Abstract
The low sulfur amino acid content of legume seeds restricts their nutritive value for animals. We have investigated the limitations to the accumulation of sulfur amino acids in the storage proteins of narrow leaf lupin (Lupinus angustifolius) seeds. Variation in sulfur supply to lupin plants affected the sulfur amino acid accumulation in the mature seed. However, when sulfur was in abundant supply, it accumulated to a large extent in oxidized form, rather than reduced form, in the seeds. At all but severely limiting sulfur supply, addition of a transgenic (Tg) sink for organic sulfur resulted in an increase in seed sulfur amino acid content. We hypothesize that demand, or sink strength for organic sulfur, which is itself responsive to environmental sulfur supply, was the first limit to the methionine (Met) and cysteine (Cys) content of wild-type lupin seed protein under most growing conditions. In Tg, soil-grown seeds expressing a foreign Met- and Cys-rich protein, decreased pools of free Met, free Cys, and glutathione indicated that the rate of synthesis of sulfur amino acids in the cotyledon had become limiting. Homeostatic mechanisms similar to those mediating the responses of plants to environmental sulfur stress resulted in an adjustment of endogenous protein composition in Tg seeds, even when grown at adequate sulfur supply. Uptake of sulfur by lupin cotyledons, as indicated by total seed sulfur at maturity, responded positively to increased sulfur supply, but not to increased demand in the Tg seeds.
Animals are unable to synthesize Met, and must therefore obtain this essential amino acid from their diet. Legume seeds such as soybean (Glycine max), pea (Pisum sativum), or lupin (Lupinus angustifolius), although rich in protein, are deficient in the sulfur amino acids, from the point of view of animal nutrition. In recent years, gene transfer technology has been used to increase seed sulfur amino acid content in several plant species, but in most cases the modified levels were still less than those required for optimal animal production (Tabe and Higgins, 1998).
The nutritional quality of narrow leaf lupin seeds has been improved by the addition of a sulfur-rich sunflower (Helianthus annuus) seed albumin (SSA). Of the 104 amino acids in SSA, 16 are Met and eight are Cys, making the protein a rich store of sulfur amino acids. SSA accumulated in mature seeds of narrow leaf lupins expressing a chimeric SSA gene controlled by the promoter from a pea seed storage protein gene. The accumulation of SSA was associated with a doubling of seed Met relative to non-transgenic (control), parental lupins, and an increase in nutritive value of the seed for rats (Rattus norvegicus) and sheep (Ovis aries; Molvig et al., 1997; White et al., 2000). However, the sulfur amino acid content of the transgenic (Tg) seeds was still less than would be necessary to balance the seed protein for optimal animal nutrition.
Because SSA contains both Cys and Met, an increase in the seed's content of both these amino acids was predicted. However, Tg seeds containing SSA and increased Met had either the same or a slightly lower content of Cys than controls. One reason for this lack of an additive effect of Cys in the foreign protein with the endogenous seed Cys could be a limitation in sulfur amino acid availability in the developing seed during storage protein synthesis.
Plants take up sulfur from the soil in the form of sulfate. Sulfate is subsequently reduced to sulfide and incorporated into Cys and then Met. These processes are thought to take place predominantly in the plastids of green tissue, utilizing reducing power generated by photosynthesis (Leustek et al., 2000; Saito, 2000). We have demonstrated recently that the assimilation of sulfur into amino acids can occur to a significant extent in the cotyledons of developing lupin seeds during maturation (Tabe and Droux, 2001). Similarly, it has been reported that seed tissue is the dominant site of ATP sulfurylase activity during reproductive growth of soybean (Sexton and Shibles, 1999). These findings provide evidence that biosynthesis of Cys and Met in the developing seed itself is an important source of sulfur amino acids for storage protein synthesis, at least in the case of grain legumes.
In the current study, we aimed to identify limitations to the accumulation of Cys and Met in lupin seed protein. We investigated the effects of plant sulfur nutrition and the addition of SSA, as a sink for sulfur, on the accumulation of sulfur amino acids in lupin seed protein. The pathway of sulfur assimilation was surveyed in control and Tg SSA lupin seeds grown under three regimes of sulfur supply. The activities of four enzymes of sulfur amino acid biosynthesis were measured, along with the sizes of various sulfur pools during and at the completion of seed maturation. It was concluded that, except in conditions of sulfur stress, demand for organic sulfur, which is itself normally regulated by sulfur supply, limited Cys and Met accumulation in non-Tg lupin seed protein. In soil-grown Tg seeds with an artificially increased sink for organic sulfur, decreases in metabolite pools indicated that flux through the sulfur amino acid biosynthetic pathway in the cotyledon became limiting. Sulfur uptake by lupin seeds, as indicated by total seed sulfur at maturity, increased with increasing sulfur supply, but did not respond to increased demand for sulfur amino acids in the Tg seeds.
RESULTS
Composition of Mature Lupin Seeds Expressing SSA
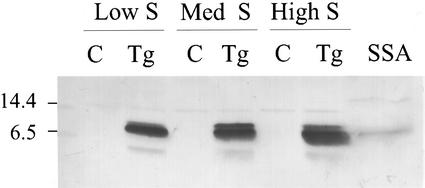
SSA accumulated in mature Tg lupin seeds grown in conditions of controlled mineral nutrition with three different sulfur nutrition regimes. The amount of SSA was only slightly less in Tg seeds grown in severely limiting sulfur than it was in Tg seeds grown at a luxurious level of sulfur supply (Fig. 1). The presence of SSA protein in the Tg seeds grown at low sulfur (Tg:Low group) indicates that the chimeric SSA gene was not subject to down-regulation in conditions of sulfur stress, as has been reported for many genes encoding sulfur-rich seed storage proteins (Chandler et al., 1984, and refs. therein). The plants grown at low sulfur showed symptoms of sulfur stress including yellowing, and smaller size than plants in the other groups. Two SSA polypeptides of slightly different mobility were resolved in protein extracts from all the Tg seeds. N-terminal amino acid sequencing showed one to be a truncated polypeptide (data not shown), possibly resulting from some degradation or aberrant processing of the SSA primary translation product.
Figure 1.
SSA in mature lupin seeds grown at different levels of sulfur supply. The figure shows a western blot probed with an SSA-specific antibody. Each track contains 10 μg of total seed protein from non-Tg lupin (C) or Tg (Tg) grown at low sulfur, medium sulfur (Med S), or high sulfur (for growth conditions, see “Materials and Methods”). Track 7 (SSA) contains 40 ng of SSA purified from sunflower seeds. Mr marker sizes (×10−3) are shown at the left.
X-ray fluorescence spectrometry (XRFS) was used to quantify sulfur in flour from mature seeds of control and Tg lupins grown under the three sulfur regimes. The technique was used to differentiate between sulfur in the +6 oxidation state (oxidized) and sulfur with an oxidation number of less than zero (reduced). These two fractions correspond mainly to sulfate and sulfur amino acids, respectively, in plant material (Pinkerton et al., 1989). The oxidized sulfur quantified in seed flours in this study by XRFS was shown by ion chromatography to correspond to sulfate, as expected (data not shown). The total sulfur contents of the seeds varied widely with different sulfur nutrition of the plants (Table I). At all levels of sulfur nutrition, seeds of Tg lupins expressing SSA had no more total sulfur than seed from the corresponding control group of lupin plants. However, at all except the lowest level of sulfur nutrition, the Tg seeds contained significantly more reduced sulfur and correspondingly less oxidized sulfur than the control seeds grown under the same conditions. Tg seeds contained approximately the same concentration of nitrogen as control seeds, so the ratios of reduced sulfur to nitrogen were higher in the Tgs than in the controls (data not shown). Average seed weight varied relatively little among all but the Tg plants grown in low sulfur, although there was a tendency for Tg seeds to be slightly lighter than controls grown in the same conditions (data not shown).
Table I.
Sulfur in mature lupin seeds
| Plant Sulfur Nutrition | Lupin Genotype | Sulfur in Mature Seed
|
||
|---|---|---|---|---|
| Reduced | Oxidized | Total | ||
| μmol sulfur g−1 dry wt | ||||
| High sulfur nutrient | C | 73.4 ± 1.9 | 52.2 ± 2.4 | 123.4 ± 5.2 |
| Tg | 87.8 ± 1.6 | 28.4 ± 0.6 | 111.6 ± 5.8 | |
| Tg − C | 14.4 | −23.8 | −11.9 | |
| Medium sulfur nutrient | C | 58.8 ± 2.4 | 12.8 ± 0.2 | 68.8 ± 2.1 |
| Tg | 71.6 ± 1.1 | 1.3 ± 1.1 | 68.8 ± 2.3 | |
| Tg − C | 12.8 | −11.6 | 0 | |
| Low sulfur nutrient | C | 26.3 ± 1.2 | 0 | 25.0 ± 0.9 |
| Tg | 27.8 ± 1.9 | 0 | 25.9 ± 1.8 | |
| Tg − C | 1.6 | 0 | 0.9 | |
| Soil with sulfur supplement | C | 70.6 ± 1.4 | 48.8 ± 0.8 | 116.3 ± 1.3 |
| Tg | 89.7 ± 0.8 | 22.5 ± 4.2 | 108.4 ± 4.4 | |
| Tg − C | 19.1 | −26.3 | −7.8 | |
| Field | C | 73.4 ± 2.4 | 26.6 ± 3.8 | 98.8 ± 5.4 |
| Tg | 85.6 ± 2.7 | 12.8 ± 3.1 | 97.5 ± 5.5 | |
| Tg − C | 12.2 | −13.8 | −1.3 | |
Values shown are the mean ± sd of the means of duplicate determinations on flour from pools of 6 g of mature seed from each of either two or three plants for each group grown in soil or sand/perlite in the greenhouse. For plants grown in the field, figures are the mean of the means of duplicate determinations on flour from pooled samples of approximately 40 seeds, n = 4. Total sulfur was determined separately; therefore, the total sulfur figures are not necessarily the perfect sum of the other two figures. C, Control; Tg–C, difference between Tg and control.
At the lowest level of sulfur nutrition, Tg seeds contained approximately the same concentration of reduced sulfur as controls (Table I). Control seeds grown under these conditions were in the normal weight range for the cultivar (mean seed weight 171 mg), and contained the same concentration of nitrogen as the control seeds grown with the higher levels of sulfur nutrition (nitrogen = approximately 5% of seed dry weight). The Tg seeds grown with low sulfur had a similar concentration of nitrogen to those in the other groups, but weighed less (mean seed weight 116 mg). Seeds from all experimental groups except the Tg:Low group germinated readily when sown in soil. Seeds of all plants of the latter group had a shrunken appearance and failed to germinate in soil (0 of 10 seeds from one Tg:Low plant germinated compared with 10 of 10 seeds of one plant from each of the other groups grown in controlled mineral nutrition).
The total sulfur amino acid concentrations in flour from mature control and Tg seeds grown in sand/perlite with the three levels of sulfur nutrition were determined (Table II). In seeds from Tg plants grown at high sulfur (Tg:High) and Tg plants grown at medium sulfur (Tg:Med), total Met was doubled, whereas total Cys was slightly less, compared with the corresponding controls. These effects of the transgene on seed sulfur amino acid composition were similar to those observed when the two lupin genotypes were grown in the field (Molvig et al., 1997). At low sulfur supply, a slight increase in seed Met in the Tg compared with the control was almost completely offset by a corresponding decrease in seed Cys. At all three levels of sulfur supply, Met plus Cys accounted for approximately 65% to 70% of the reduced sulfur measured in the mature seed by XRFS. The balance of the reduced sulfur was probably in the form of metabolites such as vitamins and cofactors or modified amino acids such as S-adenosyl-Met. Some of the difference between the reduced sulfur quantified by XRFS and the total amino acid content of the seeds may simply be because of a discrepancy between the two analytical techniques.
Table II.
Total sulfur amino acid contents of mature lupin seeds grown in sand/perlite at three levels of sulfur nutrition
| Sulfur Nutrition | Lupin Genotype | Met | Cys | Met/Cys Ratio |
|---|---|---|---|---|
| μmol g−1 dry wt | ||||
| High | C | 14.4 ± 0.5 | 33.0 ± 1.2 | 0.44 |
| Tg | 26.8 ± 0.9 | 31.0 ± 2.9 | 0.86 | |
| Medium | C | 13.9 ± 0.2 | 25.6 ± 0.0 | 0.54 |
| Tg | 26.8 ± 0.9 | 22.7 ± 1.8 | 1.18 | |
| Low | C | 9.7 ± 0.5 | 9.5 ± 1.8 | 1.02 |
| Tg | 12.7 ± 0.9 | 7.4 ± 1.2 | 1.72 | |
Separate samples of approximately 40 mature seeds from each of two plants per experimental group were milled to fine flour. Sulfur amino acids were measured after performic oxidation and acid hydrolysis. Figures are the mean ± sd of a single determination on each of the two samples. C, Control.
Pools of Sulfur Metabolites in Developing Lupin Seeds
We measured the concentrations of three major, reduced sulfur metabolites in developing seeds and in other organs of control lupins and Tg SSA-lupins grown in soil with supplementary sulfur. Free Cys and Met and the tri-peptide glutathione were all more abundant in cotyledons of developing seeds than in the leaves, pods, or testa of developing seeds (Table III). Leaves, pods, and testa of Tg plants with the seed-expressed SSA gene contained approximately the same levels of the sulfur metabolites as the corresponding organs of control plants. However, the Tg cotyledons expressing SSA contained smaller pools of all three metabolites in comparison with the control cotyledons (Table III). The difference was particularly notable for free Met, whose concentration in Tg cotyledons was approximately 12% of the concentration of Met in the control cotyledons. However, in terms of the number of moles of reduced sulfur, the largest difference between the Tg and control cotyledons was in the level of glutathione.
Table III.
Pools of sulfur metabolites in organs of control lupins and Tg lupins grown in soil supplemented with sulfur
| Genotype | Organ | Sulfur Metabolite
|
||
|---|---|---|---|---|
| Glutathione | Free Cys | Free Met | ||
| nmol g−1 dry wt | ||||
| Control | Cotyledon | 3,140 ± 42 | 223 ± 6 | 1,108 ± 160 |
| Testa | 390 ± 9 | 35 ± 2 | 111 ± 11 | |
| Pod | 241 ± 11 | 23 ± 3 | n.d. | |
| Leaf | 493 ± 17 | 19 ± 3 | 29 ± 5 | |
| Tg | Cotyledon | 1,211 ± 66 | 90 ± 7 | 139 ± 30 |
| Testa | 321 ± 20 | 31 ± 10 | 55 ± 6 | |
| Pod | 218 ± 11 | 21 ± 5 | n.d. | |
| Leaf | 450 ± 52 | 16 ± 2 | 17 ± 1 | |
Each figure is the mean ± sd of measurements on two separate extracts from the same pool of organs representing approximately 40 seeds, 12 pods, or 30 leaves. All organs were harvested when the seeds were at mid-maturation (approximately 30 d after flowering [DAF]). n.d., Not detected.
It was not possible to measure oxidized sulfur in the same cotyledon samples as above. However, oxidized sulfur was determined in pooled seeds, ranging in age from early to late maturation, from plants grown in sand/perlite with high sulfur nutrient. Developing control lupin seeds contained 32.4 μmol oxidized sulfur g−1 dry weight compared with 20.7 μmol oxidized sulfur g−1 dry weight in Tg seeds expressing SSA. Thus, the difference between the oxidized sulfur pools in control and Tg seeds exceeded the differences in reduced sulfur metabolites between the two genotypes during maturation.
Enzymes of the Sulfur Assimilation Pathway in Developing Seeds of Control and Tg Lupins at Three Levels of Sulfur Supply
The activities of four enzymes of the pathway of sulfur amino acid biosynthesis were assayed in low-salt extracts from developing seeds of control and Tg lupins grown in soil or in sand/perlite at the three levels of sulfur nutrition. The enzymes assayed were: Ser acetyltransferase (SAT), which supplies carbon and nitrogen skeletons for Cys biosynthesis; O-acetyl-Ser (thiol) lyase (OASTL), which catalyzes the combination of O-acetyl-Ser and sulfide to form Cys; cystathionine γ-synthase (CGS), the first enzyme of the pathway committed to Met biosynthesis, and cystathionine β-lyase (CBL), the second enzyme in the trans-sulfuration pathway leading to Met. Developing seeds were harvested at an early (21 DAF), middle (30 DAF), or late (40 DAF) stage of seed maturation (Tabe and Droux, 2001). The activities of SAT, OASTL, CBL, and CGS were assayed in low-salt extracts from the whole developing seeds (i.e. including both cotyledon and testa).
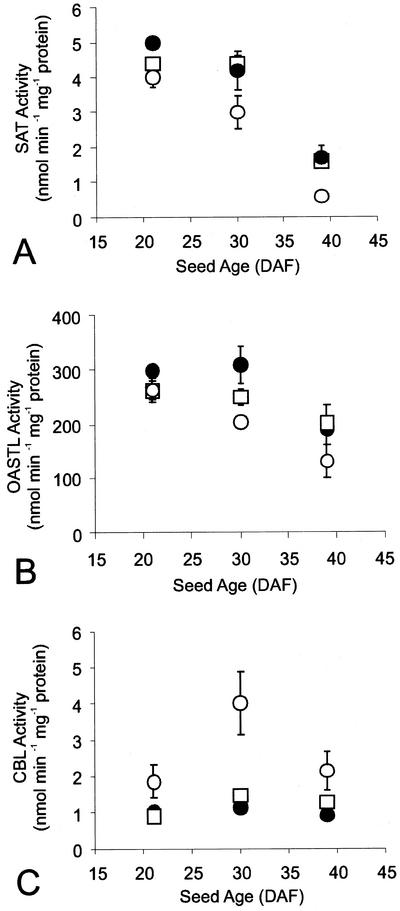
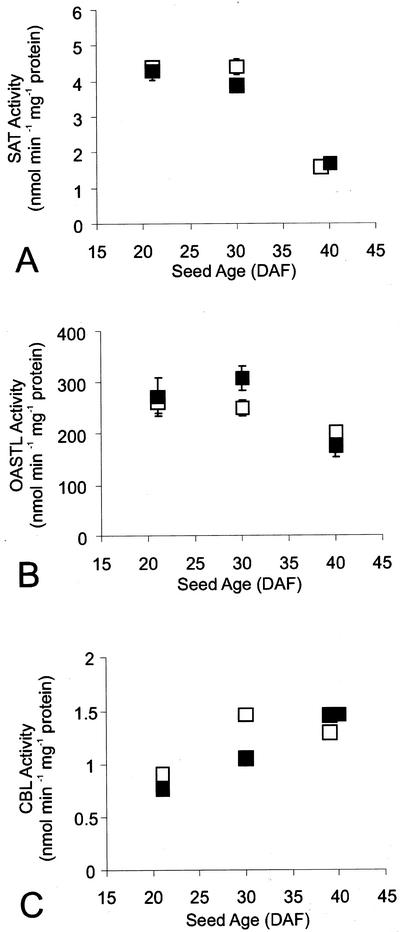
Figure 2 shows the effects of sulfur nutrition on the enzyme levels in the control lupins. The specific activities of SAT and OASTL showed similar patterns through seed development and a tendency to be lowest in control:Low seeds, but they were not particularly sensitive to sulfur nutrition of the plants. The specific activity of CBL showed the opposite trend, with the highest activities found in control:Low seed extracts (Fig. 2), indicating that the amount of enzyme in seeds is up-regulated to some extent by sulfur nutritional stress. The activities of SAT, OASTL, or CBL did not differ consistently between control seeds and Tg seeds expressing SSA at any of the three levels of S nutrition (Fig. 3, and data not shown for high and low sulfur nutrient).
Figure 2.
Activities of enzymes of the sulfur amino acid biosynthetic pathway in developing seeds of narrow leaf lupins grown at three levels of sulfur supply. Activities of SAT, A; OASTL, B; and CBL, C; in extracts from developing whole seeds of lupins grown at high (●), medium (□), or low (○) sulfur supply. Values are means ± sd of two (CBL) or four (SAT and OASTL) measurements on aliquots of a single extract from a pooled sample representing approximately 40 seeds from a total of six plants.
Figure 3.
Activities of enzymes of the sulfur amino acid biosynthetic pathway in developing seeds of control and SSA-Tg lupins grown with medium sulfur nutrient. Activities of SAT, A; OASTL, B; and CBL, C; in extracts from developing seeds of control lupins (□) or Tg lupins expressing SSA (▪). Values are means ± sd of two (CBL) or four (SAT and OASTL) measurements on aliquots of a single extract from a pooled sample representing approximately 40 seeds from a total of six plants. The error bars are too small to show on the SAT and CBL graphs.
CGS activity was measured in whole developing seeds at mid-maturation (30 DAF) from plants grown at either low or high sulfur nutrition. CGS activity was also quantified in extracts from mid-maturation developing cotyledons (approximately 30 DAF) from plants grown in soil with a sulfur supplement (Table IV). CGS activity showed little difference between the seeds grown in low or high levels of sulfate. However, CGS activity in Tg whole seeds was substantially higher than the activity found in the control seeds. The CGS activity extracted from developing Tg cotyledons was double that from cotyledons of control soil-grown seeds. Thus, the increased sink for sulfur amino acids in the Tg seeds was consistently associated with an increase in CGS activity in the developing seed.
Table IV.
CGS activity in 30-DAF developing cotyledons of lupins grown in soil supplemented with sulfur, or in 30-DAF developing whole seeds of lupins grown in sand/perlite with low or high sulfur nutrient
| Lupin Genotype | CGS Activity
|
||
|---|---|---|---|
| Cotyledon
|
Whole seed
|
||
| Soil | High sulfur nutrient | Low sulfur nutrient | |
| nmol min−1 mg−1 protein | |||
| Control | 0.17 ± 0.01 | 0.15 ± 0.01 | 0.22 ± 0.01 |
| Tg | 0.38 ± 0.01 | 0.25 ± 0.01 | 0.30 ± 0.01 |
Values represent the means ± sd of two separate determinations on a single extract from a pooled sample of approximately 40 seeds or 80 cotyledons representing six plants.
We have calculated previously that the maximum, in vitro activities of SAT, OASTL, and CBL from developing lupin seeds are theoretically sufficient to account for all the reduced sulfur that accumulates in the mature seed (Tabe and Droux, 2001). Similarly, the theoretical maximum activity of CGS during seed development was calculated by multiplying the CGS activity measured in vitro by the time period over which storage proteins accumulate in the developing seeds. The calculated CGS maximum activity was theoretically more than sufficient to account for all the accumulated Met in the mature seed (Table V).
Table V.
Maximum activity of CGS in lupin seeds throughout storage protein accumulation
| Lupin Genotype | CGS Activity at Mid-Maturation | Total Maximum CGS Activity during Seed Maturation | Total Met in Mature Seed |
|---|---|---|---|
| nmol product min−1 mg−1 protein | μmol product g dry wt−1 | μmol g dry wt | |
| Control | 0.15 | 119 | 14.4 |
| Tg | 0.25 | 198 | 26.8 |
Storage proteins accumulated between 20 and 40 DAF in narrow leaf lupin seeds grown in sand/ perlite and watered with high sulfur nutrient (Tabe and Droux, 2001). It was assumed that the CGS activity measured in whole developing seeds at mid-maturation (30 DAF) represented a mean activity over this period. A theoretical maximum amount of product that could be generated by the enzyme over the entire period was calculated by multiplying the maximum enzyme activity by time. It was assumed that the enzyme operated at maximum activity for 12 h a day, and seed flours contained an average of 55 mg low salt extractable protein g−1 dry wt. One mol of cystathionine gives rise to 1 mol of Met; therefore mol of product calculated in column 2 can be compared directly with mol of Met in column 3.
Protein Composition of Mature Control and Tg Lupin Seeds
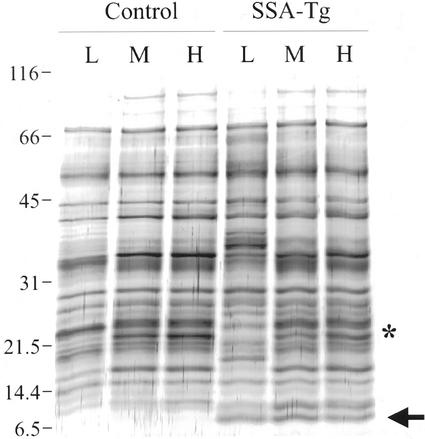
Total protein was extracted from mature seeds of control and Tg lupins grown either in soil or in sand/perlite at each of the three levels of sulfur supply. There were subtle differences in the profiles of proteins visualized by silver staining in seed extracts of the two different genotypes in each of the growth conditions. In the low sulfur conditions, the protein profile of the Tg seeds was noticeably different from that of the control seeds (Fig. 4). Some of these differences were probably because of incomplete proteolytic processing of seed storage protein precursors in the severely stressed Tg:Low seeds. Some proteins seemed more abundant in the control seeds grown at high or medium sulfur nutrition than in either the control seeds grown in low sulfur or in the Tg seeds at any of the levels of sulfur nutrition (for example, see asterisk in Fig. 4). These proteins probably correspond to endogenous sulfur-rich proteins whose abundance is controlled by sulfur availability (Blagrove et al., 1976).
Figure 4.
Total protein from mature narrow leaf lupin seeds grown in low, medium, or high sulfur. Each track contains 5 μg of total protein from finely ground flour from mature seeds of either control or Tg lupins grown with low (L), medium (M), or high (H) sulfur nutrient. Proteins were visualized by silver staining; the asterisk marks an example of a protein that was more abundant in control seeds grown in sulfur adequate conditions than in control seeds grown at low sulfur, or in Tg seeds at any of the levels of sulfur nutrition, the arrow marks the position of SSA. Mr marker sizes (×10−3) are shown at the left.
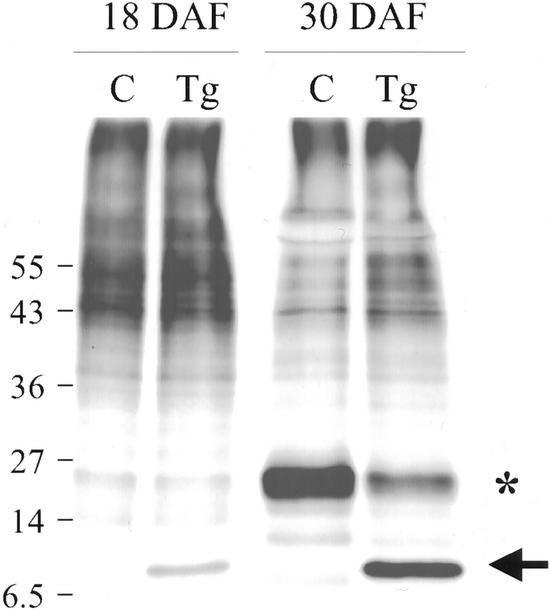
Developing cotyledons at early (18 DAF) and mid-maturation (30 DAF), from soil-grown plants, were labeled with 35SO4 to investigate specifically the sulfur-rich proteins synthesized in the two genotypes. Early in the phase of storage protein synthesis, sulfur-labeled proteins from the two genotypes were very similar, except for the appearance of SSA in the Tg cotyledons (arrow in Fig. 5). By mid-maturation, SSA was the most strongly labeled protein in the Tg cotyledons. Comparison with an equal loading of labeled protein from control cotyledons revealed that some endogenous 35S-containing proteins were diminished in the Tg seeds relative to the controls (for example, see asterisk in Fig. 5).
Figure 5.
35S-labeled proteins in developing lupin cotyledons. Cotyledons at 18 DAF or 30 DAF from control or Tg lupins grown in soil were labeled with 35SO4 for 4 h. Total proteins were subjected to SDS-PAGE and fluorography. Equal numbers of trichloroacetic acid (TCA)-insoluble counts were loaded in each track. The arrow indicates SSA; the asterisk indicates proteins that are less abundant in Tg than in the corresponding control seed protein extract. Mr marker sizes (×10−3) are shown at the left.
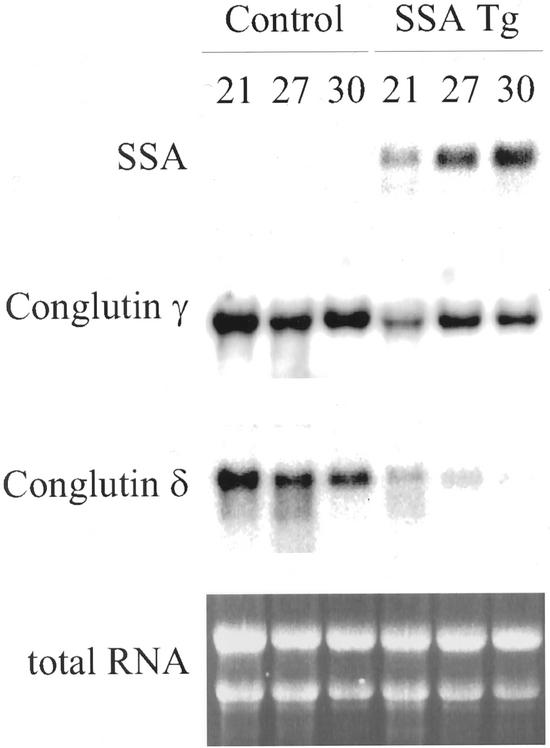
Conglutins gamma and delta are sulfur-rich storage proteins of lupin seeds (Blagrove et al., 1976; Lilley and Inglis, 1986). Northern-blot analysis revealed that the levels of mRNA coding for conglutin-δ were strongly diminished in Tg:Med cotyledons expressing SSA, compared with control:Med cotyledons (Fig. 6). Levels of conglutin-γ mRNA in the Tg:Med cotyledons showed similar but much less pronounced trends to those of the conglutin-δ mRNA (Fig. 6).
Figure 6.
Sulfur-rich storage protein-encoding mRNAs in control lupins, and Tg lupins expressing SSA. Total RNA was isolated from control or Tg developing lupin cotyledons aged 21 DAF (21), 27 DAF (27), or 30 DAF (30) from plants grown with medium sulfur nutrient. Ten micrograms of total RNA was loaded in each track. The same nylon membrane was sequentially probed with 32P-labeled DNA probes specific for SSA, conglutin-γ, or conglutin-δ. Ethidium bromide-stained RNA in the original gel is shown at the bottom.
DISCUSSION
We have investigated the factors that constrain the accumulation of sulfur amino acids in seed protein of narrow leaf lupins. We examined the effects of manipulating the sulfur supply to the plant, and the effect of introducing a seed specifically expressed sulfur-rich protein as an added sink for Met and Cys.
Effects of Manipulating Sulfur Supply
The results presented in Table I confirm previous reports that the reduced sulfur content of narrow leaf lupin seeds can vary over a wide range in response to sulfur supply to the plant. In conditions of sulfur limitation, lupin seeds accumulate increased amounts of sulfur-poor storage proteins, and decreased amounts of relatively sulfur-rich proteins like conglutins-α and -γ (Blagrove et al., 1976). Similarly, we have observed that mRNA coding for the very Cys-rich conglutin-δ is diminished in control lupin seeds grown with low and medium sulfur compared with those grown in high sulfur medium (data not shown). These conglutins contain more Cys than Met; therefore, their decreased abundance is reflected in an increase in the ratio of total Met to total Cys in seeds grown with progressively less sulfur in the nutrient (Table II). Thus, developing lupin seeds were able to sense sulfur availability and adjust their protein composition or sink strength for organic sulfur, according to sulfur supply. Little or no changes were observed in the levels of four enzymes of sulfur amino acid biosynthesis in lupin seeds grown with widely differing sulfur supply.
Effects of Increasing the Demand for Cys and Met in the Developing Lupin Seed
The presence of a high proportion of oxidized sulfur in mature seeds of control plants in most growing conditions (Table I) indicated that the sulfur supply to the seed exceeded either the demand for sulfur amino acids for storage protein synthesis, or the capacity of the seed to metabolize oxidized sulfur into sulfur amino acids. Expression of a transgene encoding a sulfur-rich protein was associated with an increase in total sulfur amino acid in the seed, consistent with the view that it was demand from storage protein synthesis that limited Met and Cys accumulation in the non-Tg seeds.
The higher levels of reduced sulfur in the Tg:Med and Tg:High mature seeds compared with the control:Med and control:High mature seeds, respectively, were entirely because of increases in seed Met, despite the fact that 8% of the amino acid residues in SSA are Cys and 16% are Met. At all three levels of sulfur nutrition, SSA constituted a comparable proportion of total protein in Tg seeds (Fig. 1). Thus, similar quantities of Cys and Met were sequestered in SSA in each nutritional treatment. The lack of an increase in Cys content of Tg seeds in any of the groups implies that a decrease in the accumulation of Cys in some other pool must compensate for the allocation of some Cys to SSA. Such a response would seem to imply the existence of a limitation in the supply of sulfur amino acids for protein synthesis in the developing lupin seeds expressing SSA. Consistent with this suggestion, pools of free Cys, glutathione, and free Met were all found to be significantly smaller in the cotyledons of developing soil-grown Tg seeds compared with controls (Table III). Similar but less pronounced differences were seen when comparing the pools of free Cys and glutathione in developing whole seeds or cotyledons of Tg versus control nutrient-fed plants (data not shown).
Limits to the Accumulation of Sulfur Amino Acids in Tg Lupin Seeds with an Increased Sink for Organic Sulfur
The processes that precede, and could potentially limit the accumulation of sulfur amino acids in seed storage proteins can be summarized as follows. Sulfate enters the cotyledon cell, then the plastid; it is reduced to sulfide, incorporated into Cys, then Met, transferred to tRNA, and finally incorporated into the polypeptide chains encoded by the seed mRNA.
In all but the low sulfur growing conditions, mature, Tg seeds expressing the sulfur-rich SSA contained less oxidized sulfur than the corresponding control seed. The decreases in moles of sulfate were of the same order of magnitude as the increases in moles of sulfur amino acid, resulting in little change in total seed sulfur in Tg compared with control mature seeds (Table I). Therefore, the increased demand for sulfur amino acids in the Tgs did not lead to an increase in sulfur import into the developing seeds. This is perhaps not surprising in the cases of the lupins grown in conditions of abundant sulfur supply. In high sulfur nutrient, in soil with a sulfur supplement, and in the field, even Tg lupin seeds containing SSA had large reserves of oxidized sulfur at maturity (Table I). In the medium sulfur treatment, oxidized sulfur constituted as much as 20% of the total sulfur in control mature seeds. However, the Tg:Med seeds contained essentially no oxidized sulfur at maturity. Thus, even the complete depletion of oxidized sulfur in the seed by the Tg sulfur sink did not lead to an increase in sulfur uptake, despite the existence of significant pools of oxidized sulfur in other plant organs, particularly stems (29 μmol oxidized sulfur g−1 dry weight in the case of Tg:Med stems). It was previously demonstrated that the majority of the oxidized sulfur in lupin seeds was in the cotyledon rather than the testa (Tabe and Droux, 2001). Therefore, uptake into the cotyledon over the course of seed maturation represented the ultimate limit to sulfur amino acid accumulation in lupin seed storage protein when demand was increased by addition of a Tg sink for sulfur. In the control:Low, Tg:Low, and Tg:Med experimental treatments, the absence of detectable sulfate in the mature seeds indicated that sulfur uptake into the cotyledon was also the proximate limit to sulfur amino acid accumulation in these seeds. If there was no more oxidized sulfur available in the seed, no more sulfur amino acid could be produced and incorporated into storage protein.
In the cases where seeds still contained significant quantities of sulfate at maturity, analysis of developing seeds was performed to look for proximate limits to sulfur amino acid accumulation. Mid-maturation seeds of the lupins grown with high sulfur nutrient already contained large pools of oxidized sulfur, which were diminished in the Tg (20.7 μmol oxidized sulfur g−1 dry weight) compared with control (32.4 μmol oxidized sulfur g−1 dry weight). Thus, the rate of import of sulfate into the developing seed appeared to exceed the rate of its reduction, even in the lupins with the added Tg sulfur sink. The first potential limitation after the import of sulfate into the cotyledon cells of the developing seeds is the rate of transport of sulfate across intracellular membranes; for example, from the cytoplasm into the plastid. Depending on relative fluxes across different membranes in the cell, sulfate may accumulate first in the vacuole before transport to the plastid. Therefore, either import into the plastid, or efflux from the vacuole, could limit the supply of sulfate for reduction in the plastids of the developing seed. It is relevant to note that transport out of the vacuole has been suggested to limit the remobilization of sulfate within whole plants in conditions of sulfur deficiency (Clarkson et al., 1983).
The next potential limit to sulfur amino acid accumulation is the rate of sulfur reduction to sulfide in the developing cotyledons, either because of a limitation in ATP sulfurylase, adenosine 5′-phosphosulphate reductase, or sulfite reductase, or because of a limit in the supply of reductant in the cotyledon. We were not able to assay the activity of enzymes in this part of the sulfur metabolic pathway; however, our finding that free Cys and glutathione pools were diminished in soil-grown Tg lupin cotyledons at mid-maturation pointed to a bottleneck upstream of Cys in the pathway of sulfur amino acid biosynthesis in these growth conditions (Table III). Such a bottleneck could be in the intracellular transport or reduction of sulfate, or in the supply of the amino acid backbone of Cys by the action of SAT and OASTL.
We have calculated that the maximum total activities of SAT, OASTL, CBL (Tabe and Droux 2001), and CGS, based on in vitro measurements, would be more than sufficient to synthesize all of the Cys and Met that accumulated in control or Tg lupin seeds during maturation. On the basis of this reasoning, it is unlikely that the amounts of SAT or OASTL are limiting in the developing cotyledon. However, it is possible that specific subcellular isoforms of OASTL, or more probably SAT, could become limiting.
Downstream of Cys formation, CGS and CBL are expected to be single isoforms in lupin, as they are in Arabdopsis (Ravanel et al., 1998). CGS was the only enzyme to vary between control and Tg developing seeds. Its activity was doubled in extracts from developing cotyledons from Tg plants compared with control plants grown in soil (Table IV). This correlated with a doubling of total Met in the mature Tg seeds. This suggests a role for CGS abundance in determining seed Met content. A significant percentage of the flux through the pathway between Cys and Met flows through to S-adenosyl-Met (Ravanel et al., 1998); therefore, measuring total seed Met may underestimate the requirement of the seed for CGS and CBL activity. Thus, CGS may be limiting for lupin seed Met synthesis in both control and in Tg seeds with an increased sink for sulfur amino acids. We cannot exclude the possibility that tRNAs charged with Cys or Met become limiting in the Tg seeds; however, a bottleneck at this late step would seem to be inconsistent with the observed decreases in pools of sulfur metabolites in the cotyledons of the soil-grown Tg lupins.
The mechanisms that limit the Cys content of Tg lupin seeds expressing SSA are likely to be the same as those that mediate the responses of seed protein composition to conditions of sulfur deficiency. There are many reports of sulfur limitation evoking specific changes in protein profiles of seeds (Chandler et al., 1984; Gayler and Sykes, 1985), including narrow leaf lupin (Blagrove et al., 1976). In general, the accumulation of sulfur-rich proteins is down-regulated, whereas sulfur-poor proteins become overrepresented. Both transcriptional and posttranscriptional mechanisms mediating such effects have been described (Beach et al., 1985; Hirai et al., 1995). In the case of the β-subunit of soybean conglycinin, O-acetyl-Ser was proposed to be the signaling molecule that stimulated the transcription of the β-conglycinin gene when nitrogen supply was sufficient and sulfur supply was limiting (Kim et al., 1999). The end results of such changes are viable seeds that contain the same amount of nitrogen, but less sulfur than seeds grown in conditions of sufficient sulfur supply.
In narrow leaf lupin, up to 70% of the sulfur amino acid in the mature seed resides in conglutin-δ (in which 9% of amino acid residues are Cys and 0% are Met; Lilley and Inglis, 1986). Our results show that conglutin-δ mRNA was strongly diminished in SSA-expressing, Tg lupin cotyledons grown in medium sulfur conditions (Fig. 6). A similar, but less pronounced decrease was observed in conglutin-δ mRNA in developing Tg versus control cotyledons of lupins grown with the high sulfur nutrient (results not shown). Some 35S-containing lupin proteins were also shown to be less strongly labeled in sulfur-adequate, soil-grown Tg cotyledons expressing SSA than in controls. SSA sequestered a large proportion of the total 35S label incorporated by mid-maturation cotyledons in this experiment (Fig. 5). We conclude that the lack of increase in Cys in total seed protein of Tg SSA lupin seeds grown in adequate sulfur was mediated by down-regulation of the accumulation of endogenous, Cys-rich lupin proteins. A similar, apparent reallocation of sulfur from endogenous proteins to a heterologous sulfur-rich protein has been seen in Tg corn (Zea mays) expressing a sulfur-rich zein (Anthony et al., 1997) and in Tg soybeans expressing a Brazil nut Bertholletia excelsa albumin (Jung et al., 1997). The mechanisms of these effects are probably the same as those that allow wild-type seeds to modulate their Cys and Met content in response to the availability of sulfur in the environment. The SSA gene was able to escape such control by virtue of possessing the promoter from a pea vicilin gene whose activity is not down-regulated by sulfur deficiency (Beach et al., 1985).
In conclusion, our results show that under most growing conditions, the Cys and Met content of narrow leaf lupins is limited by the sulfur sink strength of the seed, which is regulated by a conservative mechanism that senses the availability of sulfur in the environment. In Tg lupin seeds with an artificially increased sink for sulfur amino acids, uptake of sulfate into the cotyledon was the ultimate limit to sulfur accumulation. In soil-grown plants, decreases in the pools of organic sulfur metabolites in Tg cotyledons were indicative of a proximate limit in the pathway of sulfur assimilation in the seeds. Considering our results in the context of the literature, the most likely steps limiting the accumulation of sulfur amino acids in Tg lupin seeds with an increased sink strength for organic sulfur are transport of sulfate across intracellular membranes, or the amounts of adenosine 5′-phosphosulphate reductase, a specific subcellular isoform of SAT, or CGS.
MATERIALS AND METHODS
Plant Material
Narrow leaf lupins (Lupinus angustifolius) of two genotypes were grown under three different conditions to generate material for this study. The lupins were either cv Warrah (referred to as control), or a homozygous, Tg line of lupins from cv Warrah (line 55-38, referred to as Tg) expressing SSA (Molvig et al., 1997).
Soil-Grown Plants
Lupins of each genotype were grown in soil containing 0.6g L−1 of slow-release fertilizer (“Aboska,” containing 15.2% [w/w] nitrogen, 6.9% [w/w] phosphorus, and 5.2% [w/w] potassium sulfate) in 25-cm pots in a controlled temperature greenhouse. Each pot, containing 9 L of soil, received a supplement of 2 g of solid calcium sulfate (gypsum), which was applied to the surface of the soil when the plants started to flower. Pots were watered from the surface as required. These plants are referred to as soil-grown lupins. The plants from which fresh, developing seeds were harvested for 35SO4 labeling were grown as described, except that they did not receive a supplement of gypsum.
Field-Grown Plants
Lupins of each genotype were grown in the field, in natural rain-fed conditions, at Wongan Hills, Western Australia.
Nutrient-Fed Plants
Eighteen control plants and 18 Tg plants were grown in a controlled-temperature greenhouse in separate 25-cm pots in a perlite-sand mixture and watered with defined nutrient solution. All plants were watered for 4 weeks after sowing with solution containing 0.3 mm MgSO4, 4 mm KNO3, 4 mm Ca(NO3)2, 1 mm Na KH2PO4, 0.1 mm ferric citrate/EDTA, 37 μm H3BO3, 10 μm MnCl2, 1.5 μm ZnCl2, 0.6 μm CuCl2, and 0.2 μm H2MoO4. Subsequently, the plants of each genotype were divided into three groups of six. One group of six control plants and one group of six Tg plants were watered with the same nutrient as shown above, i.e. containing 0.3 mm MgSO4. These plants are referred to as nutrient-fed lupins grown at a medium level of sulfur supply (control:Med and Tg:Med). One group of control and one group of Tg plants were watered with the same basal nutrient containing 3 mm instead of 0.3 mm MgSO4. These plants are referred to as nutrient-fed lupins grown at a high level of sulfur supply (control:High and Tg:High). One group of control and one group of Tg plants were watered for a further 2 weeks with the same basal nutrient containing 0.02 mm instead of 0.3 mm MgSO4. Thereafter, this group was watered with basal nutrient containing no MgSO4. These plants are referred to as nutrient-fed lupins grown at a low level of sulfur supply (control:Low and Tg:Low). In all nutrient solutions, the concentration of Mg was maintained at 3 mm by balancing the MgSO4 concentration with MgCl2.
All pots were watered with 300 to 600 mL of nutrient once a day (until liquid started to drain from the pots) for 6 d a week. On the 7th d, plants received 600 mL of deionized water. In addition, during the second 3 months of growth, all pots were flushed with 600 mL of deionized water twice a week.
Sample Preparation
Samples of leaves, pods, or developing seeds were collected from either soil-grown or nutrient-fed plants at various stages of development, and pooled before being frozen in liquid nitrogen, then freeze dried. Some of the seeds were dissected into cotyledons and testa. Each sample consisted of approximately 50 individual leaves or seeds. Pod material represented approximately 12 to 20 individual pods at the same stage of development as the appropriate seed sample. The leaf samples used for enzyme analysis consisted of 10 to 15 g fresh weight of fully expanded leaves harvested on the same day as the 30-DAF seed samples. All samples were frozen in liquid nitrogen immediately after harvest, then stored for several days at −80°C before being freeze dried. The dried samples were conserved in a sealed container, with silica gel, at 4°C, for between 6 and 10 weeks, then at room temperature for 1 week, before analysis.
Determination of Sulfur by XRFS
Mature lupin seed samples weighing approximately 6 g (approximately 40 seeds) were milled to fine flour using a Cyclone sample mill (Udy, Collins, CO) with a 0.5-mm screen. Powdered samples were pressed into aluminum planchettes, then total sulfur, reduced sulfur, and oxidized sulfur were determined using a PW 1404 spectrometer (Philips, Eindhoven, The Netherlands) as previously described (Pinkerton et al., 1989; Tabe and Droux, 2001).
Protein Extraction and Enzyme Assays
Protein extraction from the dried samples and assays for SAT (EC 2.3.1.30), OASTL (EC 4.2.99.8), and CBL (EC 4.4.1.8) were performed as previously described (Tabe and Droux, 2001). CGS (EC 4.2.99.9) was assayed by measuring the production of cystathionine using derivatization with O-phthaldialdehyde (OPA) and separation by HPLC (Ravanel et al., 1995). Reactions contained 20 mm MOPS-NaOH, pH 7.5; 1 mm l-Cys; 10 mm O-phosphohomo-Ser; 1 mm dithiothreitol; and 50 μL of lupin extract (approximately 400 μg of protein) in a volume of 100 μL. After incubation at 25°C for between 10 and 30 min, the reaction was stopped by the addition of 50 μL of 20% (w/v) TCA. The precipitated protein was removed by centrifugation at 15,000g for 5 min. Cystathionine in the supernatant was quantified by HPLC of OPA adducts.
Analysis of Amino Acids and Thiols
Samples of approximately 20 mg of dried, powdered plant material were extracted into 1 mL of 25 mm HCl at 100°C for 8 min. Insoluble material was removed by centrifugation in a bench microfuge at 10,000g for 5 min. For quantification of Cys and glutathione, a sample of the acid supernatant was reacted with monobromobimane, then analyzed by reversed phase HPLC as described previously for homo-Cys (Droux et al., 1995; Tabe and Droux, 2001).
For quantification of free amino acids, a sample of the supernatant was reacted with OPA immediately before separation by reversed-phase HPLC using an Uptisphere 250- × 4.6-mm C-18 column (Bio-Tek, Winooski, VT). Mobile phases used for elution of the OPA adducts were as follows: A, 50 mm sodium acetate and 3% (v/v) tetrahydrofuran, adjusted to pH 5.7 with acetic acid; and B, methanol containing 5% (v/v) tetrahydrofuran. The elution protocol (1 mL min−1) employed a linear gradient as follows: initial, 16% (v/v) B in A, 0 to 30 min; 16% to 45% (v/v) B in A, 30 to 35 min; 45% to 55% (v/v) B in A, 35 to 55 min; 55% to 75% (v/v) B in A, 55 to 56 min; 75% to 100% (v/v) B in A, 56 to 59 min; 100% (v/v) B; and re-equilibration of the column for 10 min in 16% (v/v) B in A before new injection. Fluorescence of the OPA adducts was detected using a SFM25 fluorimeter (Bio-Tek) at 455 nm, upon excitation at 340 nm. Met was quantified by measuring peak areas using the Kromasystem 2000 software (Bio-Tek) and comparing with OPA-Met standards.
The total amino acid composition of mature seeds was determined after complete hydrolysis of finely ground flour as previously described (Tabe and Droux, 2001).
35SO4 Labeling and SDS-PAGE
Developing seeds were harvested from lupins grown in soil at early (approximately 18 DAF) and middle (approximately 30 DAF) stages of seed storage protein accumulation (Gayler et al., 1984). Cotyledons were removed from the seeds within 30 to 60 min of the pods being harvested. The cotyledons from two seeds of each age were incubated with carrier-free Na2[35S]O4 as described previously (Tabe and Droux, 2001). Protein samples were electrophoresed on SDS-polyacrylamide gradient minigels (15% to 30% [w/v] acrylamide) with equal numbers of TCA-insoluble counts loaded in each track. The gels were fluorographed after soaking in 20% (w/v) naphthalene and 0.5% (w/v) 2,5-diphenyloxazole in dimethylsulfoxide (Gill et al., 1981). Total protein from mature seeds was electrophoresed on the same type of SDS-polyacrylamide gradient minigel and protein was visualized by silver staining using a kit (Bio-Rad, Hercules, CA).
Western and Northern Blotting
SSA was quantified in total protein extracted from lupin seeds using techniques described by Molvig et al. (1997). Total protein was extracted from flour from whole, mature lupin seeds milled as for XRFS analysis. Ten micrograms of total seed protein was electrophoresed in each lane. SSA purified from sunflower (Helianthus annuus) seeds was used as a standard for quantification.
Northern-blot analysis was performed on total RNA extracted from developing cotyledons by grinding plant tissue to a fine power in liquid nitrogen using a mortar and pestle then extracting into the following buffer: 1 m Tris-HCl, pH 9.0; 1% (w/v) SDS; and 0.5% (v/v) β-mercaptoethanol. Protein was removed by extraction with 2 volumes of a mixture of phenol:chloroform:isoamyl alcohol at a ratio of 25:24:1 (v/v). After 5 min of incubation on ice, the extracts were centrifuged in a bench microfuge at 10,000g for 10 min at room temperature. The aqueous phase was re-extracted twice with an equal volume of phenol:chloroform:isoamyl alcohol, then nucleic acids were precipitated with isopropanol. The pellet was dissolved in water and then RNA was precipitated with LiCl, final concentration 0.2 m, on ice for 4 h. After centrifugation at 10,000g in a bench microfuge at room temperature for 10 min, the RNA pellet was dissolved in 200 μL of 0.3 m Na2CH3COO, pH 4.5, then precipitated with ethanol. After a second centrifugation as described above, the resulting pellet of RNA was washed with 70% (v/v) ethanol, then dissolved in water. Ten-microgram samples of RNA were electrophoresed on a 1.2% (w/v) agarose gel containing 5% (v/v) formaldehyde. RNA was transferred to Hybond N+ nylon membrane (Amersham Pharmacia Biotech, Piscataway, NJ) by capillary blotting.
32P-Labeled DNA probes were prepared using a megaprime kit (Amersham). Membranes were hybridized at 42°C overnight according to the method of Khandjian (1987) and membranes were washed at high stringency (twice in 2× SSC and 0.1% [w/v] SDS at 65°C for 15 min, then once in 0.1× SSC and 0.1% [w/v] SDS at 65°C for 15 min). Membranes were sealed in plastic bags and exposed to x-ray film for between 2 and 10 d.
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. Francis Pierre and staff (Aventis Animal Nutrition, Commentry, France) for the analysis of total sulfur amino acid composition of lupin flour samples. We thank Belinda Schouten (Commonwealth Scientific and Industrial Research Organization, Plant Industry, Canberra, Australian Capital Territory, Australia) for excellent technical assistance and Dr. T.J. Higgins (Commonwealth Scientific and Industrial Research Organization, Plant Industry) for helpful comments on the manuscript. Sincere thanks to Dr. Geoff Anderson (Commonwealth Scientific and Industrial Research Organization Plant Industry Centre for Mediterranean Agriculture Research, Perth, Western Australia, Australia) for confirming the presence of sulfate in lupin seed flour by ion chromatography, and to Dr. Ken Gayler (University of Melbourne) for the conglutin clones.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010935.
LITERATURE CITED
- Anthony J, Brown W, Buhr D, Ronhovde G, Genovesi D, Lane T, Yingling R, Aves K, Rosato M, Anderson P. Transgenic maize with elevated 10 kD zein and methionine. In: Cram WJ, De Kok LJ, Stulen I, Brunold C, Rennenberg H, editors. Sulfur Metabolism in Higher Plants: Molecular, Ecophysiological and Nutritional Aspects. Leiden, The Netherlands: Backhuys Publishers; 1997. pp. 295–297. [Google Scholar]
- Beach LR, Spencer D, Randall PJ, Higgins TJV. Transcriptional and post-transcriptional regulation of storage protein gene expression in sulfur-deficient pea seeds. Nucleic Acids Res. 1985;13:999–1013. doi: 10.1093/nar/13.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagrove RJ, Gillespie PJ, Randall PJ. Effect of sulfur supply on the seed globulin composition of Lupinus angustifolius. Aust J Plant Physiol. 1976;3:173–184. [Google Scholar]
- Chandler PM, Spencer D, Randall PJ, Higgins TJV. Influence of sulfur nutrition on developmental patterns of some major pea seed proteins and their mRNAs. Plant Physiol. 1984;75:651–657. doi: 10.1104/pp.75.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson DT, Smith FW, Vanden Berg PJ. Regulation of sulfate transport in a tropical legume, Macroptilium atropurpureum, cv. Siratro. J Exp Bot. 1983;34:1463–1483. [Google Scholar]
- Droux M, Ravanel S, Douce R. Methionine biosynthesis in higher plants: II. Purification and characterization of cystathionine β-lyase from spinach chloroplasts. Arch Biochem Biophys. 1995;316:585–595. doi: 10.1006/abbi.1995.1078. [DOI] [PubMed] [Google Scholar]
- Gayler KR, Boadle BG, Snook M, Johnson ED. Precursors of storage proteins in Lupinus angustifolius. Biochem J. 1984;221:333–341. doi: 10.1042/bj2210333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayler KR, Sykes GE. Effects of nutritional stress on the storage proteins of soybeans. Plant Physiol. 1985;78:582–585. doi: 10.1104/pp.78.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Kumarasamy R, Symons RH. Cucumber mosaic virus-induced RNA replicase: solubilization and purification of the particulate enzyme. Virology. 1981;113:1–8. doi: 10.1016/0042-6822(81)90131-8. [DOI] [PubMed] [Google Scholar]
- Hirai MY, Fujiwara T, Chino M, Naito S. Effects of sulfate concentration on the expression of a soybean seed storage protein and its reversibility in transgenic Arabidopsis thaliana. Plant Cell Physiol. 1995;36:1331–1339. [PubMed] [Google Scholar]
- Jung R, Martino-Catt S, Townsend J, Beach L. Expression of a sulfur-rich protein in soybean seeds causes an altered seed protein composition. Plant Mol Biol Rep Suppl. 1997;15:307. [Google Scholar]
- Khandjian EW. Optimized hybridization of DNA blotted and fixed to nitrocellulose and nylon membranes. Bio/Technology. 1987;5:165–167. [Google Scholar]
- Kim H, Hirai MY, Hayashi H, Chino M, Naito S, Fujiwara T. Role of O-acetyl-l-serine in the coordinated regulation of the expression of a soybean seed storage-protein gene by sulfur and nitrogen nutrition. Planta. 1999;209:282–289. doi: 10.1007/s004250050634. [DOI] [PubMed] [Google Scholar]
- Leustek T, Martin MN, Bick JA, Davies JP. Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:141–165. doi: 10.1146/annurev.arplant.51.1.141. [DOI] [PubMed] [Google Scholar]
- Lilley GG, Inglis AA. Amino acid sequence of conglutin-δ, a sulfur-rich seed protein of Lupinus angustifolius L. FEBS Lett. 1986;195:235–241. [Google Scholar]
- Molvig L, Tabe LM, Eggum BO, Moore AE, Craig S, Spencer D, Higgins TJV. Enhanced methionine levels and increased nutritive value of seeds of transgenic lupins (Lupinus angustifolius L.) expressing a sunflower seed albumin gene. Proc Natl Acad Sci USA. 1997;94:8393–8398. doi: 10.1073/pnas.94.16.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton A, Randall PJ, Norrish K. Estimation of sulfate and amino acid sulfur in plant material by X-ray spectrometry. Commun Soil Sci Plant Anal. 1989;20:1557–1574. [Google Scholar]
- Ravanel S, Droux M, Douce R. Methionine biosynthesis in higher plants: I. Purification and characterization of cystathionine γ-synthase from spinach chloroplasts. Arch Biochem Biophys. 1995;316:572–584. doi: 10.1006/abbi.1995.1077. [DOI] [PubMed] [Google Scholar]
- Ravanel S, Gakiere B, Job D, Douce R. The specific features of methionine biosynthesis and metabolism in plants. Proc Natl Acad Sci USA. 1998;95:7805–7812. doi: 10.1073/pnas.95.13.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K. Regulation of sulfate transport and synthesis of sulfur-containing amino acids. Curr Opin Plant Biol. 2000;3:188–195. [PubMed] [Google Scholar]
- Sexton PJ, Shibles RM. Activity of ATP Sulfurylase in reproductive soybean. Crop Sci. 1999;39:131–135. [Google Scholar]
- Tabe LM, Droux M. Sulfur assimilation in developing lupin cotyledons could contribute significantly to the accumulation of organic sulfur reserves in the seed. Plant Physiol. 2001;126:176–187. doi: 10.1104/pp.126.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabe LM, Higgins TJV. Engineering plant protein composition for improved nutrition. Trends Plant Sci. 1998;3:282–286. [Google Scholar]
- White CL, Tabe LM, Dove H, Hamblin J, Young P, Phillips N, Taylor R, Gulati S, Ashes J, Higgins TJV. Increased efficiency of wool growth and live weight gain in Merino sheep fed transgenic lupin seed containing sunflower albumin. J Sci Food Agric. 2000;81:147–154. [Google Scholar]