Abstract
Systemic acquired resistance (SAR) is a plant defense state that is induced, for example, after previous pathogen infection or by chemicals that mimic natural signaling compounds. SAR is associated with the ability to induce cellular defense responses more rapidly and to a greater degree than in noninduced plants, a process called “priming.” Arabidopsis plants were treated with the synthetic SAR inducer benzothiadiazole (BTH) before stimulating two prominent cellular defense responses, namely Phe AMMONIA-LYASE (PAL) gene activation and callose deposition. Although BTH itself was essentially inactive at the immediate induction of these two responses, the pretreatment with BTH greatly augmented the subsequent PAL gene expression induced by Pseudomonas syringae pv. tomato infection, wounding, or infiltrating the leaves with water. The BTH pretreatment also enhanced the production of callose, which was induced by wounding or infiltrating the leaves with water. It is interesting that the potentiation by BTH pretreatment of PAL gene activation and callose deposition was not seen in the Arabidopsis nonexpresser of PR genes 1/noninducible immunity 1 mutant, which is compromised in SAR. In a converse manner, augmented PAL gene activation and enhanced callose biosynthesis were found, without BTH pretreatment, in the Arabidopsis constitutive expresser of pathogenesis-related genes (cpr)1 and constitutive expresser of pathogenesis-related genes 5 mutants, in which SAR is constitutive. Moreover, priming for potentiated defense gene activation was also found in pathogen-induced SAR. In sum, the results suggest that priming is an important cellular mechanism in acquired disease resistance of plants that requires the nonexpresser of PR genes 1/noninducible immunity 1 gene.
Upon infection with necrotizing pathogens, for example, many plants develop an enhanced resistance to a broad spectrum of pathogens in the area of primary infection and in the distal, uninoculated organs (Hunt and Ryals, 1996; Ryals et al., 1996; Sticher et al., 1997). This so-called systemic acquired resistance (SAR) requires the endogenous accumulation of salicylic acid (SA; Ryals et al., 1996; Dempsey et al., 1999) and can also be induced by exogenous application of SA or its synthetic analogs 2,6-dichloroisonicotinic acid (Métraux et al., 1991) and benzothiadiazole (BTH; Friedrich et al., 1996; Lawton et al., 1996). SAR is associated with the activation of genes encoding pathogenesis-related (PR) proteins, some with antimicrobial activity (Van Loon and Van Strien, 1999), and with the ability to induce cellular defense responses more rapidly and to a greater degree than in noninduced plants (Mur et al., 1996). According to the terminology for a phenotypically similar phenomenon in mammalian monocytes (Hayes and Zoon, 1993), the enhanced ability to activate cellular defense responses has been called “priming” (Katz et al., 1998). Although the PR proteins and their possible role in SAR have been the object of thorough research, the biochemical mechanism and genetic basis of priming remain largely unclear. In this context, it is important to note that a strict correlation between increased accumulation of PR proteins before pathogen attack and SAR has not always been observed. However, tools and markers for monitoring additional complex cellular plant defense responses such as the hypersensitive response or local cell wall strengthening are limited. Therefore, it is important to study further defense-associated cellular events that are induced more effectively in pathogen-attacked, systemically resistant plants such as the activation of Phe ammonia-lyase (PAL)-encoding genes and the deposition of the 1,3-β-glucan callose. PAL is a key enzyme in the phenylpropanoid pathway that leads to a variety of defense-related plant secondary metabolites such as SA, phytoalexins, and lignin-like polymers (Hahlbrock and Scheel, 1989), whereas callose deposition probably contributes to disease resistance by reinforcing the plant cell wall beneath fungal penetration sites (Kauss, 1992).
Over the past decade, a parsley cell culture/Phytophthora sojae cell wall elicitor model system has proven useful in studying cell priming and the resulting potentiation of cellular plant defense responses (for review, see Conrath et al., 2001): Preincubation with SA, 2,6-dichloroisonicotinic acid, or BTH, in a strictly time-dependent process, primed parsley cells for stronger low-dose elicitation of various of cellular defense responses (Kauss et al., 1992a, 1993; Kauss and Jeblick, 1995), including the activation of various defense-related genes (Kauss et al., 1992a).
In more detailed studies with the parsley cell culture, it was found that the effect of the SAR inducers on defense gene activation strongly depends on the gene that is being monitored (Katz et al., 1998; Thulke and Conrath, 1998). One group of parsley defense genes was found directly responsive to the treatment with the SAR inducers tested and, thus, their induction reminds to the immediate activation of the PR genes in various plants. A second group of parsley defense genes was essentially unaffected by the treatment with SAR activators. Yet, these genes displayed SAR inducer-dependent potentiation of gene activation once the cells had been treated subsequently with very low elicitor doses. These results with the parsley model system supported the previously assumed dual role for SAR inducers at the level of defense gene activation (Katz et al., 1998; Thulke and Conrath, 1998).
Although in the above mentioned studies, the parsley cell culture has proven useful as a model for studying the priming of plant cells, it cannot be used to investigate the priming phenomenon in association with SAR. This led Draper and coworkers (Mur et al., 1996) to investigate the influence of pretreatment with SA on the subsequent activation by pathogen attack and wounding of PR-10::β-glucuronidase and PAL3::β-glucuronidase chimeric genes in whole transgenic tobacco (Nicotiana tabacum) plants. By doing so, Mur et al. (1996) confirmed the proposed dual role for SA in the activation of defense genes at the level of whole tobacco plants. However, thus far, little is known about the genetic basis of priming.
Over the past decade, various Arabidopsis mutants have been identified that are affected in the SAR mechanism (Delaney, 2000). SAR-constitutive and SAR-compromised mutants have been obtained. In the first type, which includes the Arabidopsis constitutive expresser of PR genes (cpr)1 and cpr5 mutants, SAR is constitutive and plants are resistant to various virulent pathogens (Bowling et al., 1994, 1997). In a converse manner, in SAR-compromised mutants, certain avirulent bacterial and fungal isolates become virulent. The Arabidopsis nonexpresser of PR genes (npr)1 mutant (Cao et al., 1994), which has also been called noninducible immunity (nim)1 (Delaney et al., 1995), is one of these SAR-compromised mutants. The NPR1/NIM1 gene has recently been cloned (Cao et al., 1997; Ryals et al., 1997), and the predicted NPR1/NIM1 protein was found to possess some homology to the IκBα subclass of mammalian transcription factor inhibitors. Therefore, the SAR signaling mechanism of plants may have mechanistic parallels to the NF-κB signal transduction pathway in mammals (Ryals et al., 1997).
Zimmerli et al. (2000) recently reported on the potentiation of pathogen-specific defense mechanisms in Arabidopsis after prolonged treatment of the plants with β-aminobutyric acid. As this compound was fully protective against Peronospora parasitica attack in the npr1/nim1 mutant and did not cause activation of the PR-1 gene in wild-type Arabidopsis plants (Zimmerli et al., 2000), the β-aminobutyric acid-induced pathogen resistance in Arabidopsis obviously differs from SA-dependent SAR. Thus, so far there is no information about whether priming and the resulting potentiation of cellular defense responses are associated with SAR of Arabidopsis. To address this issue and to elucidate the molecular and genetic basis of priming, we investigated the influence of pretreatment with the synthetic SAR inducer BTH on PAL gene activation and callose deposition in Arabidopsis wild-type and various SAR mutant plants as a first step toward understanding the role of priming in SAR of Arabidopsis.
RESULTS
Arabidopsis Plants with SAR Are Primed for Stronger PAL Gene Activation and Enhanced Callose Deposition
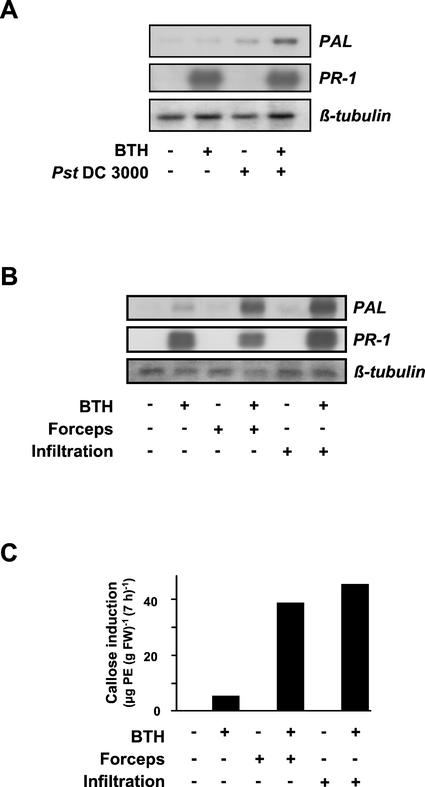
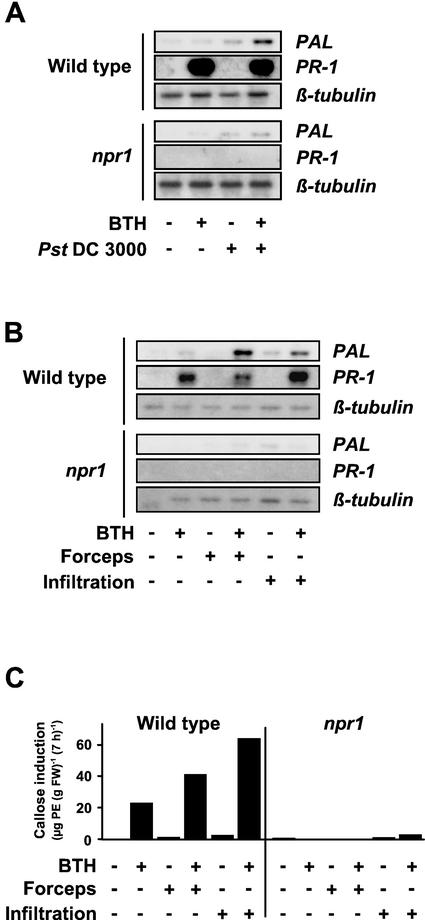
To investigate whether systemically resistant Arabidopsis is primed for stronger activation of cellular defense responses, 4-week-old plants were sprayed with the synthetic SAR inducer BTH. Three days later, leaves of the plants were inoculated with a cell suspension of Pst (strain DC3000), which causes chlorotic spots on inoculated Arabidopsis leaves (Fig. 1A), wounded by slight pressure with forceps or infiltrated with water (Fig. 1, B and C). The latter two treatments were included in the assay as systemically resistant plants were found to react with augmented defense gene expression also when subjected to abiotic stresses (Mur et al., 1996). As shown in Figure 1, A and B, there is strong PR-1 gene activation in leaves of 100 μm-BTH-pretreated plants. In contrast to PAL gene activation (see below), this response is not further enhanced upon subsequent stimulation by Pst DC3000 attack (Fig. 1A), wounding, or leaf infiltration of water (Fig. 1B). In leaves of BTH non-pretreated plants that have been inoculated with Pst DC3000 (Fig. 1A), wounded, or infiltrated with water (Fig. 1B), PR-1 gene expression was apparently absent (Fig. 1, A and B).
Figure 1.
Effect of priming by BTH on PAL and PR-1 gene activation and callose induction. Arabidopsis plants were sprayed with 100 μm BTH (+) in a wettable powder carrier or with the wettable powder carrier only (−). Three days later, leaves of the plants were left untreated (−), wounded with forceps (B and C; +), infiltrated with water (B and C; +), or inoculated with Pst DC3000 (A; +). Mock inoculations were performed by dipping plants into MgCl2/Silwet in the absence of bacteria (A; −). A and B, Total RNA was extracted from an aliquot of leaves 4 h (A) or 2 h (B) after treatment and assayed for accumulation of PAL mRNA by RNA gel-blot analysis. Another aliquot of leaves was harvested at the 24-h time point post-treatment and was analyzed for the accumulation of PR-1 transcripts (A and B). To document equal sample loading and transfer of RNA, the membranes from the PAL blots were stripped and reprobed with a 32P-labeled Arabidopsis β-tubulin cDNA. C, At the 7-h time point after wounding or infiltration of water, callose was extracted and determined from yet another aliquot of leaves. At this time point, leaves treated with the wettable powder carrier only contained 45.4 μg of pachyman equivalents (PE; g fresh weight)−1 background callose level, which supposedly is due to the high callose content observed in the leaf trichomes of Arabidopsis (A. Kohler, S. Schwindling, and U. Conrath, unpublished data). This value was subtracted from all samples. Values given are averages of two replicates. For variations in callose values obtained by the extraction method used, see Kohler et al., 2000. The establishment of SAR in the BTH-sprayed plants was confirmed in a parallel assay in which two upper leaves of four plants treated with wettable powder or BTH for 3 d were dip-inoculated with a suspension of Pst DC3000 (35 × 106 cfu mL−1). Three days later, those plants that had been pretreated with the wettable powder carrier were diseased and exhibited wet chlorotic lesions, whereas the BTH-pretreated plants remained free of visible symptoms (data not shown).
It should be noted that in about 10% of our experiments, the PR-1 gene induction by pretreatment with 100 μm BTH was apparently suboptimal. In these experiments, a stronger PR-1 gene response could be seen once the induced plants have subsequently been stimulated on their leaves by Pst DC3000-infection, wounding, or infiltration of water (data not shown).
When PAL gene expression was monitored, BTH pretreatment, wounding, and infiltration of water did not induce a response, and Pst DC3000 inoculation only weakly induced a response (Fig. 1, A and B). However, upon bacterial inoculation (Fig. 1A), wounding, or infiltration of water (Fig. 1B) into the BTH-pretreated, systemically resistant leaves, there was strong accumulation of PAL transcripts. Thus, systemically resistant Arabidopsis plants are primed for potentiated PAL gene activation, which has subsequently been induced by phytopathogenic Pst DC3000, wounding, or water infiltration.
The deposition of callose represents a quick cellular defense response presumably not induced via gene activation, but rather by membrane perturbation (Kauss, 1992). Elicited callose production is only low in BTH-pretreated plants with no further stimulation (Fig. 1C) and is even missing in leaves of control plants, independent of whether these were left untreated, wounded, or infiltrated with water (Fig. 1C). However, high amounts of callose were induced upon wounding or water-infiltrating the leaves of BTH-pretreated, systemically resistant plants (Fig. 1C). As Pst DC3000 infection per se, even at high bacterial titers, did not induce detectable callose deposition (data not shown), the influence of BTH pretreatment on Pst DC3000-induced callose production could not be investigated.
The Natural SAR Inducer SA Is Also Active at Priming for Stronger Induction of PAL Gene Activation and Callose Deposition
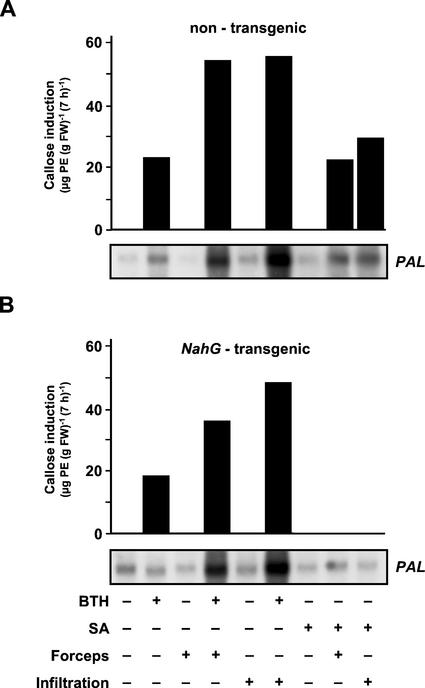
Next, we asked whether priming for enhanced activation of cellular defense responses in Arabidopsis leaves might be exclusive for BTH or whether it might also occur in response to treatment with the natural SAR activator, SA (see above). To address this question, 5-week-old Arabidopsis plants were sprayed with SA in a solution of the wettable powder carrier, the synthetic SAR inducer, BTH, also dissolved in wettable powder carrier solution (positive control) or with the wettable powder carrier only (negative control). Three days later, the leaves were left untreated, slightly squeezed with forceps, or infiltrated with water. Two and 7 h later, leaves of each plant were harvested and analyzed for PAL gene expression and callose deposition, respectively. As seen in Figure 2A, without further stimulation, SA was inactive at PAL gene induction and callose elicitation. However, when SA-pretreated leaves were slightly squeezed with forceps or infiltrated with water, there was a strong induction of both these defense responses (Fig. 2A). The potentiation by SA pretreatment of elicited PAL gene expression and callose induction is not seen in NahG-transgenic Arabidopsis plants that are unable to accumulate significant amounts of SA (Delaney et al., 1994; Ryals et al., 1996). Thus, the strong induction of PAL gene expression and callose biosynthesis in SA-pretreated and subsequently stimulated nontransgenic Arabidopsis plants (Fig. 2A) can clearly be attributed to the pretreatment with SA. Because NahG plants are unable to degrade the BTH signal (Ryals et al., 1996), BTH-mediated priming for stronger PAL gene activation and improved callose induction is still detectable in these plants (Fig. 2B). Whether our finding that the induction of callose deposition in BTH-primed and then nonstimulated or stimulated NahG-transgenic plants is somewhat smaller than in the respective nontransgenic controls (Fig. 2) is of biological relevance remains unclear.
Figure 2.
Influence of pretreatment with SA (300 μm; +) or BTH (100 μm; +) on subsequently induced PAL gene activation and callose production in nontransgenic (A) and NahG-transgenic (B) Arabidopsis plants. Controls were pretreated with the wettable powder carrier only (A and B; −). PAL gene expression was assayed 2 h after slightly squeezing the leaves with forceps (A and B; +) or infiltrating them with water (A and B; +). The respective controls were left untreated (A and B; −). Equal sample loading on the RNA gels was confirmed under UV light by visualization with ethidium bromide (not shown). Callose was extracted and quantified from respectively treated leaves at the 7-h time point post-wounding or infiltration of water. At this time point, callose content in leaves of nontransgenic and NahG-transgenic plants treated with the wettable powder carrier was 49.2 μg of PE (g fresh weight)−1 and 51.3 μg of PE (g fresh weight)−1, respectively. These values were subtracted from respective samples. Values given are averages of two replicates.
Biological Activity of SA Analogs Correlates with Ability to Prime for Stronger PAL Gene Activation
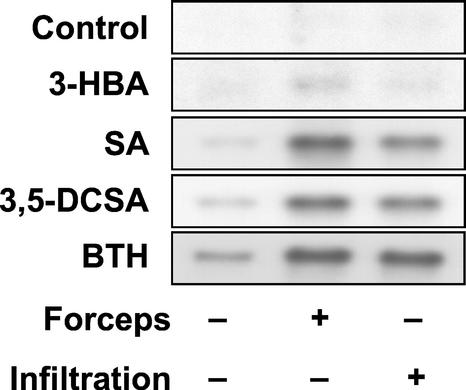
The above results have shown that pretreatment with BTH or SA primes Arabidopsis plants for improved induction of certain cellular defense responses, including activation of the PAL defense gene. Next, we decided to elucidate the priming ability of various SA analogs that differ in their ability to induce SAR. The halogenated SA derivative 3,5-dichloro-SA (for the chemical structure of the compound, see Conrath et al., 1995) has previously been shown to enhance the resistance against tobacco mosaic virus infection in tobacco, whereas 3-hydroxy-benzoic acid was found inactive in this assay (Conrath et al., 1995). As is shown in Figure 3, there was a strong induction of the PAL gene in wounded or water-infiltrated Arabidopsis leaves that had been primed before with BTH, SA, or 3,5-dichloro-SA. In contrast, PAL gene activation was only low in wounded or water-infiltrated leaves that had been pretreated with 3-hydroxy-benzoic acid, a compound that is unable to enhance the resistance of tobacco against tobacco mosaic virus (Conrath et al., 1995). Thus, there is good correlation between the ability of various compounds to induce SAR and their capability to prime Arabidopsis plants for better PAL defense gene activation.
Figure 3.
Biological activity correlates with ability to prime for stronger PAL gene activation. Arabidopsis plants were pretreated for 3 d with the indicated SA derivatives at 300 μm, with BTH at 100 μm as a positive control or with the wettable powder carrier only (control). The leaves were then left untreated (−), slightly squeezed with forceps (+), or infiltrated with water (+). After 2 h, PAL gene activation was monitored by RNA gel-blot analysis. Equal sample loading was confirmed under UV light by visualization with ethidium bromide (not shown). 3-HBA, 3-hydroxy-benzoic acid; 3,5-DCSA, 3,5-dichloro-SA.
Priming Occurs in Pathogen-Induced SAR
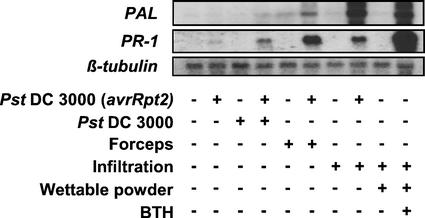
To investigate whether priming for stronger defense gene activation also occurs in pathogen-induced SAR, Arabidopsis plants were inoculated on three lower leaves with Pst DC3000 expressing the avrRpt2 avirulence gene (Whalen et al., 1991). Three days later, two upper leaves of the plants were slightly squeezed with forceps, infiltrated with water, or inoculated with virulent Pst DC3000 (Fig. 4). As is evident from Figure 4, priming for augmented PAL gene expression induced by wounding, water infiltration, or Pst DC3000 challenge inoculation indeed can be seen when SAR was established by previous infection of Arabidopsis plants with avirulent Pst DC3000 avrRpt2 (Cameron et al., 1994; legend to Fig. 4). It is interesting that in this biologically induced SAR, potentiated gene expression was also seen for PR-1 upon further stimulation with forceps, infiltration of water, or challenge infection with Pst DC3000 (Fig. 4). In BTH-induced SAR, augmented expression of PR-1 upon further stimulation by Pst DC3000-infection, wounding with forceps, or water infiltration was seen in only one out of 10 experiments (data not shown). Together, the experiment in Figure 4 demonstrates that priming, in addition to chemically induced SAR, is associated also with biologically induced SAR of Arabidopsis plants.
Figure 4.
Previous infection with avirulent Pst DC3000 avrRpt2 induces priming for enhanced PAL gene activation by virulent Pst DC3000, wounding, or infiltration of water. Three lower leaves of Arabidopsis plants were infiltrated with a cell suspension of SAR-inducing Pst DC3000 avrRpt2 (16 × 104 cfu mL−1) in MgCl2 (+). Mock inoculations were performed by infiltrating three lower leaves of control plants with MgCl2 in the absence of Pst DC3000 avrRpt2 (−). Three days later, two upper leaves of the mock-inoculated and the Pst DC3000 avrRpt2-infected plants were left untreated (−), dipped into a cell suspension of virulent Pst DC3000 (35 × 106 cfu mL−1) in MgCl2/Silwet (+), slightly squeezed with forceps (+), or infiltrated with water (+). Mock challenge treatments were performed by leaving the upper leaves untreated (−; forceps and infiltration) or by dipping two of them into a solution of MgCl2/Silwet in the absence of bacteria (−; Pst DC3000). Total RNA was extracted from the upper leaves 2 and 3 h after wounding/infiltration and bacterial inoculation, respectively, and was assayed for accumulation of PAL mRNA by RNA gel-blot analysis. Another aliquot of leaves was harvested at the 24-h time point postinoculation and was analyzed for the accumulation of PR-1 transcripts. The accumulation of PAL mRNA (2-h time point) and PR-1 transcripts (24-h time point) in plants that had been pretreated for 3 d with BTH (+) and then infiltrated on two leaves with water (+) served as a positive control. To document equal sample loading and transfer of RNA, the membrane of the PAL blot was stripped and reprobed with a 32P-labeled Arabidopsis β-tubulin cDNA. The establishment of SAR in Pst DC3000 avrRpt2-inoculated plants was confirmed in a parallel assay in which two upper leaves of three mock-preinoculated or Pst DC3000 avrRpt2-preinfected plants were infiltrated, 3 d post-treatment, with a suspension of virulent Pst DC3000. Three days later, the mock-preinoculated plants were diseased and exhibited wet chlorotic lesions, whereas the Pst DC3000 avrRpt2-preimmunized plants remained free of visible symptoms (data not shown).
Priming Is Not Seen in an SAR-Deficient Arabidopsis Mutant
From the above results, we concluded that the augmentation of cellular defense responses by priming may contribute to SAR of Arabidopsis. If this assumption holds true, one should expect that priming was lower or even absent in SAR-deficient Arabidopsis plants. To address this issue, we included the Arabidopsis npr1/nim1 mutant in our priming experiments. Although this mutant is able to accumulate wild-type levels of SA in response to treatment with avirulent pathogens, it does not express biologically or chemically induced SAR (Cao et al., 1994; Delaney et al., 1995). Figure 5, A and B, demonstrates that the activation of the PR-1 gene and the potentiated accumulation of PAL transcripts that are clearly seen upon Pst DC3000 infection (Fig. 5A), wounding (Fig. 5B), or infiltrating water (Fig. 5B) into leaves of BTH-pretreated wild-type plants were not seen in the SAR-deficient npr1/nim1 mutant. However, upon severe wounding with forceps, infiltrating the leaves with the fungal elicitor compound chitosan, or high titer Pst DC3000 infection, the PAL gene was induced in the npr1/nim1 mutant to a same degree as it was in the wild type (data not shown). Thus, the lack of augmented PAL gene expression in the npr1/nim1 mutant (Fig. 5, A and B) is not due to a defect in the mechanism that leads to PAL gene activation in these plants.
Figure 5.
Influence of pretreatment with BTH on the subsequent induction of PAL and PR-1 gene activation and callose deposition in leaves of Arabidopsis wild-type and npr1/nim1 mutant plants. Wild-type and npr1/nim1 mutant plants were pretreated with 100 μm BTH (+) or with the wettable powder carrier only (−). After 3 d, leaves of the plants were left untreated (−), dipped into a solution of MgCl2/Silwet in the absence (−) or presence (+) of Pst DC3000 (A), slightly squeezed with forceps (+; B and C), or infiltrated with water (+; B and C). A and B, Three hours (A) and 2 h (B) post-treatment, total RNA was extracted from an aliquot of leaves and was analyzed for the accumulation of PAL transcripts by RNA gel blotting. PR-1 gene activation was assayed at the 24-h time point. To check for equal sample loading, the membranes of the PAL blots were stripped and rehybridized with an Arabidopsis β-tubulin cDNA probe. C, Another aliquot of wounded or water-infiltrated leaves was analyzed for the accumulation of callose at the 7-h time point post-stimulation. At this time point, callose content in leaves treated with the wettable powder carrier only was 44.8 μg of PE (g fresh weight)−1. This value was subtracted from all samples. Values given are averages of two replicates.
Potentiation was also absent when callose induction was assayed in BTH-pretreated and subsequently wound/water infiltration-stimulated npr1/nim1 mutant plants (Fig. 5C). When, for example, subtracting the BTH-caused callose production from that of BTH-primed leaves that were subsequently slightly squeezed with forceps or infiltrated with water, it becomes obvious that in primed and subsequently stimulated wild-type plants, elicited callose production was about 10- or 15-fold higher than was the callose response to the respective stimulus alone in nonprimed wild-type plants (Fig. 5C). In contrast, in the SAR-deficient npr1/nim1 mutant, callose induction by BTH alone was absent, as was potentiation of the low callose deposition induced by wounding or the infiltration of water (Fig. 5C). It should be noted that upon severe wounding by harshly squeezing the leaves with forceps or infiltrating them with chitosan, callose deposition was induced in the npr1/nim1 mutant to a same degree as in the wild type (data not shown). Thus, the lack of potentiated callose production in the npr1/nim1 mutant (Fig. 5C) is not due to a defect in the callose depositing mechanism of these plants.
Permanent Priming in Arabidopsis Mutants with Constitutive SAR
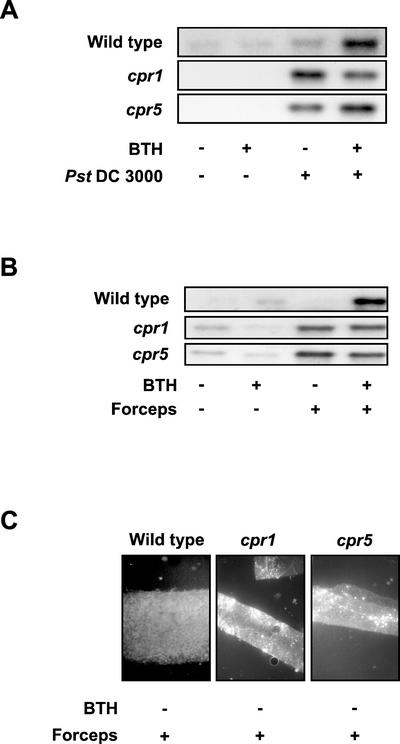
To further elucidate whether priming is associated with SAR of Arabidopsis, we next included the cpr1 and cpr5 mutants in our studies. Both cpr mutants have been shown to express constitutively enhanced resistance against various fungal and bacterial pathogens (Bowling et al., 1994, 1997). As is seen in Figure 6, A and B, in leaves of the cpr1 and cpr5 mutant plants, there was strong induction of PAL upon Pst DC3000 infection (Fig. 6A) or wounding (Fig. 6B). In the case of Pst DC3000 infection, a pretreatment with BTH only slightly enhanced (cpr5) or scarcely countered (cpr1) PAL gene activation (Fig. 6A). In contrast to PAL gene activation, PR-1 expression was optimal in BTH-non-pretreated and BTH-pretreated cpr1 and cpr5 mutant plants and was not further enhanced upon Pst DC3000 attack or wounding with forceps (data not shown).
Figure 6.
Arabidopsis cpr1 and cpr5 mutant plants are constitutively primed for enhanced PAL gene activation and augmented callose deposition. Wild-type and cpr1 and cpr5 mutant plants were treated with the wettable powder carrier solution (−) or with 100 μm BTH (+) for 3 d. Pretreated plants were then left untreated (A and B; −), mock inoculated (A; −), infected with Pst DC3000 (A; +), or wounded by slightly squeezing with forceps (B and C; +). Due to the small size of the two cpr mutants (for photographs, see Bowling et al., 1994; 1997), leaf infiltration of water could not be included as an additional stimulus. A and B, Accumulation of PAL transcripts by RNA gel-blot analysis was monitored after 3 h (A) or 2 h (B). Equal sample loading and transfer of RNA were confirmed under UV light by visualization with ethidium bromide (data not shown). C, Another aliquot of leaves from the same plants as in B was assayed for callose deposition at the 7-h time point post-wounding. Callose/sirofluor complexes are visible in the cpr1 and cpr5 mutant leaf slices as bright fluorescent spots.
Constitutive priming for stronger activation of a wound-induced defense response in cpr1 and cpr5 was also seen when the deposition of callose was assayed (Fig. 6C). Due to the small size of the two cpr mutants (for photographs, see Bowling et al., 1994, 1997), in this case callose was monitored only microscopically in leaf slices by complex formation with sirofluor. As shown in Figure 6C, there was strong induction of callose deposition upon slightly squeezing leaves of the cpr1 or cpr5 mutants in the absence of BTH pretreatment, whereas in the wounded wild-type plants, significant callose elicitation could not be observed. Thus, the SAR-constitutive Arabidopsis cpr1 and cpr5 mutants are permanently primed for stronger induction of defense responses, as is exemplarily shown for PAL gene activation and callose elicitation in Figure 6.
DISCUSSION
In these experiments, we investigated the influence of pretreatment of Arabidopsis with BTH on the activation of two representative cellular defense responses, PAL gene activation and callose deposition. By doing so, we found that whereas directly activating (Figs. 1, A and B, and 5, A and B) or augmenting (Fig. 4) the PR-1 gene, BTH was not, or only slightly, active at the immediate induction of the PAL gene (Figs. 1, A and B; 2, A and B; 3; 5, A and B; and 6, A and B), even at concentrations of up to 1 mm (data not shown). Also, the BTH treatment only slightly activated callose production (Figs. 1C; 2, A and B; and 5C). However, a pretreatment with BTH prepared the Arabidopsis plants for stronger PAL gene activation (Figs. 1, A and B; 2, A and B; 3; 4; 5, A and B; and 6, A and B) and improved callose deposition (Figs. 1C; 2, A and B; and 5C), thus demonstrating a dual role for BTH at the activation of defense responses in Arabidopsis: a direct one by immediately inducing PR-1, and an indirect one by priming the plants for stronger stimulation of callose biosynthesis and PAL gene activation. As PAL is a key enzyme in the phenylpropanoid metabolism, which is thought to include the biosynthesis pathway of SA (Chong et al., 2001), the potentiated activation of the PAL gene in primed Arabidopsis plants may further amplify the induction of SA biosynthesis to better mediate disease resistance.
Because BTH, wounding, or water infiltration do not cause production of SA in various plants, including Arabidopsis (Malamy et al., 1990; Friedrich et al., 1996; Lawton et al., 1996), we can exclude the possibility that pretreatment with BTH allows a critical level of SA to be reached, thus leading to augmented defense response activation upon further stimulation by wounding or infiltration of water. In contrast to wounding and water infiltration, Pst DC3000 infection causes some accumulation of SA in Arabidopsis (Cameron et al., 1999). However, SA biosynthesis requires prolonged activation of PAL, which was not significantly induced by Pst DC3000 in our experiments (Figs. 1A, 4, 5A, and 6A). As we assayed PAL gene expression as early as 3 to 4 h post-Pst DC3000 inoculation, it is unlikely that the effects seen after infection with Pst DC3000 are due to a synergistic action of BTH and Pst DC3000-induced SA.
BTH-pretreated Arabidopsis plants show stronger PAL gene activation or/and enhanced callose deposition upon Pst DC3000 infection (Figs. 1A, 5A, and 6A), slightly squeezing the leaves with forceps, or infiltrating them with water (Figs. 1, B and C; 2–4; 5, B and C; and 6B). In analogy to the situation in water-infiltrated rice (Oryza sativa) leaves, which display enhanced activity of genes encoding the stress marker enzyme phospholipase D (Yang et al., 1996), we assume that our water infiltration method may cause cell damage in infiltrated Arabidopsis leaves. The latter may explain why infiltration of water and wounding with forceps cause some identical responses in primed Arabidopsis leaves (Figs. 1, B and C; 2–4; and 5, B and C). If this assumption holds true, the present study demonstrates that primed Arabidopsis plants are in an alerted state that improves the induction of the pathogen defense and wound responses.
Though the identity of the common step(s) in the regulation of pathogen and wound/water infiltration responses still remains unknown, our data indicate that the NPR1/NIM1 gene and priming may be common components that mediate crosstalk between these types of defense responses in Arabidopsis (Maleck and Dietrich, 1999). This conclusion is drawn from our finding that the improvement of responses to wounding, water infiltration, and pathogen attack cannot be induced in the Arabidopsis npr1/nim1 mutant (Fig. 5), whereas priming for the wound reaction and the pathogen response was found constitutively present in cpr1 and cpr5 mutant plants (Fig. 6). In an alternate manner, feedback from products of BTH/SA-responsive genes downstream of NPR1/NIM1 may act to modify defense responses that are located upstream of BTH/SA in the cell's disease resistance mechanism (Delaney, 1997), perhaps even PAL gene activation.
Priming and the resulting potentiation of cellular defense responses are absent in the Arabidopsis npr1/nim1 mutant (Fig. 5), which also is defective in the expression of certain defense-related genes and SAR (Cao et al., 1994; Delaney et al., 1995). Priming and defense response potentiation were permanently and consistently present in the cpr1 and cpr5 mutants (Fig. 6) in which PR gene expression and SAR are constitutive (Bowling et al., 1994, 1997). Also, there is a close correlation between the ability of various compounds to activate certain PR genes and to elicit SAR and their capability to potentiate defense gene activation in Arabidopsis (Fig. 3). Therefore, it is very likely that priming, in addition to the immediate activation of certain PR genes, is an important mechanism in SAR of plants. This conclusion is further supported by the finding that Arabidopsis plants with pathogen-induced SAR are also primed for enhanced defense gene activation subsequently induced by Pst DC3000 challenge infection, wounding the leaves with forceps, or infiltrating them with water (Fig. 4). It is interesting that the constitutive induction of SAR in the Arabidopsis defense, no death1 mutant has been assumed to substitute for hypersensitive cell death in potentiating the gene-for-gene defense response (Yu et al., 1998). Moreover, the enhanced disease resistance of the Arabidopsis cpr5-2 mutant has been ascribed to the potentiated induction of the PR-1 gene in these plants (Boch et al., 1998). Finally, an attenuation of priming for potentiated induction of the oxidative burst has been associated with a loss of resistance to avirulent bacterial pathogens in tobacco (Mur et al., 2000).
The Arabidopsis cpr1 and cpr5 mutants constitutively display enhanced disease resistance, and they permanently accumulate PR proteins (Bowling et al., 1994, 1997). We cannot completely exclude the possibility that constitutive priming in these mutants might be caused by the activation of various stress response mechanisms besides the SAR pathway (Bowling et al., 1994, 1997). However, we speculate that due to the enhanced levels of SA in the cpr mutants (Bowling et al., 1994, 1997), these are permanently in a primed state that leads to constitutive PR gene expression and also keeps the plants on the alert. This situation is similar to the one in wild-type plants that have been previously infected by an avirulent pathogen or pretreated with SA or BTH. Due to constitutive priming, the cpr1 and cpr5 mutants are able to rapidly and effectively activate their various cellular defense responses once attacked by a pathogen (Fig. 6A) or stimulated by wounding (Fig. 6, B and C).
SAR, PR gene expression, and priming for augmented pathogen, wound, and water infiltration responses in Arabidopsis obviously require the intact NPR1/NIM1 protein. This is concluded from previous results demonstrating absence of SAR and PR gene expression in npr1/nim1 mutant plants, despite the accumulation of SA (Cao et al., 1994; Delaney et al., 1995), and from the finding that the potentiation of cellular defense responses is not detected in mutants with a defective NPR1/NIM1 gene (Fig. 5).
The Dong group (Zhang et al., 1999), Klessig and coworkers (Zhou et al., 2000), as well as Després et al. (2000) reported that the Arabidopsis NPR1/NIM1 protein may interact with transcription factors of the TGA/octopine synthase element binding factor basic Leu zipper protein family to activate the PR-1 gene in Arabidopsis. Based on our data, we conclude that during pretreatment with inducers of SAR, there might be synthesis and/or activation of one or more cellular factors, some of which may represent defense-related gene products, that shift the plants to the primed state. This factor(s) may then play a role in the immediate activation of certain other defense-related genes, such as the Arabidopsis PR-1 gene. By binding to the promoter of defense genes, such as the PAL and PR-1 gene of Arabidopsis, the cellular factor(s) might be able to also prepare their target gene(s) for better expression once stimulated by pathogen attack, wounding, or water infiltration.
It should be noted that in other plants, priming for enhanced induction of defense responses can also be induced by pretreatment with the signaling molecule methyl jasmonate (Kauss et al., 1992b). In addition, Zimmerli et al. (2000) recently demonstrated potentiated accumulation of PR-1 transcripts in Pst DC3000-infected Arabidopsis plants that had been primed with β-aminobutyric acid. Moreover, preincubation with the wound-generated peptide messenger systemin enhanced a rapid H2O2 burst induced by the addition of oligogalacturonides or water to tomato (Lycopersicon esculentum) cell suspension cultures (Stennis et al., 1998). Together, these observations indicate a complex, multi-entrance nature for plant cell priming.
MATERIALS AND METHODS
Biological Material
Arabidopsis plants used throughout this study were wild-type Columbia (Col-O; from the Arabidopsis Biological Resource Center, Ohio State University, Columbus), NahG-transgenic plants (in Col-O background; provided by Kay Lawton, Syngenta, Research Triangle Park, NC), or npr1-1, cpr1, or cpr5 mutant plants (in Col-O background; provided by Xinnian Dong, Duke University, Durham, NC). Plants were grown at an 8-h photoperiod at a temperature of 22°C with 60% humidity. One and one-half weeks post-sowing, seedlings were transferred, in groups of nine plants, to fresh pots and were watered once with an aqueous solution of the insecticide Confidor (50 mg L−1; Bayer, West Haven, CT) to prevent infestation of the plants by greenflies. The Confidor treatment had no effect on the outcome of the experiments (data not shown).
Pseudomonas syringae pv. tomato (strain DC3000) with and without the avrRpt2 avirulence gene was provided by Brian Staskawicz (University of California, Berkeley, CA) and was grown at 30°C in King's B media for 1 d. After centrifugation, bacterial cells were washed and resuspended to 35 × 106 cfu mL−1 (Pst DC3000) or 16 × 104 cfu mL−1 (Pst DC3000 avrRpt2) in 10 mm MgCl2. Before leaf infection, only the Pst DC3000 cell suspension was supplemented with 0.01% (v/v) of the surfactant Silwet L-77 (provided by H. Köhle, BASF, Ludwigshafen, Germany).
Plant Treatment and Harvest of Tissue
Four- to 6-week-old Arabidopsis plants were sprayed with 0.5 to 1 mL plant−1 of 100 μm BTH (Syngenta), 300 μm SA (Sigma, St. Louis), 300 μm 3,5-dichloro-SA (Sigma), or 300 μm 3-hydroxy-benzoic acid (Sigma). All these compounds were dissolved in a solution of a wettable powder carrier (Syngenta). Control plants were treated with the wettable powder carrier only. Three days later, leaves of the plants were infiltrated, from the lower surface, with water using a 1-mL plastic syringe or they were slightly squeezed with forceps. Infections with Pst DC3000 were performed by dipping whole plants into the bacterial suspension. Mock inoculations were done by dipping the plants into a solution of MgCl2/Silwet in the absence of bacteria.
To investigate the presence of priming in pathogen-induced SAR, three lower leaves of 4-week-old Arabidopsis plants were infiltrated by a syringe with a suspension of Pst DC3000 avrRpt2 prepared as described above. Mock inoculations were performed by infiltrating three lower leaves of Arabidopsis plants with MgCl2 in the absence of bacteria. After 3 d, two upper leaves of the plants were infiltrated with water, slightly squeezed with forceps, or inoculated with Pst DC3000 by dipping into a bacterial cell suspension (35 × 106 cfu mL−1) in MgCl2/Silwet. For extraction and analysis of RNA, two upper leaves of respectively treated plants were collected at the 2-h (wound/infiltration-induced PAL gene expression analysis), the 3- to 4-h (Pst DC3000-induced PAL gene activation studies), or the 24-h (PR-1 gene expression analysis) time point after wounding, infiltration of water, or Pst DC3000 infection. For the determination of callose induction, leaves were harvested at the 7-h time point after wounding or infiltration of water.
RNA Gel-Blot Analysis
Total RNA was isolated from frozen leaves using TRI-Reagent (Molecular Research Center, Cincinnati, OH) according to the manufacturer's instructions. For RNA gel-blot analysis, 5 to 10 μg of total RNA was denatured and separated on a 1.2% (w/v) agarose-2.5% (v/v) formaldehyde gel essentially as described (Thulke and Conrath, 1998). Ethidium bromide was included in the loading buffer to confirm equal sample loading. After blotting to a positively charged nylon membrane (Nytran-Plus; Schleicher & Schuell, Dassel, Germany) by downstream capillary transfer using 10× 1.5 m sodium chloride and 0.15 m sodium citrate, pH 7.0, RNA was crosslinked to the membrane by UV irradiation. Prehybridization and hybridization were performed at 65°C in 0.25 m NaHPO4, pH 7.2, 1 mm EDTA, 7% (w/v) SDS, and 1% (w/v) bovine serum albumin. Hybridization with 32P-labeled cDNA probes was for 16 h. After hybridization, the membranes were washed at 65°C for 1 h with two changes of the washing solution (40 mm NaHPO4, pH 7.2, 1 mm EDTA, 5% [w/v] SDS, and 0.5% [w/v] bovine serum albumin). Finally, blots were exposed to x-ray film (Kodak MS; Eastman-Kodak, Rochester, NY) at −70°C. For rehybridization of membranes, these were stripped of the hybridized probe by agitation in boiling 0.5% (w/v) SDS, the solution was cooled down to room temperature, and the membranes checked for any remaining radioactivity with a Geiger counter. The membranes were air dried and then hybridized to a labeled β-tubulin cDNA probe as described above.
cDNA Clones
Clones for the Arabidopsis PAL and PR-1 gene were provided by Dan Klessig (Rutgers University, New Brunswick, NJ). The expressed sequence tag clone ATTS3906 encoding β-tubulin (GenBank accession no. Z37487) was from the Arabidopsis Biological Resource Center (Ohio State University). Plasmid-DNA was harvested from respective clones, digested with restriction enzymes, and the resulting cDNA fragments were isolated by agarose gel electrophoresis. After extraction of the cDNAs from excised gel slices, they were stored at −20°C until random priming labeling and use in the hybridization experiments.
Extraction and Quantitative Determination of Callose
Extraction and measurement of callose from two to three Arabidopsis leaves was done as described (Kohler et al., 2000). Callose quantification was based on comparison with the fluorescence of known amounts of the commercial β-1,3-glucan pachyman (Calbiochem, Bad Soden, Germany). Therefore, callose concentration is given as PE g−1 leaf fresh weight.
Histochemical Examination of Callose Deposition
For visualization of callose, slices of respectively treated Arabidopsis leaves were stained with 0.1% (w/v) aniline blue (containing a β-glucan-interacting fluorochrome, sirofluor) in 1 m Gly/NaOH, pH 9.5, for 3 to 5 min. Fluorescence of callose/sirofluor complexes was detected in the tissue with a epifluorescence microscope (Carl Zeiss GmbH, Jena, Germany) using a filter set 18 (Carl Zeiss; excitation 390–420 nm, color splitter 425 nm, and secondary filter 450 nm).
All experiments shown in this study were performed at least three times with similar results.
ACKNOWLEDGMENTS
We would like to thank Xinnian Dong and Kay Lawton for providing Arabidopsis mutants and NahG-transgenic plants, respectively. We thank The Arabidopsis Biological Resource Center for providing wild-type Col-O seeds and the β-tubulin cDNA clone. We appreciate provision of bacterial stocks by Kees van Loon, Brian Staskawicz, and Jurriaan Ton. We thank Urs Neuenschwander and Bob Dietrich for supporting us with BTH and the wettable powder carrier. We are thankful to Keith Davis and Dan Klessig for providing Arabidopsis PAL- and PR-1-specific cDNA clones, respectively, and we appreciate provision of Silwet L-77 by Harald Köhle. We thank Vera Katz and Heinrich Kauss for valuable comments on the manuscript.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010744.
LITERATURE CITED
- Boch J, Verbsky ML, Robertson TL, Larkin JC, Kunkel BN. Analysis of resistance gene-mediated defense responses in Arabidopsis thaliana plants carrying a mutation in CPR5. Mol Plant-Microbe Interact. 1998;12:1196–1206. [Google Scholar]
- Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell. 1997;9:1573–1584. doi: 10.1105/tpc.9.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong X. A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell. 1994;6:1845–1857. doi: 10.1105/tpc.6.12.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron RK, Dixon RA, Lamb C. Biologically induced systemic acquired resistance in Arabidopsis thaliana. Plant J. 1994;5:715–725. [Google Scholar]
- Cameron RK, Paiva NL, Lamb CJ, Dixon RA. Accumulation of salicylic acid and PR-1 gene transcripts in relation to the systemic acquired resistance (SAR) response induced by Pseudomonas syringae pv. tomato in Arabidopsis. Physiol Mol Plant Pathol. 1999;55:121–130. [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong X. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell. 1994;8:1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell. 1997;88:57–63. doi: 10.1016/s0092-8674(00)81858-9. [DOI] [PubMed] [Google Scholar]
- Chong J, Pierell M-A, Atanassova R, Werck-Reichhart D, Fritig B, Saindenan P. Free and conjugated benzoic acid in tobacco plants and cell cultures: induced accumulation upon elicitation of defense responses and role as salicylic acid precursors. Plant Physiol. 2001;125:318–328. doi: 10.1104/pp.125.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U, Chen Z, Ricigliano JR, Klessig DF. Two inducers of plant defense responses, 2, 6-dichloroiso-nicotinic acid and salicylic acid, inhibit catalase activity in tobacco. Proc Natl Acad Sci USA. 1995;92:7143–7147. doi: 10.1073/pnas.92.16.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U, Thulke OU, Katz VA, Schwindling S, Kohler A. Priming as a mechanism in induced systemic resistance of plants. Eur J Plant Pathol. 2001;107:113–119. [Google Scholar]
- Delaney TP. Genetic dissection of acquired resistance to disease. Plant Physiol. 1997;113:5–12. doi: 10.1104/pp.113.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP. New mutants provide clues into the regulation of systemic acquired resistance. Trends Plant Sci. 2000;5:49–51. doi: 10.1016/s1360-1385(99)01552-6. [DOI] [PubMed] [Google Scholar]
- Delaney TP, Friedrich L, Ryals J. Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc Natl Acad Sci USA. 1995;92:6602–6606. doi: 10.1073/pnas.92.14.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E et al. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1249. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- Dempsey DA, Shah J, Klessig DF. Salicylic acid and disease resistance in plants. Crit Rev Plant Sci. 1999;18:547–575. [Google Scholar]
- Després C, DeLong C, Glaze S, Liu E, Fobert PR. The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell. 2000;12:279–290. [PMC free article] [PubMed] [Google Scholar]
- Friedrich L, Lawton K, Ruess W, Masner P, Specker N, Gut-Rella M, Meier B, Dincher S, Staub T, Uknes S et al. A benzothiadiazole derivative induces systemic acquired resistance in tobacco. Plant J. 1996;10:61–70. [Google Scholar]
- Hahlbrock K, Scheel D. Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:347–369. [Google Scholar]
- Hayes MP, Zoon KC. Priming of human monocytes for enhanced lipopolysaccharide responses: expression of α-interferon, interferon regulatory factors, and tumor necrosis factor. Infect Immun. 1993;61:3222–3227. doi: 10.1128/iai.61.8.3222-3227.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt MD, Ryals JA. Systemic acquired resistance signal transduction. Crit Rev Plant Sci. 1996;15:583–606. [Google Scholar]
- Katz VA, Thulke OU, Conrath U. A benzothiadiazole primes parsley cells for augmented elicitation of defense responses. Plant Physiol. 1998;117:1333–1339. doi: 10.1104/pp.117.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H. Callose and callose synthase. In: Gurr SJ, McPherson MJ, Bowles DJ, editors. Molecular Plant Pathology. Vol. 2. Oxford: Oxford University Press; 1992. pp. 1–8. [Google Scholar]
- Kauss H, Franke R, Krause K, Conrath U, Jeblick W, Grimmig B, Matern U. Conditioning of parsley (Petroselinum crispum) suspension cells increases elicitor-induced incorporation of cell wall phenolics. Plant Physiol. 1993;102:459–466. doi: 10.1104/pp.102.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H, Jeblick W. Pretreatment of parsley suspension cultures with salicylic acid enhances spontaneous and elicited production of H2O2. Plant Physiol. 1995;108:1171–1178. doi: 10.1104/pp.108.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H, Theisinger-Hinkel E, Mindermann R, Conrath U. Dichloroisonicotinic and salicylic acid, inducers of systemic acquired resistance, enhance fungal elicitor responses in parsley cells. Plant J. 1992a;2:655–660. [Google Scholar]
- Kauss H, Krause K, Jeblick W. Methyl jasmonate conditions parsley suspension cells for increased elicitation of phenylpropanoid defense responses. Biochem Biophys Res Commun. 1992b;189:304–308. doi: 10.1016/0006-291x(92)91558-8. [DOI] [PubMed] [Google Scholar]
- Kohler A, Schwindling S, Conrath U. Extraction and quantitative determination of callose from Arabidopsis leaves. BioTechniques. 2000;28:1084–1086. doi: 10.2144/00286bm06. [DOI] [PubMed] [Google Scholar]
- Lawton KA, Friedrich L, Hunt M, Weymann K, Delaney T, Kessmann H, Staub T, Ryals J. Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J. 1996;10:71–82. doi: 10.1046/j.1365-313x.1996.10010071.x. [DOI] [PubMed] [Google Scholar]
- Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Maleck K, Dietrich RA. Defense on multiple fronts: How do plants cope with diverse enemies? Trends Plant Sci. 1999;4:215–219. doi: 10.1016/s1360-1385(99)01415-6. [DOI] [PubMed] [Google Scholar]
- Métraux J-P, Ahl-Goy P, Staub T, Speich J, Steinemann A, Ryals J, Ward E. Induced resistance in cucumber in response to 2, 6-dichloroisonicotinic acid and pathogens. In: Hennecke H, Verma DPS, editors. Advances in Molecular Genetics of Plant-Microbe Interactions. Vol. 1. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. pp. 432–439. [Google Scholar]
- Mur LA, Brown IR, Darby RM, Bestwick CS, Bi Y-M, Mansfield JW, Draper J. A loss of resistance to avirulent bacterial pathogens in tobacco is associated with the attenuation of a salicylic acid-potentiated oxidative burst. Plant J. 2000;23:609–621. doi: 10.1046/j.1365-313x.2000.00825.x. [DOI] [PubMed] [Google Scholar]
- Mur LA, Naylor G, Warner SAJ, Sugars JM, White RF, Draper J. Salicylic acid potentiates defense gene expression in tissue exhibiting acquired resistance to pathogen attack. Plant J. 1996;9:559–571. [Google Scholar]
- Ryals J, Weymann K, Lawton K, Friedrich L, Ellis D, Steiner H-Y, Johnson J, Delaney TP, Jesse T, Vos P et al. The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor IκB. Plant Cell. 1997;9:425–439. doi: 10.1105/tpc.9.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner H-Y, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stennis MJ, Chandra S, Ryan CA, Low P. Systemin potentiates the oxidative burst in cultured tomato cells. Plant Physiol. 1998;117:1031–1036. doi: 10.1104/pp.117.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticher L, Mauch-Mani B, Métraux J-P. Systemic acquired resistance. Annu Rev Phytopathol. 1997;35:235–270. doi: 10.1146/annurev.phyto.35.1.235. [DOI] [PubMed] [Google Scholar]
- Thulke OU, Conrath U. Salicylic acid has a dual role in the activation of defense-related genes in parsley. Plant J. 1998;14:35–42. doi: 10.1046/j.1365-313X.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- Van Loon LC, Van Strien EA. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol. 1999;55:85–97. [Google Scholar]
- Whalen MC, Innes RW, Bent AF, Staskawicz BJ. Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell. 1991;3:49–59. doi: 10.1105/tpc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SA, Wang X, Leach JE. Changes in the plasma membrane distribution of rice phospholipase D during resistant interactions with Xanthomonas oryzae pv. oryzae. Plant Cell. 1996;8:1079–1090. doi: 10.1105/tpc.8.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I-C, Parker J, Bent AF. Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc Natl Acad Sci USA. 1998;95:7819–7824. doi: 10.1073/pnas.95.13.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fan W, Kinkema M, Li X, Dong X. Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene. Proc Natl Acad Sci USA. 1999;96:6523–6528. doi: 10.1073/pnas.96.11.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J-M, Trifa Y, Silva H, Pontier D, Lam E, Shah J, Klessig DF. NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Mol Plant-Microbe Interact. 2000;13:191–202. doi: 10.1094/MPMI.2000.13.2.191. [DOI] [PubMed] [Google Scholar]
- Zimmerli L, Jakab G, Métraux J-P, Mauch-Mani B. Potentiation of pathogen-specific defense mechanisms in Arabidopsis by β-aminobutyric acid. Proc Natl Acad Sci USA. 2000;97:12920–12925. doi: 10.1073/pnas.230416897. [DOI] [PMC free article] [PubMed] [Google Scholar]