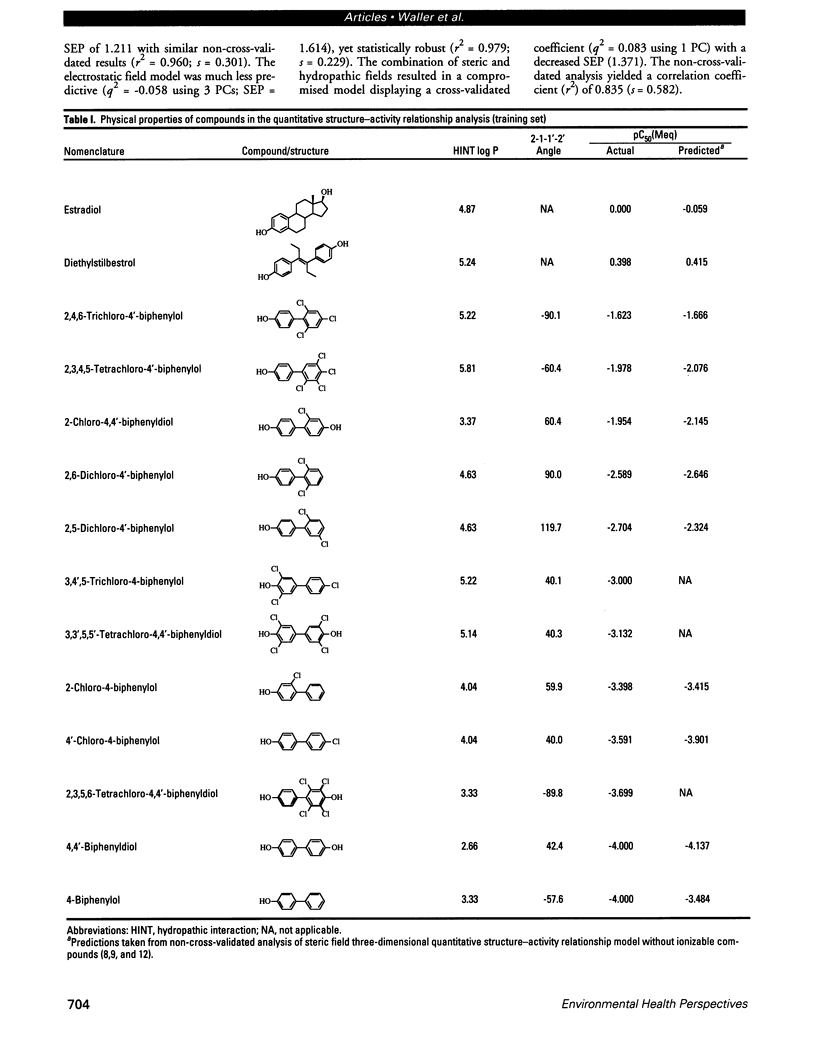
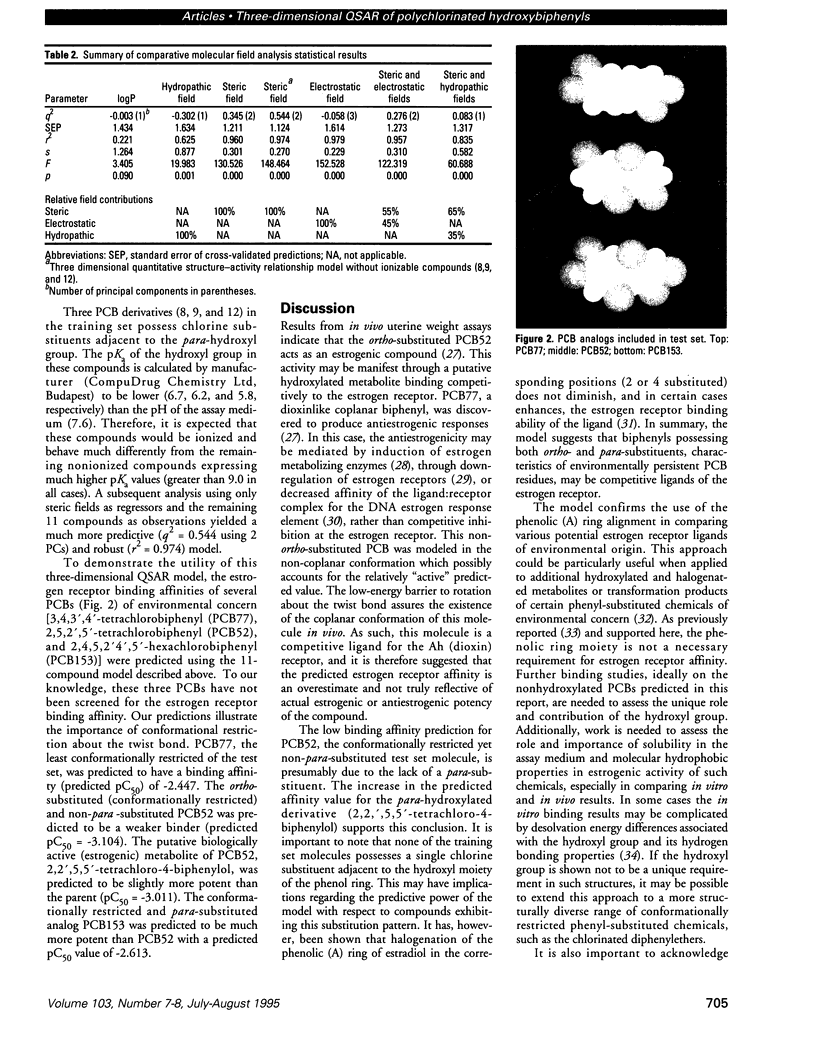
Abstract
Certain phenyl-substituted hydrocarbons of environmental concern have the potential to disrupt the endocrine system of animals, apparently in association with their estrogenic properties. Competition with natural estrogens for the estrogen receptor is a possible mechanism by which such effects could occur. We used comparative molecular field analysis (CoMFA), a three-dimensional quantitative structure-activity relationship (QSAR) paradigm, to examine the underlying structural properties of ortho-chlorinated hydroxybiphenyl analogs known to bind to the estrogen receptor. The cross-validated and conventional statistical results indicate a high degree of internal predictability for the molecules included in the training data set. In addition to the phenolic (A) ring system, conformational restriction of the overall structure appears to play an important role in estrogen receptor binding affinity. Hydrophobic character as assessed using hydropathic interaction fields also contributes in a positive way to binding affinity. The CoMFA-derived QSARs may be useful in examining the estrogenic activity of a wider range of phenyl-substituted hydrocarbons of environmental concern.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman A., Klasson-Wehler E., Kuroki H. Selective retention of hydroxylated PCB metabolites in blood. Environ Health Perspect. 1994 May;102(5):464–469. doi: 10.1289/ehp.94102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito M. J., Thomas T., Martin E., Umbreit T. H., Gallo M. A. Antiestrogenic action of 2,3,7,8-tetrachlorodibenzo-p-dioxin: tissue-specific regulation of estrogen receptor in CD1 mice. Toxicol Appl Pharmacol. 1992 Apr;113(2):284–292. doi: 10.1016/0041-008x(92)90126-d. [DOI] [PubMed] [Google Scholar]
- Gantchev T. G., Ali H., van Lier J. E. Quantitative structure-activity relationships/comparative molecular field analysis (QSAR/CoMFA) for receptor-binding properties of halogenated estradiol derivatives. J Med Chem. 1994 Nov 25;37(24):4164–4176. doi: 10.1021/jm00050a013. [DOI] [PubMed] [Google Scholar]
- Goldstein R. A., Katzenellenbogen J. A., Luthey-Schulten Z. A., Seielstad D. A., Wolynes P. G. Three-dimensional model for the hormone binding domains of steroid receptors. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9949–9953. doi: 10.1073/pnas.90.21.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton S. B. Estrogenicity of environmental PCBs. Environ Health Perspect. 1995 Jan;103(1):12–13. doi: 10.1289/ehp.9510312a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen H. T., Cooke P. S., Porcelli J., Liu T. C., Hansen L. G. Estrogenic and antiestrogenic actions of PCBs in the female rat: in vitro and in vivo studies. Reprod Toxicol. 1993 May-Jun;7(3):237–248. doi: 10.1016/0890-6238(93)90230-5. [DOI] [PubMed] [Google Scholar]
- Jordan V. C. Biochemical pharmacology of antiestrogen action. Pharmacol Rev. 1984 Dec;36(4):245–276. [PubMed] [Google Scholar]
- Kellogg G. E., Semus S. F., Abraham D. J. HINT: a new method of empirical hydrophobic field calculation for CoMFA. J Comput Aided Mol Des. 1991 Dec;5(6):545–552. doi: 10.1007/BF00135313. [DOI] [PubMed] [Google Scholar]
- Korach K. S., Fox-Davies C., Quarmby V. E., Swaisgood M. H. Diethylstilbestrol metabolites and analogs. Biochemical probes for differential stimulation of uterine estrogen responses. J Biol Chem. 1985 Dec 15;260(29):15420–15426. [PubMed] [Google Scholar]
- Korach K. S., Sarver P., Chae K., McLachlan J. A., McKinney J. D. Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls: conformationally restricted structural probes. Mol Pharmacol. 1988 Jan;33(1):120–126. [PubMed] [Google Scholar]
- Lundkvist U. Clinical and reproductive effects of Clophen A50 (PCB) administered during gestation on pregnant guinea pigs and their offspring. Toxicology. 1990 Apr 30;61(3):249–257. doi: 10.1016/0300-483x(90)90175-g. [DOI] [PubMed] [Google Scholar]
- McKinney J. D., Waller C. L. Polychlorinated biphenyls as hormonally active structural analogues. Environ Health Perspect. 1994 Mar;102(3):290–297. doi: 10.1289/ehp.94102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. A., Struck R. F., James R. Estrogenic activities of chlorinated hydrocarbons. J Toxicol Environ Health. 1978 Mar-May;4(2-3):325–339. doi: 10.1080/15287397809529664. [DOI] [PubMed] [Google Scholar]
- Pedersen L. G., Darden T. A., Oatley S. J., McKinney J. D. A theoretical study of the binding of polychlorinated biphenyls (PCBs), dibenzodioxins, and dibenzofuran to human plasma prealbumin. J Med Chem. 1986 Dec;29(12):2451–2457. doi: 10.1021/jm00162a006. [DOI] [PubMed] [Google Scholar]
- Peterson R. E., Theobald H. M., Kimmel G. L. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit Rev Toxicol. 1993;23(3):283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- Safe S. H. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 1994;24(2):87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Spink D. C., Lincoln D. W., 2nd, Dickerman H. W., Gierthy J. F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes an extensive alteration of 17 beta-estradiol metabolism in MCF-7 breast tumor cells. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6917–6921. doi: 10.1073/pnas.87.17.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. M., Levin W., Conney A. H. Estrogenic action of DDT and its analogs. Toxicol Appl Pharmacol. 1969 Mar;14(2):358–367. doi: 10.1016/0041-008x(69)90117-3. [DOI] [PubMed] [Google Scholar]




