Abstract
Winter wheat (Triticum aestivum L. cv Norin No. 61) was grown at 25°C until the third leaves reached about 10 cm in length and then at 15°C, 25°C, or 35°C until full development of the third leaves (about 1 week at 25°C, but 2–3 weeks at 15°C or 35°C). In the leaves developed at 15°C, 25°C, and 35°C, the optimum temperature for CO2-saturated photosynthesis was 15°C to 20°C, 25°C to 30°C, and 35°C, respectively. The photosystem II (PS II) electron transport, determined either polarographically with isolated thylakoids or by measuring the modulated chlorophyll a fluorescence in leaves, also showed the maximum rate near the temperature at which the leaves had developed. Maximum rates of CO2-saturated photosynthesis and PS II electron transport determined at respective optimum temperatures were the highest in the leaves developed at 25°C and lowest in the leaves developed at 35°C. So were the levels of chlorophyll, photosystem I and PS II, whereas the level of Rubisco decreased with increasing temperature at which the leaves had developed. Kinetic analyses of chlorophyll a fluorescence changes and P700 reduction showed that the temperature dependence of electron transport at the plastoquinone and water-oxidation sites was modulated by the temperature at which the leaves had developed. These results indicate that the major factor that contributes to thermal acclimation of photosynthesis in winter wheat is the plastic response of PS II electron transport to environmental temperature.
Photosynthesis in plants native to areas with large seasonal variations in temperature during their growth exhibits an ability to acclimate to growth temperature (Berry and Björkman, 1980). Plants that are grown at cold temperature regimes show maximum rates of photosynthesis at lower temperatures than do plants grown under warm temperature regimes, and an increase in growth temperature results in an increase in optimal temperature for photosynthesis. This enables plants to perform a high rate of photosynthesis at the growth temperature, provided that a shift in optimum temperature is not accompanied with counteracting changes in the photosynthetic capacity. The acclimation potential of photosynthesis to temperature greatly varies with the plant species and ecotypes. Although a shift in the optimum temperature for photosynthesis is generally less than one-half that in the growth temperature (Berry and Björkman, 1980), several plants show dramatic changes in the temperature-response curve of photosynthesis. The optimum temperature for photosynthesis in winter wheat (Triticum aestivum L. cv Norin No. 61) grown at different seasons of the year increased with increase in the mean air temperature at a rate of about 3°C increase for each 4°C increase in the growth temperature (Sawada, 1970). A 15°C increase in the growth temperature resulted in a 15°C increase in optimum temperature for photosynthesis in Pinus taeda (Strain et al., 1976) and acclimation of Saxifraga cernua to a 10°C higher temperature was accompanied with about a 10°C upward shift in the optimum temperature (Mawson et al., 1986).
Several mechanisms for thermal acclimation of photosynthesis have been proposed. Plants grown at low temperatures had higher levels of Rubisco and other enzymes, which are involved in carbon metabolism compared with plants grown at high temperatures (Badger et al., 1982; Maruyama et al., 1990; Holaday et al., 1992; Hurry et al., 1995; Strand et al., 1999). Growth at low temperatures also resulted in higher leaves of cytosolic Fru 1,6-bisphosphatase and Suc-phosphate synthases, which regenerate orthophosphate during Suc synthesis (Badger et al., 1982; Crespi et al., 1991; Holaday et al., 1992; Makino et al., 1994; Hurry et al., 1995). Photosynthetic acclimation to low temperature was, therefore, suggested to involve an increase in the capacity of enzymatic reactions that limit photosynthesis at low temperature. However, a downward shift in the optimum temperature of photosynthesis, which results in not only enhanced photosynthetic performance at low temperatures but also reduced photosynthetic performance at high temperatures, cannot be explained only in terms of quantitative increases in the level of enzymes. Growth at high temperatures resulted in an increase in the threshold temperature above which irreversible heat inactivation of photosynthesis occurs (Pearcy, 1977; Badger et al., 1982). Acclimation to high temperatures was, therefore, related to increased heat-tolerance of the photosynthetic apparatus (Berry and Björkman, 1980; Badger et al., 1982). The extent of thermal stabilization was, however, limited and not large enough to account for an upward shift in the optimum temperature for photosynthesis in the plants with a high acclimation potential.
Farquhar and von Caemmerer (1982) reported that a shift in the optimum temperature for photosynthesis also results from a change in balance between the carboxylation capacity of ribulose-1,5-bisphosphate (RuBP; which is determined from the initial slope of the response curve of photosynthetic rate to intercellular CO2 concentration) and the regeneration capacity of RuBP (which is estimated from rate of CO2-saturated photosynthesis). Recent experiments showed that growth temperature affects not only the ratio of carboxylation capacity to the regeneration capacity of RuBP but also the temperature dependence of the two capacities (Hikosaka et al., 1999; Bunce, 2000). Changes in temperature dependence of photosynthesis in eight plants were more closely related to changes in temperature dependence of the two capacities than to changes in their ratio (Bunce, 2000). RuBP regeneration is mediated by electron transport whose capacity and temperature dependence are influenced by growth temperature. At a low temperature, plants grown at low temperatures showed higher rates of photosynthetic electron transport than those grown at high temperatures (Huner, 1985; Mitchell and Barber, 1986; Mawson and Cummins, 1989). An increase in growth temperature also led to an upward shift in optimum temperature for electron transport, although the extent of shift varied greatly with the plant species (Tieszen and Helgager, 1968; Armond et al., 1978; Badger et al., 1982; Mawson and Cummins, 1989).
In the present study, acclimation of photosynthesis to high and low temperatures was investigated in winter wheat, which has been reported to have a particularly high acclimation potential (Sawada, 1970). The third leaves, which had partially developed at 25°C, were allowed to develop further at 15°C and 35°C, and, after full maturation, the temperature dependence of photosynthetic activity and levels of several functional components of photosynthesis were analyzed with the leaves that had been kept at 25°C throughout the growth period as a reference. The effects of the temperature during leaf development on the capacity and temperature dependence of electron transport were also investigated by polarographically measuring the evolution or uptake of oxygen in isolated thylakoid membranes and monitoring the modulated chlorophyll a fluorescence in the leaves. The results indicated that the temperature dependence of photosynthesis in the leaves developed at different temperatures was closely related to temperature dependence of PS II electron transport. Then, the kinetics of oxidation and reduction of QA and reduction of P700 were analyzed to determine a region of PS II electron transport, which is responsible for the observed changes in temperature dependence.
RESULTS
Temperature Dependence of Photosynthesis in Wheat Grown at Different Seasons of Year
The effect of growth temperature on temperature dependence of photosynthesis was investigated with plants that had been grown outdoors at different seasons of year. Plants were grown in summer (July–September), autumn (October–November), and winter (December–February) until full development of the third leaves. Photosynthetic oxygen evolution from the leaves was determined in the presence of a saturating concentration of CO2 to minimize the effects of stomatal conductance and photorespiration. The photosynthetic capacity of leaves varied considerably with plant even in the plants grown in the same season, and it was low in the plants grown in the rainy period, indicating that the photosynthetic capacity was influenced not only by temperature but also by other environmental factors such as sunshine. Table I shows, however, that temperature dependence of photosynthesis was mainly controlled by the growth temperature. Plants grown in the summer, autumn, and winter periods showed a maximal photosynthetic rate at about 30°C, 25°C, and 10°C, respectively. Thus, the difference in growth season of the year caused about a 20°C difference in the optimal temperature for photosynthesis. The results are consistent with the experiments of Sawada (1970) who showed that winter wheat has a large potential for thermal acclimation of photosynthesis.
Table I.
Optimum temperatures of photosynthesis in wheat leaves grown at different seasons of the year (n ≥ 3)
| Growth Periods | Average of the Maximum Temperature of a Day | Average of the Minimum Temperature of a Day | Average of Optimum Temperature (±sd) |
|---|---|---|---|
| °C | |||
| Summer | 32.7 | 25.9 | 30 ± 6 |
| Autumn | 18.1 | 11.7 | 25 ± 4 |
| Winter | 11.8 | 4.9 | 10 ± 5 |
Temperature Dependence of Photosynthesis in Leaves Grown at Different Temperatures
Experiments were also performed with plants that had been grown under controlled conditions. Plants were grown at 25°C for about 2 weeks until the third leaves developed to about 10 cm long, then transferred to 15°C or 35°C, or held at 25°C. The third leaves fully developed after 1 week at 25°C, but after 2 to 3 weeks at 15°C and 35°C. The leaves kept at 15°C and 25°C, hereafter 15C leaves and 25C leaves, respectively, were both healthy green in appearance, but 15C leaves were smaller than 25C leaves. Mid-segments (3 cm long) of the leaves were used for measurement of photosynthesis. The leaves kept at 35°C (35C leaves) showed poor chlorophyll formation compared with the other leaves, and the region of the leaf blade that had developed at this temperature was pale green. Therefore, the photosynthetic activity of the 35C leaves was determined using the apical green region of the leaf blades that had developed at 25°C before exposure to 35°C.
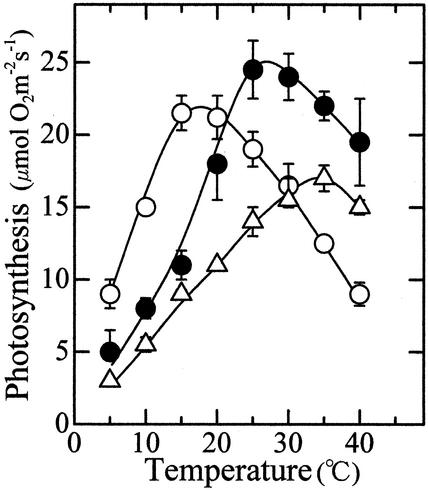
In the 15C, 25C, and 35C leaves, the optimum temperature for photosynthesis was 15°C to 20°C, 25°C to 30°C, and around 35°C, respectively (Fig. 1). At 5°C to 15°C, the photosynthetic rate in the 15C leaves was higher than that in the 25C leaves, even though the photosynthetic rate at an optimum temperature was lower in the 15C leaves than in the 25C leaves. The photosynthetic activity in the 15C leaves decreased as the temperature increased beyond 20°C. However, this cannot be ascribed to irreversible heat inactivation of the photosynthetic apparatus, because the leaves again exhibited high photosynthetic activity when returned to 15°C (not shown, see Badger et al., 1982).
Figure 1.
Temperature dependence of photosynthesis in leaves grown or treated at three different temperatures. ○,15C leaves; ●, 25C leaves; ▵, 35C leaves, n = 3. Vertical bars, sd.
The 35C leaves showed a lower photosynthetic rate than the 25C leaves did over the entire range of measurement temperature but had a higher optimum temperature than 25C leaves. This suggests that the mechanisms underlying the thermal acclimation of photosynthesis operates even at high temperatures where the photosynthetic activity gradually decreased. Because the activity was determined with the apical region of the leaf blades that had developed at 25°C before exposure to 35°C, this result also indicated that development of a leaf is not required for acclimation to temperature.
Quantum Yield of PS II Photochemistry
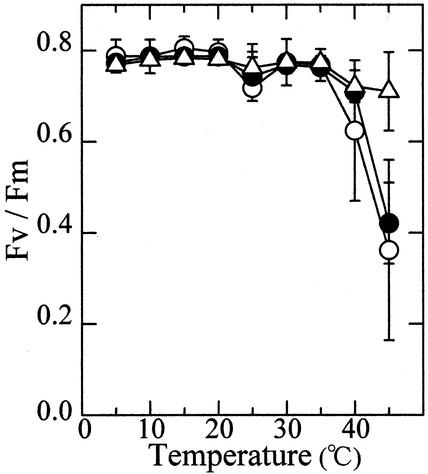
The maximum quantum yield of the PS II photochemistry (ΦII) was estimated by measuring the modulated chlorophyll a fluorescence in dark-adapted leaves (Genty et al., 1989). Irrespective of the temperature to which leaves had been exposed, ΦII was close to 0.8 between 5°C and 35°C (Fig. 2). Thus, the difference in the maximum photosynthetic rate among the 15C, 25C, and 35C leaves may not be attributed to the difference in the magnitude of photoinhibition. ΦII decreased sharply at temperatures above 35°C in the 15C and 25C leaves, whereas the 35C leaves showed a high quantum yield even at 45°C. This indicates that treatment of leaves at 35°C led to an increase in the heat stability of PS II photochemistry.
Figure 2.
Temperature dependence of the ΦII in leaves grown or treated at three different temperatures. ○, 15C leaves; ●, 25C leaves; ▵, 35C leaves, n = 6. Vertical bars, sd.
Contents of Functional Components of Photosynthesis
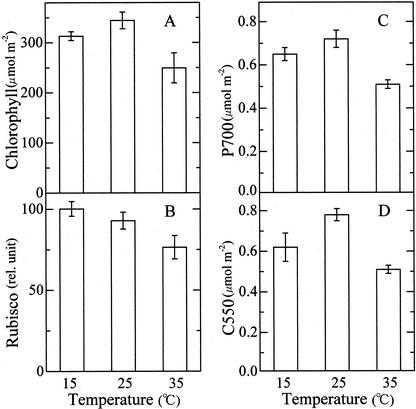
Figure 3 shows levels of several functional components of photosynthesis in the 15C, 25C, and 35C leaves. The 15°C leaves had a slightly lower level of chlorophyll than the 25C leaves, and the 35C leaves contained about 30% less pigment than the 25C leaves (Fig. 3A). Levels of other components were quantified on the basis of chlorophyll but shown in Figure 3 on the basis of leaf area for comparison with the photosynthetic activity. The lower the temperature to which the plants had been exposed, the higher was the level of Rubisco (Fig. 3B). Although, as stated in introduction, photosynthetic acclimation to low temperatures was often related to an increase in level of enzymes of carbon metabolism, no quantitative correlation was observed between the level of Rubisco and photosynthetic performance at low temperatures. The photosynthetic rate at 10°C was doubled by transfer from 25°C to 10°C (see Fig. 1), but the enzyme (Rubisco) level increased only 10% (Fig. 3B). Photosystem I (PS I) was quantified by measuring the light-induced absorption change of P700 (Fig. 3C) and the level of PS II was estimated from the light-induced absorption change of C550 (Fig. 3D). The contents of PS I and PS II were the highest in 25C leaves followed by 15C and 35C leaves, in this order, roughly in parallel to the photosynthetic rate determined at respective optimum temperatures (see Fig. 1). These results suggest that the difference in the photosynthetic capacity among the three populations of leaves is attributed to the difference in the level of functional components bound to the thylakoid membranes.
Figure 3.
Contents of chlorophyll (n = 6), Rubisco (n = 6), P700 (n = 3), and C550 (n = 3) in leaves grown at three different temperatures. Vertical bars, sd.
Temperature Dependence of Electron Transport
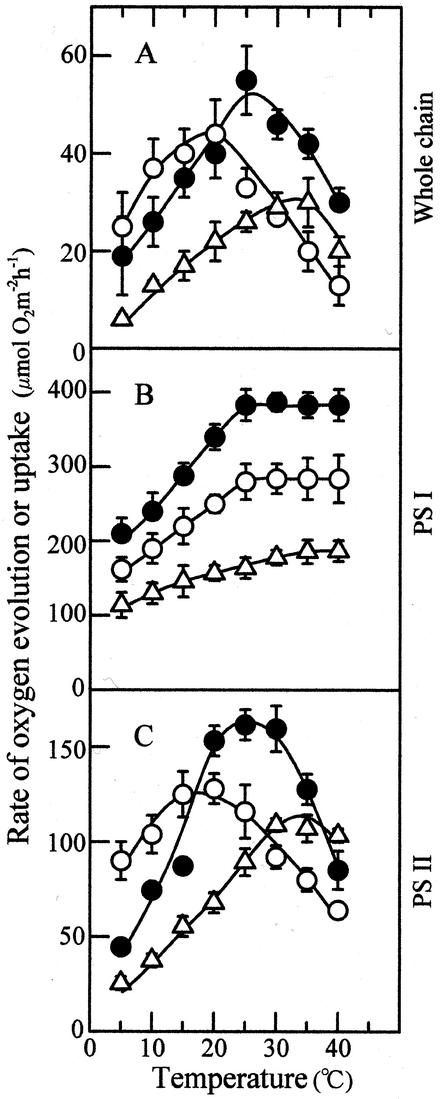
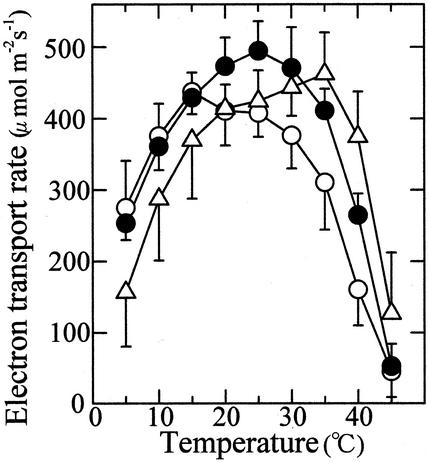
Figure 4 shows the temperature response curves of whole-chain, PS I, and PS II electron transport that were determined with thylakoid membranes isolated from leaves developed at 15°C, 25°C, and 35°C. The electron transport rate determined on the basis of chlorophyll was converted into the rate per unit leaf area using leaf chlorophyll content. The 15C, 25C, and 35C leaves showed an optimum temperature for the whole-chain electron transport at 20°C, 25°C, and 35°C, respectively (Fig. 4A). The maximum rate of the electron transport was the highest in 25C leaves, followed by the 15C and 35C leaves, in this order.
Figure 4.
Temperature dependence of electron transport determined with thylakoid membranes isolated from leaves grown at the three different temperatures. A, Whole-chain electron transport (H2O → methyl viologen); B, PS I electron transport (DCIPH2 → methyl viologen); C, PS II electron transport (H2O → phenyl-p-benzoquinone); ○, 15C leaves; ●, 25C leaves; ▵, 35C leaves, n = 3. Vertical bars, sd.
The rate of electron transport mediated by PS I increased as measurement temperature increased and tended to become constant above 25°C (Fig. 4B). The 25C leaves showed the highest rates of electron transport and the 35C leaves the lowest at all measurement temperatures. The temperature response curves of PS II electron transport were similar to those of whole-chain electron transport showing the maxima at or near the optimum temperatures for photosynthesis in all 15C, 25C, and 35C leaves (Fig. 4C). Moreover, the maximum rate of PS II electron transport decreased in the order of 25C, 15C, and 35C leaves. This shows that a major factor that contribute to thermal acclimation of CO2-saturated photosynthesis is PS II electron transport.
The rate of PS II electron transport in leaves under light was estimated using the following equation (Genty et al., 1989):
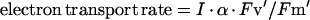 |
where Fv′/Fm′ is the quantum yield of photochemistry in open PS II reaction centers, I is photon flux density, and a was assumed to be 0.4 (Schreiber, 1994). The gas phase was air. The temperature response curves of PS II electron transport in leaves (Fig. 5) were broader than those of PS II electron transport determined with isolated thylakoids (see Fig. 4C). The growth temperature had only minor effects on the maximum rate of PS II electron transport in leaves. Thus, the temperature response curves of PS II electron transport in leaves were not so markedly different from each other among the three populations of leaves as those of PS II electron transport in isolated thylakoids. In the 25C leaves, however, the maximum rate of electron transport was observed at 25°C, and in 15C and 35C leaves, the maximum rate was observed at a 10°C lower and 10°C higher temperature, respectively. Thus, temperature dependence of PS II electron transport in leaves was also modulated so as to show the maximum rate at the temperatures at which the leaves had developed.
Figure 5.
Temperature dependence of electron transport determined by analysis of modulated chlorophyll a fluorescence changes in leaves grown at three different temperatures. ○, 15C leaves (n = 6); ●, 25C leaves (n = 4); ▵, 35C leaves (n = 3). Vertical bars, sd.
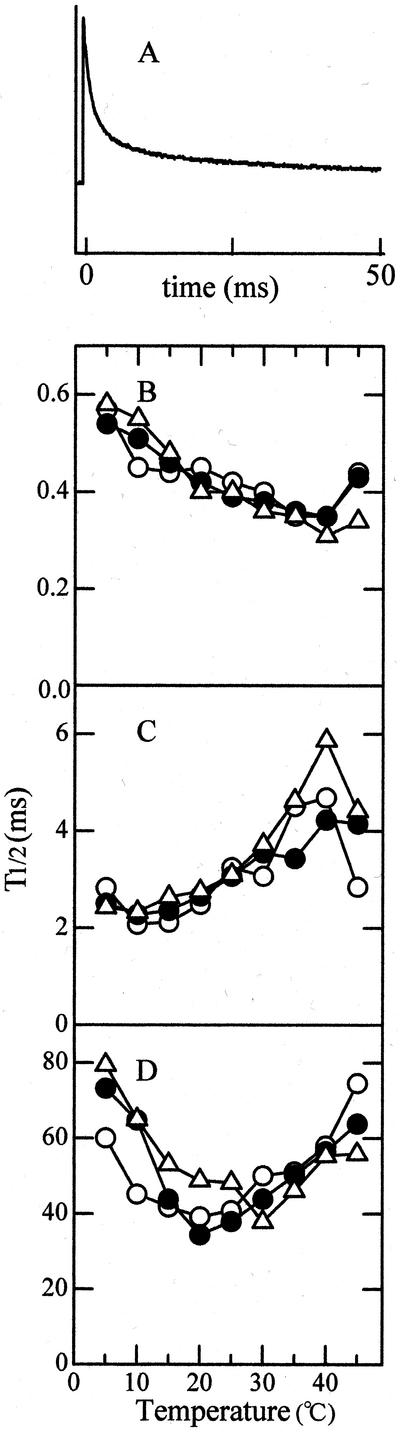
Temperature Dependence of QA Oxidation
Attempts were further made to determine which region of PS II electron transport is responsible for the observed changes in temperature dependence. Figure 6A shows the change with the lapse of time of chlorophyll a fluorescence that was induced by a short flash at 25°C. Decay kinetics of fluorescence was composed of three exponential phases with half-times of about 0.4, 3, and 30 ms (not shown). The first phase represents electron flow from QA to QB (Bowes and Crofts, 1980; Haumann and Junge, 1994), and the middle phase can be related to turnover of plastoquinone molecules at the QB site (Cao and Govindjee, 1990). The growth treatment temperature had no significant effects on the temperature dependence of the decay rates of the two phases (Fig. 6, B and C). The slow decay component with a half-time of several tens of milliseconds is attributed to oxidation of QA by PS I (Bukhov et al., 1992). The temperature response curve for the half-decay time of this component shifted to a higher temperature as the preconditioning temperature increased. The 15C and 25C leaves showed the maximum decay rates at about 20°C and the 35C leaves at 30°C (Fig. 6D). Although the effect of growth temperature was not dramatic, these results suggest that electron transport between QA and PS I involves a reaction whose temperature dependence is altered depending upon environmental temperature.
Figure 6.
Temperature dependencies of three kinetic components of fluorescence decay. A, Time course of fluorescence changes in 25C leaves induced by a short flash at 25°C. Flash was fired at time 0. B, Fast component; C, middle component; D, slow component. ○, 15C leaves; ●, 25C leaves; ▵, 35C leaves, n = 3.
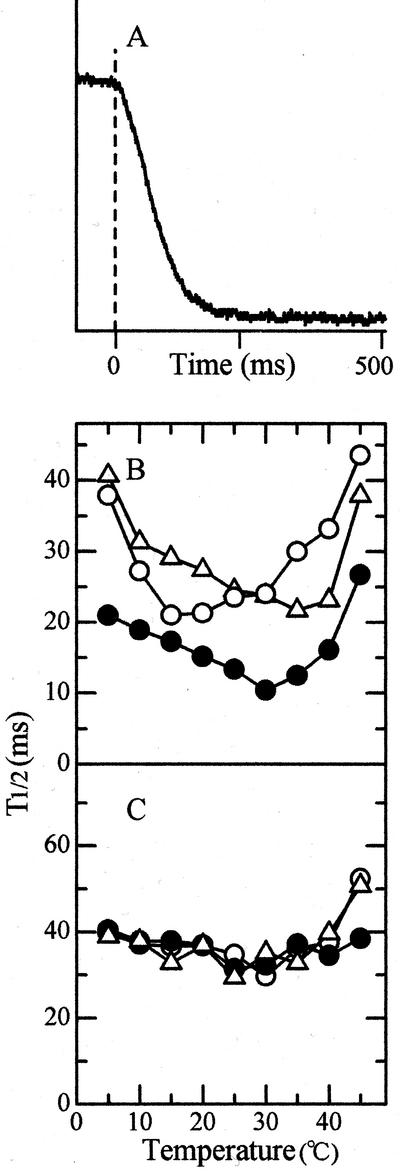
Temperature Dependence of P700 Reduction
The temperature response curves of electron transport between PS I and PS II in the three populations of leaves were also investigated by measuring reduction kinetics of P700 after oxidation by strong light. P700 was reduced with a single exponential kinetic except for a small lag that occurred immediately after cessation of irradiation (Fig. 7A). Because plastocyanin and cytochrome f were completely oxidized during irradiation, P700 reduction with a half-time of 15 ms is ascribed to electron transfer from the plastoquinone pool (Harbinson and Hedley, 1989). The 25C leaves showed a faster rate of P700 reduction than did the 15C and 35C leaves over the entire range of measurement temperature, indicating that the capacity of electron transfer from plastoquinone to P700 was influenced by growth temperature (Fig. 7B). In addition, the preconditioning temperature affected the temperature response curves of P700 reduction. The 15C and 25C leaves showed a maximum reduction rate at 15°C to 25°C and 30°C, respectively, whereas the 35C leaves did so at 35°C. By contrast, the temperature dependence of P700 reduction with the 2,6-dichlorophenolindophenol (DCIP)/ascorbate couple as electron donor was little affected by preconditioning temperature (Fig. 7C). These results show that electron transport in the plastoquinone region, the rate-limiting step between PS I and PS II, is regulated so as to show the maximum rate at the temperatures to which plants had been exposed.
Figure 7.
Temperature dependence of P700 reduction. A, Time course of reduction of P700, which was oxidized by strong white light for 2 s in 25C leaves at 25°C. Light was turned off at time 0. B, Temperature dependence of P700 reduction. C, Temperature dependence of P700 reduction in isolated thylakoids with DCIP/ascorbate as electron donor. Experimental conditions were as described for quantification of P700. ○, 15C leaves; ●, 25C leaves; ▵, 35C leaves, n = 3.
Temperature Dependence of Electron Transport on the Water Side of PS II
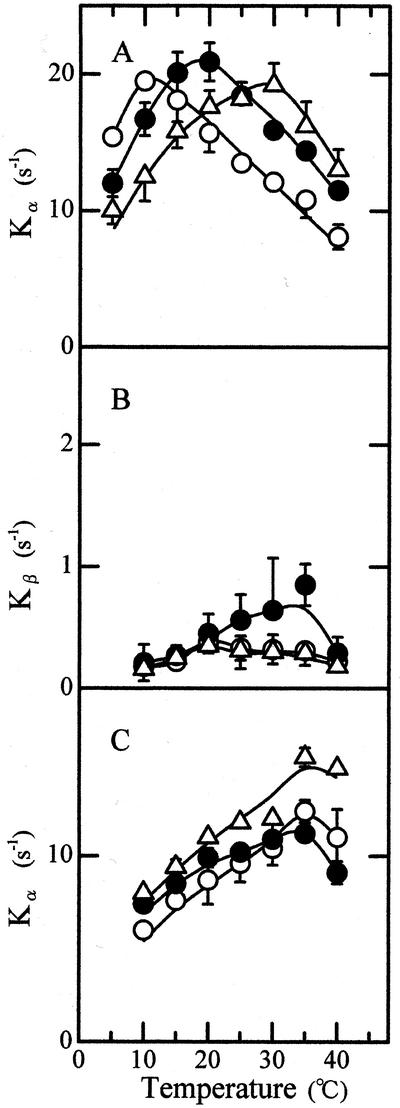
Temperature dependence of PS II electron transport (Fig. 4C) was determined with phenyl-p-benzoquinone, which receives electrons predominantly at the QB site (Satoh et al., 1995). Thus, it is unlikely that temperature dependence of PS II electron transport reflects that of electron transport at the plastoquinone site. Experiments were, therefore, performed to search another region of PS II electron transport that is sensitive to growth temperature. The induction of chlorophyll a fluorescence was determined by illuminating dark-adapted thylakoids to weak continuous light in the presence of 3-(3′, 4′-dichlorophenyl)-1,1-dimethylurea (DCMU). Growth of the area over the fluorescence induction curve was analyzed by the method of Melis and Homann (1975), which distinguishes between a fast nonexponential α-component and a slow exponential β-component. The α- and β-components represent photoreduction of QA in the PS II center that is functional in QB reduction or electron transport (PS IIα) and in the PS II center that is nonfunctional (PS IIβ), respectively. Temperature dependence of Kα, a rate constant of QA photoreduction in PS IIα, was affected by temperatures at which the leaves had developed (Fig. 8A). The 25C leaves showed a maximum rate constant at 20°C, and the 15C and 35C leaves did at 10°C lower and 10°C higher temperature, respectively. No clear effect of preconditioning temperature was observed on the temperature response curve of Kβ, a rate constant of QA photoreduction in PS IIβ (Fig. 8B). When oxygen evolution was inactivated by washing in Tris buffer and QA photoreduction was determined with hydroxylamine as electron donor, maximum Kα values were obtained at 35°C irrespective of temperatures at which the leaves had developed (Fig. 8C). The results show that temperature dependence of electron transport on the water side of the PS II reaction center is also affected by growth temperature.
Figure 8.
Temperature dependence of rate constant of QA photoreduction. A, Kα; B, Kβ; C, Kα determined in the presence of hydroxylamine after Tris-wash. ○, 15C leaves; ●, 25C leaves; ▵, 35C leaves, n = 3. Vertical bars, sd.
DISCUSSION
The present study showed that winter wheat has an extremely high potential for thermal acclimation of photosynthesis. The 25C leaves showed the maximum rate of CO2-saturated photosynthesis at 25°C to 30°C, whereas growth at 15°C led to about 10°C decrease in optimum temperature of photosynthesis. For winter wheat, 35°C is a nonphysiological temperature where development of leaves was retarded and synthesis of chlorophyll was strongly suppressed. It is remarkable, therefore, that exposure of the apical region of the leaf blades developed at 25°C to 35°C resulted in a shift in optimum temperature for CO2-saturated photosynthesis to 35°C. A question arises as to whether the upward shift in optimum temperature is indeed an adaptive response, because the 35C leaves showed a lower photosynthetic rate than the 25C leaves even at 35°C (see Fig. 1). Loss of the activity at 35°C, however, was ascribed to enhanced leaf senescence, because the levels of Rubisco, chlorophyll, P700, and C550 decreased roughly in parallel (Jiang et al., 1999). Thus, the thermal acclimation of photosynthesis seems to occur even at a nonphysiological temperature such as 35°C and in senescing leaves. A large shift in optimum temperature was observed in plants grown outdoors at different seasons of year (Table I; Sawada, 1970).
Photosynthetic acclimation to low temperature has been considered to involve an increase in the amount or activity of enzymes that limit photosynthesis and acclimation to a high temperature was related to increased heat-stability of the photosynthetic apparatus (Berry and Björkman, 1980; Badger et al., 1982). We also observed that the level of Rubisco increased with decreasing preconditioning temperature, and the exposure to 35°C resulted in enhanced heat-stability of PS II photochemistry in winter wheat. It is, however, difficult to explain such a large (more than 15°C) shift in optimum temperature for CO2-saturated photosynthesis as observed in winter wheat only in terms of changes in the level of active enzymes and heat-stability of the photosynthetic apparatus. Our results show that the temperature dependence of CO2-saturated photosynthesis in leaves grown at different temperatures is closely related to the temperature dependence of PS II electron transport. This reflects a situation in which a temperature that is optimum for electron transport is also optimum for RuBP regeneration and, hence, for CO2-saturated photosynthesis. Thus, temperature acclimation of CO2-saturated photosynthesis in winter wheat strongly depends on the plastic response of PS II electron transport to environmental temperature.
There are a few experiments in which temperature dependencies of photosynthesis and electron transport were compared. A large shift in the optimum temperature for photosynthesis was accompanied with a large shift in optimum temperature for electron transport in the arctic plant S. cernua. Exposure of the plants grown at 10°C to 20°C for 10 d resulted in 9°C to 10°C increase in the optimum temperature for gross photosynthesis (Mawson et al., 1986) and a comparable upward shift in optimum temperature for whole-chain and PS II electron transport (Mawson and Cummins, 1989). No correlation was found, however, between the temperature dependence of photosynthesis and electron transport in the two desert shrubs even when photosynthesis was determined in the presence of a saturating CO2 concentration. Larrea divaricata (Armond et al., 1978; Mooney et al., 1978) and Nerium oleander (Badger et al., 1982) grown at 45°C showed thermal optima of both CO2-saturated photosynthesis and whole-chain electron transport at about 45°C, whereas a 25°C decrease in growth temperature resulted in only about a 10°C decrease in the temperature optimum for CO2-saturated photosynthesis and even a less distinct change in the optimum temperature for electron transport. Thus, mechanisms underlying the thermal acclimation of photosynthesis vary with the plant species, and a high photosynthetic acclimation potential to temperature is associated with a highly plastic response of electron transport to temperature.
The temperature response curve of photosynthesis in normal air, which is limited by CO2 concentration and affected by photorespiration, is flatter than that of CO2-saturated photosynthesis (Berry and Björkman, 1980). Analysis of photosynthesis at various concentrations of CO2 suggested, however, that RuBP regeneration (i.e. electron transport) is an important factor that affects temperature dependence of photosynthesis at the atmospheric concentration of CO2 (Hikosaka et al., 1999; Bunce, 2000). In this respect, of particular interest is the temperature dependence of PS II electron transport in leaves (Fig. 5). The rate of PS II electron transport in leaves is strongly linked to the rate of CO2 fixation, although a part of electrons from PS II are partitioned to photorespiration and other processes (Sharkey et al., 1988; Genty et al., 1989; Oberhuber et al., 1993). Thus, the finding that leaves showed the maximum rates of PS II electron transport at or near the preconditioning temperatures (Fig. 5) indicates that the temperature dependence of photosynthesis in the air was also influenced by that of PS II electron transport. Our results are consistent with the early study, which showed that optimum temperature for gross photosynthesis at the atmospheric level of CO2 shifted from 10°C to 28°C as growth temperature of winter wheat raised from 4°C to 28°C (Sawada, 1970).
Evidence was obtained indicating that two regions of PS II electron transport are responsible for the adaptive changes in the temperature dependence of photosynthesis. Kinetic analysis of the fluorescence induction revealed that QA reduction in PS IIα was modulated to show the maximum rate near the temperatures at which the leaves had been exposed. The fluorescence induction was measured in the presence of DCMU, which inhibits electron transfer from QA to QB, but in the absence of hydroxylamine, which blocks electron transfer from QA to an oxidized intermediate on the water side of the PS II reaction center. The rate constant Kα is a function of photochemical parameters such as rate of photon trapping by chlorophylls and carotenoids, efficiencies of excitation energy transfer to and charge separation in the PS II reaction center and a thermochemical parameter, namely, rate of back electron transfer from QA to the oxidized intermediate. Therefore, Kα is maximized when electron transfer from water to the oxidized intermediate is maximized and hence rate of the back electron transfer is minimized. The results show that the temperature dependence of electron transport at the water oxidation site is affected by the temperature at which the leaves had been exposed. This conclusion was supported by the observation that the growth temperature had no effect on the temperature dependence of QA photoreduction in PS IIα, which was determined with hydroxylamine instead of water as electron donor.
Electron transport on the reducing side of PS II reaction center also involves a reaction whose temperature dependence is influenced by the growth temperature. The slow component of QA oxidation, which represents electron transfer from QA to PS I and reduction of P700 with electrons from the plastoquinone pool, showed maximum rate at or near the growth or treatment temperature. The temperature dependence of electron flow from DCIPH2 to P700 was insensitive to the preconditioning temperature. Thus, it is electron transport at the plastoquinone site, the rate-limiting site of electron transport from PS I to PS II, which was modulated to show maximum rates near the growth temperature. Because plastoquinone is located in the lipid phase of the thylakoid membranes, the fluidity of membrane lipids is a crucial factor that governs the temperature response curve of electron transport at the plastoquinone site. A possibility is that prolonged exposure of leaves to different temperatures would result in different compositions of lipids upon which the temperature dependence of membrane fluidity strongly depends. Whether electron transport at the water oxidation site is under the control of membrane fluidity remains to be investigated. At any event, the coordinated changes in the temperature dependence of electron transport on both sides of the PS II reaction center are the major factors contributing to the photosynthetic acclimation to temperature in winter wheat.
MATERIALS AND METHODS
Plant Materials and Temperature Treatments
Seeds of winter wheat (Triticum aestivum L. cv Norin No. 61) were germinated on moistened filter paper at 25°C in darkness for 2 d. Seedlings were planted in vermiculite in plastic trays (32 × 40 cm) at a density of 81 plants per tray. Plants were grown in a growth chamber at 25°C and a relative humidity of 70% under a photoperiod of 12 h with a photon flux density of 170 μmol m−2 s−1. The light source was fluorescent tubes (FL40SEX-N, Toshiba, Tokyo) and photon flux density was measured with a quantum sensor (LI-COR 185, LI-COR, Lincoln, NE). Plants were watered every day and fertilized with a nutrition solution (1:500 Hyponex 5–10−5, Hyponex, Oosaka, Japan) once a week. When the third leaves reached about 10 cm in length, temperature of the chamber was shifted to 15°C or 35°C or maintained at 25°C, and then the plants were grown until full development of the third leaves. In the experiments shown in Table I, however, plants were grown in the open air under a transparent plastic cover on the campus of Toho University (35o 42′ N) at different seasons of year. Plants were watered and fertilized as described above.
Preparation of Thylakoid Membranes
For preparation of thylakoid membranes, leaves were homogenized with a blender in a solution containing 0.4 m Suc, 10 mm NaCl, 5 mm MgCl2, and 50 mm HEPES-NaOH (pH 7.5). The homogenate was filtered through a layer of Miracloth (Calbiochem) and the filtrate was centrifuged at 2,000g for 30 s. The supernatant was again centrifuged at 6,000g for 10 min and thylakoid membranes were suspended in the above medium. For inactivation of oxygen evolution, thylakoid membranes were washed with 0.8 m Tris-HCl, pH 8.0 (Yamashita and Butler, 1968).
Measurement of Photosynthetic Activities
Photosynthetic oxygen evolution was determined with a Hansatech leaf disc oxygen electrode in air containing 4% (w/v) CO2 at indicated temperatures. Leaf segments (3 cm long) were exposed to saturating light (2,000 μmol m−2 s−1) supplied from a 150-W halogen lamp (Luminar Ace LA-150SE, Hayashi, Tokyo) and passed through a Hoya HA heat-absorbing filter. Temperature was controlled by circulating temperature-controlled water through the water jacket, and leaf temperature was monitored with a calibrated thermocouple. Outlines of leaf segments were inputted into a microcomputer with an image scanner for analysis of leaf area.
Electron transport activities in the thylakoid membranes (10 μg chlorophyll mL−1) suspended in the medium described above were measured with a Clark-type oxygen electrode (model YS, YSI Inc., Yellow Springs, OH) under white light at a saturating intensity. For measurement of whole-chain electron transport activity, 10 mm methylamine, 1 mm sodium azide, and 1 mm methyl viologen were added to the suspension. The assay medium for PS I electron transport activity contained 10 mm methylamine, 10 μm DCMU, 1 mm sodium azide, 500 μm DCIP, 2 mm sodium ascorbate, and 1 mm methyl viologen. Activity of PS II electron transport was determined using 1 mm phenyl-p-benzoquinone as electron acceptor, in the presence of 0.5 μm 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone.
The ΦII [the ratio of variable to maximum fluorescence (Fm − Fo)/Fm = Fv/Fm] was determined by measuring chlorophyll a fluorescence with a PAM fluorometer (Walz, Eichenring 6, Germany) according to the protocol described by Genty et al. (1989). Leaf segments were placed in an aluminum cuvette dipped in a temperature-controlled water bath for 5 min before measurement. The initial fluorescence level Fo was excited by a weak red light (0.5 μmol m−2 s−1) modulated at 1.6 kHz and measured at wavelengths longer than 700 nm. The maximum fluorescence level Fm was determined with the leaves that had been kept in the dark for 30 min and induced by an 800-ms pulse of strong white light (>1,500 μmol m−2 s−1). The quantum yield of photochemistry in open PS II reaction centers [(Fm′ − Ft′)/Fm′ = Fv′/Fm′] was also determined (Genty et al., 1989). Fm′ is the fluorescence level induced by an 800-ms pulse in the light (377 μmol m−2 s−1) and Ft is the fluorescence level determined without the 800 ms pulse. Signals were accumulated 64 times and averaged with a digital oscilloscope (VP-5710A, Panasonic, Tokyo).
The PAM fluorometer was also used for measurement of redox changes of QA and P700 in the leaves. Oxidation kinetics of QA was monitored by measuring the decay of chlorophyll a fluorescence, which was excited by a short flash (half-time, 8 μs), from a XST 103 Xenon flash lamp. Signals were transferred to the digital oscilloscope and analyzed with a homemade BASIC program installed in a microcomputer. Reduction kinetics of P700 were determined by measuring absorption changes at 810 nm using a dual-wavelength P700 unit, ED-P700DW. P700 was oxidized by exposure to white light of 1,500 μmol m−2 s−1 for 2 s. Signals were transferred to the digital oscilloscope and analyzed.
Induction of chlorophyll a fluorescence was determined with a laboratory-constructed apparatus. Concentration of thylakoid membranes was equivalent to 10 μg chlorophyll mL−1 and 20 μm DCMU was added. The temperature of the suspension was controlled by circulating water though a water-jacket of the cuvette and monitored with a thermometer. Samples were excited with a green light of 100 μmol m−2 s−1, which passed through a 4–96 filter (Corning, Corning, NY) and a Toshiba VO-54 filter, and fluorescence, which passed through a 680 nm cut-off filter was measured with an R446 photomultiplier (Hamamatsu, Bridgewater, NJ). Signals were stored in a Riken Denshi transient recorder (TCDC-12–8000) and inputted into a microcomputer for analysis.
Measurement of Functional Components
P700 was quantified by measuring light-induced absorption changes at 700 nm with a Hitachi 356 spectrophotometer. Blue actinic light at 80 μmol m−2 s−1 was obtained from a 150-W halogen lamp passed through a Corning 4–96 filter and the photomultiplier was protected with a Toshiba R-66 filter. Thylakoid membranes equivalent to 10 μg chlorophyll mL−1 were suspended in 50 mm HEPES-NaOH (pH 7.5) containing 10 mm NaCl, 1 mm methyl viologen, 1 mm sodium ascorbate, 5 μm DCIP, 10 μm DCMU, and 0.05% (w/v) Triton X-100. PS II was quantified by measuring light-induced absorption changes of C550, an electrochronic shift of pheophytin that is associated with photoreduction of QA (Knaff and Arnon, 1969; McCauley and Melis, 1986) at 550 nm with reference wavelength of 542 nm. The reaction mixture contained 10 mm NaCl, 4 mm ferricyanide, 10 μm DCMU, 20 μm gramicidin D, 50 mm HEPES-NaOH (pH 7.5), 0.1% (w/v) Triton X-100, and thylakoid membranes (equivalent to 75 μg chlorophyll mL−1). Red actinic light at 65 μmol m−2 s−1 was obtained by passing the light from a halogen lamp through a Toshiba R-66 cut-off filter, and the photomultiplier was guarded with a Corning 4–96 filter.
For determination of the contents of Rubisco and chlorophyll, leaves of designated areas were cut into small pieces, frozen with liquid nitrogen, and ground with a chilled mortar and pestle to fine powder. The powder was suspended in ice-cold 100 mm phosphate buffer (pH 7.5) containing 1 mm phenylmethylsulfonylfluoride, 5 mm iodoacetate, and 10 mm MgCl2 at a ratio of 2 cm2 leaf blade mL−1, and thoroughly ground. Chlorophyll was extracted from 1 mL of the homogenate with 80% (w/v) acetone and determined by the procedure of Arnon (1949). The rest of the homogenate was used for quantification of Rubisco. The homogenate was centrifuged at 36,000g for 30 min, and a part of the supernatant was incubated with 5% (w/v) SDS, 8 m urea, and 0.1% (w/v) 2-mercaptoethanol for 60 min at room temperature and then applied to 10% to 15% (w/v) acrylamide gradient gels containing 6 m urea. After electrophoresis, gels were stained with Coomassie Brilliant Blue R-250 for polypeptides. Polypeptide patterns were photographed and analyzed with a microcomputer. Relative contents of Rubisco were estimated from the peak height of the large subunit bands resolved.
Footnotes
This work was supported in part by the Ministry of Education, Science, Sport and Culture of Japan (grant no. 11440238 to K.S.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010919.
LITERATURE CITED
- Armond PA, Schreiber U, Björkman O. Photosynthetic acclimation to temperature in the desert shrub, Larrea divaricata: II. Light-harvesting efficiency and electron transport. Plant Physiol. 1978;61:411–415. doi: 10.1104/pp.61.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Björkman O, Armond PA. An analysis of photosynthetic response and adaptation to temperature in higher plants: temperature acclimation in the desert evergreen Nerium oleander L. Plant Cell Environ. 1982;5:85–99. [Google Scholar]
- Berry J, Björkman O. Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol. 1980;31:491–543. [Google Scholar]
- Bowes JM, Crofts A. Binary oscillation in the rate of reoxidation of the primary acceptor of photosystem II. Biochim Biophys Acta. 1980;590:373–384. doi: 10.1016/0005-2728(80)90208-x. [DOI] [PubMed] [Google Scholar]
- Bukhov NG, Mohanty P, Rakhimberdieva MG, Karapetyan NV. Analysis of dark-relaxation kinetics of variable fluorescence in intact leaves. Planta. 1992;187:122–127. doi: 10.1007/BF00201633. [DOI] [PubMed] [Google Scholar]
- Bunce JA. Acclimation of photosynthesis to temperature in eight cool and warm climate herbaceous C3 species: temperature dependence of parameters of a biochemical photosynthesis model. Photosynth Res. 2000;63:59–67. doi: 10.1023/A:1006325724086. [DOI] [PubMed] [Google Scholar]
- Cao J, Govindjee Chlorophyll a fluorescence transient as an indicator of active and inactive photosystem II in thylakoid membranes. Biochim Biophys Acta. 1990;1015:180–188. doi: 10.1016/0005-2728(90)90018-y. [DOI] [PubMed] [Google Scholar]
- Crespi MD, Zabaleta EJ, Pontis HG, Salerno GL. Sucrose synthase expression during cold acclimation in wheat. Plant Physiol. 1991;96:887–891. doi: 10.1104/pp.96.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S. Modelling of photosynthetic response to environmental conditions. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Physiological Plant Ecology II: Water Relations and Carbon Assimilation. Encyclopedia of Plant Physiology. 12B. Berlin: Springer-Verlag; 1982. pp. 550–587. [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Harbinson J, Hedley CL. The kinetics of P-700-positive reduction in leaves a novel in situ probe of thylakoid functioning. Plant Cell Environ. 1989;12:357–369. [Google Scholar]
- Haumann M, Junge W. The rates of proton uptake and electron transfer at the reducing side of photosystem II in thylakoids. FEBS Lett. 1994;347:45–50. doi: 10.1016/0014-5793(94)00495-1. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Murakami A, Hirose T. Balancing carboxylation and regeneration of ribulose-1,5-bisphosphate in leaf photosynthesis: temperature acclimation of an evergreen tree, Quercus myrsinaefolia. Plant Cell Environ. 1999;22:841–849. [Google Scholar]
- Holaday AS, Martindale W, Alred R, Brooks AL, Leegood RC. Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature. Plant Physiol. 1992;98:1105–1114. doi: 10.1104/pp.98.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huner NPA. Acclimation of winter rye to clod-hardening temperatures results in an increased capacity for photosynthetic electron transport. Can J Bot. 1985;63:506–511. [Google Scholar]
- Hurry VM, Strand Å, Tobiæson M, Gardeström P, Öquist G. Cold hardening of spring and winter wheat and rape results in differential effects on growth, carbon metabolism, and carbohydrate content. Plant Physiol. 1995;109:697–706. doi: 10.1104/pp.109.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CZ, Ishihara K, Satoh K, Katoh S. Loss of the photosynthetic capacity and proteins in senescence leaves at top positions of two cultivars of rice in relation to the source capacities of the leaves for carbon and nitrogen. Plant Cell Physiol. 1999;40:496–503. [Google Scholar]
- Knaff DB, Arnon DI. Spectral evidence for a new photoreactive component of the oxygen-evolving system in photosynthesis. Proc Natl Acad Sci USA. 1969;63:963–969. doi: 10.1073/pnas.63.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Nakano H, Mae T. Response of ribulose-1,5-bisphosphate carboxylase, cytochrome f, and sucrose synthesis enzymes in rice leaves to leaf nitrogen and their relationships to photosynthesis. Plant Physiol. 1994;105:173–179. doi: 10.1104/pp.105.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama S, Yatomi M, Nakamura Y. Response of rice leaves to low temperature: I. Changes in basic biochemical parameters. Plant Cell Physiol. 1990;31:303–309. [Google Scholar]
- Mawson BT, Cummins RW. Thermal acclimation of photosynthetic electron transport activity by thylakoid of Saxifraga cernua. Plant Physiol. 1989;89:325–332. doi: 10.1104/pp.89.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawson BT, Svoboda J, Cummins RW. Thermal acclimation of photosynthesis by the arctic plant Saxifraga cernua. Can J Bot. 1986;64:71–76. [Google Scholar]
- McCauley SW, Melis A. Quantitation of photosystem II in spinach chloroplasts. Biochim Biophys Acta. 1986;849:175–182. [Google Scholar]
- Melis A, Homann PH. Kinetic analysis of the fluorescence induction in 3-(3,4-dichlorophenyl)-1,1-dime-thylurea poisoned chloroplasts. Photochem Photobiol. 1975;21:431–437. [Google Scholar]
- Mitchell RAC, Barber J. Adaptation of photosynthetic electron-transport rate to growth temperature in pea. Planta. 1986;169:429–436. doi: 10.1007/BF00392141. [DOI] [PubMed] [Google Scholar]
- Mooney HA, Björkman O, Collatz GJ. Photosynthetic acclimation to temperature in the desert shrub, Larrea divaricata: I. Carbon dioxide exchange characteristics of intact leaves. Plant Physiol. 1978;61:406–410. doi: 10.1104/pp.61.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhuber W, Dai ZY, Edwards GE. Light dependence of quantum yields of photosystem II and CO2 fixation in C3 and C4 plants. Photosynth Res. 1993;35:265–274. doi: 10.1007/BF00016557. [DOI] [PubMed] [Google Scholar]
- Pearcy RW. Effects of growth temperature on the thermal stability of the photosynthetic apparatus of Atriplex lentiformis (Torr.) Wats. Plant Physiol. 1977;59:873–878. doi: 10.1104/pp.59.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Oh-hashi M, Kashino Y, Koike H. Mechanism of electron flow through the QB site in photosystem II: 1. Kinetics of the reduction of electron acceptors at the QB and plastoquinone sites in photosystem II particles from the cyanobacterium Synechococcus vulcanus. Plant Cell Physiol. 1995;36:597–605. [Google Scholar]
- Sawada S. An ecophysiological analysis of the difference between the growth rates of young wheat seedlings grown in various seasons. J Fac Sci Univ Tokyo III. 1970;10:233–263. [Google Scholar]
- Schreiber U. New emitter-detector-cuvette assembly for measuring modulated chlorophyll fluorescence of highly diluted suspension in conjunction with the standard PAM fluorometer. Z Naturforsch. 1994;49:646–656. [Google Scholar]
- Sharkey TD, Berry JA, Sage RF. Regulation of photosynthetic electron-transport in Phaseolus vulgaris L., as determined by room-temperature chlorophyll a fluorescence. Planta. 1988;176:415–424. doi: 10.1007/BF00395423. [DOI] [PubMed] [Google Scholar]
- Strain BR, Higginbotham KO, Mulroy JC. Temperature preconditioning and photosynthetic capacity of Pinus taeda L. Photosynthetica. 1976;10:47–53. [Google Scholar]
- Strand Å, Hurry V, Henkes S, Huner N, Gustafsson P, Gardeström P, Stitt M. Acclimation of Arabidopsis leaves developing at low temperature: Increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin cycle and in the sucrose-biosynthesis pathway. Plant Physiol. 1999;119:1387–1397. doi: 10.1104/pp.119.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieszen LL, Helgager JA. Genetic and physiological adaptation in the Hill reaction of Deschampsia caespitosa. Nature. 1968;219:1066–1067. doi: 10.1038/2191066a0. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Butler WL. Photoreduction and photophosphorylation with Tris-washed chloroplasts. Plant Physiol. 1968;43:1978–1986. doi: 10.1104/pp.43.12.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]