Abstract
γ-Glutamyl transpeptidase (γ-GT) is a ubiquitous enzyme that catalyzes the first step of glutathione (GSH) degradation in the γ-glutamyl cycle in mammals. A cDNA encoding an Arabidopsis homolog for γ-GT was overexpressed in tobacco (Nicotiana tabacum) plants. A high level of the membrane-bound γ-GT activity was localized outside the cell in transgenic plants. The overproduced enzyme was characterized by a high affinity to GSH and was cleaved post-translationally in two unequal subunits. Thus, Arabidopsis γ-GT is similar to the mammalian enzymes in enzymatic properties, post-translational processing, and cellular localization, suggesting analogous biological functions as a key enzyme in the catabolism of GSH.
Glutathione (GSH) is involved in a number of important cellular functions, particularly in the storage and transport of reduced S, protection of cells against oxidative stress, detoxification of xenobiotics and heavy metals, redox regulation of gene expression, and progression through the cell cycle (Meister and Anderson, 1983; May et al., 1998; Noctor et al., 1998; den Boer and Murray, 2000). Because GSH participates directly in the cellular protection against oxidative stress, modification of the GSH metabolism by changing the production of the enzymes involved in its regulation can be a useful approach to obtaining stress-tolerant plants (Noctor et al., 1998).
In mammals, the GSH metabolism is mediated by the so-called γ-glutamyl cycle (Meister and Anderson, 1983) that includes two ATP-dependent GSH synthesis steps, catalyzed by γ-glutamyl Cys synthetase and GSH synthetase, and a specific GSH degradation pathway, which allows the reutilization of the amino acids for further GSH resynthesis. Because GSH is considered to be the main storage compound of reduced S, GSH degradation is an important process to make S available for an organism in the form of Cys (Lieberman et al., 1996).
The first enzyme of the GSH breakdown pathway is γ-glutamyl transpeptidase (γ-GT; EC 2.3.2.2). γ-GT catalyzes transfer of the γ-glutamyl moiety from GSH to amino acids, peptides (including GSH itself), or water (Meister et al., 1981; Taniguchi and Ikeda, 1998). In contrast to GSH synthesis, GSH breakdown occurs on the outer surface of the plasma membrane and includes the γ-GT reaction followed by the action of a dipeptidase:
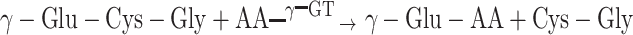 |
 |
γ-Glutamyl amino acids formed as a result of the γ-GT action are then transported back into cells where they become substrates for a γ-glutamyl cyclotransferase, an enzyme converting them into 5-oxo-Pro and the corresponding amino acids. Furthermore, a 5-oxo-prolinase converts 5-oxo-Pro to l-Glu acid, completing the γ-glutamyl cycle:
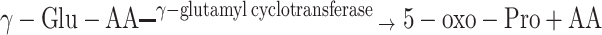 |
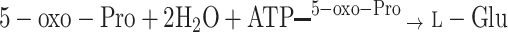 |
The mammalian γ-GT is highly active in organs with a secretory or absorptive function. These organs are characterized by a high rate of GSH export outside the cells (Meister and Anderson, 1983). γ-GT is considered to be the main enzyme for Cys reabsorption and transport. Knock-out mice lacking a functional γ-GT lose Cys in the form of GSH because they cannot reuse the GSH that has been exported outside the cells. This leads to the development of glutathionemia and glutathionuria, and animals eventually die from Cys starvation. This phenotype can be partially rescued by Cys administration (Lieberman et al., 1996).
Because GSH conjugates are also substrates for γ-GT, the enzyme plays a role both in detoxification of poisonous compounds (Meister, 1988; Ishikawa, 1992) and the normal metabolism of biologically active compounds such as leukotrienes and prostaglandins (Cagen et al., 1976; Anderson et al., 1982; Meister, 1988; Ishikawa, 1992; Carter et al., 1997).
γ-GT is also an important enzyme that probably modulates the redox status of thiols in the plasma membrane proteins (Del Bello et al., 1999; Dominici et al., 1999), because one of the products of the γ-GT reaction, Cys-Gly, contains a highly reactive thiol capable of producing active oxygen species by participating in the Fenton reaction with iron ions (Halliwell and Gutteridge, 1989).
In plants, enzymes with γ-GT activity are believed to be involved in secondary metabolism and to catalyze the synthesis of a range of γ-glutamyl dipeptides, which are formed during fruit ripening and accumulate in storage tissues such as seeds or bulbs in certain plants. Such peptides include hypoglycin B in the ackee plant (Blighia sapida; Kean and Hare, 1980); γ-Glu linked to Asp, Phe, and Tyr in soybean (Glycine max; Ishikawa et al., 1967); Asp, Glu, and Tyr in asparagus (Asparagus officinalis; Kasai et al., 1982); d-Ala and homo-Ser in pea (Pisum sativum) seedlings (Kawasaki et al., 1982); and some others. In onion, γ-GT is supposed to catalyze the last step in the formation of volatile compound precursors by cleaving the γ-glutamyl moiety off γ-glutamyl alk(en) yl-Cys sulfoxides (Lancaster and Shaw, 1994).
Whether plant γ-GT participates in GSH metabolism is still an open question. It is not clear whether GSH is a true substrate in vivo for the plant γ-GT, because, in some cases, GSH has been reported to be a poor in vitro substrate for the plant γ-GT (Steinkamp and Rennenberg, 1984; Lancaster and Shaw, 1994). Moreover, the existence of the γ-glutamyl cycle in plants is not evident. Although the GSH biosynthetic pathway is relatively well studied (Noctor et al., 1998), very little is known about the GSH degradation mechanism(s). Results obtained in experiments with tobacco (Nicotiana tabacum) suspension cultures indicated that GSH degradation is initiated by a carboxypeptidase (Steinkamp and Rennenberg, 1985; Rennenberg and Lamoureux, 1990). The γ-Glu-Cys dipeptide produced as a result of this reaction is further degraded to Glu and Cys by the consecutive actions of γ-glutamylcyclotransferase and 5-oxo-prolinase (Rennenberg and Lamoureux, 1990). On the other hand, Cys-Gly found in soybean (Bergmann and Rennenberg, 1993) and heterotrophic tobacco cultures (Schneider and Rennenberg, 1992) suggests that the first step in GSH degradation can be catalyzed by γ-GT, followed by a dipeptidase activity.
γ-GT activities and the purified enzymes have been characterized from a number of plant species (Thompson et al., 1964; Goore and Thompson, 1967; Kasai et al., 1982; Kawasaki et al., 1982; Martin and Slovin, 2000). However, the reported molecular masses and composition, cellular localization, and biochemical properties vary considerably.
A cDNA (D22) from Arabidopsis has been cloned that confers resistance to oxidative stress when expressed in yeast (Saccharomyces cerevisiae; Kushnir et al., 1995). The cDNA encoded a protein with extensive homology to the mammalian γ-GT. This finding prompted us to overexpress the cDNA in tobacco plants to characterize further the encoded protein and to study the possible effects of its overproduction on the GSH metabolism.
RESULTS
D22 cDNA Is Homologous to the Mammalian γ-GT
Alignment of the deduced amino acid sequence of the protein encoded by the D22 cDNA (Kushnir et al., 1995) with the mammalian γ-GT revealed that the putative Arabidopsis γ-GT (AtGGT) is very similar to the animal enzymes (Fig. 1). The AtGGT shares 41% identical and 48% similar amino acids with the human (Homo sapiens) γ-GT. A putative hydrophobic membrane-anchoring domain was found at the N terminus of the plant protein but was shorter than that of the mammalian enzyme. All amino acid residues, which are necessary for the catalytic activity of the mammalian γ-GT, are conserved in the plant enzyme: Arg-107, Asp-422, and Asp-423 (positions correspond to the human enzyme), which are believed to be important for binding of the donor substrate, and Ser-451 and Ser-452, which are of critical importance in both enzyme catalysis and in the reaction with the specific γ-GT inhibitor acivicin (Taniguchi and Ikeda, 1998). These data suggest that the D22 cDNA probably codes for an AtGGT that might have characteristics similar to those of the mammalian enzyme.
Figure 1.
Amino acid sequences of AtGGT (accession no. S58286) and other putative γ-GTs from Arabidopsis (corresponding accession nos. put as names) aligned with the human (accession no. P19440) and mouse (accession no. Q60928) enzymes. Identical amino acids in all sequences are boxed. Amino acids homologous to AtGGT are shadowed. Membrane-anchoring domains and putative membrane-anchoring domains of the mammalian and plant enzymes are underlined. The open arrow points to the protease cleavage site. Amino acids important for the substrate binding and those essential for the catalytic activity are marked with asterisks and black circles, respectively. Putative glycosylated amino acids in the sequence of AtGGT are indicated by black rectangles.
γ-GT in Arabidopsis Is Encoded by a Small Gene Family
Purification of enzymes with γ-GT activity from different plant species resulted in contradictions regarding their molecular masses, subunit composition, cellular localization, and biochemical characteristics. The availability of the nearly complete genomic sequence of Arabidopsis allowed us to assess to what extent these discrepancies might be due to either artifacts of enzyme purification procedures or natural gene diversity.
Database searches revealed that the AtGGT gene (accession no. CAB80627) is localized on chromosome IV. The sequence of the gene is immediately followed by that of a putative gene (accession no. CAB80628), of which the deduced amino acid sequence of the encoded protein shares 83% identical and 87% similar residues with AtGGT. These data are in agreement with the previously obtained DNA gel-blot hybridization data (Kushnir et al., 1995) that showed the presence of at least two highly homologous genes for AtGGT. Furthermore, one additional putative gene (accession no. CAB79679) was found on the same chromosome with a deduced protein sequence with 50% identical and 59% similar amino acids to AtGGT and all of the hallmarks required for catalytic activity. This putative protein of 69.2 kD has a long N-terminal extension, which is predicted to contain a trans-membrane domain. A potential gene (accession no. AAF07391) encoding a small protein of 191 amino acids (20.9 kD) was also found on chromosome I. The sequence of the encoded protein matched the 311- to 501-amino acid fragment of AtGGT, which contains the active site of the enzyme (Taniguchi and Ikeda, 1998). The putative protein shares 85% identical and 87% similar amino acids with AtGGT in this region. Thus, in Arabidopsis, γ-GT is probably represented by at least four different isoforms that might have different cellular localizations and serve different functions.
Transgenic Tobacco Plants Producing AtGGT Possess High γ-GT Activity
To further characterize AtGGT, transgenic tobacco plants were transformed with the D22 cDNA under the control of the 35S promoter. Integration and expression of the transgenes were checked by DNA and RNA gel-blot hybridizations, respectively (data not shown). Transgenic lines that demonstrated highest transgene expression at the level of mRNA were selected for further analysis.
Total protein extracts from the leaves of transgenic plants have been shown to catalyze the release of p-nitroaniline (PNA) from γ-glutamyl-p-nitroanilide (GPNA), a typical reaction used to assay γ-GT activity (Orlowski and Meister, 1963; Fig. 2). No activity was detected in protein extracts isolated from leaves of control plants transformed with the “empty” vector under the assay conditions. This observation suggests that the activity detected is attributed to the overproduced protein. This result allowed us to conclude that AtGGT is a plant γ-GT.
Figure 2.
γ-GT activities detected in the transgenic plants expressing D22 cDNA determined by the rate of PNA release in the standard transpeptidation reaction with GPNA as donor and Gly-Gly as acceptor substrates. D22/*, Protein extracts from different transgenic lines incubated with the substrates; control, protein extract from the control plant incubated with the reaction substrates.
AtGGT Is Localized on the Plasma Membrane Outside the Cell
Because the animal γ-GT is a membrane-bound protein, anchored by its short N-terminal hydrophobic domain to the plasma membrane outside the cell, we addressed the question of where AtGGT is localized. Analysis of the AtGGT protein sequence with appropriate software predicted a similar leader sequence in the N terminus and suggested with equal likelihood that the enzyme was localized either in the plasma or in the vacuolar membranes. To find out whether AtGGT is a membrane-bound protein, we analyzed the solubilization of the enzymatic activity as a function of the ionic strength of the extraction buffer, because it had been shown previously that membrane-bound plant γ-GTs could be solubilized in a high-salt buffer (Martin and Slovin, 2000).
No γ-GT activity was detected in the soluble fraction of the leaf tissue of transgenic plants under low-ionic-strength conditions, indicating that the overproduced enzyme was exclusively membrane bound. The activity could only be solubilized in a high-salt solution, containing 0.5 to 1 m NaCl.
The mammalian γ-GT is localized on the plasma membrane outside the cell. To check whether this is the case for the plant enzyme, we incubated intact mesophyll protoplasts from the transgenic and control plants in a solution containing GPNA. Whereas no γ-GT activity was observed with protoplasts from the control plants, incubation of protoplasts from transgenic plants resulted in the release of PNA, suggesting that the overproduced enzyme is localized outside the cell. However, this result still leaves open the possibility that the substrate has been taken up by the protoplasts with further secretion of the reaction product. Furthermore, it cannot be excluded that a fraction of the enzyme is localized in the intracellular membranes, such as the vacuolar membrane or the endoplasmic reticulum.
To localize the AtGGT activity in a more direct way, leaf discs from the transgenic and control plants were infiltrated with a fluorescent γ-GT substrate, γ-glutamyl-7-amido-4-methylcoumarin (Smith et al., 1979). The fluorescence was monitored in vivo using laser confocal microscopy. As is seen in Figure 3, intense fluorescence is associated with the plasma membrane/cell wall of the AtGGT-overproducing plants. No membrane-associated fluorescence was detected in control plants. Thus, the AtGGT is a plasma membrane-bound protein and localized outside the cell.
Figure 3.
Confocal images of tobacco leaf discs infiltrated with γ-glutamyl-7-amido-4-methylcoumarin. A and B, AtGGT-overproducing plants. C and D, Control plants. Bars = 60 μm (A and C) and 25 μm (B and D).
AtGGT Consists of Two Unequal Subunits
The mammalian γ-GT is composed of two subunits derived from the processing by a protease of a single polypeptide chain precursor. When high-salt protein extracts from the transgenic and control plants were analyzed by protein gel blots with a polyclonal antibody raised against AtGGT (see “Materials and Methods”), two bands with calculated molecular masses of 29 and 41 kD in a 1:1 ratio were found to react with the antibody (Fig. 4). The sum of their calculated molecular masses, 70 kD, was roughly equal to the expected molecular mass of AtGGT (61.1 kD). The AtGGT proteolysis did probably not occur during the protein isolation, because the same bands were obtained in the protein gel blots of total protein extracts isolated under denaturing conditions (data not shown). From these data, the bands can be assumed to be products of the single-chain precursor cleavage. The discrepancy between expected and determined molecular masses can be explained by possible glycosylation of the plant γ-GT, because animal γ-GTs are known to be heavily glycosylated (Taniguchi and Ikeda, 1998). Two potential glycosylation sites were found in the large subunit sequence and one in the sequence of the small subunit (Fig. 1).
Figure 4.
Protein gel blots of protein extracts solubilized in 0.5 m NaCl from transgenic plants with polyclonal anti-AtGGT antibody. D22, Protein extract from the transgenic plant overproducing AtGGT; C, protein extract from the control plant transformed with an “empty” vector.
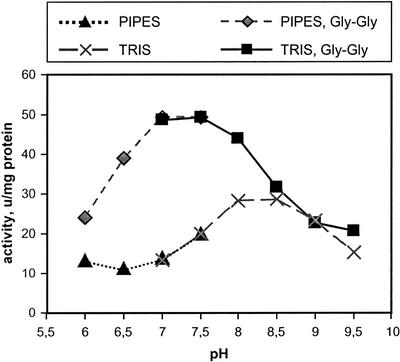
AtGGT Is Similar to the Mammalian Enzyme in Its Biochemical Characteristics
Given the similarity between the mammalian γ-GT and AtGGT sequences, processing, and extracellular localization, the question arises as to whether the plant enzyme also possesses similar biochemical characteristics. The plant enzyme efficiently hydrolyzes GPNA in a relatively wide pH range (Fig. 5), and by adding the “classical” γ-glutamyl group acceptor, dipeptide Gly-Gly, the reaction is stimulated by approximately 1.5-fold (Vmax of the hydrolysis and transpeptidation reactions, 5 and 8 μm min−1, respectively). The transpeptidation was stimulated also to a different extent by addition of different amino acid acceptors, each having a different pH optimum (data not shown). The Km value of AtGGT for GPNA as determined in the transpeptidation reaction with GPNA as donor and Gly-Gly as acceptor was in a range similar to those of the enzymes from other organisms (Table I).
Figure 5.
pH dependence of the AtGGT transpeptidation and hydrolysis. Two buffers, 1,4-piperazinediethanesulfonic acid (PIPES) and Tris, were used to cover the pH range from 6.0 to 9.5. Both activities were 1.8-fold lower in PIPES buffer. The corresponding curves were shifted to the level of the activities in Tris buffer to make a continuous curve.
Table I.
Km values of different γ-GTs for GPNA donor substrate
| Enzyme Source | Km Value | Reference |
|---|---|---|
| mm | ||
| Human | 0.9 | Visvikis et al. (1991) |
| Rat kidney | 2.0 | Thompson and Meister (1977) |
| Yeast | 1.5 | Penninckx and Jaspers (1985) |
| Onion | 0.6 | Lancaster and Shaw (1994) |
| Tomato I | 1.7 | Martin and Slovin (2000) |
| Tomato II | 2.1 | Martin and Slovin (2000) |
| Arabidopsis | 0.8 | This work |
Because one of the supposed functions of AtGGT is connected to GSH catabolism, it was important to determine whether AtGGT could readily use GSH as donor substrate. Incubation of a purified bovine γ-GT with GSH as the donor and [35S]Met as the acceptor of the γ-glutamyl group led to the formation of the 35S-labeled γ-Glu-Met, which was visualized by thin-layer chromatography (Fig. 6). Essentially the same reaction was catalyzed by the protein extract from transgenic plants but not by that from the control plants. These data prove unequivocally that GSH can be used in vitro by AtGGT as donor of the γ-glutamyl group.
Figure 6.
Radio-image of thin-layer chromatograph showing the reaction products of AtGGT with GSH as donor and [35S]Met as acceptor of the γ-Glu group. AtGGT and BovGGT reactions were catalyzed by the protein extract from AtGGT-overproducing plants and by purified bovine γ-GT, respectively. Control, Reaction was carried out in the presence of the protein extract from control plants; −, no GSH added; +, 1 mm GSH added as donor substrate.
The ability of AtGGT to use GSH as donor substrate in vitro raises another important question as to whether GSH is a high-affinity substrate that can be used by the enzyme in vivo. When GSH was added to the standard activity reaction, the release of PNA from GPNA was inhibited competitively (Fig. 7). In such a reaction in which one substrate inhibits the other substrate, the inhibition constant Ki is actually equal to the kinetic constant Km of the inhibiting substrate (Cornish-Bowden and Wharton, 1988). The kinetics of PNA release at different GSH concentrations allowed us to determine the Ki as 255 ± 4 μm. Therefore, the Km value of AtGGT for GSH is 255 ± 4 μm, which is low enough to claim that GSH is a good substrate for AtGGT in vitro and can potentially be the main in vivo substrate.
Figure 7.
Competitive inhibition by GSH of PNA release catalyzed by AtGGT, when added to the standard transpeptidation reaction. GPNA and Gly-Gly were used as donor and acceptor substrates, respectively.
Taken together, these results confirm that AtGGT has catalytic characteristics very similar to those of yeast, mammalian, and other plant γ-GTs and, more importantly, that it has a high in vitro affinity to GSH, suggesting that GSH may be the functional substrate for the plant γ-GT in vivo.
Transgenic and Control Plants Have Similar GSH Degradation Rates
By assuming that GSH is a good substrate for AtGGT, does the high γ-GT activity affect GSH metabolism in transgenic plants? Quantitative determination of GSH and oxidized glutathione (GSSG) demonstrated that the steady-state levels of these compounds (800 ± 100 pM μg−1 protein with a GSH:GSSG ratio of 9:1 [w/w]) are essentially the same in leaves of transgenic and control plants. This observation does not exclude the possibility that the GSH turnover rate can be different in transgenic plants, because the higher GSH degradation rate brought about by the AtGGT overproduction might be compensated by an increase in the rate of GSH synthesis. To check whether this is the case, leaf discs from transgenic and control plants were infiltrated with l-buthionine-[S,R]-sulfoximine, which inhibits GSH synthesis, and the time course of the GSH degradation rate was followed (Fig. 8). Upon inhibition of GSH synthesis with l-buthionine-[S,R]-sulfoximine, the level of GSH slowly dropped to approximately 10% of the initial value after 32 h of incubation in leaf discs from both transgenic and control plants. No significant differences were observed between the transgenic and control plants in the degradation rate of GSH.
Figure 8.
Time course of GSH breakdown in leaf discs of transgenic and control plants infiltrated with 1 mm l-buthionine-[S,R]-sulfoximine. To simplify the picture error bars were not added. sd in three independent experiments was not more that 15%.
DISCUSSION
In transgenic plants overproducing AtGGT, the γ-GT activity is high in the leaves, whereas no activity is detected in the leaves of control plants, at least under our assay conditions. This observation allowed us to use crude protein extracts from the transgenic plants to study the catalytic properties of AtGGT without further purification.
We show that AtGGT is a membrane-bound protein localized on the plasma membrane outside the cell. This result is consistent with most data concerning mammalian enzymes, which have been shown to have the same cellular localization (Meister et al., 1981; Taniguchi and Ikeda, 1998). In contrast, the yeast enzyme is confined not only to the plasma membrane (Payne and Payne, 1984), but also to the vacuolar membrane (Jaspers and Penninckx, 1984). Existing data on the cellular localization of plant γ-GTs are controversial. Two isoforms of γ-GT from tomato (Lycopersicon esculentum) fruit have been reported to be exclusively membrane-bound proteins (Martin and Slovin, 2000). Steinkamp and Rennenberg (1984) attributed only 30% of the total γ-GT activity in a tobacco suspension culture to the plasma membrane-bound fraction and the remaining 70% to the cytoplasm. This result was explained either by the enzyme solubilization during the tissue homogenization step or by the existence of several γ-GT isoforms in tobacco (Steinkamp and Rennenberg, 1984). The Arabidopsis database search revealed that the plant γ-GT is represented by a multigene family, in which the protein products may have different cellular localizations and serve different functions.
The protein gel-blot analysis strongly suggests that AtGGT is a heterodimer that is formed from the cleavage of the single-chain precursor with the large and small subunits of approximately 41 and 29 kD, respectively, as determined from the protein gel-blot hybridization. Moreover, the amino acid sequence of the cleavage site is well conserved in all γ-GT sequences available, and the AtGGT sequence is no exception to this rule (Fig. 1). The data are in a good agreement with other reports showing that all γ-GTs from bacteria, yeast, and mammals that have been studied to date, to our knowledge, are post-translationally cleaved into two subunits with molecular masses ranging from 38 to 72 kD for the large subunit and from 21 to 66 kD for the small subunit (Taniguchi and Ikeda, 1998). It is noteworthy that such a high variation in the molecular masses can be explained by a high glycosylation of the animal (Taniguchi and Ikeda, 1998) and plant enzymes (Lancaster and Shaw, 1994; Martin and Slovin, 2000).
Previous studies concerning purified γ-GT from plants reported that the enzymes were single polypeptides with highly variable molecular masses: 12.5 kD in the ackee plant (Kean and Hare, 1980), 43 kD in tomato fruits (Martin and Slovin, 2000), 56.7 kD in onion (Lancaster and Shaw, 1994), and 180 kD in kidney bean (Goore and Thompson, 1967). These differences can be explained by the existence of different enzyme isoforms, by partial proteolysis of the purified proteins, or by glycosylation, in the case of the very high-molecular masses.
The catalytic properties of AtGGT are very similar to those of other γ-GTs studied to date. When the artificial substrate GPNA is used, the kinetics constants are in the same range as those described for the mammalian, yeast, bacterial, and plant enzymes (Table I). However, a discrepancy in the enzyme affinity toward GSH is found in different γ-GTs of plant origin. γ-GT purified from onion has a Km value of 4.97 mm for GSH (Lancaster and Shaw, 1994). Indirect evidence presented by Steinkamp and Rennenberg (1984) also confirmed that GSH is a poor substrate for γ-GT from a tobacco suspension culture, challenging the assumption that plant γ-GT may function in vivo as an enzyme of the GSH catabolism. On the other hand, two γ-GTs isolated from tomato fruits (Martin and Slovin, 2000) have a high affinity to GSH (Km values 90 and 110 μm). Like the enzymes from tomato fruits, AtGGT has a high affinity to GSH (Km value 255 μm), which strongly suggests that GSH can be used in vivo by AtGGT as the main donor substrate.
If we assume that AtGGT can use GSH, the AtGGT-overproducing transgenic plants may be an interesting experimental system to study the role of γ-GT in GSH metabolism. We did not find a significant difference in either the steady-state levels of GSH or in the GSH to GSSG ratio in transgenic plants when compared with control plants under “normal” conditions. Moreover, the GSH degradation rate was essentially the same in transgenic and control plants.
Although seemingly surprising, this fact makes direct sense when the extracellular localization of AtGGT is borne in mind. For example, under “normal conditions” the apoplast of barley (Hordeum vulgare) does not contain detectable amounts of GSH (Vanacker et al., 1999), which probably explains why the increase in the γ-GT activity brought about by the AtGGT overproduction did not lead to a higher GSH degradation rate and why the endogenous γ-GT activity was practically undetectable in the control plants. However, the situation can dramatically change when plants are challenged by biotic or abiotic stresses. In case of barley, the hypersensitive response to the mildew pathogen results in the increased GSH synthesis rate with the amount of apoplastic GSH rising to 2% of the total GSH content. Under such conditions, AtGGT potentially can play a role in GSH salvage and recycling as does the animal enzyme.
In conclusion, AtGGT is very similar to the mammalian enzyme in its in vitro biochemical properties, molecular structure, and cellular localization. Its possible in vivo role in GSH metabolism requires further investigation.
MATERIALS AND METHODS
Bacterial Strains
For the molecular cloning procedures, the Escherichia coli strain JM109 (Promega, Madison, WI) was used, and expression of the D22–6x-His fusion was carried out in the E. coli BL21(DE3) strain (Promega). Agrobacterium tumefaciens strain C58C1RifR (pGV2260; Deblaere et al., 1985) was used for the transformation of tobacco plants.
Transformation of Tobacco (Nicotiana tabacum) Plants and Analysis of the Transgene Integration and Expression
Tobacco SR1 (Maliga et al., 1975) was used for the generation of stable transformants according to the procedure of leaf disc cocultivation (De Block et al., 1987). Potential transgenic lines were analyzed by DNA and RNA gel-blot hybridizations. In both procedures, Hybond-N membranes (Amersham Pharmacia Biotech, Little Chalfont, UK) were used, and hybridizations were done under stringent conditions according to the manufacturer's specifications. Genomic DNA was isolated using DNeasy Kit (Qiagen, Hilden, Germany). Total RNA was isolated with the TRIzol reagent (Invitrogen, Gaithersburg, MD). Probes were labeled with Redivue [32P]dCTP (3,000 Ci mmol−1) with the Rediprime DNA labeling kit (Amersham Pharmacia Biotech).
Molecular Cloning Procedures
For the expression of D22 cDNA in transgenic plants, a plant expression cassette pDH51 (Pietrzak et al., 1986) was used containing the 35S promoter and the 3′nos transcription terminator. The vector was digested with the restriction enzyme XbaI (Amersham Pharmacia Biotech). The resulting 5′-protruding ends were dephosphorylated with calf intestinal phosphatase (Promega) followed by end-filling with the Klenow fragment (Amersham Pharmacia Biotech). D22 cDNA was cut out from the pFL61 yeast (Saccharomyces cerevisiae) vector with NotI (Amersham Pharmacia Biotech) and ligated with the vector after the ends were filled in with the Klenow fragment. The resulting fusion between the promoter cDNA and the terminator was excised from the vector with EcoRI (Amersham Pharmacia Biotech) and subcloned into the plant SalI-linearized (Amersham Pharmacia Biotech) transformation vector pGSC1704 (Aventis CropScience, Gent, Belgium). Before the ligation, the cohesive ends were converted to blunt as described above. The resulting construct was used for plant transformation.
For the expression of the D22 cDNA in E. coli as a 6x-His fusion, the expression vector pET-19b (Novagen, Madison, WI) was used. The cDNA has been amplified with Pfu polymerase (Stratagene, La Jolla, CA) with a pair of specific primers containing the NdeI site in frame with the 6x-His tag sequence of the expression vector. The amplified cDNA was digested with NdeI (Amersham Pharmacia Biotech) and ligated with the correspondingly digested and dephosphorylated vector.
Antibody Production
AtGGT was purified from the E. coli-overproducing D22–6x-His fusion on His-affinity resin (Qiagen) under denatured conditions according to the manufacturer's instructions. The produced protein was insoluble and appeared in the SDS gel as a single band of the expected molecular mass of approximately 60 kD. Because the protein was insoluble, it was suspended in phosphate-buffered saline buffer to immunize rabbits according to a standard immunization protocol (Harlow and Lane, 1988) of subcutaneous antigen administration with boost injections after 14, 28, and 84 d after the first antigen administration at the Laboratory of Hormonology (Marloie, Belgium).
Protein Extraction for the Analysis of γ-GT Activity and Protein Gel-Blot Hybridization
Typically, 5 g of tobacco leaf tissue was homogenized in liquid nitrogen and resuspended in 25 mL of cold extraction buffer, containing 50 mm Tris-HCl (pH 8.0), 50 mm NaCl, and one tablet of a protein inhibitor cocktail (Roche Diagnostics, Brussels). All procedures were carried out at 4°C. At this point, an aliquot was taken and, after the cell debris had been precipitated by centrifugation at 20,000g, used to determine the activity in the soluble fraction. To obtain a final NaCl concentration of 0.5 m, 5 m NaCl solution was added to the remainder of the homogenate. The homogenate was incubated for 30 min with periodical steering to allow solubilization of membrane-bound proteins. The homogenate was cleared by centrifugation at 20,000g, and proteins were precipitated by addition of crystalline ammonium sulfate to 70% saturation. Precipitated proteins were collected by centrifugation at 20,000g and diluted in 3 mL of homogenization buffer. The protein extract was desalted on the Econo-Pac 10DG column (Bio-Rad, Hercules, CA) and used for the γ-GT activity assay. As an alternative, for the protein gel blots, total proteins were isolated by using the phenol extraction method to prevent protein degradation (Hurkman and Tanaka, 1986). Protein concentration in the samples was measured according to the method of Bradford (1976).
The protein extracts were separated by SDS-PAGE with a Mini-Protean II apparatus (Bio-Rad) and subsequently blotted onto nitrocellulose membranes (Hybond C super, Amersham Pharmacia Biotech) with a mini trans-blot electrophoretic transfer cell (Bio-Rad) according to the manufacturer's specifications. Antisera against AtGGT were used at a dilution of 1:1,000,000 (v/v). For the primary antibody detection, the secondary anti-rabbit peroxidase-linked goat antibody (Amersham Pharmacia Biotech) was used in combination with the Renaissance ECL detection kit (NEN Life Science Products, Boston).
γ-GT Activity Assay
Standard γ-GT activity assays were performed with GPNA (Sigma-Aldrich, St. Louis; Orlowski and Mister, 1963) as donor substrate and the dipeptide Gly-Gly (Sigma) as an acceptor substrate. The assay was carried out in 96-well microtiter plates. The release of PNA was monitored with a SpectraMAX 250 microplate spectrophotometer system (Amersham Pharmacia Biotech) at 405 nm (ε = 8,800). A standard reaction was carried out typically in a volume of 300 μL and contained 0.1 m Tris-HCl (pH 7.5), 1 mm GPNA, 40 mm Gly-Gly, and 200 μg of the protein extract. One unit of enzyme is defined as the amount that releases 1 μmol PNA min−1 at 20°C. For the determination of the enzyme activity in a pH range of 6.0 to 7.5, piperazine-N,N′-bis(ethanesulfonic acid) buffer was used at 0.1 m. All amino acid acceptors were used at 40 mm concentration.
To determine the kinetics parameters, a time course of the reaction at different concentrations of GPNA was recorded. Km and Vmax were determined from a Lineweaver-Burk plot as described (Wilson and Goulding, 1986). For the Ki determination, the reaction was performed at different concentrations of GSH, and a Lineweaver-Burk plot for each GSH concentration was constructed. Ki was determined as a negative intercept of a secondary plot of the slopes of the primary plots against the GSH concentration on the GSH concentration axis (Wilson and Goulding, 1986).
Reaction of AtGGT with GSH was done in a total volume of 50 μL with 0.1 mm Tris-HCl (pH 7.5), 1 mm GSH, 10 mm Met (Sigma-Aldrich), 10 μCi of Redivue [35S]Met (Amersham Pharmacia Biotech), and 200 μg of a protein extract. As a positive control and marker for γ-Glu-Met, a reaction with bovine kidney γ-GT (Sigma-Aldrich) was used. Before the reaction with GSH, the bovine enzyme was titrated in a reaction with GPNA to have approximately the same activity as the protein extract from a transgenic plant. The reaction was carried out at 20°C and was stopped after 30 min by adding an equal volume of absolute ethanol. After incubation on ice for 10 min and removal of the protein precipitate, 2 μL of the reaction was analyzed by ascending thin-layer chromatography on Polygram CEL 300 plates (Macherey-Nagel, Düren, Germany) in a pyrydine:butanol:water (1:1:1, v/v) buffer system. The reaction products were visualized on a phosphor imager (model 445SI, Amersham Pharmacia Biotech).
For the AtGGT activity tests with intact protoplasts, tobacco mesophyll protoplasts were isolated from the leaves of transgenic and control plants according to a standard protocol (Kushnir et al., 1991). Protoplasts (106 mL−1) were incubated in the standard reaction mix with GPNA supplemented with 0.4 m sorbitol. After 1 h of incubation, protoplasts were floated by centrifugation at 100g, and the underlying supernatant was used to determine the amount of PNA released.
Determination of the AtGGT Cellular Localization
For the confocal microscopy, leaf discs with a diameter of 8 mm cut from transgenic and control plants were vacuum infiltrated with a solution containing 0.1 m Tris-HCl (pH 7.5), 1 mm γ-glutamyl-7-amido-4-methylcoumarin, 40 mm Gly-Gly, briefly washed in water, and mounted in water. The leaf discs were examined with a 20×/0.5 Plan-Neo fluor lens on a LSM510 laser scanning confocal microscope (Karl Zeiss Inc., Thornwood, NY). Images were obtained with the Enterprise UV laser (351 and 364 nm) with the band-pass emission filter 385 to 470 nm. Optical sections were taken and maximal brightness was projected using the LSM510 software package supplied with the microscope. The images were converted to black and white for best contrast.
GSH Content Determination
The GSH content was analyzed according to the HPLC method described by Willekens et al. (1997).
Determination of the Rate of GSH Breakdown
To determine the GSH breakdown rate, 0.8-cm-diameter leaf discs from transgenic or control plants were vacuum infiltrated with 1 mm l-buthionine-[S,R]-sulfoximine (Sigma-Aldrich) or water (negative control). At certain time points, four leaf discs from transgenic or control plants were taken to determine the GSH content.
Computer Analysis
For computer analyses, software from the Genetics Computer Group (Madison, WI) was used. Multiple sequences were aligned with the PILEUP Program and the aligned sequences were edited with the SeqVu program (The Garvan Institute of Medical Research, Sydney). Pair-wise amino acid sequence similarities were calculated with the GAP program (Genetics Computer Group). For the homology searches, the BLAST program was used. The searches were done at http://www.bork.embl-heidelberg.de and http://Arabidopsis.org. Prediction of the cellular localization was made by using the PSORT program at http://psort.nibb.ac.jp/form.html.
ACKNOWLEDGMENTS
We thank Gilbert Engler and Tom Beeckman for γ-GT cellular localization determination, Srikrishnan Mani for thin-layer chromatography and fruitful discussions, Martine De Cock for help in preparing the manuscript, and Rebecca Verbanck for excellent artwork.
Footnotes
This work was supported by the Fund for Scientific Research Flanders (grant no. G004796).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010887.
LITERATURE CITED
- Anderson ME, Allison RD, Meister A. Interconversion of leukotrienes catalyzed by purified γ-glutamyl transpeptidase: concomitant formation of leukotriene D4 and γ-glutamyl amino acids. Proc Natl Acad Sci USA. 1982;79:1088–1091. doi: 10.1073/pnas.79.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann L, Rennenberg H. Glutathione metabolism in plants. In: De Kok LJ, Stulen I, Rennenberg H, Brunold C, Rauser WE, editors. Sulfur Nutrition and Assimilation in Higher Plants: Regulatory Agricultural and Environmental Aspects. The Hague, The Netherlands: SPB Academic Publishing; 1993. pp. 109–123. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cagen LM, Fales HM, Pisano JJ. Formation of glutathione conjugates of prostaglandin A1in human red blood cells. J Biol Chem. 1976;251:6550–6554. [PubMed] [Google Scholar]
- Carter BZ, Wiseman AL, Orkiszewski R, Ballard KD, Ou C-N, Lieberman MW. Metabolism of leukotriene C4in γ-glutamyl transpeptidase-deficient mice. J Biol Chem. 1997;272:12305–12310. doi: 10.1074/jbc.272.19.12305. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A, Wharton CW. Enzyme Kinetics (In Focus Series). Oxford: IRL Press; 1988. [Google Scholar]
- Deblaere R, Bytebier B, De Greve H, Deboeck F, Schell J, Van Montagu M, Leemans J. Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res. 1985;13:4777–4788. doi: 10.1093/nar/13.13.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Block M, Botterman J, Vandewiele M, Dockx J, Thoen C, Gosselé V, Movva R, Thompson C, Van Montagu M, Leemans J. Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J. 1987;6:2513–2518. doi: 10.1002/j.1460-2075.1987.tb02537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bello B, Paolicchi A, Comporti M, Pompella A, Maellaro E. Hydrogen peroxide produced during γ-glutamyl transpeptidase activity is involved in prevention of apoptosis and maintenance of proliferation in U937 cells. FASEB J. 1999;13:69–79. doi: 10.1096/fasebj.13.1.69. [DOI] [PubMed] [Google Scholar]
- den Boer BGW, Murray JAH. Triggering the cell cycle in plants. Trends Cell Biol. 2000;10:245–250. doi: 10.1016/s0962-8924(00)01765-7. [DOI] [PubMed] [Google Scholar]
- Dominici S, Valentini M, Maellaro E, Del Bello B, Paolicchi A, Lorenzini E, Tongiani R, Comporti M, Pompella A. Redox modulation of cell surface protein thiols in U937 lymphoma cells: the role of γ-glutamyl transpeptidase-dependent H2O2 production and S-thiolation. Free Radic Biol Med. 1999;27:623–635. doi: 10.1016/s0891-5849(99)00111-2. [DOI] [PubMed] [Google Scholar]
- Goore MY, Thompson JF. γ-Glutamyl transpeptidase from kidney bean fruit: I. Purification and mechanism of action. Biochim Biophys Acta. 1967;132:15–26. doi: 10.1016/0005-2744(67)90187-8. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Clarendon Press; 1989. [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Hurkman WJ, Tanaka CK. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986;81:802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci. 1992;17:463–468. doi: 10.1016/0968-0004(92)90489-v. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Hasegawa S, Kasai T, Obata Y. Changes in amino acid composition during germination of soybean: Part IV. Identification of α- and γ-glutamylaspartic acid. Agric Biol Chem. 1967;31:490–493. [Google Scholar]
- Jaspers CJ, Penninckx MJ. Glutathione metabolism in yeast Saccharomyces cerevisiae: evidence that γ-glutamyl-transpeptidase is a vacuolar enzyme. Biochimie. 1984;66:71–74. doi: 10.1016/0300-9084(84)90193-7. [DOI] [PubMed] [Google Scholar]
- Kasai T, Ohmiya A, Sakamura S. γ-Glutamyltrans-peptidases in the metabolism of γ-glutamyl peptides in plants. Phytochemistry. 1982;21:1233–1239. [Google Scholar]
- Kawasaki Y, Ogawa T, Sasaoka K. Occurrence and some properties of a novel γ-glutamyltransferase responsible for the synthesis of γ-l-glutamyl-d-alanine in pea seedlings. Biochim Biophys Acta. 1982;716:194–200. [Google Scholar]
- Kean EA, Hare ER. γ-Glutamyl transpeptidase of the ackee plant. Phytochemistry. 1980;19:199–203. [Google Scholar]
- Kushnir S, Babiychuk E, Bannikova M, Momot V, Komarnitsky I, Cherep N, Gleba Y. Nucleocytoplasmic incompatibility in cybrid plants possessing an Atropa genome and a Nicotianaplastome. Mol Gen Genet. 1991;225:225–230. doi: 10.1007/BF00269852. [DOI] [PubMed] [Google Scholar]
- Kushnir S, Babiychuk E, Kampfenkel K, Belles-Boix E, Van Montagu M, Inzé D. Characterization of Arabidopsis thalianacDNAs that render yeasts tolerant toward the thiol-oxidizing drug diamide. Proc Natl Acad Sci USA. 1995;92:10580–10584. doi: 10.1073/pnas.92.23.10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JE, Shaw ML. Characterization of purified γ-glutamyl transpeptidase in onions: evidence for in vivorole as a peptidase. Phytochemistry. 1994;36:1351–1358. [Google Scholar]
- Lieberman MW, Wiseman AL, Shi Z-Z, Carter BZ, Barrios R, Ou C-N, Chévez-Barrios P, Wang Y, Habib GM, Goodman JC et al. Growth retardation and cysteine deficiency in γ-glutamyl transpeptidase-deficient mice. Proc Natl Acad Sci USA. 1996;93:7923–7926. doi: 10.1073/pnas.93.15.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliga P, Sz-Breznovits A, Marton L, Joo F. Non-Mendelian streptomycin-resistant tobacco mutant with altered chloroplasts and mitochondria. Nature. 1975;255:401–402. doi: 10.1038/255401a0. [DOI] [PubMed] [Google Scholar]
- Martin MN, Slovin JP. Purified γ-glutamyl transpeptidases from tomato exhibit high affinity for glutathione and glutathione S-conjugates. Plant Physiol. 2000;122:1417–1426. doi: 10.1104/pp.122.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Leaver C, Van Montagu M, Inzé D. Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot. 1998;49:649–667. [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A, Tate SS, Griffith OW. γ-Glutamyl transpeptidase. In: Jakoby WB, editor. Detoxication and Drug Metabolism. Methods in Enzymology. Vol. 77. New York: Academic Press; 1981. pp. 237–253. [DOI] [PubMed] [Google Scholar]
- Noctor G, Arisi A-CM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot. 1998;49:623–647. [Google Scholar]
- Orlowski M, Meister A. γ-Glutamyl-p-nitroanilide: a new convenient substrate for determination and study of l- and d-γ-glutamyltranspeptidase activities. Biochim Biophys Acta. 1963;73:679–681. doi: 10.1016/0006-3002(63)90348-2. [DOI] [PubMed] [Google Scholar]
- Payne GM, Payne JW. γ-Glutamyltransferase is not involved in the bulk uptake of amino acids, peptides or γ-glutamyl-amino acids in yeast (Saccharomyces cerevisiae) Biochem J. 1984;218:147–155. doi: 10.1042/bj2180147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx MJ, Jaspers CJ. Molecular and kinetic properties of purified γ-glutamyl transpeptidase from yeast (Saccharomyces cerevisiae) Phytochemistry. 1985;24:1913–1918. [Google Scholar]
- Pietrzak M, Shillito RD, Hohn T, Potrykus I. Expression in plant of two bacterial antibiotic resistance genes after protoplast transformation with a new plant expression vector. Nucleic Acids Res. 1986;14:5857–5868. doi: 10.1093/nar/14.14.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennenberg H, Lamoureux GL. Physiological processes that modulate the concentration of glutathione in plant cells. In: Rennenberg H, Brunold C, De Kok LJ, Stulen I, editors. Sulfur Nutrition and Sulfur Assimilation in Higher Plants. The Hague, The Netherlands: SPB Academic Publishers; 1990. pp. 53–65. [Google Scholar]
- Schneider A, Rennenberg H. Degradation of glutathione (GSH) in heterotrophic tobacco cells. Phyton. 1992;32:113–117. [Google Scholar]
- Smith GD, Ding JL, Peters TJ. A sensitive fluorimetric assay for γ-glutamyl transpeptidase. Anal Biochem. 1979;100:136–139. doi: 10.1016/0003-2697(79)90122-2. [DOI] [PubMed] [Google Scholar]
- Steinkamp R, Rennenberg H. γ-Glutamyl-transpeptidase in tobacco suspension cultures: catalytic properties and subcellular localization. Physiol Plant. 1984;61:251–256. [Google Scholar]
- Steinkamp R, Rennenberg H. Degradation of glutathione in plant cells: evidence against the participation of a γ-glutamyltranspeptidase. Z Naturforsch. 1985;40c:29–33. [Google Scholar]
- Taniguchi N, Ikeda Y. γ-Glutamyl transpeptidase: catalytic mechanism and gene expression. In: Purich DL, editor. Amino Acid Metabolism. Part A: Advances in Enzymology and Related Areas of Microbiology. Vol. 72. New York: John Wiley & Sons; 1998. pp. 239–278. [DOI] [PubMed] [Google Scholar]
- Thompson GA, Meister A. Interrelationship between the binding sites for amino acids, dipeptides, and γ-glutamyl donors in γ-glutamyl transpeptidase. J Biol Chem. 1977;252:6792–6798. [PubMed] [Google Scholar]
- Thompson JF, Turner DH, Gering RK. γ-Glutamyl transpeptidase in plants. Phytochemistry. 1964;3:33–46. [Google Scholar]
- Vanacker H, Foyer CH, Carver TLW. Changes in apoplastic antioxidants induced by powdery mildew attack in oat genotypes with race non-specific resistance. Planta. 1999;208:444–452. [Google Scholar]
- Visvikis A, Thioudellet C, Oster T, Fournel-Gigleux S, Wellman M, Siest G. High-level expression of enzymatically active mature human γ-glutamyltransferase in transgenic V79 Chinese hamster cells. Proc Natl Acad Sci USA. 1991;88:7361–7365. doi: 10.1073/pnas.88.16.7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inzé D, Van Camp W. Catalase is a sink for H2O2 and is indispensable for stress defense in C3plants. EMBO J. 1997;16:4806–4816. doi: 10.1093/emboj/16.16.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K, Goulding KH. A Biologist's Guide to Principles and Techniques of Practical Biochemistry: A Series of Student Texts in Contemporary Biology. Ed 3. London: E. Arnold; 1986. [Google Scholar]