Abstract
Objective
To assess the role of partial splenectomy for symptomatic children with various congenital hemolytic anemias.
Summary Background Data
The use of total splenectomy for symptomatic children with congenital hemolytic anemias is restricted by concern of postsplenectomy sepsis. A partial splenectomy is an alternative procedure, although its utility remains incompletely defined.
Methods
This longitudinal cohort study followed 25 symptomatic children with various congenital anemias who underwent partial splenectomy. Sixteen children had hereditary spherocytosis (HS), and nine children had other erythrocyte disorders. Outcome measures were clinical and laboratory hemolysis, splenic phagocytic and immune function, and splenic regrowth as measured by ultrasonography. Discrete parameters were compared using the Student t test.
Results
Partial splenectomy was successful in all 25 children, with minimal morbidity. Follow-up ranged from 7 months to 6 years (mean 2.3 ± 1.5 years). Following surgery, children with HS had increased hemoglobin values, decreased reticulocyte and bilirubin levels, and preserved splenic function. Most children without HS had decreased symptoms of hypersplenism and splenic sequestration. Over time, variable rates of splenic regrowth were noted, although regrowth did not necessarily correlate with recurrent hemolysis.
Conclusions
In children with hereditary spherocytosis, a partial splenectomy appears to control hemolysis while retaining splenic function. In children with other congenital hemolytic anemias, a partial splenectomy appears to control symptoms of hypersplenism and splenic sequestration.
Congenital hemolytic anemias frequently lead to severe hemolysis due to splenic sequestration of abnormal erythrocytes. 1 For children with hereditary spherocytosis (HS), a total splenectomy eliminates the main source of erythrocyte destruction. For children with other hemolytic anemias, a splenectomy eliminates splenic sequestration and reduces symptoms of hypersplenism.
The use of total splenectomy in children is restricted by concern of overwhelming postsplenectomy sepsis. 2–5 In children less than 5 years of age, the risk of overwhelming postsplenectomy sepsis may be increased 60- to 100-fold compared to children who have not had a splenectomy. 4 Although the risk of overwhelming postsplenectomy sepsis is reduced by use of immunizations to Streptococcus pneumoniae, Meningococcus, and Haemophilus influenzae as well as postoperative antibiotic prophylaxis, its risk is never eliminated. 5 Moreover, concerns persist of incomplete protection by pneumococcal vaccinations, antibiotic resistance, and poor compliance with antibiotic prophylaxis. 5,6
For children with congenital hemolytic anemias, a partial splenectomy has been proposed as an alternative to total splenectomy, with the goal of removing enough spleen to gain a desired hematologic effect while preserving splenic immune function. 7–10 However, the use of partial splenectomy is limited because of technical difficulties and concerns of splenic regrowth. 7,8,11,12 Recently several groups have renewed an interest in partial splenectomy. 7–10
To assess the role of partial splenectomy in symptomatic children with various congenital hemolytic anemias, we have followed 25 children with various congenital hemolytic anemias who underwent partial splenectomy. We examined the effects of partial splenectomy on hemolysis, splenic phagocytic and immune function, and splenic regrowth. Our results suggest that partial splenectomy may be beneficial for a variety of congenital hemolytic anemias.
METHODS
Patients
We have followed 25 children with various congenital hemolytic anemias who underwent partial splenectomy from 1993 to 2000 (Table 1). The children were from either the Pediatric Hematology Programs from Duke University (n = 13) or the Children’s Hospital of Wisconsin (n = 12). Sixteen children had HS (n = 16). Three children had another congenital erythrocyte membrane or enzyme disorder, including pyruvate kinase deficiency (n = 2), or congenital nonspherocytotic hemolytic anemia of unknown etiology (n = 1). Six children had a congenital hemoglobinopathy, including HgbSC disease (n = 2), HgbSS disease (n = 2), HgbCC disease (n = 1), or combined HgbS-β-thalassemia (n = 1). As the indications for surgery and expected clinical responses differ for children with HS compared to other children, we have evaluated these two groups separately. All children had signs and symptoms related to hemolysis and hypersplenism (Table 2). We defined hypersplenism as symptoms of chronic abdominal pain, activity limitations, and/or early satiety.
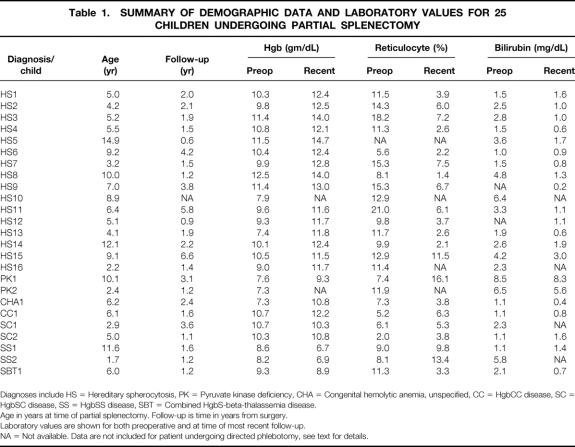
Table 1. SUMMARY OF DEMOGRAPHIC DATA AND LABORATORY VALUES FOR 25 CHILDREN UNDERGOING PARTIAL SPLENECTOMY
Diagnoses include HS = Hereditary spherocytosis, PK = Pyruvate kinase deficiency, CHA = Congenital hemolytic anemia, unspecified, CC = HgbCC disease, SC = HgbSC disease, SS = HgbSS disease, SBT = Combined HgbS-beta-thalassemia disease.
Age in years at time of partial splenectomy. Follow-up is time in years from surgery.
Laboratory values are shown for both preoperative and at time of most recent follow-up.
NA = Not available. Data are not included for patient undergoing directed phlebotomy, see text for details.
Table 2. CLINICAL FINDINGS IN CHILDREN WITH VARIOUS CONGENITAL HEMOLYTIC ANEMIAS BEFORE AND AFTER PARTIAL SPLENECTOMY

Children are grouped as having either hereditary spherocytosis (HS), or children without HS (Non-HS), including diagnoses of pyruvate kinase deficiency, non-specified hemolytic anemia, or a hemoglobinopathy (HgbSS, HgbSC, HgbCC, combined HgbS-beta thalassemia). One child with HS was lost to long-term follow up. Definitions: RBC transfusion = need for one or more transfusion, sequestration crisis = need for hospitalization for aplastic or sequestration crisis, abdominal pain = complaint of abdominal pain from hypersplenism, jaundice = bilirubin level greater than 2.0 mg/dl, cholelithiasis = sonographic evidence of gallstones.
The mean age of the children at the time of partial splenectomy was 6.6 ± 3.4 years (range 1–15 years). Mean follow-up was 2.3 ± 1.5 years (range 7 months to 6 years). One child with HS was lost to follow-up, and follow-up data on this child are not included in this report. The institutional review boards of both participating centers approved this review. As the use of partial splenectomy is accepted care for these conditions, we did not include any research study protocol as part of the operative consent. Data on children receiving a total splenectomy during this study period are not included in this report.
Surgical Procedure
After extensive discussions with each family regarding the potential risks and benefits of the procedure, a partial splenectomy was performed. The objective of each partial splenectomy was to resect 80% to 90% of normal estimated splenic volume. As our experience developed, we recognized that for children with very large spleens, more extensive parenchymal resection was required to attain this desired resection. All children were prepared for potential total splenectomy and received preoperative immunizations with polyvalent pneumococcal vaccine (Pneumovax), meningococcal vaccine (Meningovax), as well as confirmation of previous H. influenzae type b vaccine as part of routine immunization practice.
After exposure of the spleen via a left subcostal incision, the spleen was partially devascularized to maintain flow either from the short gastric arcades to the upper pole or from branches of the left gastroepiploic artery or the splenic artery to the lower pole (Fig. 1 A). The ischemic portion of the spleen was allowed to demarcate. Before splenic transection, the devascularized tissue was compressed to “autotransfuse” the remaining blood back into the patient.
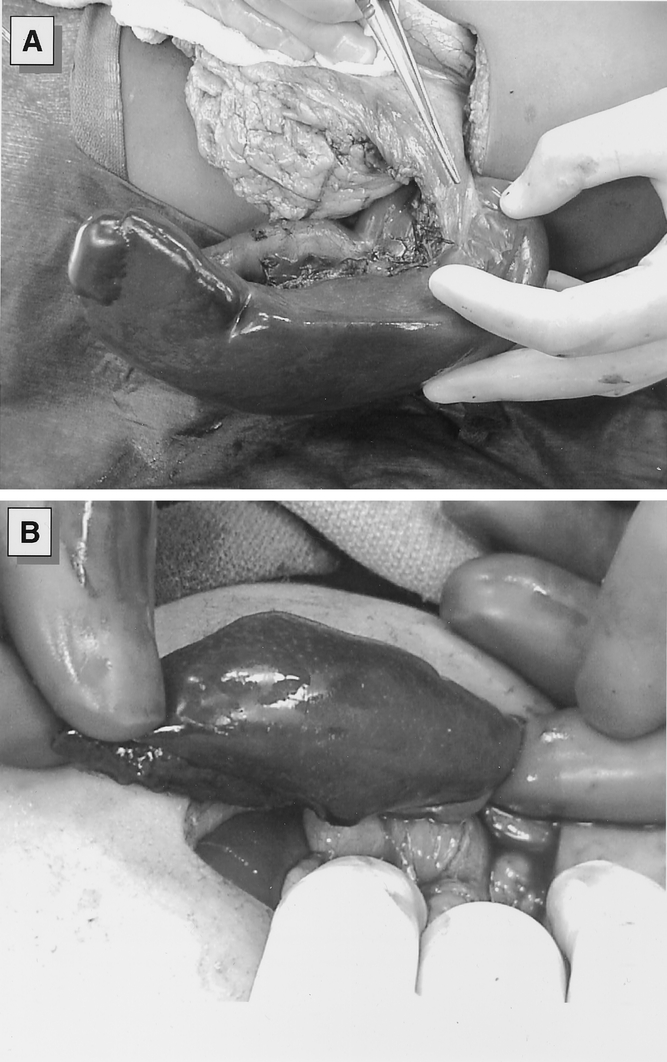
Figure 1. (A) Intraoperative photograph of the spleen following devascularization of the lower pole and the major splenic vessels. The upper pole of the spleen is perfused by retained vasculature from the short gastric vessels. (B) The spleen is transected at the transition between ischemic and perfused tissue with the use of a TA surgical stapler. Hemostasis is supported by use of argon beam coagulator and topical hemostatic agents.
In most cases, we transected the splenic parenchyma using a TA stapler with 4.5-mm staples (U.S. Surgical Corporation, Norwalk, CT) (see Fig. 1 B). In some cases, we occluded the splenic vasculature with a vascular clamp to limit blood loss during parenchyma transection. Bleeding from the splenic bed was controlled with an Argon beam coagulator (Conmed Corp, Utica, NY), suture ligation of vessels, or topical hemostatic agents. In case of a narrow vascular pedicle, the splenic remnant was fixed to the posterior abdominal wall by securing the splenic remnant within the retroperitoneum. In most cases, this fixation could be accomplished quite easily, and no prosthetic mesh was required. Children received standard postoperative care, and length of postoperative stay was generally 3 to 4 days. Children were kept at activity restrictions for 6 weeks to minimize the risk of bleeding and received antibiotic prophylaxis with oral penicillin for at least 1 year postoperatively.
Splenic Imaging
To evaluate splenic regrowth after partial splenectomy, we measured splenic volume with ultrasonography using either ATL 3000 or Ultramark 9 equipment, using broadband 7.5-MHz curved array or 10.5-MHz linear array transducers. The splenic volume was calculated using a formula for the volume of a prolate ellipsoid:13 EQUATION
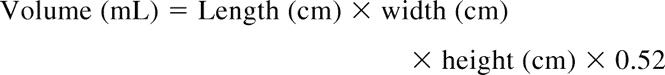 |
In 13 children, we were able to measure the splenic volume preoperatively, and postoperatively at 1 week, at 1, 3, 6, and 12 months, and at yearly intervals thereafter. In 12 children, we did not have preoperative measurements for comparison but were able to follow postoperative splenic volumes. As part of sonography, children were screened for gallstones.
Effects on Hemolysis
Clinical control of hemolysis was evaluated by serial measurement of hemoglobin levels, reticulocyte counts, and bilirubin levels. Baseline levels were measured when the child was well, and not during any acute illness or sequestration crisis. In most cases, laboratory values were measured during the first postoperative week, and at 1, 3, 6, and 12 months postoperatively, and at yearly intervals thereafter. Mean values for the study groups were compared between preoperative and postoperative levels.
Splenic Function
All patients had serial examination of peripheral blood smears by a senior hematologist. The absence of circulating Howell-Jolly bodies was interpreted as evidence of retained phagocytic function by the splenic remnant. In eight children, we measured levels of pitted red cells by interference contrast phase microscopy with Nomarski optics. 14 We recognize that the measurement of pitted red cells is limited in children with hemolytic anemias, as some of these children have pitted red cells as a result of their underlying disease. In eight children, we used nuclear medicine scintigraphy with technetium-99m-labeled sulfur colloid at intervals from 6 to 48 months after partial splenectomy. 15
In 10 children, we measured total serum IgM and IgG levels both initially and at intervals postoperatively out to 4 years of follow-up. As the spleen is the primary source of antibody production in young children, 16 the maintenance of normal immunoglobulin levels suggests at least partially retained ability for antibody synthesis. In six children, we measured specific antibody titers to 12 serotypes of S. pneumoniae using a standard laboratory ELISA assay.
Statistical Analysis
Mean laboratory values were compared between preoperative and postoperative levels. Equality of means was compared by the paired Student t test. The mean splenic volume was compared at intervals postoperatively with the baseline value for each child using the paired Student t test. A threshold of statistical significance was set at 0.05.
RESULTS
Surgical Procedure
A partial splenectomy was successfully performed in 25 children. One additional child with HgbSC disease underwent intraoperative conversion to a total splenectomy due to massive splenomegaly (>400 mL) and diminutive blood vessels to both poles, making preservation of splenic tissue technically impossible. Further data on this child are not included in this report.
The amount of spleen removed was compared in the 13 children with quantitative preoperative and postoperative splenic measurements. In these cases, the measured reduction of splenic volume closely correlated with the surgeon’s operative impression of the amount of spleen tissue removed (within 5–10% of measured splenic weights). In the 12 children without preoperative ultrasonography, the surgeon’s estimate of the amount of spleen removed was supported by measured splenic weights. In 20 children, 80% to 90% of the splenic tissue was resected. In two children, resection of only 60% to 70% of the spleen was possible due to aberrant short gastric vessels, which overlapped with the major splenic pedicle and restricted vascular preservation. In three children, ligation of the dominant vascular pedicle from the short gastric arcades resulted in loss of 90% to 97% of the splenic mass.
Perioperative complications were minimal. No child had perioperative bleeding requiring a blood transfusion. There were no infections or wound complications. One child had a postoperative bowel obstruction requiring adhesiolysis 1 week after partial splenectomy. Operative times ranged from 40 to 120 minutes, although the most recent five cases had substantially shorter operative times, ranging from 40 to 75 minutes. Most children were discharged within 3 to 4 days postoperatively. No child has required subsequent conversion to total splenectomy.
Splenic Regrowth Following Partial Splenectomy
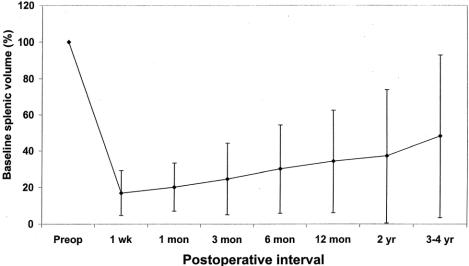
There was little early regrowth of the splenic remnant following partial splenectomy. In the 13 children with preoperative and postoperative measurements of splenic volume, the mean size of the splenic remnant remained between 15% and 30% of baseline volume throughout the first 2 years of follow-up, significantly below the preoperative size (P < .05 by paired Student t test, Fig. 2). By 4 years of follow-up, the splenic regrowth was more pronounced, averaging 40% of original splenic size, although the rate of splenic regrowth is quite variable among children. Interestingly, for two children in whom 95% to 97% of the spleen was removed, there has been regrowth of the splenic to 5% to 20% of baseline splenic volume within 2 years after surgery.
Figure 2. Evaluation of splenic volume following partial splenectomy using ultrasonography. Mean splenic volumes are expressed as a percentage of baseline splenic volume for 13 patients in whom preoperative and postoperative measurements are available. A significant decrease in splenic volume is seen out to 2 years of follow-up compared to preoperative values (P < .05 by paired Student t test). Error bars show the standard deviation.
In particular, four children with HS have regrown their spleens to 75% to 100% of their original size. However, regrowth of the spleen was not associated necessarily with recurrent hemolysis. For example, in patient HS1, the spleen regrew to 80% of its baseline size within 2 years postoperatively, although there has been no recurrent hemolysis or anemia out to 2 years of follow-up. Similarly, in patient HS6, the splenic remnant grew to 95% of baseline size within 4 years postoperatively, although there has not been any recurrent hemolysis. In contrast, in patients HS11 and HS15, the spleen regrew to 75% to 100% of estimated original size within 4 to 6 years postoperatively. Patient HS11 had a transient aplastic crisis 4 years postoperatively. Patient HS15 had mild jaundice, abdominal pain, and an elevated reticulocyte count at 6 years postoperatively. These findings suggest that hemolysis may persist in some children with excessive splenic regrowth, although the clinical importance of these findings is unclear.
Effect on Anemia and Hemolysis
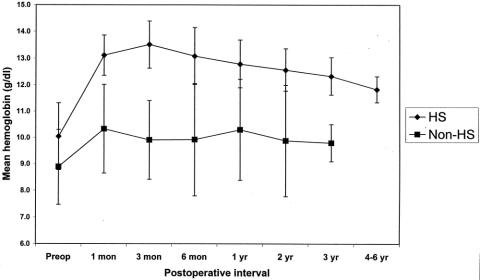
In the children with HS, there appears to be sustained control of hemolysis after partial splenectomy. Mean hemoglobin concentrations increased by at least 2 g/dL compared to baseline values, and this difference has persisted throughout 4 to 6 years of follow-up (Fig. 3, P < .05 by Student paired t test). Mean reticulocyte counts decreased following partial splenectomy, from 12.7 ± 4.2% preoperatively to 4.9 ± 2.9% at the most recent postoperative follow-up (P < .05 by paired Student t test). Mean serum bilirubin levels also decreased after partial splenectomy for these children, from a level of 2.6 ± 1.1 mg/dL preoperatively to 1.3 ± 0.7 mg/dL at the most recent postoperative follow-up (P < .05 by paired Student t test). Finally, there was a reduction in signs and symptoms of hemolysis and hypersplenism (see Table 2).
Figure 3. Mean hemoglobin levels after partial splenectomy for children with hereditary spherocytosis (HS) (n = 15, one child lost to follow-up) and for children without HS (n = 8, one child undergoing directed phlebotomy). Diagnoses for children without HS include pyruvate kinase deficiency, congenital nonspherocytotic hemolytic anemia of unknown etiology, HgbSS, HgbSC, HgbCC, and HgbS-β-thalassemia. For children with HS, hemoglobin levels increased compared to preoperative values throughout 4 to 6 years of follow-up (P < .05 by paired Student t test). For children without HS, there was no change in hemoglobin levels after partial splenectomy. Error bars show the standard deviation within each study group.
For children without HS, a partial splenectomy did not significantly affect hemoglobin levels (see Fig. 3). Similarly, there was no effect on reticulocyte counts or bilirubin levels. Importantly, however, these children had reduced symptoms of hypersplenism as well as control of splenic sequestration (see Table 2). For the two children with pyruvate kinase deficiency, a partial splenectomy increased their hemoglobin levels over 1.5 to 2 g/dL. In fact, one child with pyruvate kinase deficiency who required multiple transfusions for anemia before the partial splenectomy has begun a serial phlebotomy program to reduce her iron overload (due to this phlebotomy program, follow-up data on this child’s hemoglobin levels or reticulocyte count are not included in this analysis).
Two children had complications of cholelithiasis. In one child with HS, an episode of cholecystitis before partial splenectomy led to a concurrent cholecystectomy with the partial splenectomy. Another child with HS required a cholecystectomy 1 year after partial splenectomy for symptomatic cholelithiasis. In this child, no gallstones were noted by ultrasound before the partial splenectomy, which raises the possibility of continued hemolysis. We found a relatively low incidence of gallstones in this study, which raises the possibility that we may have intervened with splenectomy early in the onset of hemolysis.
Splenic Function
Splenic phagocytic function appears to be preserved in children with HS following partial splenectomy. In the first month postoperatively, most children with HS had circulating Howell-Jolly bodies. However, this finding was transient, and 13 of 14 children had no Howell-Jolly bodies during any future examination. Five children with HS were tested postoperatively for pitted red cells; three had normal levels (<2%) and two had mildly elevated levels (3–6%). Three children with HS had technetium 99m-sulfur colloid scans postoperatively, including one child who had elevated postoperative levels of pitted red cells. All three of these children had normal radionuclide uptake, and the size of the radionuclide image approximated the spleen size as determined by ultrasonography.
Children without HS had less preservation of splenic phagocytic function after partial splenectomy, especially those with sickle hemoglobinopathies. One child with HgbS-β-thalassemia had elevated pitted red cell levels as well as no radionuclide uptake after surgery, although this child had increased pitted red cell levels before surgery. One child with HgbSS disease had increased pitted red blood cells and evidence of Howell-Jolly bodies after surgery, and this child had low radionuclide uptake of the splenic remnant. Another child with HgbSS had no radionuclide uptake in the splenic remnant. However, one child with pyruvate kinase deficiency had a normal radionuclide scan after surgery.
All seven children tested at intervals out to 3 years have retained normal serum levels of IgG and IgM. Six other children were tested out to 3 years after surgery for specific antibody titers to S. pneumoniae. Five of these six children have retained normal antibody titers to S. pneumoniae, and one child with HgbS-β-thalassemia had low antibody titers to several serotypes. No child has developed a severe postoperative infection or sepsis.
DISCUSSION
Partial splenectomy in children with congenital hemolytic anemia can be performed with minimal operative risks and generally results in a desired hematologic effect. For children with HS, we observed a persistent reduction in hemolysis and sustained splenic function. For children with pyruvate kinase deficiency, a partial splenectomy similarly reduced hemolysis and preserved splenic function. In children with sickle hemoglobinopathies, a partial splenectomy controlled symptoms of hypersplenism and splenic sequestration, although splenic function was less well preserved.
Classic indications for splenectomy in children with HS include repeated transfusions or anemic crises. 1 However, many children have less severe symptoms of hemolysis, including fatigue, cholelithiasis, and growth failure. Similarly, many children have symptoms of hypersplenism, including pain, early satiety, or activity limitations. 1,8 For children with milder symptoms of hemolysis or hypersplenism, an open total splenectomy has been difficult to justify, and a partial splenectomy may offer some benefit. Recent innovations for total splenectomy in children include the use of laparoscopy, which may decrease operative morbidity compared to an open approach. 17 At this time, we do not perform partial splenectomy by laparoscopic techniques, although further refinements may allow this approach.
For partial splenectomy, our goal of removing 80% to 90% of splenic tissue is designed to gain the desired hematologic effect while preserving enough tissue for phagocytic and immune function. Animal models suggest that 25% of splenic mass will preserve the splenic phagocytic response to a challenge of S. pneumoniae. 18–20 Although our analysis was limited, it appears that at least partial splenic phagocytic function is maintained for children with HS after partial splenectomy, as normal immunoglobulin levels and specific responses to pneumococcal antigen appear to be preserved. Previous reports have found decreased immunoglobulin levels and impaired pneumococcal antibody responses in children undergoing total splenectomy for a variety of hematologic disorders. 21,22 Although the spleen is the principal site of immunoglobulin production, the significance of preserved antibody levels following partial splenectomy is unclear given the multiple sites of antibody production.
The risk of overwhelming postsplenectomy sepsis after partial splenectomy is impossible to estimate. We follow common practice guidelines that advocate immunoprophylaxis against pneumococcus, meningococcus, and H. influenzae as well as antibiotic prophylaxis for the child undergoing total splenectomy, although the efficacy of these measures has not been proven. 5 Given the low rate of overwhelming postsplenectomy sepsis with proper use of immunizations and antibiotic prophylaxis, any risk reduction for partial splenectomy compared to total splenectomy would require a prohibitively large clinical trial.
The role for partial splenectomy in children with other congenital hemolytic anemias is quite different than in children with HS. In sickle hemoglobinopathies, a partial splenectomy appears to control symptoms of hypersplenism and splenic sequestration but does not always preserve splenic function. We suspect that children with sickle hemoglobinopathies often progress to autoinfarct the splenic remnant following partial splenectomy, consistent with the high rate of functional asplenia in these conditions. 23 The splenic remnants in these children in our study generally appear to become nonfunctional over time. However, the preservation of splenic function after partial splenectomy, even for a limited period, may be beneficial for young children. In contrast to sickle hemoglobinopathies, it appears that hemolysis from pyruvate kinase deficiency is readily controlled by partial splenectomy. Our two children with pyruvate kinase deficiency showed both reduced hemolysis and retained splenic function following partial splenectomy, in contrast to the experience of Sandoval et al. 24
Our study complements the report of Bader-Meunier et al., who summarized their experience in Europe with partial splenectomy for children with HS. 10 In both their and our experience, there appears to be a variable rate of splenic regrowth following partial splenectomy, although importantly this regrowth does not necessarily correlate with recurrent hemolysis. 8,10 The reasons for the discrepancy between splenic regrowth and hematologic status are unclear, although it may be due to altered blood flow or parenchymal remodeling after partial resection.
We found that the hematologic improvement after subtotal splenectomy, particularly for children with HS, approaches the hematologic responses observed after total splenectomy. 25–27 For example, children with HS undergoing total splenectomy generally achieve an increase in hemoglobin of 1.5 to 3.0 g/dL, 25–27 slightly higher than the response seen in our patients. Similarly, persistent hemolysis following partial splenectomy is reflected in the response of reticulocyte count and bilirubin, as they are not of the degree seen following a total splenectomy, in which the reticulocyte count is generally below 3% and the bilirubin levels are less than 1.0 mg/dL. 25–27 The hematologic responses following a partial splenectomy appear to be sustained over a prolonged period and in most cases do not lead to significant recurrent symptoms.
In the European experience, which has limited follow-up out to 14 years, a small number of children undergoing partial splenectomy have required later conversion to total splenectomy. 8,10 No child in our experience has required conversion to total splenectomy to date, although there appears to be a trend toward splenic regrowth at 4 to 6 years of follow-up. We closely follow these children to identify symptoms that would warrant secondary conversion to a total splenectomy. Although there are no clear indications for secondary conversion to a total splenectomy following a partial splenectomy, we suggest that general clinical guidelines practiced for consideration of total splenectomy in these conditions, such as multiple transfusion requirements, severe anemia, jaundice, and fatigue, be used to guide consideration for secondary conversion to total splenectomy. Longer follow-up will define the need for later surgical procedures. In any case, we believe that the potential benefits of retaining functional splenic tissue in a young child, even if only for a few years, outweigh the risks of a possible secondary surgical procedure.
In summary, for symptomatic children with various congenital hemolytic anemias, a partial splenectomy can be performed with minimal morbidity and generally results in a desired hematologic effect. For children with HS and pyruvate kinase deficiency, a partial splenectomy reduces the rate of hemolysis and preserves splenic function. For children with other hemoglobinopathies, a partial splenectomy reduces symptoms of hypersplenism and splenic sequestration, although splenic function is less well preserved than for children with spherocytosis. We recognize that a partial splenectomy is a technically challenging operation, and its role compared to a total splenectomy for the treatment of young children with various hemolytic congenital anemias continues to be defined. We suggest that a prospective, long-term analysis of this approach will help determine its proper role in the care of these children.
Footnotes
Correspondence: Henry E. Rice, MD, Division of Pediatric Surgery, Box 3815, Duke University Medical Center, Durham, NC, 27710.
E-mail: rice0017@mc.duke.edu
Accepted for publication June 13, 2002.
References
- 1.Delaunay J. Genetic disorders of the red cell membrane. Crit Rev Oncol Hematol 1995; 19: 79–110. [DOI] [PubMed] [Google Scholar]
- 2.King H, Shumacker HB Jr. Susceptibility to infection after splenectomy performed in infancy. Ann Surg 1951; 136: 239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch AM, Kapila R. Overwhelming postsplenectomy infection. Infect Dis Emerg 1996; 10: 693–707. [DOI] [PubMed] [Google Scholar]
- 4.Leonard AS, Giebink GS, Baesl TJ, et al. The overwhelming postsplenectomy sepsis problem. World J Surg 1980; 4: 423–432. [DOI] [PubMed] [Google Scholar]
- 5.Brigden ML, Pattullo AL. Prevention and management of overwhelming postsplenectomy infection: an update. Crit Care Med 1999; 27: 836–842. [DOI] [PubMed] [Google Scholar]
- 6.Gold HS, Moellering RCJ. Antimicrobial drug resistance. N Engl J Med 1996; 335: 1445–1453. [DOI] [PubMed] [Google Scholar]
- 7.Tchernia G, Gauthier F, Mielot F, et al. Initial assessment of the beneficial effect of partial splenectomy in hereditary spherocytosis. Blood 1993; 81: 2014–2020. [PubMed] [Google Scholar]
- 8.Tchernia G, Bader-Meunier B, Berterottiere P, et al. Effectiveness of partial splenectomy in hereditary spherocytosis. Curr Opin Hematol 1997; 4: 136–141. [DOI] [PubMed] [Google Scholar]
- 9.Freud E, Cohen IJ, Mor C, et al. Splenic “regeneration” after partial splenectomy for Gaucher disease: histological features. Blood Cell Mol Dis 1998; 24: 309–316. [DOI] [PubMed] [Google Scholar]
- 10.Bader-Meunier B, Gauthier F, Archambaud F, et al. Long-term evaluation of the beneficial effect of subtotal splenectomy for management of hereditary spherocytosis. Blood 2001; 97: 399–403. [DOI] [PubMed] [Google Scholar]
- 11.Svarch E, Vilorio P, Nordet I, et al. Partial splenectomy in children with sickle cell disease and repeated episodes of splenic sequestration. Hemoglobin 1996; 20: 393–400. [DOI] [PubMed] [Google Scholar]
- 12.Guzzetta PC, Ruley EJ, Merrick HFW, et al. Elective subtotal splenectomy: indications and results in 33 patients. Ann Surg 1990; 211: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Odorico I, Spaulding KA, Pretorius DH, et al. Normal splenic volumes estimated using three-dimensional ultrasonography. J Ultrasound Med 1999; 18: 231–236. [DOI] [PubMed] [Google Scholar]
- 14.Reinhart WH, Chien S. Red cell vacuoles: their size and distribution under normal conditions and after splenectomy. Am J Hematol 1988; 27: 265–271. [DOI] [PubMed] [Google Scholar]
- 15.Sty JR, Conway J. The spleen: development and functional evaluation. Semin Nucl Med 1985; 15: 276–298. [DOI] [PubMed] [Google Scholar]
- 16.Spirer Z. The role of the spleen in immunity and infection. Adv Pediatr 1980; 27: 55–88. [PubMed] [Google Scholar]
- 17.Farah RA, Rogers ZR, Thompson WR, et al. Comparison of laparoscopic and open splenectomy in children with hematologic disorders. J Pediatr 1997; 131: 41–46. [DOI] [PubMed] [Google Scholar]
- 18.Malangoni MA, Dawes LG, Droege EA, et al. Splenic phagocytic function after partial splenectomy and splenic autotransplantation. Arch Surg 1985; 120: 275–278. [DOI] [PubMed] [Google Scholar]
- 19.Goldthorn JF, Schwartz AD, Swift AJ, et al. Protective effect of residual splenic tissue after subtotal splenectomy. J Pediatr Surg 1978; 13: 587–590. [DOI] [PubMed] [Google Scholar]
- 20.Witte MH, Witte CL, Van Wyck DB, et al. Preservation of the spleen. Lymphology 1983; 16: 128–137. [PubMed] [Google Scholar]
- 21.Koren A, Haasz R, Tiatler A, et al. Serum immunoglobulin levels in children after splenectomy. A prospective study. Am J Dis Child 1984; 138: 53–55. [PubMed] [Google Scholar]
- 22.Cohn DA, Schiffman G. Immunoregulatory role of the spleen in antibody responses to pneumococcal polysaccharide antigens. Infect Immun 1987; 55: 1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown AK, Sleeper LA, Miller ST, et al. Reference values and hematologic changes from birth to 5 years in patients with sickle cell disease. Arch Pediatr Adol Med 1994; 148: 796–804. [DOI] [PubMed] [Google Scholar]
- 24.Sandoval C, Stringel G, Weisberger J, et al. Failure of partial splenectomy to ameliorate the anemia of pyruvate kinase deficiency. J Pediatr Surg 1997; 32: 641–642. [DOI] [PubMed] [Google Scholar]
- 25.Schilling RF. Hereditary spherocytosis: a study of splenectomized persons. Semin Hematol 1976; 13: 169–176. [PubMed] [Google Scholar]
- 26.Mehta J, Harjai K, Vansani J, et al. Hereditary spherocytosis: experience of 145 cases. Indian J Med Sci 1992; 46: 103–110. [PubMed] [Google Scholar]
- 27.Agre P, Asimos A, Casella JF. inheritance pattern and clinical response to splenectomy as a reflection of erythrocyte spectrin deficiency in hereditary spherocytosis. N Engl J Med 1986; 315: 1579–1583. [DOI] [PubMed] [Google Scholar]