Abstract
Objective
To investigate the degree and mechanism of hepatic sinusoidal injury in different graft sizes in right lobe live donor liver transplantation (LDLT).
Summary Background Data
Liver grafts from living donors are likely to be small-for-size for adult recipients. Graft injury after reperfusion is common, but the mechanism and degree of injury remain unclear. The hepatic sinusoidal injury in different graft sizes and its relationship with portal hemodynamics and intragraft gene response at the early phase after reperfusion have not been studied in right lobe LDLT.
Methods
From May 2000 to November 2001, 40 adults receiving right lobe LDLT had portal pressure measured continuously before and after reperfusion. Liver biopsies were taken before and after reperfusion for detection of vasoregulatory genes (endothelin-1 and endothelial nitric oxide synthase) and heat shock genes (heat shock protein 70 and heme oxygenase-1), and electron microscope examination. Blood samples from the portal vein and suprahepatic inferior vena cava were taken for the measurement of plasma nitric oxide level.
Results
The recipients were grouped according to the ratio of graft weight to estimated standard liver weight: group 1 (n = 10), less than 40%; group 2 (n = 21), 40% to 60%; and group 3 (n = 9), more than 60%. The portal pressures recorded after reperfusion in group 1 were significantly higher within 30 minutes of reperfusion than those in groups 2 and 3. After reperfusion, the intragraft endothelin-1 mRNA level in group 1 increased by 161% of the basal level but decreased by 31.5% and 62% of the basal level in groups 2 and 3, respectively. The intragraft mRNA level of heme oxygenase-1 in groups 1 and 2 decreased by 75.5% and 25.3% of the basal level respectively but increased by 41% of basal level in group 3. The intragraft protein level of heat shock protein 70 decreased by 50 ng/mL after reperfusion in group 1 but increased by 12.4 ng/mL and 0.6 ng/mL in groups 2 and 3, respectively. The portal vein plasma nitric oxide level decreased more significantly after reperfusion in group 1 than in group 2. Electron microscope examination of liver biopsies in group 1 showed tremendous mitochondrial swelling as well as irregular large gaps between the sinusoidal lining cells. There were two hospital deaths in group 1 and none in the other two groups.
Conclusions
Patients implanted with grafts less than 40% of standard liver weight suffered from transient portal hypertension early after reperfusion. The phenomenon was accompanied by intragraft upregulation of endothelin-1 and ultrastructural evidence of sinusoidal damage. The transient portal hypertension after reperfusion, subsequent endothelin-1 overexpression, and plasma nitric oxide level reduction, together with downregulation of heme oxygenase-1 and heat shock protein 70, may account for the small-for-size graft injury.
The major limitation of live donor liver transplantation (LDLT) is the graft size. Grafts from the live donors are likely to be small-for-size for the recipients, especially when the recipient is larger than the donor. Small-for-size graft injuries have been frequently observed, 1–3 but the exact mechanism remains unclear. The majority of previous studies have focused on the hepatic functional analysis and light microscopy, 4,5 but no study has investigated the portal hemodynamics and intragraft gene response at the acute phase after reperfusion in relation to graft size. Although our previous animal study showed that damage of hepatic sinusoids resulted from excessive blood flow and transient portal hypertension at the early phase after reperfusion, 6 the conclusion could not be extrapolated to humans. This is because in the rat model, preexisting portal hypertension is absent, while in the clinical situation, cirrhosis and portal hypertension are usually severe. To clarify the relationship of sinusoidal injury and graft size, we investigated the portal hemodynamics at the early phase after reperfusion together with intragraft gene detection and electron microscope examination in patients receiving right lobe LDLT.
METHODS
From May 2000 to November 2001, 40 adults underwent right lobe LDLT at Queen Mary Hospital, the University of Hong Kong. The donor and recipient operations were performed according to the technique described previously. 7,8 The middle hepatic vein was included in the graft in all cases. Venovenous bypass was not used. The inferior vena cava was cross-clamped during implantation of the graft. Before total hepatectomy of the diseased liver in the recipients, the inferior mesenteric vein was cannulated by a catheter with the tip placed in the portal vein. This catheter was connected to a transducer and the Quad Bridge Amp (ML118 Quad Bridge Amp, PowerLab System, AD Instruments Pty Ltd., Australia) and a multichannel data-recording unit (ML500 PowerLab/800, PowerLab System, AD Instruments) for continuous pressure monitoring and recording. The recipient’s portal pressure was monitored before the total hepatectomy of the diseased liver until 2 hours after reperfusion. The central venous pressure and pulmonary artery pressure were also monitored.
The body weight and body height of the recipients were used to calculate the body surface area according to the DuBois formula. 9 The standard liver weight of the recipient was determined according to the Urata equation. 10
The graft weight ratio (GWR) was the weight of the liver graft divided by the estimated standard liver weight of the recipient.
The study protocol was approved by the Ethics Committee of the University of Hong Kong. Informed consents were obtained from every patient before the operation.
Intragraft Gene Detection by Real-Time Quantitative RT-PCR
Liver biopsies were taken before liver harvesting from the donors and 2.5 hours after reperfusion from the recipients, and immersed in liquid nitrogen immediately. The samples were stored at −80°C until total RNA extraction. The total RNA was extracted using the Rneasy Midi Kit (QIAGEN Company GmbH, Germany) and the quality was examined by electrophoresis using 1% agarose gel. The quantity of the total RNA was detected by the spectrophotometer (DU-65, Beckham, Germany). About 1 μg total RNA from each sample was used to perform reverse transcription (RT) reaction. Taqman Reverse Transcription Reagents (PE Applied Biosystem, Foster City, CA) were used according to the manufacturer’s instruction (25°C × 10 minutes, 48°C × 30 minutes, 95°C × 5 minutes). RT product (1 μL) was used to perform real-time quantitative polymerase chain reaction (PCR) with a reaction volume of 50 μL (TaqMan PCR Core Reagent Kit, PE Applied Biosystem) by the ABI PRISM 7700 Sequence Detection System (PE Applied Biosystem). The probes and primers of endothelin-1 (ET-1), endothelial nitric oxide synthase (eNOS), and heme-oxygenase-1 (HO-1) were designed under the Primer Express software according to the criteria for real-time PCR (PE Applied Biosystem) (Table 1). The Taqman Ribosomal RNA Control Reagent (18S RNA probe [VIC] and primer pair, PE Applied Biosystem) was used for internal control in the same PCR plate well to normalize the target gene amplification copies. The PCR protocol was in accord with the manufacturer’s recommendations (50°C × 2 minutes, 95°C × 10 minutes, [95°C × 15 seconds, 60°C × 1 minute] × 50 cycles). All the samples were detected in triplicate, and the readings from each sample and its internal control were used to calculate the gene expression level. After normalization with the internal control, the gene expression levels after hepatectomy were calculated as the percentage of the basal levels before hepatectomy.
Table 1. PROBES AND PRIMER PAIRS FOR REAL-TIME QUANTITATIVE RT-PCR
Intragraft Expression of Heat Shock Protein 70 by Enzyme Immunoassay
The whole-cell protein of the liver biopsies was isolated from the liver tissues as described previously. 11 The protein expression of heat shock protein 70 (Hsp-70) was detected using Hsp-70 EIA kit (StressGen Biotechnologies Corp., Canada).
Intracellular Expression of ET-1, eNOS, and iNOS by Immunostaining
The paraffin sections of the liver biopsies were immunochemically stained for inducible nitric oxide synthase (iNOS), eNOS, and ET-1, using the Dako EnVision system (Dako, Glostrup, Denmark). In brief, after deparaffinization, endogenous peroxidase activity was quenched by immersing the sections for 30 minutes in absolute methanol containing 0.3% H2O2. The sections were processed to unmask the antigens by conventional microwave oven heating in 10 mol/L citric acid buffer (pH 6.0) for 12 minutes. The sections were then treated with 30% normal goat serum for 30 minutes to reduce the background staining, followed by treatment of primary monoclonal antibody iNOS, eNOS (Transduction Laboratories, Lexington, KY), and ET-1 (Oncogene Research Products, Darmstadt, Germany) at 4°C overnight. After washing, the sections were incubated with EnVision for 30 minutes at room temperate and then visualized with chromogenic substrate solution for 2 minutes. The slides were examined under a light microscope.
Measurement of NO in Plasma by Chemiluminescence
Three milliliters of blood was taken from the portal vein and suprahepatic inferior vena cava before the diseased livers were removed and 2.5 hours after reperfusion in the recipients. The plasma was collected from each sample by centrifuge at 2,500 g for 10 minutes at 4° and stored at −70°C until detection. Thawed samples (100 μL) were diluted fourfold with deionized water and deproteinated by zinc sulfate. They were centrifuged at 10,000 g for 5 minutes at room temperature and 5 μL supernatant was injected into a chemiluminescence machine (Sievers 280 NO Analyzer, Sievers Instruments, Inc., Boulder, CO) as described previously. 12 Since NO2−/NO3−, in particular nitrate, are end stable products of nitric oxide, this method gives a very accurate representation of NO in the blood.
Electron Microscope Examination
The liver biopsies were taken before graft harvesting from the donors and 2.5 hours after reperfusion from the recipients. The liver tissue was immediately cut into cubes of less than 1 mm and fixed in 2.5% glutaraldehyde in cacodylate buffer (0.1 mol/L sodium cacodylate-HCl buffer, pH 7.4) overnight at 4°C for electron microscope section. The sections were examined under a transmission electron microscope (Philips EM208S, Eindhoven, Holland).
Statistical Analysis
Continuous variables were expressed as medians and ranges. The Mann-Whitney test was used for statistical comparison of continuous variables. The chi-square test was used to compare discrete variables. Significance was defined as P < .05. Calculations were made using the SPSS computer software (SPSS Inc., Chicago, IL).
RESULTS
Clinical Data
The range of GWR was 32.3% to 81.8%. The recipients were grouped according to the GWR: group 1 (n = 10), less than 40%; group 2 (n = 21), 40% to 60%; and group 3 (n = 9), more than 60%. The grouping was made in this way because we demonstrated previously that grafts less than 40% GWR had a worse outcome, 13 while grafts more than 60% GWR were similar to full-size cadaveric liver grafts. Cadaveric liver grafts were not included in this study because the duration of cold ischemia was distinctly different.
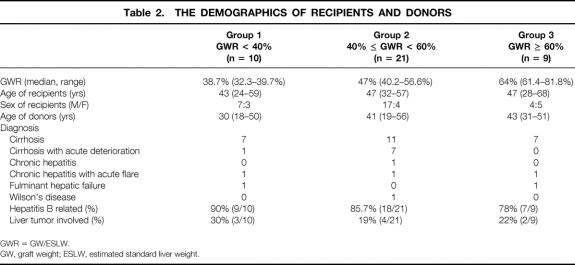
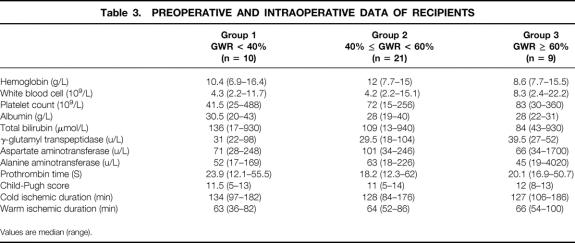
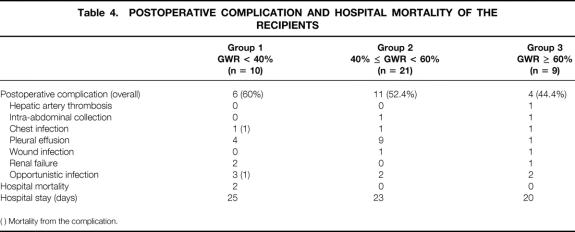
There was no significant difference in the demographic data, preoperative hematology, renal and liver biochemistry, Child-Pugh score, duration of cold and warm ischemia of theliver grafts, postoperative complications, and duration of hospital stay among the three groups of patients (Tables 2–4).
Table 2. THE DEMOGRAPHICS OF RECIPIENTS AND DONORS
GWR = GW/ESLW.
GW, graft weight; ESLW, estimated standard liver weight.
Table 3. PREOPERATIVE AND INTRAOPERATIVE DATA OF RECIPIENTS
Values are median (range).
Table 4. POSTOPERATIVE COMPLICATION AND HOSPITAL MORTALITY OF THE RECIPIENTS
( ) Mortality from the complication.
There were two hospital deaths in group 1 (P = .058 vs. groups 2 and 3). The causes of hospital deaths were systemic fungal infection and Legionnaires’ disease.
Although there was no statistical difference in the postoperative liver biochemistry and hematology among the three groups, group 1 had a higher total bilirubin level during the first postoperative week (Fig. 1).
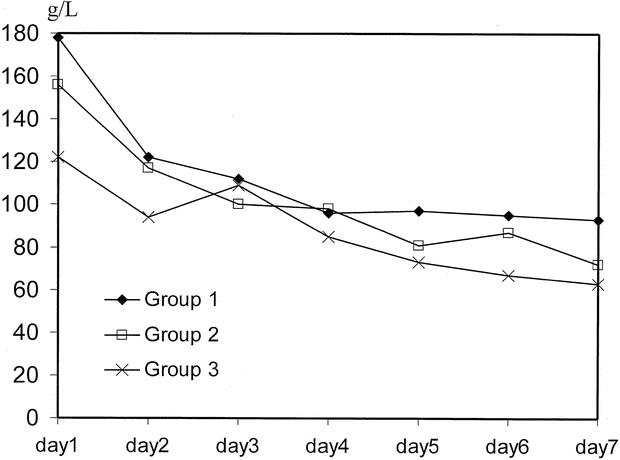
Figure 1. Time course of total bilirubin. Day 1, postoperative day 1.
Hemodynamics
The time course of portal pressure before and after reperfusion in the recipients is shown in Figure 2. There was no significant difference in the portal pressure before the removal of the diseased liver and after portal vein clamping among the three groups. Since more than 85% of the recipients suffered from liver cirrhosis, the basal level of portal pressure was high (30–35 cm H2O). The portal pressure increased to 60 to 70 cm H2O after portal vein clamping and decreased precipitously immediately after reperfusion of the graft. The portal pressures recorded after reperfusion in group 1 were significantly higher at three time points than those in group 2: immediately after reperfusion (34 vs. 25 cm H2O, P = .002), 5 minutes (28 vs. 25 cm H2O, P = .012), and 30 minutes (34 vs. 25 cm H2O, P = .004). The portal pressures during the first 30 minutes after reperfusion in group 1 were significantly higher than those in group 3: immediately after reperfusion (34 vs. 26.5 cm H2O, P = .004), 10 minutes (27 vs. 22.5 cm H2O, P = .031), 15 minutes (27 vs. 22 cm H2O, P = .019), 20 minutes (28 vs. 22 cm H2O, P = .024), and 30 minutes (29 vs. 21 cmH2O, P = .014). There was no statistical difference in the portal pressure between groups 2 and 3.
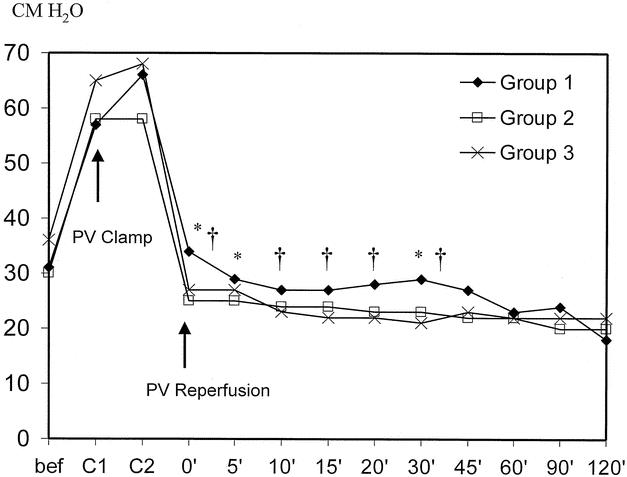
Figure 2. Time course of portal pressure. *P < .05, group 1 vs. group 2; †P < .05, group 1 vs. group 3.
The time course of the mean arterial pressure of the recipients is shown in Figure 3. Patients in group 1 had a lower mean arterial pressure at 5 minutes (79 vs. 90 mm Hg, P = .006), 15 minutes (69 vs. 75 mm Hg, P = .034), and 20 minutes (68 vs. 77 mm Hg, P = .009) after reperfusion compared to group 3 patients. At 20 minutes after reperfusion, the mean arterial pressure of the patients in group 2 was lower than that of the patients in group 3 (75 vs. 86 mm Hg, P = .03; 69 vs. 77 mm Hg, P = .045).
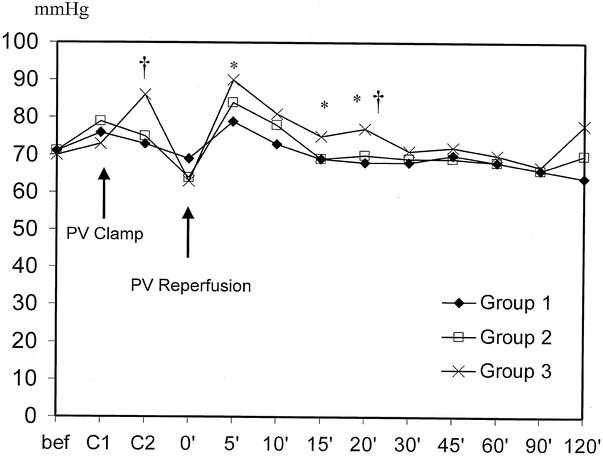
Figure 3. Time course of mean arterial pressure. *P < .05, group 1 vs. group 3; †P < .05, group 2 vs. group 3.
There was no significant difference in pulmonary arterial pressure and central venous pressure among the three groups before and after reperfusion.
Intragraft Expression of ET-1, eNOS, HO-1, and Hsp-70
The intragraft mRNA levels of ET-1 in group 1 increased by 161% of the basal level and were significantly higher than those in groups 2 and 3 (Fig. 4). The intragraft mRNA levels of eNOS increased in all three groups. Group 3 patients had a higher expression of eNOS, but there was no significant difference among the three groups. As for the intragraft expression of HO-1, the mRNA level of patients in group 1 decreased by 75.5% of the basal level and was significantly lower than that of patients in groups 2 and 3. The changes of intragraft protein levels of Hsp-70 were also different among the three groups (Fig. 5). The protein levels of Hsp-70 decreased by 50 ng/mL after reperfusion in group 1, while they increased by 12.4 ng/mL in group 2 (P = .04).
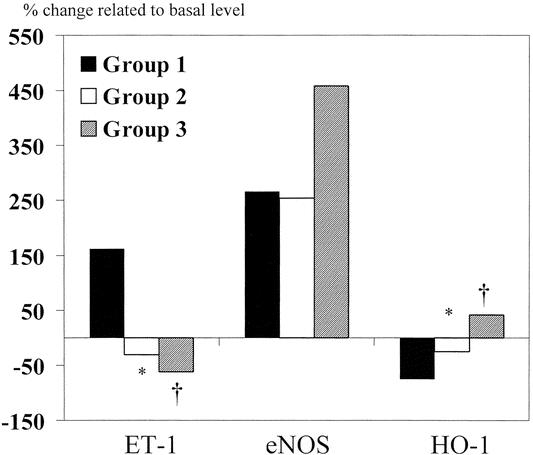
Figure 4. Intragraft mRNA levels of ET-1, eNOS, and HO-1 after reperfusion. *P < .05, group 1 vs. group 2; †P < .05, group 1 vs. group 3.
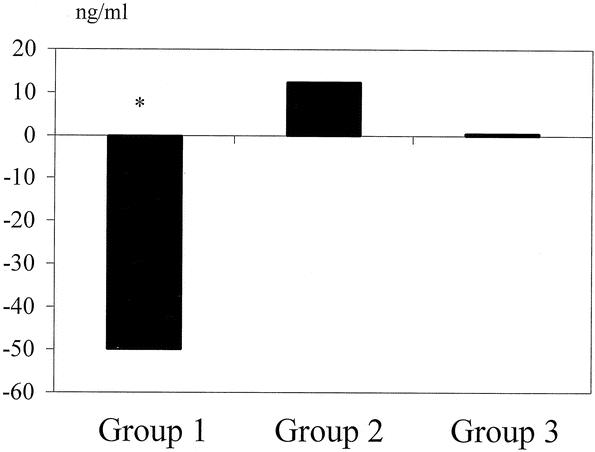
Figure 5. Intragraft protein levels of Hsp-70. *P < .05, group 1 vs. group 2.
Intracellular Expression of ET-1, eNOS, and iNOS
ET-1 was primarily expressed in hepatocytes. Consistent with the intragraft mRNA levels, group 1 had a relatively higher expression of ET-1 after reperfusion (Fig. 6). eNOS protein was expressed mainly in hepatic sinusoids and overexpressed in groups 2 and 3 (Fig. 7). Group 1 had a relatively higher expression of iNOS after reperfusion, accompanied by slight dilation of hepatic sinusoids (Fig. 8).
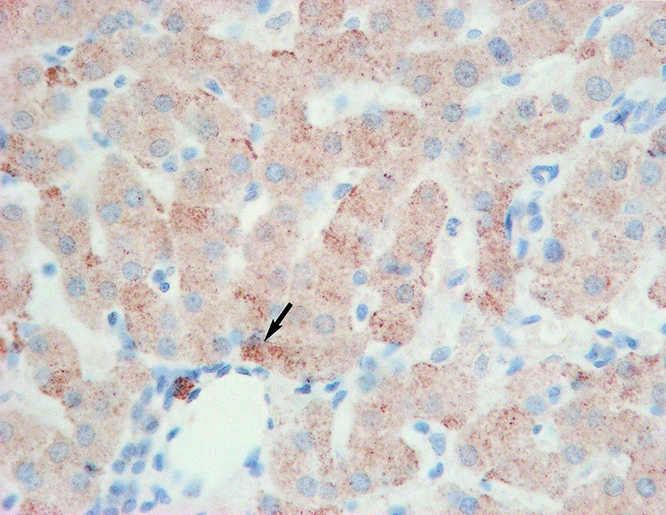
Figure 6. Intracellular ET-1 expression in group 1 patients (arrow) (×400).
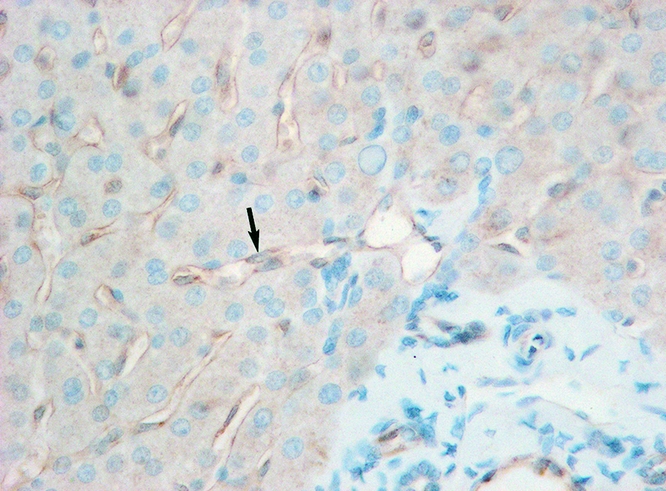
Figure 7. Intracellular eNOs expression in group 3 patients (arrow) (×400).
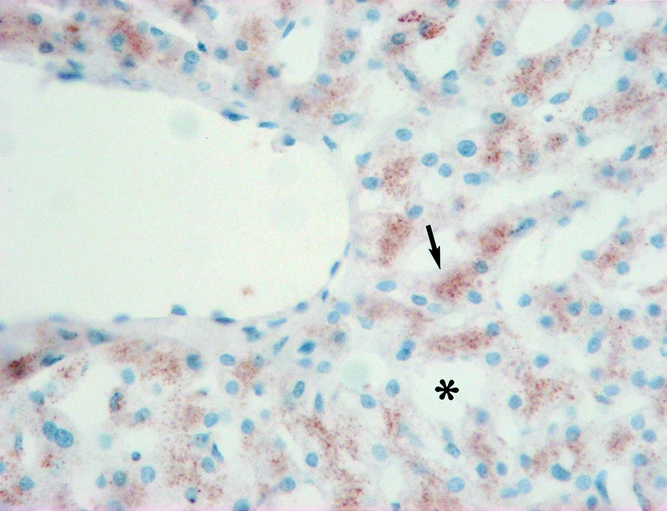
Figure 8. Intracellular iNOS expression in group 1 patients (arrow). Dilation of sinusoids was found (*) (×400).
Plasma Level of NO
In group 1, the plasma levels of NO from the portal vein blood samples decreased by 17.4 μmol/L after reperfusion compared to the levels before portal vein clamping. As for groups 2 and 3, the portal vein NO levels decreased by 2.3 μmol/L (P = .02 vs. group 1) and 4.6 μmol/L, respectively. The NO level of the blood drawn from the suprahepatic inferior vena cava decreased by 7.6 μmol/L, 3.3 μmol/L, and 1.8 μmol/L in groups 1, 2, and 3, respectively (P = NS).
Electron Microscopy
Hepatocytes and sinusoidal cells had a normal appearance under transmission electron microscopy in the donor biopsies. Chromatin in the nucleus appeared normal. Mitochondria were elliptical, with well-visualized cristae. The endoplasmic reticulum was intact, and there were numerous microvilli in the space of Disse. The sinusoidal lining cells were intact.
In group 1, tremendous mitochondrial swelling was found in the hepatocytes in 80% of the patients (Fig. 9). Collapse of the space of Disse and an irregular large gap between the sinusoidal lining cells also occurred in group 1.
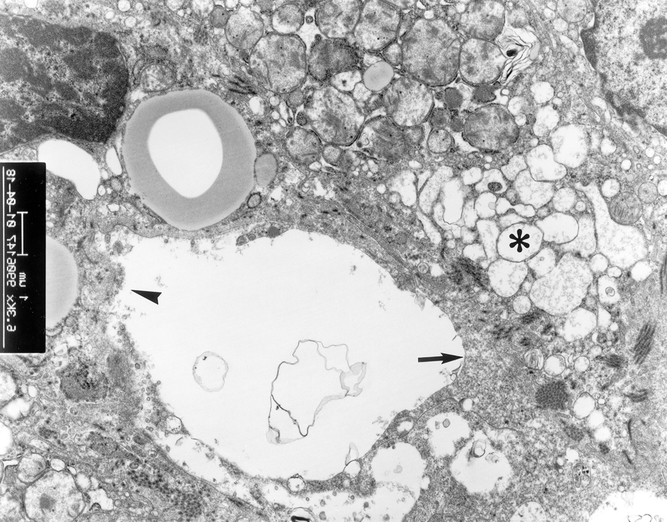
Figure 9. Electron microscopy examination of liver grafts in patients in group 1. Extensive swelling of hepatocyte mitochondria (*) and collapse of space of Disse (arrow) were found. Irregular large gap of sinusoidal lining cells was present (arrowhead) (×6,300).
In contrast, although slight mitochondrial swelling was found in three patients (14%) in group 2, the sinusoidal lining cells were intact (Fig. 10). All patients in group 3 had normal ultrastructural architecture (Fig. 11).
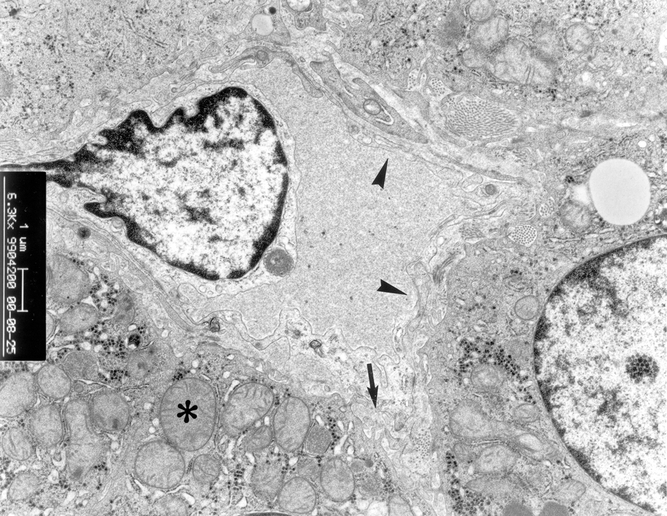
Figure 10. Electron microscopy examination of liver graft in patients in group 2. Slight mitochondrial swelling in hepatocyte was found (*). There were microvilli in the space of Disse (arrow). The sinusoidal lining cells were intact (arrowhead) (×6,300).
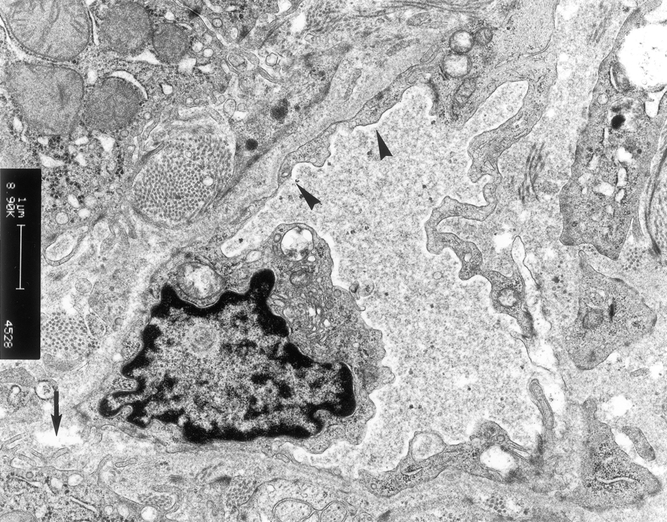
Figure 11. Electron microscopy examination of liver graft in patients in group 3. There were microvilli in the space of Disse (arrow). The sinusoidal lining cells were intact (arrowhead) (×6,300).
DISCUSSION
In the present study, transient portal hypertension was found in the patients implanted with a graft less than 40% standard liver weight during the first 30 minutes after reperfusion. It was accompanied by a relatively low mean arterial pressure. The portal hypertension resulting from excessive portal blood flow into a small-for-size graft was probably the major cause of the mechanical injury to the hepatic sinusoids. 6,14,15 In addition, the imbalance of intragraft vasoregulatory gene expression shown in this study probably plays an important role in sinusoidal damage. The maintenance of hepatic microcirculation depends mainly on the balance of vasoconstriction and vasodilation. ET-1 is a potent constrictor of the vascular smooth muscle 16 and modulates intrahepatic resistance by directly constricting the hepatic sinusoid. 17 ET-1 has been identified in the endothelial cells of the hepatic sinusoids as well as in preterminal portal venules. 16 Increased production of ET-1 and its increased response to ischemia/reperfusion are thought to be involved in the pathogenesis of ischemia/reperfusion liver injury. 18–20 On the other hand, eNOS plays an important role in the maintenance of vasodilation through the production of endogenous NO. 21,22 The endogenous NO from eNOS regulates the blood flow under physiologic conditions. In genetically engineered models, eNOS knockout mice will develop the phenotype vulnerable to increased ischemic injury and inflammatory injury. 23 In the present study, the lower expression of eNOS and less production of plasma NO in group 1 accelerated the imbalance of sinusoidal constriction and relaxation. In addition, the relatively lower expression of the two intracellular stress genes, Hsp-70 and HO-1, also plays an important role in the graft injury of group 1. HO-1 is a protective gene in the vascular response to injury by reducing vasoconstriction. 24,25 Both Hsp-70 and HO-1 are responsible for tissue repair and intracellular homeostasis. 26,27
The homeostasis of hepatic microcirculatory environment is crucial for the early recovery of graft function after reperfusion. 28 The liver sinusoids play an important role in hepatic microcirculation, especially at the early phase after reperfusion. Due to a combination of a higher portal pressure and sinusoidal constriction induced by an imbalance of vasoregulatory genes at the acute phase after reperfusion, the hepatic sinusoidal lining cells are disrupted. The injured hepatic sinusoids thus impair recovery of hepatic synthetic function, which is reflected by the lower expression of heat shock proteins.
The understanding of the mechanism of small-for-size graft injury not only is helpful to develop novel therapeutic strategies to reduce small-for-size graft damage to the recipient, but also prevents small-for-size syndrome of living donors after a right lobe liver donation. To avoid the small-for-size graft injury, several methods such as splenic artery ligation 29 and portocaval shunting 30 have been applied to attenuate the portal hypertension after reperfusion. Gradual controlled release of the portal vein clamp at the time of reperfusion may be useful in ameliorating the adverse effect of a large volume of blood flow into the liver, but the actual efficacy is not yet known.
Several drugs are probably beneficial for the maintenance of hepatic microcirculation. FK409, an NO donor, has been shown to attenuate ischemia/reperfusion injury in a rat heart transplantation model. 31 The somatostatin 32 and ET-1 antagonist 33 might be other solutions to attenuate transient portal hypertension after liver transplantation. These strategies may be essential in extending the donor pool in right lobe LDLT. However, all the aforementioned strategies are unlikely to be successful unless the venous drainage of the right lobe graft is uniform and sufficient. In this study, all patients received a right lobe graft that contained the right and middle hepatic veins. Thus, a satisfactory result was obtained. Even among patients receiving a graft that was less than 40% GWR, the survival rate has reached 80%. In other series, the middle hepatic vein was not included in the graft. In the absence of adequate and uniform venous drainage, a larger graft is probably needed for survival.
CONCLUSIONS
Patients implanted with a graft less than 40% of the standard liver weight suffered transient portal hypertension early after reperfusion, which was accompanied by intragraft upregulation of ET-1 and ultrastructural evidence of endothelial injury. Transient portal hypertension after reperfusion with subsequent ET-1 overexpression and NO level reduction (leading to sinusoidal constriction), together with downregulation of HO-1 and Hsp-70, may lead to the small-for-size graft injury.
Acknowledgments
The authors thank Mr. Bosco Yau, Ms. Amy Wong, and Mr. W.S. Lee for their assistance in the work of electron microscopy in the Electron Microscope Unit, the University of Hong Kong.
Footnotes
Supported by CRCG grant and Distinguished Research Achievement Award of the University of Hong Kong.
Correspondence: Sheung-Tat Fan, MS, MD, PhD, FRCS (Edin & Glasg), FACS, Department of Surgery, Center for the Study of Liver Disease, University of Hong Kong Medical Center, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong.
E-mail: hrmsfst@hkucc.hku.hk
Accepted for publication May 15, 2002.
References
- 1.Kiuchi T, Kasahara M, Uryuhara K, et al. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation 1999; 67: 321–327. [DOI] [PubMed] [Google Scholar]
- 2.Ku Y, Fukumoto T, Nishida T, et al. Evidence that portal vein decompression improves survival of canine quarter orthotopic liver transplantation. Transplantation 1995; 59: 1388–1392. [DOI] [PubMed] [Google Scholar]
- 3.Xu HS, Pruett TL, Jones RS. Study of donor-recipient liver size match for transplantation. Ann Surg 1994; 219: 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emond JC, Renz JF, Ferrell LD, et al. Functional analysis of grafts from living donors. Implications for the treatment of older recipients. Ann Surg 1996; 224: 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugawara Y, Makuuchi M, Takayama T, et al. Small-for-size grafts in living-related liver transplantation. J Am Coll Surg 2001; 192: 510–513. [DOI] [PubMed] [Google Scholar]
- 6.Man K, Lo CM, Ng IO, et al. Liver transplantation in rats using small-for-size grafts: a study of hemodynamic and morphological changes. Arch Surg 2001; 136: 280–285. [DOI] [PubMed] [Google Scholar]
- 7.Lo CM, Fan ST, Liu CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg 1997; 226: 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan ST, Lo CM, Liu CL. Technical refinement in adult-to-adult living donor liver transplantation using right lobe graft. Ann Surg 2000; 231: 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989; 5: 303–311. [PubMed] [Google Scholar]
- 10.Urata K, Kawasaki S, Matsunami H, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 1995; 21: 1317–1321. [PubMed] [Google Scholar]
- 11.Mayer MP, Bukau B. Hsp70 chaperone systems: diversity of cellular functions and mechanism of action. Biol Chem 1998; 379: 261–268. [PubMed] [Google Scholar]
- 12.Choi IC, Fung PC, Leung AY, et al. Plasma nitric oxide is associated with the occurrence of moderate to severe acute graft-versus-host disease in haemopoietic stem cell transplant recipients. Haematologica 2001; 86: 972–976. [PubMed] [Google Scholar]
- 13.Lo CM, Fan ST, Chan JK, et al. Minimum graft volume for successful adult-to-adult living donor liver transplantation for fulminant hepatic failure. Transplantation 1996; 62: 696–698. [DOI] [PubMed] [Google Scholar]
- 14.Sinclair RA, Antonovych TT, Mostofi FK. Renal proliferative arteriopathies and associated glomerular changes: a light and electron microscopic study. Hum Pathol 1976; 7: 565–588. [DOI] [PubMed] [Google Scholar]
- 15.Schmucker DL, Curtis JC. A correlated study of the fine structure and physiology of the perfused rat liver. Lab Invest 1974; 30: 201–212. [PubMed] [Google Scholar]
- 16.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988; 332: 411–415. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JX, Pegoli W, Clemens MG. Endothelin-1 induces direct constriction of hepatic sinusoids. Am J Physiol 1994; 266: G624–G632. [DOI] [PubMed] [Google Scholar]
- 18.Sonin NV, Garcia-Pagan JC, Nakanishi K, et al. Patterns of vasoregulatory gene expression in the liver response to ischemia/reperfusion and endotoxemia. Shock 1999; 11: 175–179. [DOI] [PubMed] [Google Scholar]
- 19.Goto M, Takei Y, Kawano S, et al. Endothelin-1 is involved in the pathogenesis of ischemia/reperfusion liver injury by hepatic microcirculatory disturbances. Hepatology 1994; 19: 675–681. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Pagan JC, Zhang JX, Sonin N, et al. Ischemia/reperfusion induces an increase in the hepatic portal vasoconstrictive response to endothelin-1. Shock 1999; 11: 325–329. [DOI] [PubMed] [Google Scholar]
- 21.Bredt DS. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res 1999; 31: 577–596. [DOI] [PubMed] [Google Scholar]
- 22.Goligorsky MS, Abedi H, Noiri E, et al. Nitric oxide modulation of focal adhesions in endothelial cells. Am J Physiol 1999; 276: C1271–C1281. [DOI] [PubMed] [Google Scholar]
- 23.Mashimo H, Goyal RK. Lessons from genetically engineered animal models. IV. Nitric oxide synthase gene knockout mice. Am J Physiol 1999; 277: G745–G750. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz SM. A protective player in the vascular response to injury. Nat Med 2001; 7: 656–657. [DOI] [PubMed] [Google Scholar]
- 25.Duckers HJ, Boehm M, True AL, et al. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med 2001; 7: 693–698. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto K, Honda K, Kobayashi N. Protective effect of heat preconditioning of rat liver graft resulting in improved transplant survival. Transplantation 2001; 71: 862–868. [DOI] [PubMed] [Google Scholar]
- 27.Amersi F, Buelow R, Kato H, et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest 1999; 104: 1631–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menger MD, Richter S, Yamauchi J, Vollmar B. Role of microcirculation in hepatic ischemia/reperfusion injury. Hepato-Gastroenterology 1999; 46 (Suppl 2): 1452–1457. [PubMed] [Google Scholar]
- 29.Sahin M, Tekin S, Aksoy F, et al. The effects of splenic artery ligation in an experimental model of secondary hypersplenism. J R Coll Surg Edinb 2000; 45: 148–152. [PubMed] [Google Scholar]
- 30.Nishizaki T, Ikegami T, Hiroshige S, et al. Small graft for living donor liver transplantation. Ann Surg 2001; 233: 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katori M, Tamaki T, Takahashi T, et al. Prior induction of heat shock proteins by a nitric oxide donor attenuates cardiac ischemia/reperfusion injury in the rat. Transplantation 2000; 69: 2530–2537. [DOI] [PubMed] [Google Scholar]
- 32.Reynaert H, Vaeyens F, Qin H, et al. Somatostatin suppresses endothelin-1-induced rat hepatic stellate cell contraction via somatostatin receptor subtype 1. Gastroenterology 2001; 121: 915–930. [DOI] [PubMed] [Google Scholar]
- 33.Ricciardi R, Schaffer BK, Shah SA, et al. Bosentan, an endothelin antagonist, augments hepatic graft function by reducing graft circulatory impairment following ischemia/reperfusion injury. J Gastrointest Surg 2001; 5: 322–329. [DOI] [PubMed] [Google Scholar]