Abstract
We investigated the role of transcription factors (R, SN, C1, and PL) in the regulation of anthocyanin biosynthesis by different light qualities (white, red, blue, and ultraviolet) and by cytokinin in maize (Zea mays). We analyzed anthocyanin accumulation, structural gene expression, and regulatory gene expression in the seed aleurone and the seedling mesocotyl. In the mesocotyl, white, blue, and ultraviolet-B light strongly induced anthocyanin accumulation and expression of two key structural genes. In contrast, red light had little effect. Cytokinin enhanced the response to light but was not sufficient to induce anthocyanin accumulation in darkness. Plants with the pl-bol3 allele showed high levels of anthocyanin accumulation in response to light, whereas those with the pl-W22 allele did not, demonstrating the importance of pl1 in the light response. The expression of the pl-bol3 gene, encoding an MYB-related transcription factor, was induced by light and enhanced by cytokinin in a very similar manner to the structural genes and anthocyanin accumulation. Expression of the bHLH (basic helix-loop-helix) Sn1-bol3 gene was stimulated by several light qualities, but not enhanced by cytokinin, and was less well correlated with the induction of anthocyanin biosynthesis. In the aleurone, white, red, and blue light were effective in stimulating anthocyanin accumulation and expression of the MYB-related gene C1. The bHLH R gene was constitutively expressed. We conclude that specific members of the MYB-related c1/pl1 gene family play important roles in the regulation of anthocyanin synthesis in maize in response to different light qualities and cytokinin.
Anthocyanins represent one of the most widespread classes of pigment in higher plants. They are important secondary metabolites produced in a range of organs. Anthocyanins are involved in a variety of processes during plant development and during interactions between the plant and its environment (for review, see Mol et al., 1996; Chalker-Scott, 1999).
The biochemical pathway leading to the synthesis of anthocyanin is well understood and the structural and regulatory genes involved have been cloned from many plants (for review, see Martin and Paz-Ares, 1997; Mol et al., 1998; Winkel-Shirley, 2001). In maize (Zea mays), at least 20 genes are involved in the synthesis as well as in the determination of the amount, type, and distribution of anthocyanins (Dooner et al., 1991). The structural genes, encoding enzymes catalyzing the different steps of the biosynthetic pathway and including c2, chi, f3h, a1, a2, bz1, and bz2, are controlled in a coordinated manner by the action of at least two families of regulatory genes, r1/b1 and c1/pl1, responsible for the developmental and tissue-specific pigmentation of plant and seed tissues. The r1/b1 family encodes functionally exchangeable proteins with sequence homology to the basic helix-loop-helix (bHLH) DNA-binding/dimerization domain found in the MYC oncoproteins. This family comprises the b1 and r1 genes and in certain accessions additional members such as sn1, Lc, and Hopi, distal to r1 (Chandler et al., 1989; Ludwig et al., 1989; Tonelli et al., 1991; Consonni et al., 1993; Petroni et al., 2000). The c1/pl1 family encodes proteins with sequence homology to the DNA-binding domain of the MYB-related oncoproteins (Cone et al., 1986, 1993a; Paz-Ares et al., 1986, 1987). This family shows less allelic diversity than r1/b1 and its members are characterized by functional and structural similarity (Cone et al., 1993a). c1 is required for anthocyanin synthesis only in seeds tissues such as the aleurone, the scutellum, and the embryo, whereas pl1 is necessary for the pigmentation of several tissues of the plant body and of the pericarp, the outer seed integument.
Anthocyanin biosynthesis is modulated by environmental stimuli such as light, temperature, and nutrient supply, as well as by internal stimuli such as growth regulators, metabolites, and the particular developmental stage of the competent tissue (Mol et al., 1996). Light is one of the most important environmental stimuli regulating anthocyanin accumulation and acts both as an essential stimulus and as a factor that modulates the intensity of pigmentation. Scheffler et al. (1994) demonstrated that the C1 active allele is necessary for light induction of the C2 structural gene in the aleurone of germinating seeds. In addition, expression of the Sn1-bol3 gene is modulated by light in the pericarp layer of the seed (Procissi et al., 1997) and in the mesocotyl, according to the sn1 allele studied (Tonelli et al., 1991, 1994). The Pl gene is unaffected by light (i.e. Pl-Rh allele), whereas some pl1 alleles are induced by light (i.e. “sun red” pl; Cone et al., 1993b). Analogously, the C1 gene appears to be constitutively activated in the aleurone during seed development, although some c1 alleles show light inducibility during seed germination (Scheffler et al., 1994). Moreover, in pericarp, both Sn1-bol3 and pl expression are light modulated, whereas in aleurone R-sc is constitutively expressed and C1 shows light inducibility (Procissi et al., 1997). The light-induced expression of the MYB genes C1 and pl was found to be the limiting factor for conferring the developmental competence of the pericarp and the aleurone layers to respond to light (Procissi et al., 1997). The expression of the r1 gene Hopi in scutellum is not enhanced by light and is limited to the germination phase, whereas the accumulation of C1 transcript is under both developmental and light control (Petroni et al., 2000).
Little is known about the role of different light qualities in the modulation of anthocyanin synthesis and accumulation in maize. Mereghetti et al. (1991) determined the kinetics of light-induced pigment accumulation in pericarp and aleurone. The aleurone responds to white, red, and blue light by increasing its pigment content up to 72 h of irradiation. Pericarp tissue responds to light to a lesser extent reaching the highest value between 24 and 48 h of continuous illumination with blue and white light; red light, on the other hand, induces only a negligible response. A similar analysis has been performed in maize roots. Irradiation of seedling root tissues with different light qualities resulted in a significant increase in anthocyanin only in response to blue light (Galbiati et al., 1994).
Plant growth regulators are also important in controlling anthocyanin biosynthesis (Mol et al., 1996). For instance, gibberellins stimulate anthocyanin accumulation in petunia (Petunia hybrida) corolla tissue (Weiss et al., 1992) and abscisic acid modulates anthocyanin accumulation in maize seeds by its ability to regulate C1 gene expression (Kao et al., 1996). Cytokinin treatment stimulates anthocyanin accumulation in tissue culture and plant organs. In Arabidopsis seedlings, this increase is due to the coordinate increased accumulation of mRNAs encoded by four genes in the anthocyanin biosynthetic pathway that also appear to be controlled by a circadian clock (Deikman and Hammer, 1995).
Here, we address the regulatory mechanisms underlying the accumulation of anthocyanin in maize aleurone and mesocotyl tissues in response to different light qualities and cytokinin. We define the effects of different light qualities, show that the two tissues differ markedly in their responsiveness to red light, and demonstrate that cytokinin enhances the effect of light in mesocotyls. We report the regulation by light qualities and cytokinin of MYB-related and bHLH maize genes involved in the control of anthocyanin biosynthesis. We conclude that the accumulation of anthocyanin, and induction of anthocyanin structural genes, is most closely correlated with expression of the relevant MYB regulatory genes.
RESULTS
Different Effects of Red Light on Anthocyanin Gene Expression in Seeds and Seedlings
Because light regulation of anthocyanin biosynthesis is mediated through transcriptional activation of the biosynthetic genes, the question we addressed is whether light induces structural gene expression through the same transcription factors that control tissue-specific pigment accumulation, or through a different set of regulatory genes. Moreover, we asked whether genes of the r1/b1 and c1/pl1 families were themselves light regulated because this would implicate them as effectors of light signal transduction.
Although responses to light occur throughout the life of the plant, they are especially evident in the young seedling. We determined anthocyanin content in homozygous r-Δ Sn1-bol3 pl-bol3 and homozygous r-Δ Sn1-bol3 pl-W22 mesocotyls after exposure to different light qualities: white, blue, and red light (Table I). In each case, we measured pigment accumulation over 72 h illumination. In r-Δ Sn1-bol3 pl-bol3 mesocotyls, no anthocyanin accumulation was detected in darkness, whereas both white and blue light induced a strong increase in pigment content. There was a difference in the effect of these light qualities because after 72 h of exposure, the anthocyanin content in white light exceeded that in blue light. In contrast, red light induced only a very weak response. Homozygous r-Δ Sn1-bol3 pl-W22 mesocotyls showed a much smaller response to all three types of light treatment than r-Δ Sn1-bol3 pl-bol3 mesocotyls, confirming a higher accumulation in white- and blue-light treatments than in the red one (Table I).
Table I.
Anthocyanin accumulation in the mesocotyl of 5-d-old dark-grown r-Δ Sn1-bol3 pl-bol3 and r-Δ Sn1-bol3 pl-W22 seedlings after 72 h subsequent treatment with different light quality
| Line | Light Treatment
|
|||||
|---|---|---|---|---|---|---|
| Dark | White | Blue | Red | UV-A | UV-B | |
| pl-bol3 | 0.04 | 9.01 | 4.88 | 0.86 | 2.42 | 6.98 |
| pl-W22 | 0.08 | 1.17 | 1.22 | 0.11 | 0.41 | 1.31 |
Mean values are expressed as A530 per mesocotyl. Mean ses are below 5%. For light treatments, see “Materials and Methods.”
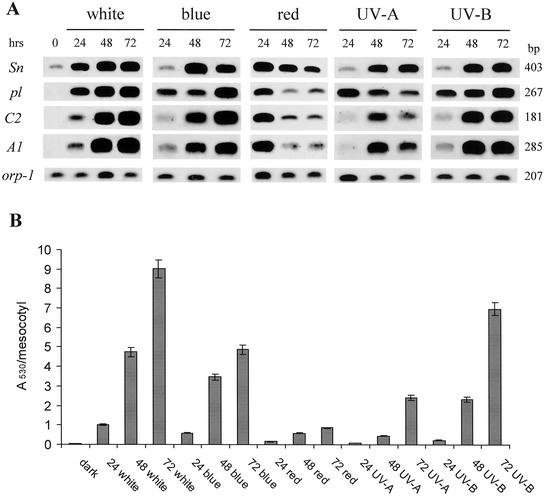
Homozygous lines used in the experiments differed only in their pl1 locus constitution (pl-bol3 versus pl-W22). Therefore, the anthocyanin accumulation data suggest that the regulatory gene pl1 could be the main factor determining the response of the tissue to different light qualities. It is known that sn1 and pl1 genes mediate the transcriptional control of structural genes involved in anthocyanin deposition (Procissi et al., 1997). To analyze anthocyanin gene expression, we measured transcript levels of two key structural genes, C2 (chalcone synthase) and A1 (dihydroflavonol reductase), and of sn1 and pl1 regulatory genes by reverse transcriptase (RT)-PCR in homozygous r-Δ Sn1-bol3 pl-bol3 mesocotyls after exposure to white, red, and blue light for 24, 48, and 72 h (Fig. 1A). The expression of the C2 gene was well correlated with anthocyanin accumulation after white- and blue-light treatments (Fig. 1B). Both treatments induced an increase in the steady-state level of the C2 transcript up to 72 h of illumination. In contrast, after 24 h of exposure to red light, we observed a strong induction of the C2 transcript that was followed by a severe reduction after 48 and 72 h. No expression was observed in the absence of light. Analysis of A1 gene expression gave very similar results.
Figure 1.
A, RT-PCR analysis of mRNA accumulation of anthocyanin structural and regulatory genes in r-Δ Sn1-bol3 pl-bol3 seedlings exposed to continuous white, blue, red, UV-A, and UV-B light for 0, 24, 48, or 72 h. cDNA was made from total RNA extracted from mesocotyls. Specific primers for the Sn, pl, C2, and A1 genes were used to amplify the cDNA (see “Materials and Methods”). Amplifications were carried out for 20 cycles. The amplification of the orp-1 transcript was used as an internal control. The blots were hybridized with the different probes (see “Materials and Methods”). B, Anthocyanin accumulation in mesocotyl was measured in each treatment as described in A. Values are expressed as A530 per mesocotyl. Mean values represent 10 independent replicates. Mean ses are below 5%.
Analysis of the expression pattern of the MYB gene pl-bol3 highlighted that the pl-bol3 transcript was absent in the dark but was strongly induced after 24 h of exposure to white, blue, and red light. We observed a further increase of pl-bol3 gene expression up to 72 h of white and blue illumination. On the contrary, after red treatment, the initial induction was followed by a strong subsequent decrease in transcript level. Analysis of Sn1-bol3 expression showed that it was expressed in the dark at a low level. After exposure to white light, its transcript increased up to 72 h of treatment. Treatment with blue light did not have any effect at 24 h, whereas 48 and 72 h of illumination induced mRNA accumulation. In contrast, red-light treatment strongly induced Sn1-bol3 expression after 24 h, followed by a subsequent decrease in mRNA level. In this case, the decrease in transcript level was less pronounced compared with pl-bol3. Therefore, from this RT-PCR analysis it appeared that, even if the bHLH-like Sn1-bol3 gene is necessary for the full transactivation of the A1 and C2 structural genes, the ability of the mesocotyl to respond to different light qualities was most closely correlated with the expression of the MYB-related regulatory gene. This result is strengthened by the observation that in r-Δ Sn1-bol3 pl-W22 mesocotyl, the pl-W22 allele is less induced by white light than pl-bol3 and this lower expression is well correlated to the structural genes transcript levels (data not shown).
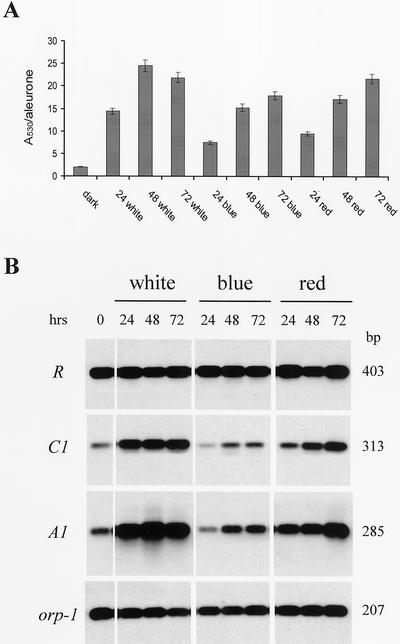
To discover whether this observation was also valid for other members of the bHLH and MYB regulatory gene families, the same analysis was performed in the aleurone of R-sc C1 homozygous seeds. Pigmentation of the external layer of the endosperm is due to the concomitant expression of the r1 and c1 genes. Figure 2A shows that different results were obtained for the aleurone compared with the mesocotyl. White-, blue-, and red-light treatments were all able to induce anthocyanin accumulation, although to slightly different extents. It is interesting that red light-induced anthocyanin accumulation almost as much as white light. Weak but detectable anthocyanin accumulation was observed in darkness. The expression pattern of the structural gene A1 was well correlated with anthocyanin accumulation (Fig. 2B). A1 was feebly expressed in the absence of light but was strongly active after white- and red-light treatments. Peaks of expression were observed after 48 h of white-light exposure and after 72 h of red-light treatment. In contrast, blue light induced only a moderate expression of the structural gene. Analysis of regulatory gene activity showed that expression of the R-sc gene was constitutive in all treatments performed. In contrast, the C1 gene expression pattern was very similar to that of A1, being weak in darkness and strongest in white light. Red light stimulated expression over 72 h, whereas blue light had only a slight effect on C1 expression after 48 and 72 h.
Figure 2.
A, RT-PCR analysis of mRNA accumulation of anthocyanin structural and regulatory genes in R-sc sn C1 aleurones of 30-d after pollination seeds exposed to continuous white, blu,e and red light for 0, 24, 48, or 72 h. cDNA was made from total RNA extracted from aleurones. Specific primers for the R, C1, and A1 genes were used to amplify the cDNA (see “Materials and Methods”). Amplifications were carried out for 20 cycles. The amplification of the orp-1 transcript was used as an internal control. The blots were hybridized with the different probes (see “Materials and Methods”). B, Anthocyanin accumulation in aleurones was measured in each treatment as described in A. Values are expressed as A530 per aleurone. Mean values represent 10 independent replicates. Mean ses are below 5%.
We emphasise that red light was able to induce anthocyanin accumulation in aleurone but much less so in mesocotyl. Both structural and regulatory genes were strongly expressed in red light in the seed, whereas their expression was transient in the young plant, decreasing to a very low level after the initial induction.
UV-B Light Greatly Induces Anthocyanin Accumulation in Mesocotyls
One of the most important abiotic stresses that plants experience is UV irradiation. With the aim to understand how different light qualities can modulate anthocyanin biosynthesis, we analyzed the response of the mesocotyl to UV-A and UV-B light treatments. As shown in Table I and Figure 1B, UV light is able to induce anthocyanin accumulation in homozygous r-Δ Sn1-bol3 pl-bol3 mesocotyls; in particular, UV-B light was very effective. The UV-B light induction was, in fact, almost comparable with that in white light. However, UV-A light induced a moderate response only after 72 h of treatment (Fig. 1B). Also, in r-Δ Sn1-bol3 pl-W22 mesocotyls, the UV-B light is more effective than UV-A light in inducing anthocyanin accumulation, although the levels are still much lower than in r-Δ Sn1-bol3 pl-bol3 as already observed for the other light treatments (Table I).
Analysis of mRNA accumulation of the structural A1 and C2 genes revealed very low expression after 24 h of exposure to UV-A or UV-B light (Fig. 1A). Considerable transcript accumulation was observed only after 48 and 72 h of illumination. Moreover, UV-B light treatment was able to induce stronger A1 and C2 expression compared with UV-A at each time analyzed. The MYB-related gene pl-bol3 was expressed at a high level even after 24 h of treatment with both light qualities. Subsequently, the pl-bol3 transcript level slightly increased after exposure to UV-B light, whereas it decreased after exposure to UV-A light. In contrast, Sn1-bol3 was only feebly expressed after 24 h, whereas its transcript level increased after longer exposure. This increase was to a similar extent in both light qualities. We conclude that pigment accumulation and structural gene mRNA levels in the mesocotyl are most closely correlated with expression of pl-bol3.
MYB-Related Genes Mediate the Effects of Cytokinin on Anthocyanin Accumulation in Maize
To test the effects of cytokinin on maize anthocyanin accumulation, homozygous r-Δ Sn1-bol3 pl-bol3 and homozygous r-Δ Sn1-bol3 pl-W22 plantlets were grown in the presence of different concentrations (0.5 and 25 μm) of the synthetic cytokinin benzyladenine (BA) for 10 d in darkness and then exposed to continuous white light for 48 h. Even at the lower concentration of BA tested, pigments accumulated more in r-Δ Sn1-bol3 pl-bol3-treated plants than in the controls (Table II). At 25 μm BA, the anthocyanin amount in treated plants was 5-fold greater than in the controls. In contrast, both treatments were unable to induce a detectable response in homozygous r-Δ Sn1-bol3 pl-W22 mesocotyls. Therefore, the pl gene seems to be the key factor for pigment accumulation in response to cytokinin application.
Table II.
Anthocyanin accumulation in the mesocotyl of r-Δ Sn1-bol3 pl-bol3 and r-Δ Sn1-bol3 pl-W22 plantlets grown for 10 d in the dark with BA at 0, 0.5, and 25 μm, followed by 2 d in the light
| Line | BA Treatment
|
||
|---|---|---|---|
| Control | 0.5 | 25 | |
| μm | |||
| pl-bol3 | 0.47 | 0.78 | 2.32 |
| pl-W22 | 0.11 | 0.11 | 0.12 |
Mean values are expressed as A530 per mesocotyl. Mean ses are below 5%. For hormone treatments, see “Materials and Methods.”
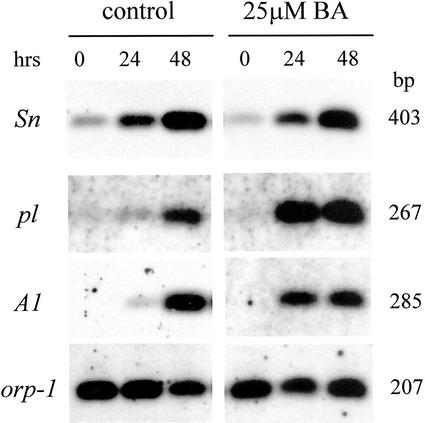
To analyze anthocyanin gene expression in response to cytokinin treatment, we performed a RT-PCR analysis (Fig. 3) on mesocotyls maintained in darkness for 7 d, watered with BA solution (25 μm), and then exposed for increasing periods of time to continuous white light (0, 24, and 48 h). First, we found that no expression of the A1 structural gene was observed in the absence of light in control and cytokinin-treated plants. Therefore, cytokinin alone is insufficient to induce the response. Second, BA treatment induced an increase in the steady-state level of the transcripts of both structural (A1) and MYB-related regulatory (pl-bol3) genes. This effect was particularly evident after 24 h of exposure to light. An effect of cytokinin was not observed for Sn1-bol3. A similar increase in Sn1-bol3 transcripts was observed in treated and control plants after exposure to white light. Therefore, cytokinin acts to enhance the effect of the light stimulus in maize mesocotyls and this effect is correlated with the ability of the hormone to affect the level of the MYB-related regulatory gene transcript.
Figure 3.
RT-PCR analysis of mRNA accumulation of anthocyanin structural and regulatory genes in r-Δ Sn1-bol3 pl-bol3 seedlings treated with a 25 μm BA solution for 7 d in darkness and subsequently exposed to continuous white light for 0, 24, and 48 h. Control plants were maintained in the same conditions. cDNA was made from total RNA extracted from mesocotyls. Specific primers for the Sn, pl, and A1 genes were used to amplify the cDNA (see “Materials and Methods”). Amplifications were carried out for 20 cycles. The amplification of the orp-1 transcript was used as an internal control. The blots were hybridized with the different probes (see “Materials and Methods”).
DISCUSSION
Our aim was to investigate the role of specific transcription factors in the regulation of anthocyanin biosynthesis by different light qualities and by cytokinin in maize. Different maize tissues are characterized by the expression of different combinations of r1/b1 and c1/pl1 regulatory genes, and the corresponding transcription factors mediate the tissue-specific regulation of anthocyanin biosynthesis (Cone et al., 1993b; Consonni et al., 1993). Moreover, several studies have indicated a role for specific transcription factors in light-induced anthocyanin accumulation in maize (Scheffler et al., 1994; Procissi et al., 1997). However, the function of these transcription factors in responses to different light qualities and to cytokinin was unknown. Therefore, we analyzed anthocyanin accumulation, anthocyanin structural and regulatory gene expression in different tissues, a seed tissue (the aleurone), and a plant shoot tissue (the mesocotyl) after light and hormone treatments.
In the mesocotyl, light strongly induces anthocyanin accumulation and expression of two key structural genes, C2 and A1. There is no detectable accumulation in darkness. The induction is strongest in white, blue, and UV-B light. This is similar to the regulation of anthocyanin biosynthesis in several species; for instance, mature Arabidopsis leaf tissue (Jackson et al., 1995; Fuglevand et al., 1996). Blue light is likely to be detected by a cryptochrome photoreceptor in maize, whereas UV-B is not detected by cryptochromes, at least in Arabidopsis (Fuglevand et al., 1996; Wade et al., 2001). The relatively small effect of red light on anthocyanin accumulation in the mesocotyl indicates a low level of responsiveness to light-stable phytochrome. Although not examined here, it is possible that far-red light, detected by a light-labile phytochrome, would have been effective. In contrast, the aleurone has detectable anthocyanin accumulation and structural gene expression in darkness and a strong response to red light, presumably mediated by phytochrome. A similar observation was reported by Mereghetti et al. (1991). The response to blue light in the aleurone could be mediated by cryptochrome, or phytochrome, but this is unknown at present. It is interesting that 24 h of illumination with red light strongly induces A1 and C2 transcript accumulation in the mesocotyl, but these accumulations are transient and no significant anthocyanin accumulation ensues. The possibility of a translational or posttranslational control merits investigation. Similarly, Noh and Spalding (1998) reported that an anion channel blocker inhibited anthocyanin accumulation in response to blue light in Arabidopsis, but did not prevent light induction of transcripts or protein of several biosynthetic enzymes, suggesting a posttranslational control.
The pl-bol3 allele encoding the MYB-related transcription factor is strongly light induced in the mesocotyl. The pattern of pl-bol3 regulation by different light qualities, for instance in blue versus red light and UV-B versus UV-A, closely correlates with that of the structural genes and anthocyanin accumulation. A sustained increase in pl-bol3 transcript accumulation is seen in white, blue, and UV-B light, in parallel with continued anthocyanin accumulation, whereas in red light pl-bol3 transcript accumulation is transient. The much weaker white, blue, and UV-B light response of plants possessing the pl-W22 allele demonstrates the importance of the MYB transcription factor in mediating light induction. We hypothesize that cryptochrome and UV-B light signal transduction pathways promote rapid induction of the pl-bol3 gene and that the encoded MYB-related factor mediates expression of the anthocyanin biosynthetic genes. An MYB-related transcription factor also has a key role in regulating anthocyanin structural gene expression in other species, such as parsley (Petroselinum crispum; Feldbrugge et al., 1997) and Arabidopsis (Hartmann et al., 1998; Borevitz et al., 2000; Harmer et al., 2000). In these species, the identities of the specific MYB-related genes that mediate light induction are not yet clear. MYBs are encoded by large gene families and light is reported to induce the expression of several MYB-related genes (e.g. in Arabidopsis; Kranz et al., 1998), so identification of the genes mediating specific responses is difficult. The effect of cytokinin is to enhance the response to light. Exogenous cytokinin is insufficient to stimulate A1 gene expression and anthocyanin accumulation in darkness in the mesocotyl. Similar results were found with Arabidopsis seedlings grown in a light/dark cycle: Cytokinin enhanced anthocyanin accumulation and biosynthetic gene expression during the photoperiod (Deikman and Hammer, 1995). However, in contrast, the addition of cytokinin to dark-grown Arabidopsis seedlings stimulated activity of the chalcone synthase gene promoter (Chory et al., 1991). The effect of cytokinin in the maize mesocotyl appears to be mediated by the pl1 allele. We observed a hyper-stimulation of pl-bol3 expression in the light in the presence of cytokinin. In contrast, there was no effect of cytokinin on light induced expression of the Sn1-bol3 allele. Moreover, the much reduced cytokinin response of plants possessing the pl-W22 allele highlights the importance of the MYB-related gene in mediating the response to cytokinin.
In the aleurone, the C1 gene is important in mediating the effects of light (Scheffler et al., 1994; Petroni et al., 2000). In darkness, C1 shows a low level of expression and there is a small amount of anthocyanin accumulation. White and red light promote a strong, sustained increase in C1 transcripts, and these treatments produce the highest levels of structural gene expression and anthocyanin accumulation. Blue light elicits the least response in terms of C1 expression and anthocyanin biosynthesis. Nevertheless, substantial levels of anthocyanin are formed despite the small increase in C1 expression. In fact, after 24 h of blue light, anthocyanin accumulates in the absence of any increase in C1, or A1, expression. This observation suggests that the response to blue light in this tissue may involve additional factors, such as the posttranslational control of preexisting enzymes.
Although the importance of the pl1/c1 regulatory gene family in responses to light and cytokinin is highlighted by our findings, the role of the r1/sn1 genes should not be diminished because at least one member of this family must be expressed to activate the structural genes. In aleurone tissue, expression of the R allele is not affected by light, in contrast to C1. Previous studies have shown that Sn1-bol3 gene expression is modulated by light in several tissues (Tonelli et al., 1991, 1994; Procissi et al., 1997). Here, we show that Sn1-bol3 gene expression is light induced in mesocotyls somewhat differently to pl-bol3. Moreover, Sn1-bol3 expression is less well correlated with structural gene expression and anthocyanin accumulation than pl-bol3 expression; this is evident, for example, in red versus blue light, UV-B versus UV-A light, and in the response to cytokinin. Furthermore, the presence of Sn1-bol3 transcripts in darkness in the mesocotyl is insufficient to induce anthocyanin accumulation.
In summary, our research extends previous studies in maize of the effects of light on anthocyanin accumulation and the light regulation of transcription factors controlling anthocyanin biosynthesis. We report the effects of different light qualities on the expression of specific transcription factors and correlate these with the biosynthesis of anthocyanin in both the mesocotyl and aleurone. Our findings include the first data on the mechanisms underlying the UV-B induction and cytokinin regulation of anthocyanin accumulation in maize. The results point to a key role for MYB-related transcription factors in mediating the responses.
MATERIALS AND METHODS
Plant Materials
All maize (Zea mays) seed stocks used in this study were in the W22 background and were homozygous dominant for the color factors a1, a2, c1, c2, bz1, and bz2, and homozygous recessive for the b1 gene. However, they differed in r1, sn1, and pl1 gene constitution. r1, sn1, and pl1 were collected from diverse sources and incorporated by backcrossing into the background of inbred W22. R-sc is self-colored aleurone from green plants, a germinal derivative of the R-st, composed of (Sc)(I-R)(Nc) and obtained by loss of the (I-R) component (Kermicle, 1984; Ronchi et al., 1995). sn1 is a factor lying two map units distal to r1 conferring specific pigmentation, after light exposure, to the scutellar node, mesocotyl tissue, leaf base, midrib, and to seed integuments (glumes and pericarp). Three independent accessions (bol1, bol2, and bol3) have been identified in separate Bolivian populations. Sn1-bol3 differs from the others in that it confers, after light exposure, a higher pigmentation level to mesocotyls. Detailed descriptions of the origin, phenotypes, and structural characteristics of the pl1 locus can be found in Cone et al. (1993a, 1993b). The genetic stocks used are as follows: (a) R-sc Sn1-bol3 C1 is a line homozygous for R-sc and C1 and devoid of the Sn1-bol3 gene. This line allows the detection of pigment in the aleurone. R-sc and C1 are expressed in the aleurone where they lead to homogeneous pigmentation. (b) r-Δ Sn1-bol3 pl-bol3 is a line homozygous for r-Δ, Sn1-bol3, and pl-bol3 genes. r-Δ indicates an interstitial deletion involving a region of the long arm of chromosome 10 containing the r1 locus. Plant and seed tissues homozygous for the deficiency are totally devoid of pigment (Alleman and Kermicle, 1993) unless they contain a functional sn1 allele. r-Δ Sn1-bol3 plants have been obtained by crossing heterozygous r-r Sn1-bol3/r-Δ females to r-Δ/r-Δ males (Consonni et al., 1997). pl-bol3 is a pl1 allele conferring high mesocotyl pigmentation upon light treatment and recessive to Pl-Rh (Ronchi et al., 1998). (c) r-Δ Sn1-bol3 pl-W22 is a homozygous line bearing the pl-W22 allele. pl-W22 is the recessive pl1 allele residing in the W22 line.
Light Treatment
Immature ears at 30 d after pollination were cut longitudinally into two halves and placed in plastic boxes layered with 0.9% (w/v) agar. They were then exposed to continuous white, blue, and red light for 0, 24, 48, or 72 h at 22°C. At the end of the light treatments, seeds were excised and anthocyanin or total RNA was extracted.
For mesocotyl analysis, seeds were allowed to germinate in darkness for 5 d at 25°C until a mesocotyl approximately 3 cm long had developed. Seedlings were then exposed to continuous light for 0, 24, 48, and 72 h at 21°C. At the end of the treatment with white, blue, red, UV-A, and UV-B light, mesocotyls were sampled and anthocyanin and total RNA was extracted. Illumination was performed in controlled-environment rooms at 21°C.
White light was provided by cool-white (F36T12/CW/HO) fluorescent tubes (21 W m−2) from GTE Sylvania (Lighting Products Group, Danvers, MA). Red light was obtained by covering the special phosphor red (F36T12/236/HO) fluorescent lamps from GTE Sylvania with one layer of RESCOLUX number 27 red filter (Rosco, Port Chester, NY), which emit light between 610 and 690 nm with a λmax of 660 nm. The fluence rate was 125 μmol m−2 s−1. Blue light was obtained by covering the special phosphor blue (F36T12/246/HO) fluorescent lamps from GTE Sylvania with one layer of RESCOLUX N° 83 blue filter (Rosco), which emit light between 400 and 490 nm with a λmax of 434 nm. The fluence rate was 88 μmol m−2 s−1. UV-A light was provided by TLK 40W/10R UV-A lamps (Philips, London), which emit light between 350 and 400 nm with a λmax of 370 nm. The fluence rate was 21 μmol m−2 s−1. UV-B light was provided by TL 20W/12RS UV-B lamp (Philips), covered with a cellulose acetate filter, and changed each 24 h to remove UV-C wavelenghts. The fluence rate was 5 μmol m−2 s−1 (280–320 nm).
Cytokinin Treatment
For cytokinin treatment, the synthetic hormone BA was dissolved initially in a small volume of 1 n KOH and then diluted to the final concentration with water. Plants were grown on 3 m filter paper (Whatman, Clifton, NJ) and watered with different BA solutions (0.5 and 25 μm) for 10 d in darkness at 25°C. Plantlets were then exposed to continuous white light for 48 h and anthocyanins were extracted. Control plants were watered with the same final concentration of KOH and maintained in the same conditions.
For kinetic experiments, plants were grown for 7 d in darkness at 25°C in the presence of the BA solution (25 μm) and then exposed to continuous white light for 0, 24, and 48 h. Control plants were maintained in the same conditions. At the end of the treatment, mesocotyls were sampled and total RNA was extracted.
Anthocyanin Determination
Anthocyanins were extracted by grinding a single seed or mesocotyl in a precooled mortar with 1 mL of cold ethanol containing 1% (v/v) HCl. Extracts were centrifuged twice and absorption determined spectrophotometrically at 530 nm. Anthocyanin concentration is expressed as absorbance value at 530 nm per seed or per mesocotyl. Mean values represent 10 independent replicates. ses of means are below 5%.
RNA Isolation and RT-PCR Analysis
Total RNA was isolated from mesocotyls and aleurones as previously described (van Tunen et al., 1988). All RNA samples were treated with DNaseI (Boehringer, Mannheim, Germany) before cDNA synthesis. First strand cDNA synthesis was carried out from 5 μg of total RNA with an oligo(dT) and RT SuperscriptII as recommended by the manufacturers (Life Technologies, Gaithersburg, MD). The primer used was a 35-base oligonucleotide with 17dT residues and a sequence adapter (5′-GGGAATTCGTCGACAAGC-3′; Frohman, 1990). First strand cDNA was used as a template for PCR amplification. Amplification reactions containing an aliquot of cDNA; 1× Promega (Madison, WI) polymerase buffer; 2.5 mm MgCl2; 200 μm each of dATP, dCTP, dGTP, and dTTP; 0.1 μm of each primer; and 1 unit of Taq DNA polymerase (Promega) were performed in a final volume of 50 μL. After the first denaturation step (5 min at 94°C), the reaction mix underwent 20 cycles of denaturation at 94°C for 45 s, annealing at 60°C for 1 min and extension at 72°C for 2 min. A final extension at 72°C for 5 min was performed to complete the reaction.
A set of primers specific for the orp-1 (orange pericarp-1) gene, which encodes the β-subunit of Trp synthase (Wright et al., 1992), were used to standardize the concentration of different samples. An orp-1-specific sequence was amplified using the following primers: upstream primer, 5′-AAGGACGTGCACACCGC-3′; and downstream primer, 5′-CAGATACAGAACAACAACTC-3′. The length of the amplified product was 207 bp. Several cycles of successive cDNA dilution and orp1 amplification and hybridization were done to obtain a similar signal of amplification among the different samples. To ensure that amplification reactions were within linear range, the reactions were carried out for 20 cycles.
PCR products were fractionated on 1.2% (w/v) agarose gels, transferred onto Hybond N+ nylon membranes (Amersham, Buckinghamshire, UK), and hybridized with random primed fluorescein fragments (Amersham) according to the manufacturer's protocols.
For mRNA detection of the genes under analysis, the following specific primer sets were used: for R-sc and Sn1-bol3, OR31 (upstream primer 5′-ATGGCTTCATGGGGCTTAGATAC-3′) and OR32 (downstream primer 5′-GAATGCAACCAAACACCTTATGCC-3′); for C1, PL6 (upstream primer 5′-TCGGACGACTGCAGCTCGGC-3′) and AC1 (downstream primer 5′-CACCGTGCCTAATTTCCTGTCCGA-3′); for pl-bol3, PL6 (upstream primer 5′-TCGGACGACTGCAGCTCGGC-3′) and PL8 (downstream primer 5′-GATTATATTGTTTACACGATGAAG-3′); for A1, A1 (upstream primer 5′-TTCTCGTCCAAGAAGCTCCAGGA-3′) and A2 (downstream primer 5′-CAATTCGTTGAACATGGAAGTAAG-3′); and for C2, CHS1 (upstream primer 5′-TCGACGAGATGCGCAAGCGCT-3′) and CHS2 (downstream primer 5′-GAATTTGATCGTTGATGAATC-3′).
The sizes of the amplified products were 403 bp for R-sc and Sn1-bol3, 313 bp for C1, 267 bp for pl-bol3, 285 bp for A1, and 181 bp for C2. The R-sc and Sn1-bol3 PCR products were hybridized with the 1.4-kb PstI-EcoRI fragment of Sn1-bol3 cDNA (Tonelli et al., 1991), the C1 products with the 1.2-kb EcoRI fragment of Pl-Rh cDNA (Cone et al., 1993b), the pl-bol3 products with the XhoI-DraI fragment of Pl-Rh cDNA (Cone et al., 1993b), the A1 products with a 700-bp BamHI fragment of the A1 gene (Schwarz-Sommer et al., 1987), and the C2 products with the PCR fragment obtained by amplification of a C2 genomic clone using the CHS1 and CHS2 primers.
ACKNOWLEDGMENT
The authors are very grateful to Cristina Bandera for help in this project.
Footnotes
This work was supported by Ministero Delle Politiche Agricole E Forestali Progetto Biotecnologie Vegetali (Area 1, Progetto N. 2) and by Ministero Dell' Istruzione, Dell' Università E Della Ricerca (Italy) Progetto Strategico Biotecnologie (to C.T.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010799.
LITERATURE CITED
- Alleman M, Kermicle JL. Somatic variegation and germinal mutability reflect the position of transposable element Dissociation within the maize R gene. Genetics. 1993;135:189–203. doi: 10.1093/genetics/135.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell. 2000;12:2383–2393. doi: 10.1105/tpc.12.12.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker-Scott L. Environmental significance of anthocyanins in plant stress. Photochem Photobiol. 1999;70:1–9. [Google Scholar]
- Chandler VR, Radicella PJ, Robbins TP, Chen J, Turks D. Two regulatory genes of the maize anthocyanin pathway are homologous: isolation of B utilizing R genomic sequences. Plant Cell. 1989;1:1175–1183. doi: 10.1105/tpc.1.12.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J, Aguilar N, Peto CA. The phenotype of Arabidopsis thaliana det1 mutants suggests a role for cytokinins in greening. Symp Soc Exp Biol. 1991;45:21–29. [PubMed] [Google Scholar]
- Cone KC, Burr FA, Burr B. Molecular analysis of the maize anthocyanin regulatory locus C1. Proc Natl Acad Sci USA. 1986;83:9631–9635. doi: 10.1073/pnas.83.24.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone KC, Cocciolone MS, Burr AF, Burr B. Maize anthocyanin regulatory gene pl is a duplicate of c1 that functions in the plant. Plant Cell. 1993a;5:1795–1805. doi: 10.1105/tpc.5.12.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone CK, Cocciolone MS, Moehlenkamp CA, Weber T, Drummond BJ, Tagliani LA, Bowen BA, Perrot GH. Role of the regulatory gene pl in the photocontrol of maize anthocyanin pigmentation. Plant Cell. 1993b;5:1807–1816. doi: 10.1105/tpc.5.12.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consonni G, Geuna F, Gavazzi G, Tonelli C. Molecular homology among members of the R gene family in maize. Plant J. 1993;3:335–346. doi: 10.1111/j.1365-313x.1993.tb00185.x. [DOI] [PubMed] [Google Scholar]
- Consonni G, Ronchi A, Pilu R, Gavazzi G, Dellaporta SL, Tonelli C. Ectopic anthocyanin pigmentation in maize as a tool for defining interactions between homologous regulatory factors. Mol Gen Genet. 1997;256:265–276. doi: 10.1007/s004380050569. [DOI] [PubMed] [Google Scholar]
- Deikman J, Hammer PE. Induction of anthocyanin accumulation by cytokinins in Arabidopsis thaliana. Plant Physiol. 1995;108:47–57. doi: 10.1104/pp.108.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooner HK, Robbins TP, Jorgensen RA. Genetic and developmental control of anthocyanin biosynthesis. Ann Rev Genet. 1991;25:173–199. doi: 10.1146/annurev.ge.25.120191.001133. [DOI] [PubMed] [Google Scholar]
- Feldbrugge M, Sprenger M, Halbrock K, Weisshar B. PcMYB1, a novel plant protein containing a DNA-binding domain with one MYB repeat, interacts in vivo with a light-regulatory promoter unit. Plant J. 1997;11:1079–1093. doi: 10.1046/j.1365-313x.1997.11051079.x. [DOI] [PubMed] [Google Scholar]
- Frohman MA. RACE, rapid amplification of cDNA ends. In: Innis MA, Gelfand DH, Sninsky J, White TJ, editors. PCR Protocols: A Guide to Methods and Applications. San Diego: Academic Press, Inc.; 1990. pp. 28–38. [Google Scholar]
- Fuglevand G, Jackson AJ, Jenkins GI. UV-B, UV-A and blue light signal transduction pathways interact synergistically to regulate chalcone synthase gene expression in Arabidopsis. Plant Cell. 1996;8:2347–2357. doi: 10.1105/tpc.8.12.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati M, Chiusi A, Peterlongo P, Mancinelli A, Gavazzi G. Photoinduction of anthocyanin in maize: a genetic approach. Maydica. 1994;39:89–95. [Google Scholar]
- Harmer SC, Hogenesc JB, Straume M, Chang H-S, Han B, Zhu T, Wang X, Kreps JA, Kay SA. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science. 2000;290:2110–2213. doi: 10.1126/science.290.5499.2110. [DOI] [PubMed] [Google Scholar]
- Hartmann U, Valentine WJ, Christie JM, Hays J, Jenkins GI, Weisshaar B. Identification of UV/blue light-response elements in the Arabidopsis thaliana chalcone synthase promoter using a homologous protoplast transient expression system. Plant Mol Biol. 1998;36:741–754. doi: 10.1023/a:1005921914384. [DOI] [PubMed] [Google Scholar]
- Jackson JA, Fuglevand G, Brown BA, Shaw MJ, Jenkins GI. Isolation of Arabidopsis mutants altered in the light-regulation of chalcone synthase gene expression using a transgenic screening approach. Plant J. 1995;8:369–380. doi: 10.1046/j.1365-313x.1995.08030369.x. [DOI] [PubMed] [Google Scholar]
- Kao C, Cocciolone SM, Vasil IK, McCarty DR. Localization and interaction of the cis-acting elements for abscissic acid, VVIPAROUS1 and light activation of the C1 gene of maize. Plant Cell. 1996;8:1171–1179. doi: 10.1105/tpc.8.7.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermicle JL. Recombination between components of a mutable gene system in maize. Genetics. 1984;107:489–500. doi: 10.1093/genetics/107.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz HD, Denekamp M, Greco R, Jin H-L, Leyva A, Meissner RC, Petroni K, Urzainqui A, Bevan M, Martin C et al. Towards functional characterization of the members of the R2R3-MYB gene family from Arabidopsis thaliana. Plant J. 1998;16:263–276. doi: 10.1046/j.1365-313x.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- Ludwig SR, Habera LF, Dellaporta SL, Wessler SR. Lc, a member of the maize R gene family responsible for tissue-specific anthocyanin production encodes a protein similar to transcriptional activators and contains the myc-homology region. Proc Natl Acad Sci USA. 1989;86:7092–7096. doi: 10.1073/pnas.86.18.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Paz-Ares J. MYB transcription factors in plants. Trends Genet. 1997;13:67–73. doi: 10.1016/s0168-9525(96)10049-4. [DOI] [PubMed] [Google Scholar]
- Mereghetti M, Tonelli C, Gavazzi G. Developmental expression of light inducible genes of the R family in immature seeds of maize. Maydica. 1991;36:337–342. [Google Scholar]
- Mol J, Grotewold E, Koes R. How genes paint flowers and seeds. Trends Plant Sci. 1998;3:212–217. [Google Scholar]
- Mol J, Jenkins GI, Schafer E, Weiss D. Signal perception, transduction and gene expression involved in anthocyanin biosynthesis. Crit Rev Plant Sci. 1996;15:525–557. [Google Scholar]
- Noh B, Spalding EP. Anion channels and the stimulation of anthocyanin accumulation by blue light in Arabidopsis seedlings. Plant Physiol. 1998;116:503–509. doi: 10.1104/pp.116.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares J, Ghosal D, Wienand U, Peterson PA, Saedler H. The regulatory C1 locus of Zea mays encodes a protein with homology to MYB-related protooncogene products and with structural similarities to transcriptional activators. EMBO J. 1987;6:3553–3558. doi: 10.1002/j.1460-2075.1987.tb02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares J, Wienand U, Peterson PA, Saedler H. Molecular cloning of the c locus of Zea mays: a locus regulating the anthocyanin pathway. EMBO J. 1986;5:829–833. doi: 10.1002/j.1460-2075.1986.tb04291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroni K, Cominelli E, Consonni G, Gusmaroli G, Gavazzi G, Tonelli C. The tissue specific expression of the maize regulatory gene Hopi determines germination-dependent anthocyanin accumulation. Genetics. 2000;155:323–336. doi: 10.1093/genetics/155.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procissi A, Dolfini S, Ronchi A, Tonelli C. Light-dependent spatial and temporal expression of pigment regulatory genes in developing maize seed. Plant Cell. 1997;9:1547–1557. doi: 10.1105/tpc.9.9.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchi A, Petroni K, Tonelli C. The reduced expression of endogenous duplications (REED) in the maize R gene family is mediated by DNA methylation. EMBO J. 1995;14:5318–5328. doi: 10.1002/j.1460-2075.1995.tb00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchi A, Pilu R, Tonelli C. Silencing of gene expression in the anthocyanin regulatory gene families. In: Lo Shiavo F, Last RL, Morelli G, Raikhel NV, editors. Cellular Integration of Signaling Pathways in Plant Development: NATO ASI Series. H 104. Berlin: Springer Verlag; 1998. pp. 93–102. [Google Scholar]
- Scheffler B, Franken P, Schutt E, Schrell A, Saedler H, Wienand U. Molecular analysis of the C1 alleles in Zea mays defines regions involved in the expression of this regulatory gene. Mol Gen Genet. 1994;242:40–48. doi: 10.1007/BF00277346. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z, Shepherd N, Tacke E, Gierl A, Rohde W, Leclercq L, Mattes M, Berndtgen R, Peterson PA, Saedler H. Influence of transposable elements on the structure and function of A1 gene of Zea mays. EMBO J. 1987;6:287–294. doi: 10.1002/j.1460-2075.1987.tb04752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli C, Consonni G, Faccio Dolfini S, Dellaporta SL, Viotti A, Gavazzi G. Genetic and molecular analysis of Sn, a light-inducible, tissue specific regulatory gene in maize. Mol Gen Genet. 1991;225:401–410. doi: 10.1007/BF00261680. [DOI] [PubMed] [Google Scholar]
- Tonelli C, Faccio Dolfini S, Ronchi A, Consonni G, Gavazzi G. Light inducibility and tissue specificity of the R gene family in maize. Genetica. 1994;94:225–234. [Google Scholar]
- van Tunen AJ, Koes RE, Spelt CE, van der Krol AR, Stuitje AR, Mol JNM. Cloning of two chalcone flavanone isomerase genes from Petunia hybrida: coordinate, light regulated and differential expression of flavonoid genes. EMBO J. 1988;7:1257–1263. doi: 10.1002/j.1460-2075.1988.tb02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade HK, Bibikova TN, Valentine WJ, Jenkins GI. Interactions within a network of phytochrome, cryptochrome and UV-B phototransduction pathways regulate chalcone synthase gene expression in Arabidopsis leaf tissue. Plant J. 2001;25:675–685. doi: 10.1046/j.1365-313x.2001.01001.x. [DOI] [PubMed] [Google Scholar]
- Weiss D, Van Blokand R, Kooter IM, Mol JNM, Van Tunen AJ. Giberellic acid regulates chalcone synthase gene trasnscription in the corolla of Petunia hybrida. Plant Physiol. 1992;107:695–702. doi: 10.1104/pp.98.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel-Shirley B. Flavonoid biosynthesis: a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126:485–493. doi: 10.1104/pp.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AD, Moehlenkamp CA, Perrot GH, Neuffer MG, Cone KC. The maize auxotrophic mutant orange pericarp is defective in duplicate genes for tryptophan synthase. Plant Cell. 1992;4:711–719. doi: 10.1105/tpc.4.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]