Abstract
We investigated the mechanism of amylose synthesis in Arabidopsis leaves using 14C-labeling techniques. First, we tested the hypothesis that short malto-oligosaccharides (MOS) may act as primers for granule-bound starch synthase I. We found increased amylose synthesis in isolated starch granules supplied with ADP[14C]glucose (ADP[14C]Glc) and MOS compared with granules supplied with ADP[14C]Glc but no MOS. Furthermore, using a MOS-accumulating mutant (dpe1), we found that more amylose was synthesized than in the wild type, correlating with the amount of MOS in vivo. When wild-type and mutant plants were tested in conditions where both lines had similar MOS contents, no difference in amylose synthesis was observed. We also tested the hypothesis that branches of amylopectin might serve as the primers for granule-bound starch synthase I. In this model, elongated branches of amylopectin are subsequently cleaved to form amylose. We conducted pulse-chase experiments, supplying a pulse of ADP[14C]Glc to isolated starch granules or 14CO2 to intact plants, followed by a chase period in unlabeled substrate. We detected no transfer of label from the amylopectin fraction to the amylose fraction of starch either in isolated starch granules or in intact leaves, despite varying the time course of the experiments and using a mutant line (sex4) in which high-amylose starch is synthesized. We therefore find no evidence for amylopectin-primed amylose synthesis in Arabidopsis. We propose that MOS are the primers for amylose synthesis in Arabidopsis leaves.
Starch is composed of two glucan polymers: amylopectin and amylose. Amylopectin accounts for 70% or more of the starch from wild-type plants. It is a large, highly branched molecule, whereas amylose is smaller and much less branched. Amylopectin molecules become organized to form the semicrystalline matrix of the starch granule and amylose molecules exist in an unorganized state within this matrix (French, 1984). Amylose and amylopectin are synthesized simultaneously during starch granule biosynthesis. Mutational and antisense analysis has shown that the enzyme granule-bound starch synthase I (GBSS) is exclusively responsible for the synthesis of amylose (Shure et al., 1983; Hovenkamp-Hermelink et al., 1987; Hseih, 1988; Denyer et al., 1995; Nakamura et al., 1995). The isoforms of starch synthase responsible for the synthesis of amylopectin are located primarily in the soluble phase of the plastid with only a fraction of these proteins contained within the granule matrix. However, even when bound to the granule these isoforms do not synthesize amylose (Denyer et al., 1999).
GBSS catalyzes the transfer of the glucosyl residue of ADP-Glc onto the non-reducing end of a glucan primer, but the nature of this primer in vivo is not known. Two possibilities have been suggested. First, soluble malto-oligosaccharides (MOS) may act as primers for amylose synthesis. When supplied to isolated starch granules from pea (Pisum sativum), potato (Solanum tuberosum), and the unicellular green alga Chlamydomonas reinhardtii, MOS between two and seven Glc units in length are elongated by the addition of Glc from ADP-Glc to form amylose within the granule matrix (Denyer et al., 1996, 1999; Van de Wal et al., 1998). Second, amylopectin branches within the matrix may be elongated by GBSS then cleaved off to form amylose. Recent work with starch granules isolated from C. reinhardtii supports this idea. Van de Wal et al. (1998) found that [14C]Glc from ADP[14C]Glc was incorporated initially into the amylopectin fraction but during prolonged incubation of the granules 14C was transferred to the amylose fraction. There was also an increase in amylose content in the granules during these incubations. These results are consistent with the idea that amylopectin is the primer for GBSS and that amylose is formed by the cleavage of the elongated chain by an as-yet-unidentified enzymatic activity (Ball et al., 1998). GBSS within starch granules isolated from potato, sweet potato (Ipomoea batatas), and pea embryos can also transfer Glc from ADP-Glc to amylopectin branches (Baba et al., 1987; Denyer et al., 1996). However, there is so far no evidence for these species that the branches are cleaved off to form amylose (Denyer et al., 1999).
Both models for the priming and synthesis of amylose are based on experiments carried out in vitro. Although providing vital clues, such experiments cannot identify conclusively the nature of the primer for amylose synthesis in vivo. In the in vitro experiments, the soluble enzymes of starch synthesis and the other plastid components are washed away and amylose synthesis occurs in isolation. This may exclude factors that would influence amylose synthesis in vivo. To investigate the priming of amylose synthesis in vivo, we used Arabidopsis leaves. Because leaf starch is made directly from carbon assimilated through photosynthesis its synthesis can be studied by supplying 14CO2 during the light period.
We tested whether MOS-primed amylose synthesis may be occurring in vivo using a mutant line of Arabidopsis that accumulates MOS (dpe1; Critchley et al., 2001). This mutant line lacks disproportionating enzyme, which is involved in the metabolism of MOS during starch degradation. MOS consequently accumulate to 15 times the wild-type levels during starch mobilization at night and then decline to wild-type levels during first 4 h of the subsequent day. In the wild type, the short MOS produced during starch degradation are metabolized by disproportionating enzyme to provide longer MOS as substrates for other starch degrading enzymes. Thus, the level of MOS is low throughout the diurnal cycle. The dpe1 mutant produces starch with a considerably higher amylose content than starch produced in wild-type leaves. If MOS act as primers for amylose synthesis, this high amylose content may be accounted for by the elevated MOS in the mutant leaf in the first few hours of the day. We supplied a pulse of 14CO2 to mutant plants under conditions where they had either high or low MOS and compared the incorporation of 14C into amylose in these plants and in wild-type plants under the same conditions. We also tested whether amylopectin-primed amylose synthesis may be occurring in Arabidopsis leaves by using pulse-chase experiments to look for transfer of label from amylopectin to amylose.
Our results are consistent with the idea that MOS can act as primers for amylose synthesis, but provide no evidence for amylopectin-primed amylose synthesis in Arabidopsis leaves.
RESULTS
Amylose Synthesis in Isolated Starch Granules
In initial experiments we investigated whether starch granules isolated from Arabidopsis displayed either the MOS-primed or the amylopectin-primed amylose synthesis reported for granules from other species. First, we determined whether GBSS activity was stable in extracted Arabidopsis starch. Granules were isolated from mature leaves of wild-type plants, midway through a 12-h photoperiod, and incubated in assay medium for up to 24 h. There was no appreciable loss of GBSS activity over the first 6 h of the incubation, but after 24 h only 30% of the initial GBSS activity remained (not shown). Subsequent pulse-chase experiments were conducted over 6 h or less.
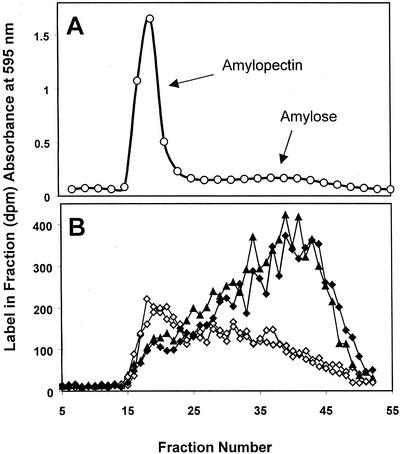
To determine whether amylose synthesis was stimulated by MOS, granules were incubated in a medium containing 1 mm ADP[14C]Glc with or without MOS (1 mm maltotriose). After 1 h, the starch granules were recovered and separated into amylose and amylopectin using Sepharose CL2B chromatography (demonstrated in Fig. 1A). The results (Fig. 1B) show that in the presence of maltotriose, incorporation of label from ADP[14C]Glc was increased and a greater proportion of the label was in the low-Mr amylose fractions than in the absence of maltotriose.
Figure 1.
The effect of malto-oligosaccharides on the incorporation of 14C from ADP[14C]Glc into isolated Arabidopsis starch granules. A, Separation of the amylopectin and amylose of wild-type starch granules using Sepharose CL2B chromatography. Fractions were mixed with an iodine solution, and the A595 was determined. B, Starch granules were incubated with ADP[14C]Glc for 1 h in the presence (black symbols) or absence (white symbols) of 1 mm maltotriose. Starch was fractionated by Sepharose CL2B chromatography and the 14C in each fraction was determined by scintillation counting. The results of two replicate experiments are shown.
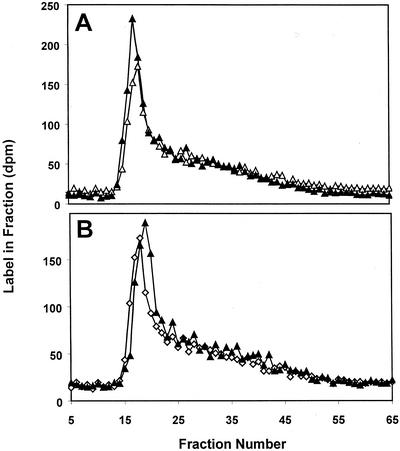
To determine whether amylopectin-primed synthesis occurred, starch granules were isolated from leaves and supplied with ADP[14C]Glc for 30 min (pulse). The ADP[14C]Glc was then removed and replaced with unlabeled ADP-Glc for a chase of either 2 or 6 h. Samples of the labeled starch granules were taken after the pulse and at the end of the chase periods. The starch was separated into amylose and amylopectin. The results (Fig. 2) show that most of the label was incorporated into the high-Mr, amylopectin-containing fractions. There was no detectable movement of label from the amylopectin fraction to the low-Mr amylose-containing fraction during the chase period.
Figure 2.
Incorporation of 14C into isolated Arabidopsis starch granules after a pulse of ADP[14C]Glc and a subsequent chase in unlabeled substrate. A, Isolated starch granules were incubated in the presence of ADP[14C]Glc for 30 min (pulse; white symbols) and for a further 2 h in unlabeled substrate (chase; black symbols). Starch was fractionated by Sepharose CL2B chromatography and the 14C in each fraction was determined by scintillation counting. The results from a representative experiment are shown. B, As described for (A) except that the 30-min pulse was followed by a 6-h chase.
Testing MOS-Primed Amylose Synthesis in Vivo
To determine whether MOS may act as primers for amylose synthesis in vivo, we compared wild-type plants with plants of the mutant line dpe1. Because this mutant contains elevated levels of MOS at the beginning of the day but normal levels later in the light period, we reasoned that it could be used to discover the effect of elevated MOS on amylose synthesis.
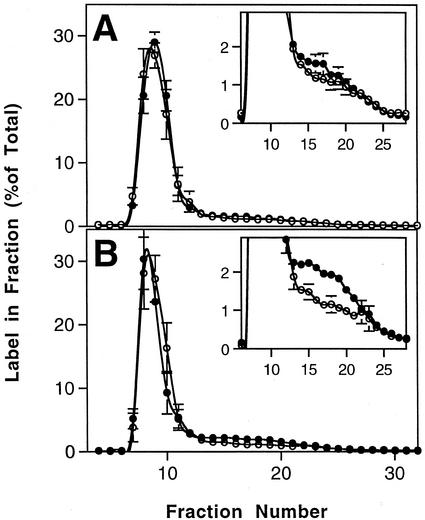
Wild-type and dpe1 plants were allowed to photosynthesize for the first 6 h of the photoperiod. One-half the plants were then transferred to darkness for 4 h (“dark-treated”), whereas the rest were left in the light (“light-treated”). Sampling plants at this stage revealed that the MOS content was low in plants in the light (11 ± 1 and 29 ± 2 μg g−1 fresh weight for the wild type and dpe1, respectively) and in the darkened wild-type plants (19 ± 4 μg g−1 fresh weight) but elevated severalfold in the darkened dpe1 plants (137 ± 2 μg g−1 fresh weight). This was specifically because of an increase in maltotriose (data not shown; Critchley et al., 2001). The darkened plants were then returned to the light, and after 15 min, all the plants were exposed to 14CO2 for a further 30 min. The plants were then harvested and frozen in liquid N2. The starch was extracted from the plants, and the amylose and amylopectin were separated by Sepharose CL2B chromatography. The percentage of label incorporated into amylose in the light-treated wild-type and dpe1 plants was the same (Fig. 3A). However, in the dark-treated plants there was significantly more incorporation of label into the amylose-containing fractions of dpe1 starch than into the same fractions of wild-type starch (Fig. 3B). Further analysis revealed that there were no statistically significant differences between the label incorporated into amylose in the wild type irrespective of light and dark treatment, but that in the darkened mutant plants, the increase in the label in amylose was significant when compared with the light-treated mutant plants. Thus, only in the darkened dpe1 plants, where there was an elevated MOS content, was there also increased synthesis of amylose.
Figure 3.
The effect of a 4-h dark pretreatment on the incorporation of 14C into starch in intact, photosynthesizing Arabidopsis plants supplied with 14CO2. A, Wild-type Arabidopsis plants were supplied with 14CO2 for 30 min after 10 h of the photoperiod (white symbols) or after 6 h of the photoperiod followed by 4 h in darkness (black symbols). Samples comprising all the leaves from a single plant were harvested and frozen in liquid N2. Starch was extracted from the leaves and fractionated by Sepharose CL2B chromatography. The 14C in each fraction was determined by scintillation counting. The results are the means and ses of four replicate samples. Label in the amylose-containing fractions is shown more clearly on an expanded y axis in the inset graph. B, Arabidopsis plants of the mutant line dpe were supplied with 14CO2 for 30 min after 10 h of the photoperiod (white symbols) or after 6 h of the photoperiod followed by 4 h in darkness (black symbols) as described in A.
A second identical experiment comparing dark-treated wild-type and mutant plants gave essentially the same results (Fig. 4A). In addition, we took replicate samples to determine the whether the amount of label incorporated into starch was different in the dark-treated wild-type and mutant plants. We found that the same amount of label was incorporated into starch in both (Table I).
Figure 4.
Analysis of the 14C-labeled, low-Mr material in the starch isolated from wild-type and dpe1 plants. A, Wild-type (white symbols) and dpe1 (black symbols) plants were supplied with 14CO2 for 30 min after 6 h of the photoperiod followed by 4 h in darkness. Samples were harvested and processed as described in Figure 3A. The results of two replicate experiments are shown. B, Low-Mr material from wild-type (white symbols) and dpe1 (black symbols) starch (fractions 12–24 in A) was collected, debranched with isoamylase, and fractionated using Sepharose CL4B chromatography.
Table I.
Assimilation of 14C from 14CO2 in leaves of wild-type and dpe1 plants
|
14C Incorporated into Each Fraction
|
||
|---|---|---|
| Wild Type | dpe1 | |
| dpm × 10−3, g−1 fresh wt | ||
| Ethanol-soluble material | 1,547 ± 125 | 1,665 ± 48 |
| Ethanol-insoluble material | 1,272 ± 91 | 1,433 ± 64 |
| Starch | 778 ± 71 | 784 ± 46 |
| Total 14C incorporated | 2,819 ± 215 | 3,098 ± 112 |
After 6 h of the photoperiod, plants were darkened for 4 h and then transferred to a sealed chamber and allowed to photosynthesize for 30 min in 14CO2. Samples comprising all the leaves of individual plants were harvested and boiled in 80% (w/v) ethanol. Total 14C in starch was determined after digestion of the insoluble material using α-amylase and amyloglucosidase. Values are the means ± se of four replicate samples.
To confirm that the labeled material synthesized in dpe1 after the dark treatment was amylose rather than a contaminating “tail” of amylopectin, fractions 12 to 24 from the Sepharose CL2B were pooled, lyophilized, and rechromatographed on the same column. Most of the labeled material from both the wild type (85%) and mutant (98%) re-eluted from the CL2B column in fractions 12 to 24. To determine if the material was largely linear, identical samples were treated with isoamylase (to hydrolyze α-1,6-linkages) and separated on Sepharose CL4B column. Amylose consists of long linear or infrequently branched chains, which would be largely unaffected by isoamylase. Any low-Mr branched material (for example nascent amylopectin molecules) would be debranched by isoamylase to yield very short chains. Most of the material from the wild type eluted late from the column (fractions 17–22), indicating that it consisted of very short chains released by the isoamylase treatment (Fig. 4B). Only 31% of the material was eluted earlier (fractions 7–16) indicating long chains. However, in the mutant, only 38% of the labeled material eluted late from the column, with 62% eluting early. These results show that the additional low-Mr material synthesized in the presence of MOS in dpe1 was amylose.
Testing Amylopectin-Primed Amylose Synthesis in Vivo
To determine whether amylopectin-primed amylose synthesis occurs in vivo, we performed pulse-chase labeling experiments, supplying 14CO2 to photosynthesizing leaves of intact plants (pulse) and then allowing photosynthesis to continue in unlabeled CO2 (chase). Transfer of label from amylopectin to amylose during the chase would imply that GBSS was elongating chains of amylopectin, which were subsequently cleaved off to form amylose. We used the wild type and the high starch mutant line sex4 (Zeeman et al., 1998; Zeeman and ap Rees, 1999). This mutant was used because it accumulates starch with a higher amylose content than the wild type. This is probably because of the increased activity of GBSS relative to soluble starch synthase in this line (S.C. Zeeman, unpublished data). Leaves of sex4 have the same MOS content as wild-type leaves (S.C. Zeeman, unpublished data).
We conducted the pulse and chase experiments over two time periods. In the first set of experiments, plants were exposed to 14CO2 for 15 min (pulse). The 14CO2 was then removed, and photosynthesis was allowed to continue in air for a further 45 min (chase). In the second set of experiments, a 1-h pulse was followed by a 5-h chase. The different time periods were used to enable detection of transfer of label over different time frames. Samples were harvested at the end of the pulse and at the end of the chase and frozen in liquid N2. Starch was extracted from the samples and the incorporation of label into amylose and amylopectin was determined using Sepharose CL2B chromatography.
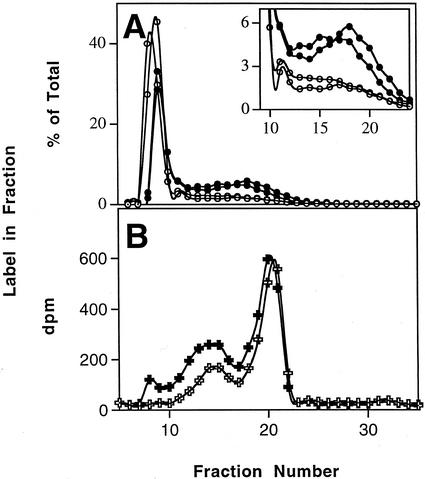
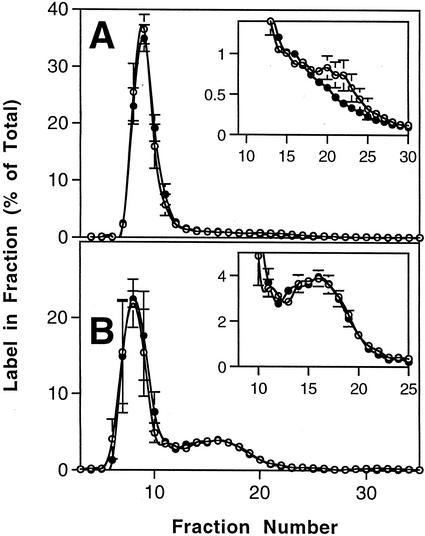
The results for the 15-min pulse and 45-min chase are presented in Figure 5. In the wild type, there was a small but statistically significant decrease in the proportion of label in the low-Mr, amylose fractions during the chase (Fig. 5A). In the mutant line sex4, the proportion of label in the amylose-containing fractions was much greater than in the wild type. In this case there was no significant change during the chase in the proportion of label in amylose (Fig. 5B). In the longer pulse-chase experiments, there were no significant changes during the chase in the proportion of label in amylose in either line (data not shown). The proportion of 14C incorporated into amylopectin and amylose in these longer experiments was similar to the proportions seen in the shorter experiments.
Figure 5.
Incorporation of 14C into starch after a pulse of 14CO2 to intact, photosynthesizing Arabidopsis plants and a subsequent chase in air. A, Wild-type Arabidopsis plants were supplied with 14CO2 for 15 min (pulse; white symbols) and then allowed to photosynthesize in air for a further 45 min (chase; black symbols). Samples were harvested and processed as described in Figure 3A. The results are the means and ses of four replicate samples. Label in the amylose-containing fractions is shown more clearly on an expanded y axis in the inset graph. B, Arabidopsis plants of the mutant line sex4 were supplied with a pulse of 14CO2 and a chase in air as described in A.
DISCUSSION
Our results, from both in vitro and in vivo experiments, indicate that maltotriose can stimulate amylose synthesis in Arabidopsis. In isolated Arabidopsis starch granules, amylose synthesis was stimulated by the presence of 1 mm maltotriose. Because similar observations have been made in isolated starch granules from pea, potato (Denyer et al., 1996), and C. reinhardtii (Van de Wal et al., 1998), it is likely that this is a widespread phenomenon. The concentration of MOS required to stimulate amylose synthesis in isolated granules is low (observed at 0.05 mm maltotriose in pea; Denyer et al., 1999) and maltose, maltotriose, and maltohexaose are all able to promote amylose synthesis (Denyer et al., 1996). However, there are few reliable measurements of the MOS concentration in plants. In developing pea embryos, the MOS content is 0.42 mg Glc equivalents g−1 fresh weight, but the nature of these MOS was not determined (Clarke, 1998). In wild-type Arabidopsis leaves, maltose comprises most of the MOS during the day, and is approximately 0.04 mg g−1 fresh weight (Critchley et al., 2001). In the present study the observed value was even lower at 0.01 mg g−1 fresh weight. Assuming that this maltose is exclusively plastidial and that the plastid compartment accounts for 8% of the Arabidopsis leaf cell volume (Leidreiter et al., 1995), this equates to a maltose concentration of 0.4 to 1.6 mm. In our in vitro experiments, a MOS concentration of 1 mm was sufficient to promote amylose synthesis. Therefore, the MOS concentration in wild-type Arabidopsis leaves is likely to be at the same level as that required to promote amylose synthesis in experiments in vitro.
In dpe1 plants with elevated MOS contents, the ratio of amylose to amylopectin synthesized was greater than in either dpe1 plants that have low levels of MOS or wild-type plants. There are two possible explanations for this observation. First, MOS may be stimulating amylose synthesis. This could result from MOS acting as a primer for amylose synthesis or from MOS stimulating GBSS without acting as a primer. The results of in vitro studies using pea embryo starch granules show that labeled maltose is elongated by GBSS to form amylose and that maltose analogs, which cannot be elongated, do not stimulate amylose synthesis (Denyer et al., 1999). This indicates that the stimulatory effect of MOS on GBSS is due to increased priming of amylose molecules. As an alternative, the elevated levels of MOS may provide additional substrates for soluble starch synthases, thereby decreasing the rate of amylopectin synthesis and giving rise to the observed increase in the proportion of label in amylose. However, our measurements of the total label in starch show that there was no decrease in incorporation into starch (per gram fresh weight of leaf tissue) in the dpe1 plants with high MOS contents, compared with the wild-type plants. Considering these data and the results from the in vitro experiments, the first explanation seems more likely. The increase in MOS in dpe1 in the present study was due specifically to an increase in maltotriose and, assuming it to be plastidial, was an increase of 2.8 mm (assuming the plastidial volume is 8% of the total cell volume).
Our results provide no evidence for amylopectin-primed amylose synthesis either in vitro or in vivo. In isolated granules supplied with a pulse of ADP[14C]Glc, significant label was incorporated into amylopectin. However, there was no detectable transfer of this label from amylopectin to the amylose during the chase in unlabeled substrate. This is similar to the situation in isolated starch granules from pea embryos (Denyer et al., 1999). In our in vivo pulse-chase experiments, very much larger amounts of label were incorporated from 14CO2 into both amylose and amylopectin, but again there was no detectable movement of this label from amylopectin to amylose. This was the case in both 1- and 6-h experiments with the wild type and the high-amylose mutant line sex4. Thus, our data indicate that amylopectin is not the primer for amylose synthesis in Arabidopsis.
These conclusions differ from those derived from experiments on C. reinhardtii by Van de Wal et al. (1998), who observed transfer of label from amylopectin to amylose in isolated granules. In our isolated granule experiments, it is possible that the amount of transfer may be too low to detect, particularly as the level of incorporation of label into starch from ADP[14C]Glc is relatively low. This may be because of the low activity of GBSS in Arabidopsis starch granules. However, in our in vivo experiments, a high level of incorporation of label into starch was achieved, indicating that a lack of sensitivity cannot account for the absence of detectable transfer. It is also possible that some transfer of label from amylopectin to amylose occurs, but it is so rapid that it is undetectable in our experiments. In our shortest pulse-chase experiment with wild-type plants (Fig. 5A), we detected transfer from the amylose-containing fractions to the amylopectin-containing fractions rather than vice versa. This may represent the formation of small low-Mr amylopectin molecules during the pulse, which are subsequently completed during the chase period.
It is possible that amylopectin-primed amylose synthesis may be particular to C. reinhardtii. The GBSS of C. reinhardtii is distinct from those of higher plants in that it has a C-terminal extension of approximately 150 amino acids in length (GenBank accession no. AAC17969). This results in a mature protein of 76 kD (Delrue et al., 1992), compared with GBSS from higher plants, which in all cases to date has a molecular mass of approximately 60 kD. This unusual protein structure may reflect a difference in the mode of action of the enzyme. On the other hand, the C. reinhardtii enzyme responds to the presence of MOS in the same manner as GBSS from higher plants and, in a mutant of C. reinhardtii that accumulates MOS (sta11), high-amylose starch is synthesized (Colleoni et al., 1999).
Our suggestion that MOS may act as primers for amylose synthesis in Arabidopsis leaves does not necessarily imply that MOS concentration is the major factor limiting amylose synthesis. It is clear from other work that several factors play a part in determining the amylose content of starch. For example, GBSS activity will affect the amylose content of starch, but in most species studied, the wild-type GBSS activity exercises very little control over the rate of amylose synthesis (Denyer et al., 2001). ADP-Glc supply is also known to affect amylose content, because the soluble isoforms of starch synthase have a higher affinity for ADP-Glc than GBSS (Van den Koornhuyse et al., 1996; Clarke et al., 1999; Lloyd et al., 1999). Finally, the space within the starch granule into which amylose can be deposited may be important. It has been proposed that amylose is primarily located in the amorphous zones of the growth rings of the starch granule (Jane et al., 1992). This consideration is particularly relevant in smaller starch granules such as those from leaves, which may not possess these higher-order structural features (S.C. Zeeman, unpublished data).
MATERIALS AND METHODS
Materials
All chemicals were obtained from Sigma Chemical Co. (Poole, Dorset, UK). Radioisotopes were supplied by Amersham Pharmacia Biotech (Amersham, Buckinghamshire, UK).
Plants and Growth Conditions
Wild-type Arabidopsis of the ecotypes Wasserilewskija (Ws) and Columbia (Col) and their mutants dpe1-1 (Ws; Critchley et al., 2001) and sex4-1 (Col; Zeeman et al., 1998; Zeeman and ap Rees, 1999) were grown in peat-based compost in a growth chamber with a 12-h light/12-h dark cycle at 20°C and 75% humidity. The irradiance was 170 μmol photons m−2 s−1. Col wild-type, Ws wild-type, and dpe1 plants were used after 4 to 5 weeks of growth, whereas sex4 plants were used after 5 to 6 weeks growth. At these ages, the plants were at equivalent developmental stages.
In Vitro Labeling, Sepharose Chromatography, and GBSS Assays
To isolate starch granules, mature leaves were harvested midway through the photoperiod and homogenized in extraction buffer containing 50 mm 3-(N-morpholino) propanesulphonic acid (MOPS), pH 7.2, and 1 mm dithiothreitol. The homogenate was filtered through Miracloth (Calbiochem, San Diego), and the starch granules were collected by centrifugation. The pellet was washed twice in extraction medium containing 0.05% (v/v) Triton X-100 and three times in extraction medium without Triton to obtain a clean starch preparation.
GBSS in isolated starch granules was assayed as described by Denyer et al. (1996). To label the products of GBSS, ADP[U-14C]Glc at a concentration of either 1 or 4 mm and a specific activity 9.3 or 2.3 GBq mol−1, respectively, was supplied in 100 μL of assay medium (Denyer et al., 1996). Each incubation contained 0.1 to 0.2 mg starch with a GBSS activity of 16 to 32 nmol min−1. The granules were incubated at 20°C in labeled ADP-Glc (“pulse”) for 30 or 60 min and with or without 1 mm maltotriose, depending on the experiment. At the end of the incubation, starch granules were collected by centrifugation, washed twice in 3 mL of aqueous 75% (v/v) methanol and 1% (w/v) KCl, and collected by centrifugation. The pellets were dried and then dissolved in 0.5 m NaOH. The amylose and amylopectin were separated by Sepharose CL2B chromatography as described in Denyer et al. (1995). For continued incubation in unlabeled substrate (“chase'), granules were washed twice and resuspended in extraction medium plus 4 mm ADP-Glc. At the end of the chase, incubations were processed in the same manner as the pulse incubations. The amount of ADP-Glc consumed during the 6-h chase was less than 25% of that supplied.
In Vivo Labeling and MOS Measurements
To label starch with 14C in vivo, intact photosynthesizing plants were exposed to 14CO2 with a specific activity between 1.23 and 2.22 MBq mmol−1 and a CO2 concentration of 1,000 μL L−1. The plants were sealed in a Perspex chamber and 14CO2 liberated by acidification of sodium [14C]bicarbonate. The light intensity was the same as that used to grow the plants, and the heat load was alleviated using a water trap (Zeeman and ap Rees, 1999). At the end of the pulse period, the 14CO2 was removed, and the pulse samples were harvested. In the pulse and chase experiments, chase samples were left in the chamber, through which air was pumped at a rate of 1.2 L min−1.
To extract the labeled starch, plants were homogenized with a pestle and mortar in ice-cold extraction medium containing 100 mm MOPS, pH 7.2, and 5 mm EDTA. SDS was added to a final concentration of 1% (w/v) and the homogenate was filtered through four layers of Miracloth and a nylon mesh (pore size, 25 μm). Starch in the filtrate was collected by centrifugation (3000g, 20°C, 5 min), washed twice with extraction medium plus SDS and five times with water. The pellets were dissolved in 0.5 m NaOH, and the amylose and amylopectin were separated by Sepharose CL2B chromatography.
Fractions from the Sepharose columns were collected, neutralized by the addition of HCl, and then lyophilized. The material was dissolved in 1 mL of 50 mm sodium acetate, pH 3.5. Then, 5,000 units of isoamylase was added, and the mixture was incubated for 16 h at 37°C. The debranched material was lyophilized, dissolved in 0.5 m NaOH, applied to a Sepharose CL4B column (8.75-mL volume, 35 cm long, and 0.56 cm in diameter), and eluted with 10 mm NaOH at a flow rate of 0.2 mL min−1. Fractions were collected every 2 min.
Malto-oligosaccharides were measured enzymatically exactly as described by Critchley et al. (2001). Between three and five replicate samples were analyzed, each comprising all the leaves of individual plants.
The distribution of total label was analyzed as follows. Plants were homogenized with a pestle and mortar in 80% (v/v) ethanol. The ethanol-insoluble material was removed by centrifugation, washed three times with 80% (v/v) ethanol, and resuspended in water. The ethanol-soluble material and the washes were pooled. To determine the total label in the insoluble material, a sample of the insoluble fraction was solubilized using Scintran tissue solubilizer (British Drug House, Poole, Dorset, UK), and the 14C was determined by scintillation counting. To determine the label in starch, samples of the insoluble material were boiled for 15 min. The starch was then digested to Glc by the addition of 100 mm sodium acetate, pH 4.8, 0.5 units of amyloglucosidase, and 6 units of α-amylase. Control samples contained no enzymes. The digests and controls were adjusted to 75% (v/v) methanol and 1% (w/v) KCl and incubated for 1 h at 4°C. Undigested starch in the control samples was precipitated, but material hydrolyzed to Glc in the digests was not. The precipitate was removed by centrifugation, and the difference in 14C in the supernatants of the digests and controls was measured.
ACKNOWLEDGMENT
We thank Dr. Kay Denyer for her valuable comments.
Footnotes
This work was funded by the Biotechnology and Biological Science Research Council (BBSRC), UK (grant no. 208/D11090) and by the Gatsby Charitable Foundation. The John Innes Centre is funded by a competitive strategic grant from the BBSRC.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010640.
LITERATURE CITED
- Baba T, Yoshii M, Kainuma K. Acceptor molecule for granule bound starch synthase from sweet potato roots. Starch-Stärke. 1987;39:52–56. [Google Scholar]
- Ball S, Van de Wal M, Visser R. Progress in understanding the biosynthesis of amylose. Trends Plant Sci. 1998;3:462–467. [Google Scholar]
- Clarke BR. The rate of starch synthesis as a determinant of starch composition. PhD thesis. UK: University of East Anglia; 1998. [Google Scholar]
- Clarke BR, Denyer K, Jenner CF, Smith AM. The relationship between the rate of starch synthesis, the adenosine 5′-diphosphoglucose concentration and the amylose content of starch in developing pea embryos. Planta. 1999;209:324–329. doi: 10.1007/s004250050639. [DOI] [PubMed] [Google Scholar]
- Colleoni C, Dauvillée D, Mouille G, Buléon A, Gallant D, Bouchet B, Morell M, Samuel M, Delrue B, d'Hulst C et al. Genetic and biochemical evidence for the involvement of α-1,4 glucanotransferases in amylopectin synthesis. Plant Physiol. 1999;120:993–1003. doi: 10.1104/pp.120.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley JH, Zeeman SC, Takaha T, Smith AM, Smith SM. A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant J. 2001;26:89–100. doi: 10.1046/j.1365-313x.2001.01012.x. [DOI] [PubMed] [Google Scholar]
- Delrue B, Fontaine T, Routier F, Decq A, Wieruszeski J-M, Van den Koornhuyse N, Maddelein M-L, Fournet B, Ball S. Waxy Chlamydomonas reinhardtii: monocellular algal mutants defective in amylose biosynthesis and granule-bound starch synthase activity accumulate a structurally modified amylopectin. J Bacteriol. 1992;174:3612–3620. doi: 10.1128/jb.174.11.3612-3620.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer K, Barber LM, Burton R, Hedley CL, Hylton CM, Johnson S, Jones DA, Marshall J, Smith AM, Tatge H et al. The isolation and characterization of novel, low-amylose mutants of Pisum sativumL. Plant Cell Environ. 1995;18:1019–1026. [Google Scholar]
- Denyer K, Clarke B, Hylton C, Tatge H, Smith AM. The elongation of amylose and amylopectin chains in isolated starch granules. Plant J. 1996;10:1135–1143. [Google Scholar]
- Denyer K, Johnson P, Zeeman S, Smith AM. The control of amylose synthesis. J Plant Physiol. 2001;158:479–487. [Google Scholar]
- Denyer K, Waite D, Motawia S, Møller BL, Smith AM. Granule-bound starch synthase I in isolated starch granules elongates malto-oligosaccharides processively. Biochem J. 1999;340:183–191. [PMC free article] [PubMed] [Google Scholar]
- French D. Organisation of starch granules. In: Whistler RL, BeMiller JN, Paschall FF, editors. Starch: Chemistry and Technology. Orlando, FL: Academic Press; 1984. pp. 183–247. [Google Scholar]
- Hovenkamp-Hermelink JHM, Jacobsen E, Ponstein AS, Visser RGF, Vos-Scheperkeuter GH, Bijmolt EW, de Vries JN, Witholt B, Feenstra WJ. Isolation of an amylose-free starch mutant of potato (Solanum tuberosum L.) Theor Appl Genet. 1987;75:217–221. [Google Scholar]
- Hseih J-S. Genetic studies of the Wx gene of sorghum (Sorghum bicolor[L.] Moench) Bot Bull Academia Sinica. 1988;29:293–299. [Google Scholar]
- Jane JL, Xu A, Radosavljevic M, Seib PA. Location of amylose in normal starch granules: I. Susceptibility of amylose and amylopectin to cross-linking reagents. Cereal Chem. 1992;69:405–409. [Google Scholar]
- Leidreiter K, Kruse A, Heineke D, Robinson DG, Heldt HW. Subcellular volumes and metabolite concentrations in potato (Solanum tuberosumcv Desiree) leaves. Bot Acta. 1995;108:439–444. [Google Scholar]
- Lloyd JR, Springer F, Buléon A, Müller-Röber B, Willmitzer L, Kossmann J. The influence of alterations in ADP glucose pyrophosphorylase activities on starch structure and composition in potato tubers. Planta. 1999;209:230–238. doi: 10.1007/s004250050627. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yamamori M, Hirano H, Hidaka S, Nagamine T. Production of waxy (amylose free) wheats. Mol Gen Genet. 1995;248:253–259. doi: 10.1007/BF02191591. [DOI] [PubMed] [Google Scholar]
- Shure M, Wessler S, Federoff N. Molecular identification and isolation of the waxylocus in maize. Cell. 1983;35:225–233. doi: 10.1016/0092-8674(83)90225-8. [DOI] [PubMed] [Google Scholar]
- Van de Wal M, D'Hulst C, Vincken JP, Buléon A, Visser R, Ball S. Amylose is synthesised in vitroby extension and cleavage from amylopectin. J Biol Chem. 1998;273:22232–22240. doi: 10.1074/jbc.273.35.22232. [DOI] [PubMed] [Google Scholar]
- Van den Koornhuyse N, Libessart N, Delrue B, Zabawinski C, Decq A, Iglesias A, Carton A, Preiss J, Ball S. Control of starch composition and structure through substrate supply in the monocellular alga Chlamydomonas reinhardtii. J Biol Chem. 1996;271:16281–16288. doi: 10.1074/jbc.271.27.16281. [DOI] [PubMed] [Google Scholar]
- Zeeman SC, ap Rees T. Changes in carbohydrate metabolism and assimilate partitioning in starch-excess mutants of Arabidopsis. Plant Cell Environ. 1999;22:1445–1453. [Google Scholar]
- Zeeman SC, Northrop F, Smith AM, ap Rees T. A starch-accumulating mutant of Arabidopsis thalianadeficient in a chloroplastic starch-hydrolyzing enzyme. Plant J. 1998;15:357–365. doi: 10.1046/j.1365-313x.1998.00213.x. [DOI] [PubMed] [Google Scholar]