Abstract
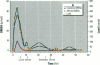
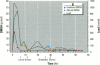
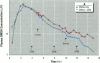
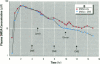
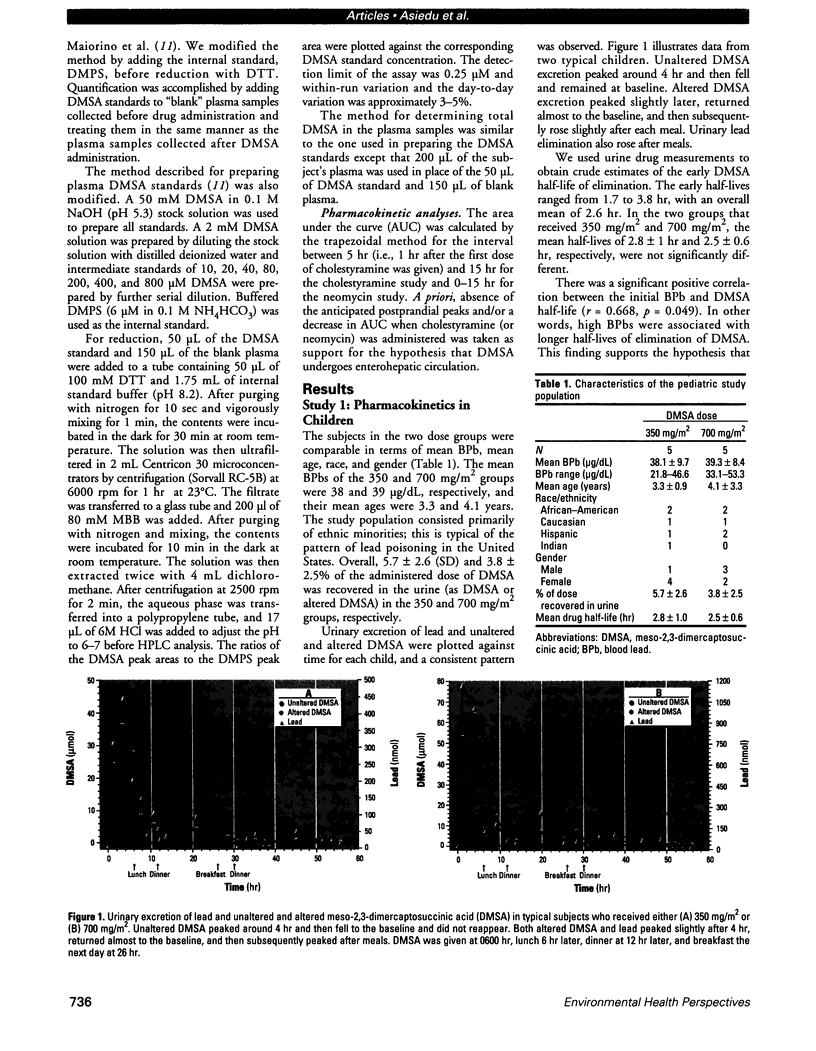
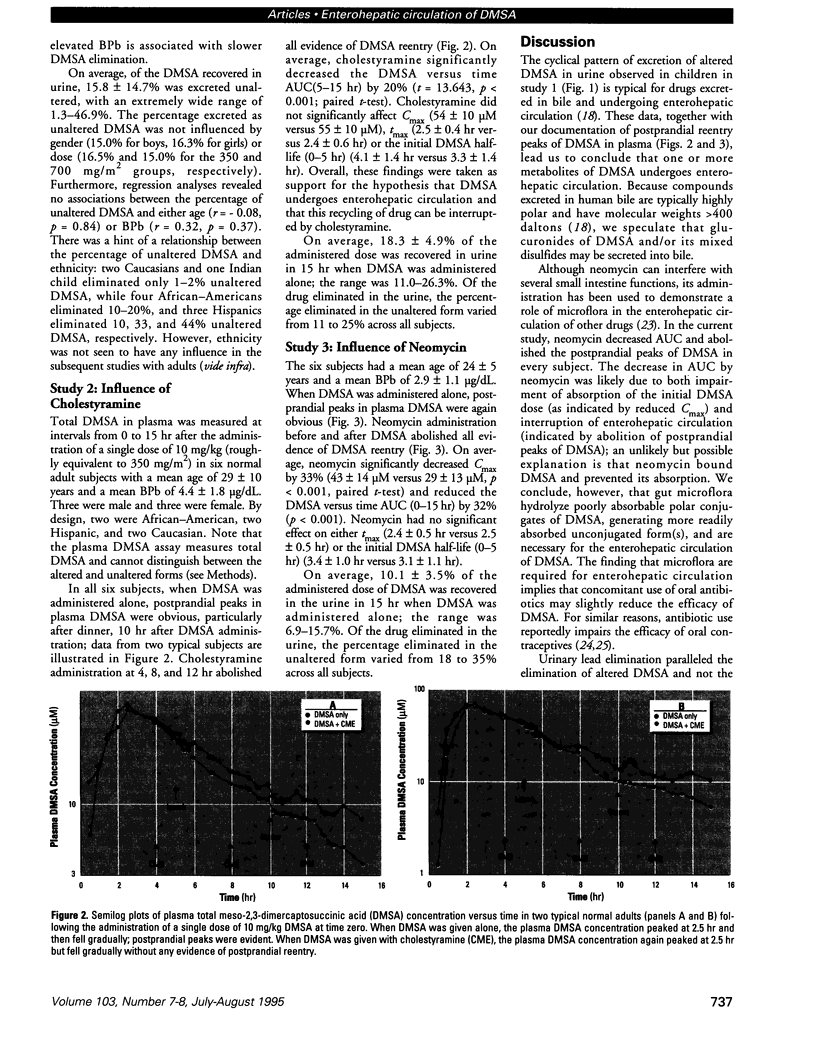
Meso-2,3-dimercaptosuccinic acid (DMSA, or succimer) is an oral chelating agent for heavy-metal poisoning. While studying the urinary elimination of unaltered DMSA, altered DMSA (i.e., its mixed disulfides), and lead in children with lead poisoning, we observed a pattern of urinary drug elimination after meals suggestive of enterohepatic circulation. The excretion of lead in urine patterned the elimination of altered DMSA rather than the parent molecule. In addition, the half-life of elimination of DMSA via the kidney was positively associated with blood lead concentration. Two additional crossover studies of DMSA kinetics were conducted in normal adults to confirm the presence of enterohepatic circulation of DMSA after meals. In one, increases in plasma total DMSA concentration were observed after meals in all six subjects; these increases were prevented by cholestyramine administration 4, 8, and 12 hr after DMSA. In the second, the administration of neomycin also prevented increases in DMSA after meals. These studies indicate that 1) a metabolite(s) of DMSA undergoes enterohepatic circulation and that microflora are required for DMSA reentry; 2) in children, moderate lead exposure impairs renal tubular drug elimination; and 3) a metabolite of DMSA appears to be an active chelator.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvares A. P., Kapelner S., Sassa S., Kappas A. Drug metabolism in normal children, lead-poisoned children, and normal adults. Clin Pharmacol Ther. 1975 Feb;17(2):179–183. doi: 10.1002/cpt1975172179. [DOI] [PubMed] [Google Scholar]
- Aposhian H. V., Maiorino R. M., Dart R. C., Perry D. F. Urinary excretion of meso-2,3-dimercaptosuccinic acid in human subjects. Clin Pharmacol Ther. 1989 May;45(5):520–526. doi: 10.1038/clpt.1989.67. [DOI] [PubMed] [Google Scholar]
- Back D. J., Grimmer S. F., Orme M. L., Proudlove C., Mann R. D., Breckenridge A. M. Evaluation of Committee on Safety of Medicines yellow card reports on oral contraceptive-drug interactions with anticonvulsants and antibiotics. Br J Clin Pharmacol. 1988 May;25(5):527–532. doi: 10.1111/j.1365-2125.1988.tb03341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon J. F., Shenfield G. M. Pregnancy attributable to interaction between tetracycline and oral contraceptives. Br Med J. 1980 Feb 2;280(6210):293–293. doi: 10.1136/bmj.280.6210.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns L. A., Butterworth L. F., Munson A. E. Reversal of gallium arsenide-induced suppression of the antibody response by a mixed disulfide metabolite of meso-2,3-dimercaptosuccinic acid. J Pharmacol Exp Ther. 1993 Feb;264(2):695–700. [PubMed] [Google Scholar]
- Cramér K., Goyer R. A., Jagenburg R., Wilson M. H. Renal ultrastructure, renal function, and parameters of lead toxicity in workers with different periods of lead exposure. Br J Ind Med. 1974 Apr;31(2):113–127. doi: 10.1136/oem.31.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart R. C., Hurlbut K. M., Maiorino R. M., Mayersohn M., Aposhian H. V., Hassen L. V. Pharmacokinetics of meso-2,3-dimercaptosuccinic acid in patients with lead poisoning and in healthy adults. J Pediatr. 1994 Aug;125(2):309–316. doi: 10.1016/s0022-3476(94)70217-9. [DOI] [PubMed] [Google Scholar]
- Dobrinska M. R. Enterohepatic circulation of drugs. J Clin Pharmacol. 1989 Jul;29(7):577–580. doi: 10.1002/j.1552-4604.1989.tb03385.x. [DOI] [PubMed] [Google Scholar]
- Friedheim E., Graziano J. H., Popovac D., Dragovic D., Kaul B. Treatment of lead poisoning by 2,3-dimercaptosuccinic acid. Lancet. 1978 Dec 9;2(8102):1234–1236. doi: 10.1016/s0140-6736(78)92103-7. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Jacobsen I. A., Jørgensen P. J. Chronic lead poisoning treated with dimercaptosuccinic acid. Pharmacol Toxicol. 1991 Apr;68(4):266–269. doi: 10.1111/j.1600-0773.1991.tb01236.x. [DOI] [PubMed] [Google Scholar]
- Graziano J. H., Lolacono N. J., Meyer P. Dose-response study of oral 2,3-dimercaptosuccinic acid in children with elevated blood lead concentrations. J Pediatr. 1988 Oct;113(4):751–757. doi: 10.1016/s0022-3476(88)80396-2. [DOI] [PubMed] [Google Scholar]
- Graziano J. H., Lolacono N. J., Moulton T., Mitchell M. E., Slavkovich V., Zarate C. Controlled study of meso-2,3-dimercaptosuccinic acid for the management of childhood lead intoxication. J Pediatr. 1992 Jan;120(1):133–139. doi: 10.1016/s0022-3476(05)80618-3. [DOI] [PubMed] [Google Scholar]
- Graziano J. H., Siris E. S., LoIacono N., Silverberg S. J., Turgeon L. 2,3-Dimercaptosuccinic acid as an antidote for lead intoxication. Clin Pharmacol Ther. 1985 Apr;37(4):431–438. doi: 10.1038/clpt.1985.67. [DOI] [PubMed] [Google Scholar]
- Herman R. J., Van Pham J. D., Szakacs C. B. Disposition of lorazepam in human beings: enterohepatic recirculation and first-pass effect. Clin Pharmacol Ther. 1989 Jul;46(1):18–25. doi: 10.1038/clpt.1989.101. [DOI] [PubMed] [Google Scholar]
- Lenz K., Hruby K., Druml W., Eder A., Gaszner A., Kleinberger G., Pichler M., Weiser M. 2,3-Dimercaptosuccinic acid in human arsenic poisoning. Arch Toxicol. 1981 Jun;47(3):241–243. doi: 10.1007/BF00368684. [DOI] [PubMed] [Google Scholar]
- Maiorino R. M., Akins J. M., Blaha K., Carter D. E., Aposhian H. V. Determination and metabolism of dithiol chelating agents: X. In humans, meso-2,3-dimercaptosuccinic acid is bound to plasma proteins via mixed disulfide formation. J Pharmacol Exp Ther. 1990 Aug;254(2):570–577. [PubMed] [Google Scholar]
- Maiorino R. M., Aposhian M. M., Xu Z. F., Li Y., Polt R. L., Aposhian H. V. Determination and metabolism of dithiol chelating agents. XV. The meso-2,3-dimercaptosuccinic acid-cysteine (1:2) mixed disulfide, a major urinary metabolite of DMSA in the human, increases the urinary excretion of lead in the rat. J Pharmacol Exp Ther. 1993 Dec;267(3):1221–1226. [PubMed] [Google Scholar]
- Maiorino R. M., Bruce D. C., Aposhian H. V. Determination and metabolism of dithiol chelating agents. VI. Isolation and identification of the mixed disulfides of meso-2,3-dimercaptosuccinic acid with L-cysteine in human urine. Toxicol Appl Pharmacol. 1989 Feb;97(2):338–349. doi: 10.1016/0041-008x(89)90338-4. [DOI] [PubMed] [Google Scholar]
- Maiorino R. M., Weber G. L., Aposhian H. V. Fluorometric determination of 2,3-dimercaptopropane-1-sulfonic acid and other dithiols by precolumn derivatization with bromobimane and column liquid chromatography. J Chromatogr. 1986 Jan 24;374(2):297–310. doi: 10.1016/s0378-4347(00)83285-5. [DOI] [PubMed] [Google Scholar]
- Mortensen M. E. Succimer chelation: what is known? J Pediatr. 1994 Aug;125(2):233–234. doi: 10.1016/s0022-3476(94)70200-4. [DOI] [PubMed] [Google Scholar]
- Renowden S., Westmoreland D., White J. P., Routledge P. A. Oral cholestyramine increases elimination of warfarin after overdose. Br Med J (Clin Res Ed) 1985 Aug 24;291(6494):513–514. doi: 10.1136/bmj.291.6494.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels H. A., Boeckx M., Ceulemans E., Lauwerys R. R. Urinary excretion of mercury after occupational exposure to mercury vapour and influence of the chelating agent meso-2,3-dimercaptosuccinic acid (DMSA). Br J Ind Med. 1991 Apr;48(4):247–253. doi: 10.1136/oem.48.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetre R., Rabenstein D. L. Determination of penicillamine in blood and urine by high performance liquid chromatography. Anal Chem. 1978 Feb;50(2):278–280. doi: 10.1021/ac50024a027. [DOI] [PubMed] [Google Scholar]
- Tasende M. S., Gale G. R., Smith A. B., Jones M. M., Singh P. K. Monoisoamyl meso-2,3-dimercaptosuccinate: interaction with metallothionein-bound cadmium in vitro and evidence of active transport into renal and hepatic cells in vivo. Res Commun Chem Pathol Pharmacol. 1992 Jun;76(3):323–339. [PubMed] [Google Scholar]
- Zheng W., Maiorino R. M., Brendel K., Aposhian H. V. Determination and metabolism of dithiol chelating agents. VII. Biliary excretion of dithiols and their interactions with cadmium and metallothionein. Fundam Appl Toxicol. 1990 Apr;14(3):598–607. doi: 10.1016/0272-0590(90)90264-k. [DOI] [PubMed] [Google Scholar]