoto M, Kono H. Blockade of liver macrophages by gadolinium chloride reduces lethality in endotoxemic rats; analysis of mechanisms of lethality in endotoxemia. J Leukoc Biol 1996; 55: 723–728. [DOI] [PubMed] [Google Scholar]
- 4.Seki S, Habu Y, Kawamura T, et al. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1. 1+T cells in T helper immune responses. Immunol Rev 2000; 174: 35–46. [DOI] [PubMed] [Google Scholar]
- 5.Kohno H, Yamamoto M, Iimuro Y. Reduction of mortality in endotoxemic rats pretreatment with gadolinium chloride: relationship to suppression of superoxide production in liver macrophages. Yamanashi Med J 1993; 8: 101–112. [Google Scholar]
- 6.Smith O. Animal models of human genetic disease. Trends Genet 1993; 9: 112–116. [DOI] [PubMed] [Google Scholar]
- 7.Woodcock NP, Zeigler D, Palmer MD, et al. Enteral versus parenteral nutrition: a pragmatic study. Nutrition 2001; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 8.Ikejima K, Iimuro Y, Forman DT, et al. A diet containing glycine improve survival in endotoxin shock in the rat. Am J Physiol 1996; 27: G97-G103. [DOI] [PubMed] [Google Scholar]
- 9.Manhart N, Vierlinger K, Spittler A, et al. Oral feeding with glutamine prevents lymphocyte and glutathione depletion of Peyer’s patches in endotoxemic mice. Ann Surg 2001; 234: 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kono H, Enomoto N, Connor HD, et al. Medium-chain triglycerides inhibit free radical formation and TNF-alpha production in rats given enteral ethanol. Am J Physiol 2000; 278: G467–G476. [DOI] [PubMed] [Google Scholar]
- 11.Arai M. Effect of ethanol on the intestinal uptake of endotoxin. Nippon Shokakibyo Gakkai Zasshi 1986; 83: 1060. [PubMed] [Google Scholar]
- 12.Ozawa K, Kamiyama Y, Kimura K. Relation of mitochondrial redox potential to hepatic energy charge in animals with hepatectomy, jaundice, hemorrhagic and sepsis as well as patients with multiple organ failure. Langenbeck Arch Chir 1982; 357: 204–205. [Google Scholar]
- 13.Kono H, Fujii H, Matsuda M, et al. Gadolinium chloride prevents mortality in hepatectomized rats given endotoxin. J Surg Res 2001; 96: 204–210. [DOI] [PubMed] [Google Scholar]
- 14.Ukikusa M, Ozawa K, Shimahara Y. Changes in blood ketone body ratio. Their significance after major hepatic resection. Arch Surg 1981; 116: 781–785. [DOI] [PubMed] [Google Scholar]
- 15.Williamson DH, Mellanby J. D-(-)-3-Hydroxybuturate. In Bergmeyer HU, ed. Methods of enzymatic analysis. New York: Academic Press; 1974.
- 16.Mellanby J, Wiiliamson DH. Accetoacetate. In Bergmeyer HU, ed. Methods of enzymatic analysis. New York: Academic Press; 1974;
- 17.Ozawa K, Fujimoto T, Nakatani T. Changes in hepatic energy charge, blood ketone body ratio and indocyanine green clearance in relation to DNA synthesis after hepatectomy. Life Sci 1982; 31: 481–490. [DOI] [PubMed] [Google Scholar]
- 18.Bode JCH. Alcohol and the gastrointestinal tract. In: Advances in internal medicine and pediatrics. Heidelberg: Springer-Verlag; 1980.
- 19.Peters JM, Rusyn I, Rose ML, et al. Peroxisome proliferator-activated receptor alpha is restricted to hepatic parenchymal cells, not Kupffer cells: implications for the mechanism of action of peroxisome proliferators in hepatocarcinogenesis. Carcinogenesis 2000; 21: 823–826. [DOI] [PubMed] [Google Scholar]
- 20.Arii S, Imamura M. Physiological role of sinusoidal endothelial cells and Kupffer cells and their implication in the pathogenesis of liver injury. J Hepatobiliary Pancreat Surg 2000; 7: 40–48. [DOI] [PubMed] [Google Scholar]
- 21.French SW. Nutrition in the pathogenesis of alcoholic liver disease. Alcohol Alcoholism 1993; 28: 97–109. [PubMed] [Google Scholar]
- 22.Yin M, Ikejima K, Arteel GE, et al. Glycine accelerates recovery from alcohol-induced liver injury. J Pharmacol Exp Ther 1998; 286: 1014–1019. [PubMed] [Google Scholar]
- 23.Su GL, Rahemtulla A, Thomas P, et al. CD14 and lipopolysaccharide binding protein expression in a rat model of alcohol liver disease. Am J Physiol 1998; 152: 841–849. [PMC free article] [PubMed] [Google Scholar]
- 24.Kawada N, Mizoguchi Y, Kobyashi K, et al. Calcium-dependent prostaglandin biosynthesis by lipopolysaccharide-stimulated rat Kupffer cells. Prostaglandins Leukot Essent Fatty Acids 1992; 47: 209–214. [DOI] [PubMed] [Google Scholar]
- 25.Hijioka T, Rosenberg RL, Lemasters JJ, et al. Kupffer cells contains voltage-dependent calcium channels. Mol Pharmacol 1991; 41: 435–440. [PubMed] [Google Scholar]
- 26.Alexander JW. Immunonutrition: the role of omega-3 fatty acids. Nutrition 1998; 14: 627–633. [DOI] [PubMed] [Google Scholar]
- 27.Rusyn I, Bradham CA, Cohn L, et al. Corn oil rapidly activates nuclear factor-κB in hepatic Kupffer cells by oxidant-dependent mechanisms. Carcinogenesis 1999; 20: 2095–2100. [DOI] [PubMed] [Google Scholar]
- 28.Iimuro Y, Gallucci RM, Luster MI, et al. Antibodies to tumor necrosis factor-α attenuate hepatic necrosis and inflammation due to chronic exposure to ethanol in the rat. Hepatology 1998; 26: 1530–1537. [DOI] [PubMed] [Google Scholar]
- 29.Nanji AA, Sadrzadeh SM, Yang EK, et al. Dietary saturated fatty acids: a novel treatment for alcoholic liver disease. Gastroenterology 1995; 109: 547–554. [DOI] [PubMed] [Google Scholar]
- 30.Wolfram G. Medium-chain triglycerides–useful energy carriers in parenteral nutrition. Wien Klin Wochenschr 1989; 101: 300–303. [PubMed] [Google Scholar]
- 31.Lima LA. Neonatal parenteral nutrition with medium-chain triglycerides: rationale for research. J Parenter Enteral Nutr 1989; 13: 312–317. [DOI] [PubMed] [Google Scholar]
- 32.Famuralo G, De Simons C. A new era for carnitine. Immunol Today 1995; 16: 211–213. [DOI] [PubMed] [Google Scholar]
- 33.Ozaki N, Ringe B, Gubernatis G, et al. Changes in energy substrates in relation to arterial ketone ratio after human orthotopic liver transplantation. Surgery 1993; 113: 403–409. [PubMed] [Google Scholar]
- 34.Nakatani T, Ozawa K, Asano M, et al. Changes in predominant energy substrate after hepatectomy. Life Sci 1981; 28: 257–264. [DOI] [PubMed] [Google Scholar]
- 35.Ohtoshi M, Jikko A, Asano M, et al. Ketogenesis during sepsis in relation to hepatic energy metabolism. Res Exp Med 1984; 184: 209–219. [DOI] [PubMed] [Google Scholar]
- 36.Alscher KT, Phang PT, McDonald TE, et al. Enteral feedings decrease gut apoptosis, permeability, and lung inflammation during murine endotoxemia. Am J Physiol 2001; 281: G569–G576. [DOI] [PubMed] [Google Scholar]
- 37.Fukatsu K, Zarzaur B, Johnson CD, et al. Enteral nutrition prevents remote organ injury and death after a gut ischemic insult. Ann Surg 2001; 233: 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]