Abstract
Objective
To examine the clinical efficacy and safety of ertapenem, a novel β-lactam agent with wide activity against common pathogens encountered in intraabdominal infection.
Summary Background Data
Ertapenem has a pharmacokinetic profile and antimicrobial spectrum that support the potential for use as a once-a-day agent for the treatment of common mixed aerobic and anaerobic infections.
Methods
This prospective, randomized, controlled, and double-blind trial was conducted to compare the safety and efficacy of ertapenem with piperacillin/tazobactam as therapy following adequate surgical management of complicated intraabdominal infections.
Results
Six hundred thirty-three patients were included in the modified intent-to-treat population, with 396 meeting all criteria for the evaluable population. Patients with a wide range of infections were enrolled; perforated or abscessed appendicitis was most common (approximately 60% in microbiologically evaluable population). A prospective, expert panel review was conducted to assess the adequacy of surgical source control in patients who were failures as a component of evaluability. For the modified intent-to-treat groups, 245 of 311 patients treated with ertapenem (79.3%) were cured, as were 232 of 304 (76.2) treated with piperacillin/tazobactam. One hundred seventy-six of 203 microbiologically evaluable patients treated with ertapenem (86.7%) were cured, as were 157 of the 193 (81.2%) treated with piperacillin/tazobactam.
Conclusions
In this study, the efficacy of ertapenem 1 g once a day was equivalent to piperacillin/tazobactam 3.375 g every 6 hours in the treatment of a range of intraabdominal infections. Ertapenem was generally well tolerated and had a similar safety and tolerability profile to piperacillin/tazobactam. A formal process for review of adequacy of source control was found to be of benefit. The results of this trial suggest that ertapenem may be a useful option that could eliminate the need for combination and/or multidosed antibiotic regimens for the empiric treatment of intraabdominal infections.
Complicated intraabdominal infections (i.e., those requiring both operative drainage and antimicrobial therapy) are among the most common infections in general surgery. Antimicrobial therapy is an important element in the management of these infections; there are convincing data that absent or inadequate empiric antibiotic therapy results in both increased failure rates and increased mortality. 1–4 The infecting flora seen with community-acquired intraabdominal infections is well known and consists of aerobic, facultative, and anaerobic gram-negative bacilli, various streptococci and enterococci, and a plethora of gram-positive anaerobes. 5–7 The synergistic interactions between endotoxin-bearing gram-negative organisms and Bacteroides fragilis define both groups as important targets for antimicrobial therapy. 8
Ertapenem (formerly MK-0826, Merck & Co., Inc.) is being investigated as a once-a-day parenteral β-lactam antimicrobial agent with the potential for use as monotherapy for the treatment of community-acquired mixed flora infections. This approach is based on its spectrum of activity, previously reported clinical studies that used once-a-day dosing, pharmacodynamic studies in animals, and pharmacokinetic studies in both humans and animals. 9,10 Ertapenem is highly active in vitro against gram-positive and gram-negative aerobic, facultative, and anaerobic bacteria, including the predominant pathogens responsible for intraabdominal infections. 11–13 However, it provides limited coverage against Pseudomonas aeruginosa, Acinetobacter spp, and enterococci, organisms generally associated with nosocomial infections.
The primary objectives of this study were to determine the clinical and microbiologic efficacy and safety of ertapenem for patients with complicated intraabdominal infections. The comparative agent was piperacillin/tazobactam, a β-lactam/β-lactamase inhibitor combination agent that has been well studied and is approved in the treatment of intraabdominal infection. 14 This agent is typically administered at 3.375 g every 6 hours in the United States.
This study also provided an opportunity to analyze an additional element of clinical trial design, the adequacy of surgical source control. Inadequate control of infection, through either incomplete drainage or incomplete management of enteric perforations, is an independent risk factor for treatment failure. 15,16 An expert panel review process was conducted to examine, under blinded conditions, the adequacy of surgical source control as a component of evaluability in a prospectively generated and well-documented group of patients.
METHODS
Protocol
This was a prospective, multicenter, double-blind (also with sponsor blinding), randomized, comparative study to evaluate the safety, efficacy, and tolerability of ertapenem versus piperacillin/tazobactam in the treatment of complicated intraabdominal infection in hospitalized adults. Complicated infection is defined as an infection requiring surgical intervention and one that extends beyond the hollow viscus of origin into the peritoneal space, and is associated either with abscess formation or peritonitis. This trial was conducted in accord with Infectious Disease Society of America/Food and Drug Administration (IDSA/FDA) guidelines. 17,18 The protocol was reviewed and approved by each institutional review board/ethical review committee, and written informed consent was obtained for each patient according to the guidelines of the institution.
Inclusion Criteria
Patients were eligible for inclusion if they were at least 18 years of age and showed clinical evidence of intraabdominal infection, and operative or percutaneous drainage of an infectious focus was either planned or recently performed (within 24 hours), confirming the presence of complicated intraabdominal infection. Allowed interventions included open laparotomy, laparoscopy, and percutaneous drainage procedures. The protocol required that specimens be sent for aerobic and anaerobic cultures at the time of the initial intervention.
Prior antibiotic therapy was allowed only if the patient received less than 24 hours of prior therapy or was considered to have failed that treatment if there had been more than 24 hours of treatment. Non-study antibiotics were stopped before study treatment was begun. For patients enrolled after intervention, no more than a single dose of non-study therapy given after the intervention was allowed.
Exclusion Criteria
Patients were excluded from entry for diagnoses of traumatic bowel perforation operated on within 12 hours of injury; perforation of gastroduodenal ulcers with operation within 24 hours of perforation; simple cholecystitis; simple appendicitis; acute suppurative cholangitis; and infected, necrotizing pancreatitis; and planned management by staged abdominal repair or open abdomen technique.
Other reasons for exclusion included history of anaphylaxis or serious allergy to either study agent or antibiotic agents in the carbapenem or other β-lactam class; low likelihood of either treatment response or survival to the 4- to 6-week follow-up visit, APACHE II score more than 30;19 baseline pathogens known to be resistant to study agents at study entry; concurrent confounding infection; use of other investigational therapy within the previous 30 days; immunosuppressive therapy or diagnosis of AIDS according to current Centers for Disease Control (CDC) criteria; and previous enrollment in the trial. Patients with creatinine clearance of 30 mL/min or less or undergoing dialysis were also excluded, as were those with ALT or AST more than six times the upper limit of normal (ULN), bilirubin more than three times ULN, neutropenia (<1,000 × 106 neutrophils/L), platelets less than 75,000/mm3, or PT or PTT more than 1.5 times ULN. Pregnant or nursing women and fertile women not practicing adequate contraception were also excluded.
Randomization
The study pharmacist was unblinded and performed the randomization and treatment allocation procedures. The unblinded pharmacist also prepared the blinded study infusions but did not administer study therapy or perform any patient assessments. Blinded study personnel performed study drug administration and patient assessments. Randomization was performed using allocations determined with a block size of four.
To achieve balance in the treatment groups, patients were stratified before randomization based on the primary site of infection (complicated appendicitis without generalized peritonitis vs. all other diagnoses) and severity of illness (APACHE II score ≤15 or >15). To limit the proportion of cases with complicated appendicitis without generalized peritonitis, the protocol specified that enrollment into that stratum at all sites was to be closed when approximately 50% of target enrollment was achieved.
Study Treatment
Patients were randomized (1:1) to receive ertapenem 1 g once a day with placebo infusions at 6-hourly intervals or piperacillin/tazobactam given 3.375 g every 6 hours. For piperacillin/tazobactam, dosage adjustments for renal failure were made according to product labeling. Study therapy was given for a minimum of 4 full days, unless treatment failure was identified earlier, and the suggested maximum duration was 14 full days.
Addition of open label vancomycin was permitted if Enterococcus or methicillin-resistant Staphylococcus aureus was one of the pathogens isolated, and if, in the opinion of the investigator, specific therapy was indicated. In such cases the patient could remain evaluable provided gram-negative pathogens were also present at baseline. Antifungal therapy was permitted according to the usual practice of the investigator.
Microbiologic Procedures
Appropriate aerobic and anaerobic cultures were required for all patients at the time of the initial procedure within 24 hours of enrollment. Aerobic organisms were tested for in vitro susceptibility to ertapenem and piperacillin/tazobactam in the local microbiology laboratory using standard disk diffusion or MIC test using NCCLS guidelines and either approved (for piperacillin/tazobactam) or provisional (for ertapenem) breakpoints. Specimens of infected fluid or tissue from study sites outside the United States were sent in transport media to a central reference laboratory (Alden Laboratories, Santa Monica, CA) for anaerobic isolation and susceptibility testing.
Definitions of Patient Populations
All determinations of evaluability were made before unblinding using prespecified criteria. The analyses described below are on the clinical modified intention-to-treat (MITT) population and the microbiologically evaluable population. The clinical MITT population was composed of randomized patients who received at least one dose of study drug and met the minimal disease definition. The minimal disease definition included all intraabdominal infections, whether complicated (perforating) or not. Examples of excluded diagnoses were sterile fluid collections and necrotizing pancreatitis. Identification of baseline pathogens was not required for this population.
The microbiologically evaluable per protocol population, which was used for the primary analysis, was a subset of the clinical MITT population comprised of patients with: (1) complicated intraabdominal infection (no prior antibiotic use >24 hours in the absence of failure); (2) adequate surgical source control at the initial intervention; (3) sufficient information to determine outcomes; (4) no confounding factors that interfered with the assessment of that outcome; (5) identified baseline pathogens, one or more of which were susceptible to both study therapies; (6) adequate duration of study therapy (patients had to receive at least 4 days and not more than 17 days of study therapy to be considered evaluable cures and at least 2 days of therapy to be considered evaluable failures); (7) test-of-cure assessment; and (8) assessment of a microbiologic response. Patients receiving additional antimicrobial therapy for postoperative infection outside the abdomen before 5 days of study therapy were not included in the evaluable group; if treatment for the postoperative infection began after 5 days on study therapy, these patients were included as evaluable, as specified in the IDSA/FDA guidelines for studies in complicated intraabdominal infection. 18
Clinical and Microbiologic Outcome Assessments and Definitions
Clinical and microbiologic outcome assessments were made at the time intravenous therapy was discontinued, at 1 to 2 weeks after completion of therapy (the early follow-up visit), and at 4 to 6 weeks after completion of therapy (the test of cure visit).
Clinical cure was defined as resolution of the index infection, with no further antibiotic therapy necessary. Clinical failure was defined as one of the following: (1) persisting or recurrent infection within the abdomen, documented by findings at percutaneous or operative reintervention; (2) postsurgical wound infection; (3) death related to ongoing intraabdominal infection; or (4) treatment with additional antibiotics for undocumented intraabdominal infection during the study period. For the MITT analysis, patients with indeterminate clinical outcomes were considered treatment failures.
Expert Assessment of Adequacy of the Initial Intervention
Inadequate surgical source control was considered a reason for clinical nonevaluability in this study since inadequate surgical management of the presenting infection could obscure the assessment of antimicrobial efficacy. To assess the adequacy of surgical source control in a consistent manner, a prespecified procedure was established that enlisted a committee of eight experts to assess the adequacy of source control under blinded conditions. This committee included seven surgeons and one interventional radiologist, of whom five were study investigators. The committee reviewed all cases in two categories: all those cases scored as a failure by the local investigator, and all cases in which an unplanned second procedure was performed, to ensure there was no evidence of occult failure when these cases were considered cured by the investigator. All data were blinded during the two-step review process, and included case report form data extractions, operative notes, and narrative summaries of the cases.
For operative procedures, adequacy was defined as control of the underlying pathologic process by resection, closure, or drainage, and drainage of existing purulent collections. For percutaneous procedures, adequacy was determined by review of the imaging studies by an experienced interventional radiologist and included adequate drainage of all collections initially present. The key issue for the panel was not whether better alternative methods of management were available, but rather whether the method chosen was adequate to effect cure with adequate antimicrobial and supportive care.
In the first step, three reviewers independently judged the adequacy of the intervention for each case; panel members did not initially review cases from their own sites. If complete agreement was reached among the three reviewers that the initial procedure was adequate, or that in the case of a second procedure there was no evidence of occult failure, the case was not further discussed, and source control was considered adequate. If there was any discordance, the case was presented to the full panel for discussion and majority decision. Lavage of the peritoneal or abscess cavity with antibiotics was prohibited. The decision to use percutaneous or operative intervention was made by the treating physician. The operating surgeon made decisions regarding closure of the skin incision and the use of drains.
Statistical Analysis
Clinical Outcome and Treatment Equivalence
The primary hypothesis tested in this study was that the proportion of microbiologically evaluable patients who were cured in the ertapenem and piperacillin/tazobactam treatment groups would be equivalent at the test of cure visit. The definition of equivalence used was that the 95% (two-sided) confidence interval for the difference in response rates between the two treatment groups (test drug group minus control group) contained zero and the lower limit of the confidence interval was not less than −15% points. A categorical modeling procedure provided point estimates (means and variances) for the differences between treatment group outcomes. Adjustment was made for the effect of stratification.
Secondary analyses included comparison of favorable clinical and microbiologic response rates in microbiologically evaluable patients at the earlier time points of discontinuation of intravenous study therapy and at the 1- to 2-week early follow-up assessment. Comparison of the clinical response rates in the clinical MITT populations was performed as a supportive analysis. To examine specific subgroups of microbiologically evaluable patients, prespecified subset analyses included primary outcomes by anatomic site of infection and infectious process. Exploratory analyses were performed using outcomes across the range of APACHE II scores and for other baseline disease characteristics. Observed response rates are presented for these subgroups.
Logistic Regression
To assess possible prognostic factors for treatment failure, a post hoc logistic regression was performed using combined clinical and microbiologic outcome as the dependent variable and several possible prognostic factors as independent variables. These were treatment group; APACHE II score; presence of generalized peritonitis; antibiotic treatment of more than 2 days before study entry; initial organ site of infection (appendix vs. all other); polymicrobial versus monomicrobial infection; primary wound closure; U.S. site versus non-U.S. site; high-enrolling versus low-enrolling site; age; sex; presence of an abscess; postoperative etiology; at least one baseline resistant pathogen; and enterococcus as a baseline pathogen. Analysis was performed using SAS PROC LOGISTIC with a stepwise variable selection procedure. The significance level for entering a variable into the model was specified at 0.25.
RESULTS
Fifty-seven sites participated in this study, including centers in Argentina, Belgium, Brazil, Canada, Chile, Colombia, France, Germany, Guatemala, Italy, Mexico, Peru, Russia, South Africa, Spain, Switzerland, the United States, and Venezuela. Twenty-six sites were in the United States.
Six hundred thirty-three patients were randomized: 323 were randomized to ertapenem and 310 were randomized to piperacillin/tazobactam. Two hundred three ertapenem-treated patients and 193 piperacillin/tazobactam-treated patients were microbiologically evaluable. The reasons 237 randomized patients were considered not microbiologically evaluable for analysis are detailed in Table 1. The most common reason was absence of identified pathogens. No differences between study arms were identified.
Table 1. REASONS 237 PATIENTS WERE EXCLUDED FROM THE CLINICALLY AND MICROBIOLOGICALLY EVALUABLE PATIENT GROUP
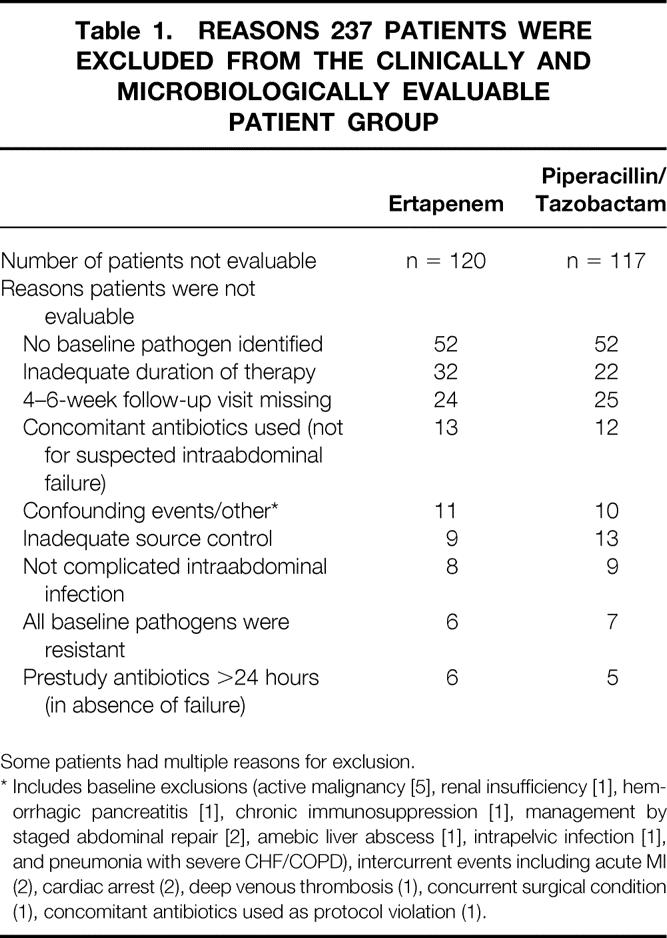
Some patients had multiple reasons for exclusion.
* Includes baseline exclusions (active malignancy [5], renal insufficiency [1], hemorrhagic pancreatitis [1], chronic immunosuppression [1], management by staged abdominal repair [2], amebic liver abscess [1], intrapelvic infection [1], and pneumonia with severe CHF/COPD), intercurrent events including acute MI (2), cardiac arrest (2), deep venous thrombosis (1), concurrent surgical condition (1), concomitant antibiotics used as protocol violation (1).
The expert panel reviewed 145 of the 633 randomized patients. Of these, 96 went to second-step face-to-face review. Of these, 22 patients (9 in the ertapenem group, 13 in the piperacillin/tazobactam group) were judged by the expert panel to have had inadequate source control in their initial interventions. Of the 145 cases reviewed, 5 patients overall who had second procedures and were rated as cures by the investigator were considered by the panel as failures, and as a result these were downgraded to failures for analysis.
Demographics, Baseline Disease Characteristics, and Infectious Pathology
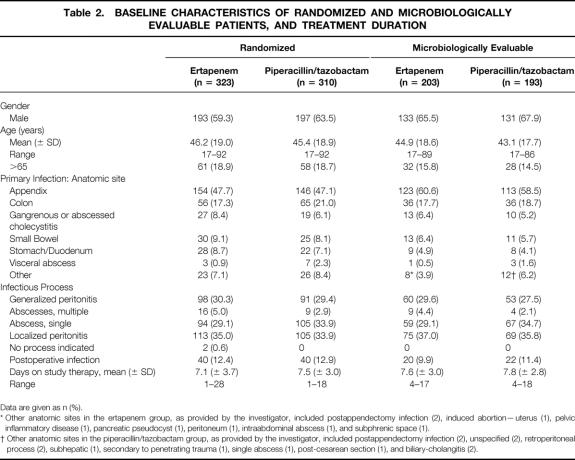
The demographic and baseline disease characteristics of the randomized and microbiologically evaluable populations are detailed in Table 2. There were no differences between the treatment groups.
Table 2. BASELINE CHARACTERISTICS OF RANDOMIZED AND MICROBIOLOGICALLY EVALUABLE PATIENTS, AND TREATMENT DURATION
Data are given as n (%).
* Other anatomic sites in the ertapenem group, as provided by the investigator, included postappendectomy infection (2), induced abortion—uterus (1), pelvic inflammatory disease (1), pancreatic pseudocyst (1), peritoneum (1), intraabdominal abscess (1), and subphrenic space (1).
† Other anatomic sites in the piperacillin/tazobactam group, as provided by the investigator, included postappendectomy infection (2), unspecified (2), retroperitoneal process (2), subhepatic (1), secondary to penetrating trauma (1), single abscess (1), post-cesarean section (1), and biliary-cholangitis (2).
Since multiple infectious processes could have been reported for a single patient, a hierarchy was established for reporting purposes (1, generalized peritonitis; 2, multiple abscess; 3, single abscess; and 4, localized disease, which included localized peritonitis and visceral perforations in the absence of frank abscess or peritonitis). Patients were scored as having the infectious process highest in this hierarchy if they had multiple processes reported. Approximately 11% of microbiologically evaluable patients had postoperative infection at entry. The duration of therapy was similar in the two treatment groups. The clinical MITT data were similar (not shown). Details of the interventional procedures performed are provided in Table 3.
Table 3. INITIAL INTERVENTIONS IN THE MICROBIOLOGICALLY EVALUABLE POPULATION
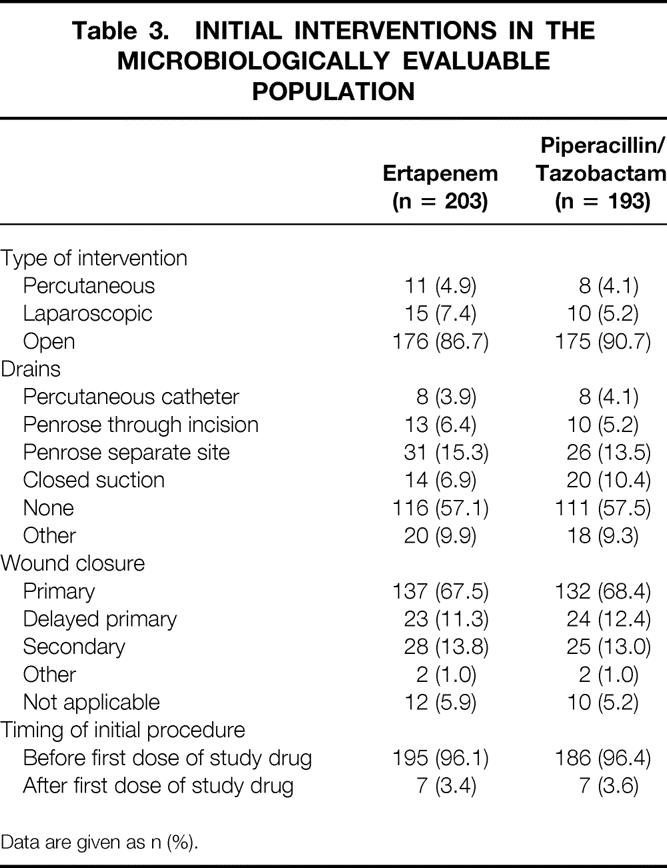
Data are given as n (%).
Approximately 4% of microbiologically evaluable patients in each treatment group received vancomycin for gram-positive baseline pathogens, as allowed per protocol.
Microbiologic Findings
Polymicrobial infection was documented in the majority of microbiologically evaluable patients (335/396 [84.6%]), with gram-negative and anaerobic organisms most prevalent. The most frequent isolates were E. coli, B. fragilis and other Bacteroides spp, and Clostridium spp. The organisms encountered in this study were similar to those reported in previous studies, and the most common are displayed in Table 4. Susceptibility data for anaerobes isolated from patients in this study enrolled at all non-U.S. sites and selected U.S. sites has been previously published; these data show excellent activity of ertapenem for a wide range of anaerobic pathogens. 11
Table 4. MICROORGANISMS ISOLATED FROM MICROBIOLOGICALLY EVALUABLE PATIENTS
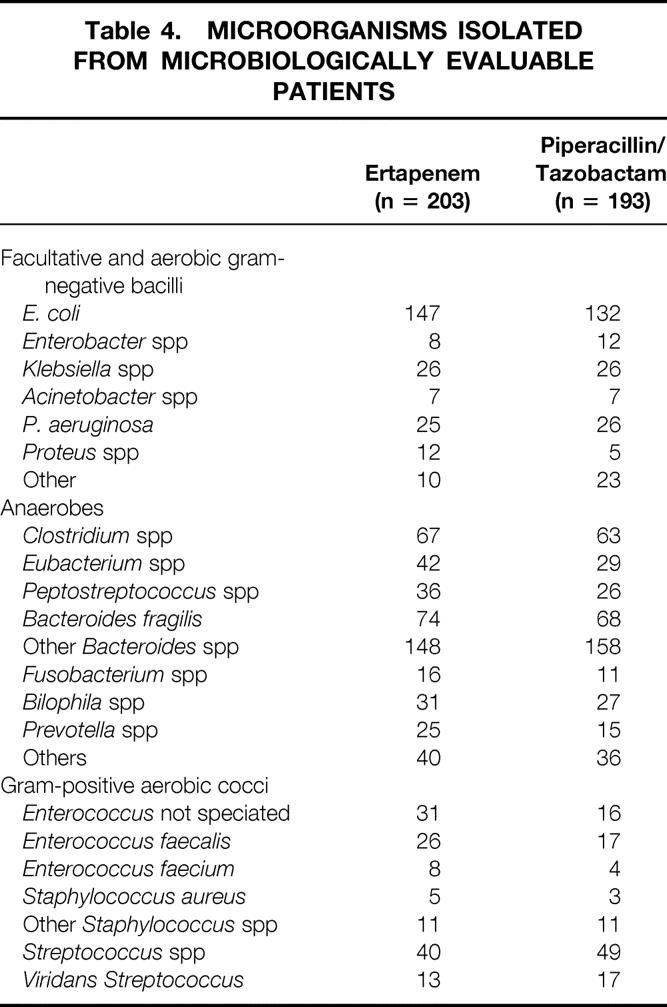
Clinical and Microbiologic Outcome and Treatment Equivalence
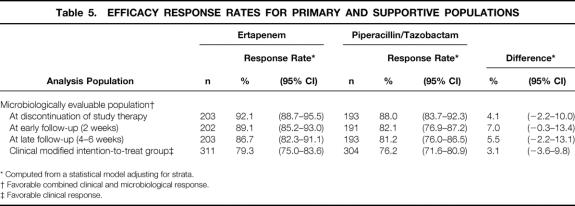
The primary and supportive efficacy analyses are shown in Table 5. In the primary efficacy analysis, 176 of the 203 (86.7%, adjusted for stratum) microbiologically evaluable patients treated with ertapenem had favorable clinical and microbiologic responses and were therefore considered cures, as did 157 of the 193 (81.2%, adjusted for stratum) treated with piperacillin/tazobactam. The result of the primary analysis meets the prespecified definition for equivalence of ertapenem and piperacillin/tazobactam; the MITT analysis confirms this result.
Table 5. EFFICACY RESPONSE RATES FOR PRIMARY AND SUPPORTIVE POPULATIONS
* Computed from a statistical model adjusting for strata.
† Favorable combined clinical and microbiological response.
‡ Favorable clinical response.
Cure rates at the time of discontinuation of intravenous therapy and at the early follow-up visit show slightly higher response rates for ertapenem and support the primary analysis. In a post hoc analysis of efficacy in patients who were evaluable at the early follow-up visit, which includes patients who were not evaluable only because they did not return for a final assessment, the cure rates were 190/212 (89.6%) for the ertapenem group and 162/196 (82.7%) for the piperacillin/tazobactam group, with a difference of 7.0%. The 95% confidence interval about the observed difference between the cure rates for this population was 0.3 to 13.7.
Categories of Treatment Failure
Categories of treatment failure are presented in Table 6. Most failures were due to documented persistent or recurrent infection or surgical site infections. The most common anatomic findings at the time of failure were abscess and deep or superficial incisional surgical site infection.
Table 6. CATEGORIES OF CLINICAL FAILURE
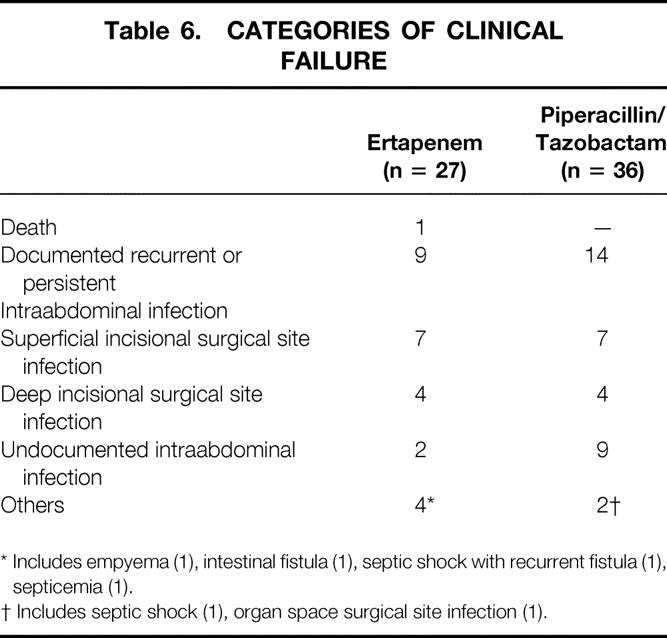
* Includes empyema (1), intestinal fistula (1), septic shock with recurrent fistula (1), septicemia (1).
† Includes septic shock (1), organ space surgical site infection (1).
Outcome Results for Patients With Severe Infections
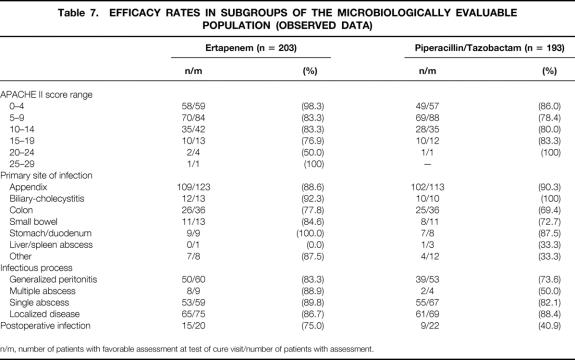
Subset analyses that were prespecified as supportive analyses and other exploratory subset analyses are shown in Table 7. Statistical analysis was not preplanned for these subsets and thus not performed. The response rates for patients with high APACHE II scores were generally lower than for patients with low APACHE II scores, but the total number of evaluable patients with high APACHE II scores was small, and there was an imbalance between the treatment groups among patients with the highest APACHE II scores (≥20).
Table 7. EFFICACY RATES IN SUBGROUPS OF THE MICROBIOLOGICALLY EVALUABLE POPULATION (OBSERVED DATA)
n/m, number of patients with favorable assessment at test of cure visit/number of patients with assessment.
Patients with complicated appendiceal infection had similar response rates in the two treatment groups. Ertapenem showed consistently higher response rates than the comparator in patients with infections that were more difficult to treat. Patients with nonappendiceal infection had response rates of 83.8% in the ertapenem group and 68.8% in the piperacillin/tazobactam group. Patients with generalized peritonitis had response rates of 83.3% in the ertapenem group and 73.6% in the piperacillin/tazobactam group. In addition, patients with postoperative infections had response rates of 75% in the ertapenem group and 40.9% in the piperacillin/tazobactam group.
Pseudomonas aeruginosa and Enterococcal Infections
Because of the anticipated differences of in vitro susceptibility to the two study agents for Pseudomonas aeruginosa and enterococcus, the outcomes associated with these pathogens are presented. For P. aeruginosa isolated as a baseline pathogen, susceptibility rates were lower, as expected, for ertapenem (60% susceptible in the ertapenem treatment group, n = 25 isolates tested) than for piperacillin/tazobactam (100% susceptible in the piperacillin/tazobactam group, n = 22 isolates tested). Clinical response rates for P. aeruginosa were 19/26 (73.1%) for the ertapenem group and 23/26 (88.5%) for the piperacillin/tazobactam group.
Enterococcal susceptibility rates were, as anticipated, lower for ertapenem (32% susceptible, n = 62 isolates tested) compared with piperacillin/tazobactam (97% susceptible, n = 33 isolates tested). Post hoc analyses compared the clinical response rates of patients with any enterococcal infection at baseline. The clinical response rates for enterococcus includes the patients who were given vancomycin, whereas eradication rates exclude the enterococcal isolates from patients who received vancomycin. Clinical response rates were 50/65 (76.9%) for ertapenem-treated patients and 24/37 (64.9%) for piperacillin/tazobactam-treated patients. Microbiologic eradication rates were also calculated and were 55/61 (90.2%) for ertapenem and 27/37 (73.0%) for piperacillin/tazobactam. The relatively high response rates for enterococcus were not an artifact of vancomycin use, since both clinical and microbiologic response rates for ertapenem were generally similar to piperacillin/tazobactam even when vancomycin use was excluded.
Mortality
While only one death occurred as a study end point in the primary analysis population, there were a total of 31 deaths in the study, 20 in the ertapenem group and 11 in the piperacillin/tazobactam group. Death was not recorded as the cause of failure because the first event defining failure (e.g., documented persistent or recurrent infection) was used to categorize the failure. Therefore, patients who developed recurrent infection or who had other bases for failure were scored as such, even if they subsequently died.
These deaths occurred in patients with significant comorbidity and/or severe baseline infection. The number of patients with APACHE II scores of at least 20 in the ertapenem treatment group (n = 13) was greater than in the piperacillin/tazobactam group (n = 6), and this was reflected in skewed mortality data. This maldistribution of mortality was primarily accounted for by the finding that five patients randomized to ertapenem died within 48 hours of study entry, a time frame too short for outcome to be affected by antimicrobial therapy. None of the deaths was considered drug-related by the investigator, and detailed review of the case records and narrative information of all patients who died revealed no evidence to suggest that their antimicrobial regimen contributed to their deaths.
Logistic Regression
A posthoc logistic regression was performed using combined clinical and microbiologic outcomes of the evaluable patient population (success/failure) as the dependent variable with a stepwise variable selection procedure. Three of the independent variables contributed significantly to prediction of the outcome. Postoperative infection (vs. absence of postoperative infection, odds ratio = 2.95, 95% confidence interval 1.22–7.19), patients enrolled in a U.S. site (vs. patients enrolled in non-U.S. sites, odds ratio = 3.38), and presence of generalized peritonitis (vs. absence of generalized peritonitis, odds ratio = 2.05) were significant predictors of failure (P < .05 for each of the three independent factors).
The model was reexamined without these three factors to identify variables highly correlated with the three significant ones from the first analysis. In this case, antibiotic treatment for more than 2 days before study entry was a significant predictor of failure (P = .0076). The odds ratio was 2.6, with a 95% confidence interval of 1.3 to 5.2, representing the odds of failure ascribed to antibiotic treatment for patients who had (vs. those who did not have) antibiotic treatment for more than 2 days before study entry. If the initial organ site of infection was the appendix (relative to all other organ sites), the odds of failure to antibiotic treatment were decreased to 0.5, with a 95% confidence interval of 0.3 to 0.9.
Adverse Events
The most common drug-related clinical adverse experiences were diarrhea and phlebitis/thrombophlebitis (incidence of 5.7% and 4.1% respectively in the ertapenem group and 7.5% and 3.3% in the piperacillin/tazobactam group); these were generally of mild to moderate severity. No patients discontinued treatment due to diarrhea. There were two patients in the ertapenem group in whom C. difficile-associated diarrhea was reported. Four patients in the ertapenem group discontinued therapy as a result of a drug-related adverse experience (one each rash, confusion, thrombocytopenia, and grand mal seizure) compared with six patients in the piperacillin/tazobactam group (two rash, one deep vein thrombosis, one infused vein complication, one patient with confusion and jaundice, and one patient with hypertension and seizure disorder).
The most common drug-related laboratory adverse experiences were increased liver transaminases (ALT and AST) and increased serum alkaline phosphatase. The incidences of adverse experience of ALT, AST, and alkaline phosphatase increase were 6.7%, 6.0%, and 7.0%, respectively, in the ertapenem group and 6.4%, 6.8%, and 6.4% in the piperacillin/tazobactam group. These elevations were generally mild to moderate and were transient, where follow-up information was available. No patients discontinued treatment due to serious laboratory adverse experiences.
DISCUSSION
Analysis of Outcome Results
The current study was undertaken to evaluate the safety and efficacy of a structurally unique carbapenem with activity against the gram-negative, gram-positive, and anaerobic organisms encountered in intraabdominal infections. This study documented the equivalence of ertapenem 1 g once a day and piperacillin/tazobactam 3.375 g every 6 hours in the population enrolled. The finding of equivalence in the primary analysis group, the microbiologically evaluable population, was supported by results with the MITT group and by a series of preplanned and post hoc subset analyses.
Use of Ertapenem in Patients With Severe Intraabdominal Infections
Two questions relating to efficacy must be answered in any phase III clinical trial of an agent intended for use in intraabdominal infections. The first concerns the efficacy of the agent against the polymicrobial flora commonly seen in such infections; the second is definition of appropriate patient groups to be treated with the agent.
Perforated or abscessed appendicitis is an accepted surrogate to test the in vivo antibacterial activity of broad-spectrum agents such as ertapenem and piperacillin/ tazobactam. The disease is common and provides certain advantages since a relatively young population, without substantial confounding disease and without risk of mortality, is studied. A relatively uniform pathology is also encountered in such patients, and source control is readily accomplished by standard and widely accepted operative techniques. This enhances the probability that the differences observed between groups are due to differences in antibiotic efficacy rather than source control. In this setting, important differences between antibiotic regimens have previously been identified. 2,20,21
There is concern, however, that this disease process does not accurately mimic the breadth of anatomic pathology and physiologic derangement (measured by APACHE II score) encountered in other forms of intraabdominal infection. These populations (appendiceal vs. nonappendiceal sources of infection) provide overlapping and complementary information regarding the antimicrobial efficacy of the agent in humans and the safety of the agent across various patient groups. To balance these objectives, we stratified enrollment by diagnosis and specified that 50% of patients enrolled across all centers be appendicitis cases. 22 The distributions of nonappendiceal diseases and of severity scores in this study were similar to those in recently reported trials.
The number of evaluable patients with high APACHE II scores was too small for statistical analysis. For APACHE II ranges below 15, the response rates were similar in both treatment groups. Consequently, the response rates in other subgroups were also examined for insight into the activity of ertapenem in patients with severe infection. Patients with nonappendiceal infection had response rates of 83.8% (67/80) in the ertapenem group and 68.8% (55/80) in the piperacillin/tazobactam group. Patients with generalized peritonitis, which has been associated with higher rates of failure in previous studies, had response rates of 83.3% (50/60) in the ertapenem group and 73.6% (39/53) in the piperacillin/tazobactam group. Patients enrolled with postoperative infections, accounting for 10.6% of infections, had response rates of 75% (15/20) in the ertapenem group and 41% (9/22) in the piperacillin/tazobactam group.
In this study, patients whose infections included enterococcus who were treated with ertapenem had outcomes similar, and indeed numerically superior to, those treated with piperacillin/tazobactam, regardless of susceptibility patterns. This finding is of note since enterococcus may be a marker for patients with infections that are more difficult to treat. 23 The consistency across these subset analyses supports the conclusion that ertapenem 1 g daily has broad efficacy in the treatment of complicated intraabdominal infections.
We believe the results of this study support the inclusion of approximately half the cases as appendicitis, the others as a range of nonappendiceal infections. Our data raise the issue of stratified randomization for patients with failure of prior therapy or postoperative infections, since these patients provide a small but important group with a high risk of failure. Stratification on this parameter would ensure even distribution between treatment arms.
The per pathogen clinical response data presented for enterococcus and P. aeruginosa suggest that specific empiric treatment for enterococcus as part of a polymicrobial nonbacteremic infection is not necessary. The allowed but infrequent use of vancomycin did not obscure failures of study therapy, since the use of vancomycin was associated with lower response rates in this study. Although P. aeruginosa is infrequent in community-acquired intraabdominal infection, specific antipseudomonal therapy may be considered when it is identified in polymicrobial infection.
Adequacy of the Initial Intervention
The key determinant of outcome is the procedure performed to deal with the anatomic source and consequence of infection. The definition of an adequate operative procedure is generally agreed on and involves drainage of all fluid collections, closure, resection or drainage of any openings into the gastrointestinal tract, and resection of inflamed tissue. The latter aspect of operative management is the most controversial, and recommendations have ranged from complete peritoneal debridement to attention only to the source of infection. The extent of such inflammation is an important factor limiting the efficacy of percutaneous drainage. 24,25
The primary concern in the context of clinical trials of antiinfectives is that the adequacy of intervention is an independent variable determining outcome. Particularly with the likely small number of patients not receiving adequate source control, there is the very real possibility that such patients would not be evenly distributed by randomization and would therefore skew results.
In the absence of clear rules for intervention, we chose to review the procedures performed by use of an expert panel. This process was defined prospectively and conducted in a blinded manner. The expert review panel ultimately identified 22 patients as having had an inadequate intervention, and 5 occult failures. It is notable that a relatively small proportion of cases were considered to have inadequate source control. Had these patients remained evaluable in the primary analysis, the difference in observed response rates would have been greater (6.8%). While it is likely that the conclusion of this study would have been the same without the benefit of the expert panel review process, the conduct of the review provided important assurance regarding the adequacy of the initial intervention in the heterogenous patient population studied in this large multicenter trial.
CONCLUSIONS
In this study, the efficacy of ertapenem 1 g once a day was statistically equivalent to piperacillin/tazobactam 3.75 g every 6 hours. Prospectively planned subset analyses demonstrated consistent activity of ertapenem in patients with postoperative infections, in patients with nonappendiceal infections, and in patients with generalized peritonitis. Ertapenem was generally well tolerated and had a similar safety and tolerability profile to the comparator agent in this study, piperacillin/tazobactam. The clinical utility of ertapenem will be in providing broad-spectrum coverage for community-acquired infections with once-a-day dosing. The results of this trial suggest that ertapenem may be a useful option that could eliminate the need for combination and/or multidosed antibiotic regimens for the empiric treatment of intraabdominal infections.
017 Study Group
Michael Aoun, MD, Brussels, Belgium
Jose Balibrea, MD, Madrid, Spain
Paul Bankey, MD, Worcester, MA, USA
Eduardo Barboza, MD, Lima, Peru
Philip Barie, MD, New York, NY, USA
J. Eric Bauwens, MD, Phoenix, AZ, USA
Robert Bennion, MD, Sylmar, CA, USA
Julian Betancurt, MD, Calle, Colombia
Markus Buechler, MD, Berne, Switzerland
John Bohnen, MD, Toronto, Canada
Jacqueline Brown, MD, Soweto, South Africa
Nicholas Christou, MD, Montreal, Canada
Larry Diebel, MD, Detroit, MI, USA
Prof. Ippolito Donini, Ferrara, Italy
Prof. Roelof Du Toit, Bloemfontein, South Africa
Alvaro Fernandez, MD, Guatemala City, Guatemala
Jurandir Filho Ribas, MD, Curitiba-Parana, Brazil
Donald Fry, MD, Albuquerque, NM, USA
Saul Fuenzalida-Gonzalez, MD, Mexico City, Mexico
Gerard Fulda, MD, Newark, DE, USA
David Gilbert, MD, Portland, OR, USA
Javier Gonzalez, MD, Barcelona, Spain
Paul Harrison, MD, Wichita, KS, USA
James Hassett, MD, Buffalo, NY, USA
Abel Jasovich, MD, Febrero, Argentina
Patricio Lorente, MD, Madrid, Spain
Jochen Lange, MD, St. Gallen, Switzerland
Luz Letelier, MD, Santiago, Chile
Jorge Garbino, MD, Geneva, Switzerland
Christopher Lucasti, DO, Somerset, NJ, USA
Prof. B. Lunstedt, Frankfurt, Germany
Stanley Klein, MD, Torrance, CA, USA
Eugene Mangiante, MD, Memphis, TN, USA
Michael Metzler, MD, Columbia, MO, USA
William Miles, MD, Charlotte, NC, USA
Prof. Yves Ozier, Paris, France
Michel Poisson, MD, Montreal, Canada
Russell Postier, MD, Oklahoma City, OK, USA
Ori Rotstein, MD, Toronto, Canada
Prof. Francesco Salvestrini, Siena, Italy
Leonid Stratchounski, MD, Smolensk, Russia
Jose Maria Tellado, MD, Madrid, Spain
Prof. Mario Trignano, Sassari, Italy
H. Hank Simms, MD, Providence, RI, USA
Joseph Solomkin, MD, Cincinnati, OH, USA
Bernardo Vainrub, MD, Caracas, Venezuela
Juan Velasquez, MD, Bogota, Colombia
Prof. Brian Warren, Parow, South Africa
Eric Wilson, MD, Orange, CA, USA
Dietmar Wittmann, MD, Milwaukee, WI, USA
Albert Yellin, MD, Los Angeles, CA, USA
References
- 1.Berne TV, Yellin AW, Appleman MD, et al. Antibiotic management of surgically treated gangrenous or perforated appendicitis. Comparison of gentamicin and clindamycin versus cefamandole versus cefoperazone. Am J Surg 1982; 144: 8–13. [DOI] [PubMed] [Google Scholar]
- 2.Yellin AE, Heseltine PN, Berne TV, et al. The role of Pseudomonas species in patients treated with ampicillin and Sulbactam for gangrenous and perforated appendicitis. Surg Gynecol Obstet 1985; 161: 303–307. [PubMed] [Google Scholar]
- 3.Solomkin JS, Dellinger EP, Christou NV, et al. Results of a multicenter trial comparing imipenem/cilastatin to tobramycin/clindamycin for intra-abdominal infections. Ann Surg 1990; 212: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosdell DM, Morris DM, Voltura A, et al. Antibiotic treatment for surgical peritonitis. Ann Surg 1991; 214: 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorber B, Swenson RM. The bacteriology of intra-abdominal infections. Surg Clin North Am 1975; 55: 1349–1354. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett JG, Onderdonk AB, Louie T, et al. A review. Lessons from an animal model of intra-abdominal sepsis. Arch Surg 1978; 113: 853–857. [DOI] [PubMed] [Google Scholar]
- 7.Nichols RL, Muzik AC. Enterococcal infections in surgical patients: the mystery continues. Clin Infect Dis 1992; 15: 72–76. [DOI] [PubMed] [Google Scholar]
- 8.Onderdonk AB, Bartlett JG, Louie T, et al. Microbial synergy in experimental intra-abdominal abscess. Infect Immun 1976; 13: 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill CJ, Jackson JJ, Gerckens LS, et al. In vivo activity and pharmacokinetic evaluation of a novel long-acting carbapenem antibiotic, MK-826 (L-749,345). Antimicrob Agents Chemother 1998; 42: 1996–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundelof JG, Hajdu R, Gill CJ, et al. Pharmacokinetics of L-749,345, a long-acting carbapenem antibiotic, in primates. Antimicrob Agents Chemother 1997; 41: 1743–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein EJ, Citron DM, Vreni MC, et al. Comparative in vitro activities of ertapenem (MK-0826) against 1,001 anaerobes isolated from human intra-abdominal infections. Antimicrob Agents Chemother 2000; 44: 2389–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs PC, Barry AL, Brown SD. In vitro activities of ertapenem (MK-0826) against clinical bacterial isolates from 11 North American medical centers. Antimicrob Agents Chemother 2001; 45: 1915–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livermore DM, Carter MW, Bagel S, et al. In vitro activities of ertapenem (MK-0826) against recent clinical bacteria collected in Europe and Australia. Antimicrob Agents Chemother 2001; 45: 1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson SE, Nord CE. Clinical trials of extended spectrum penicillin/beta-lactamase inhibitors in the treatment of intra-abdominal infections. European and North American experience. Am J Surg 1995; 169: 21S–26S. [PubMed] [Google Scholar]
- 15.Bohnen JM, Marshall JC, Fry DE, et al. Clinical and scientific importance of source control in abdominal infections: summary of a symposium. Can J Surg 1999; 42: 122–126. [PMC free article] [PubMed] [Google Scholar]
- 16.Wacha H, Hau T, Dittmer R, et al. Risk factors associated with intraabdominal infections: a prospective multicenter study. Peritonitis Study Group. Langenbecks Arch Surg 1999; 384: 24–32. [DOI] [PubMed] [Google Scholar]
- 17.Beam TR Jr, Gilbert DN, Kunin CM. General guidelines for the clinical evaluation of anti-infective drug products. Infectious Diseases Society of America and the Food and Drug Administration. Clin Infect Dis 1992; 15 Suppl 1: S5–32. [DOI] [PubMed] [Google Scholar]
- 18.Solomkin JS, Hemsell DL, Sweet R, et al. Evaluation of new anti-infective drugs for the treatment of intraabdominal infections. Infectious Diseases Society of America and the Food and Drug Administration. Clin Infect Dis 1992; 15 Suppl 1: S33–42. [DOI] [PubMed] [Google Scholar]
- 19.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 20.Heseltine PNR, Yellin AE, Appleman MD, et al. Perforated and gangrenous appendicitis: an analysis of antibiotic failures. J Infect Dis 1983; 148: 322–329. [DOI] [PubMed] [Google Scholar]
- 21.Gill MA, Cheetham TC, Chenella FC, et al. Matched case-control study of adjusted versus nonadjusted gentamicin dosing in perforated and gangrenous appendicitis. Ther Drug Monit 1986; 8: 451–456. [DOI] [PubMed] [Google Scholar]
- 22.Solomkin JS, Wilson SE, Christou NV, et al. Results of a clinical trial of clinafloxacin versus imipenem/cilastatin for intraabdominal infections. Ann Surg 2001; 233: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burnett RJ, Haverstock DC, Dellinger EP, et al. Definition of the role of enterococcus in intraabdominal infection: analysis of a prospective randomized trial. Surgery 1995; 118: 716–721. [DOI] [PubMed] [Google Scholar]
- 24.Jaques P, Mauro M, Safrit H, et al. CT features of intraabdominal abscesses: prediction of successful percutaneous drainage. AJR Am J Roentgenol 1986; 146: 1041–1045. [DOI] [PubMed] [Google Scholar]
- 25.Deveney CW, Lurie K, Deveney KE. Improved treatment of intra-abdominal abscess. A result of improved localization, drainage, and patient care, not technique. Arch Surg 1988; 123: 1126–1130. [DOI] [PubMed] [Google Scholar]
Footnotes
Supported by Merck & Co., Inc., West Point, Pennsylvania.
Correspondence: Joseph S. Solomkin, MD, Department of Surgery, University of Cincinnati College of Medicine, 231 Albert B. Sabin Way, Cincinnati, OH 45267-0558.
E-mail: joseph.solomkin@uc.edu
Accepted for publication May 10, 2002.