In this issue of PNAS, Babior et al. (1) extend the published series of experiments by Wentworth et al. (2–5) in which it was shown that antibodies catalyze the generation of ozone by a water oxidation pathway. The overall process is postulated to involve more than one equivalent of the lower-energy singlet state of molecular oxygen (1O2) and to proceed via dihydrogen trioxide (H2O3) as a key intermediate (Eq. 1). Alternatively, the involvement of a higher-energy singlet state of oxygen may be considered
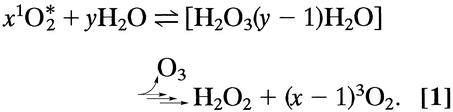 |
The presumed role of the antibody molecule is to organize the short-lived reactant 1O2 (t1/2 1O2 in water is ≈1 μs) and water into a productive complex and also stabilize the H2O3 intermediate [t1/2 H2O3 at room temperature in water is ≈20 ms; t1/2 H2O3 at room temperature in anhydrous acetone is ≈ 3 min (P. Wentworth, Jr., personal communication). Because all antibodies, regardless of source, species, or antigenic specificity, can catalyze this reaction (2), it is most properly thought of as a newly discovered effector function of antibodies. Some of the early questions about this reaction concerned the source of the 1O2 as well as its biological significance. These questions were approached in a recent study by Wentworth et al. (4) in which they demonstrated that activated white cells, which have been shown to produce 1O2 (6), could serve as a substrate source for the antibody-catalyzed reaction. In this instance, the catalysts are presumed to be the Ig molecules bound to the neutrophil plasma membrane via the FcγIII receptors. As will be discussed below, this arrangement of reactants and catalysts may comprise a highly efficient killing system.
From a chemical point of view, it must be emphasized that the antibody-catalyzed water oxidation pathway is independent of the source of 1O2 and can be initiated when the 1O2 is provided by either the thermal decomposition of endoperoxides, in a retro-4 + 2 reaction, or from photochemical sources (2). Thus, the only requirement for the reaction to occur is the juxtaposition of substrate 1O2 and an antibody molecule. The biological consequences of this reaction were underscored when it was demonstrated that: (i) products of the reaction can kill bacteria; (ii) activated white cells can initiate the reaction; and (iii) the reaction occurs in an inflammatory response in vivo (4).
Given that the ultimate product of the antibody-catalyzed process was H2O2, it was reasonable to think that it alone was responsible for the bacterial killing. However, quantitative studies revealed that, while H2O2 can kill bacteria, the amount of H2O2 produced by antibodies was far below that required for the observed killing. This circumstance led to the central hypothesis of the earlier work (4), expanded on by Babior et al. (1): killing occurs by a highly reactive oxidant formed either during the water oxidation pathway from 1O2 to H2O2 and/or when H2O2 reacts with another oxidant to generate a more highly toxic agent. On the basis of chemical reasoning and analytical data, as well as the quantum chemical calculations of Goddard's group (3, 7), one such toxic oxidant was postulated to be ozone. This was the first suggestion that this highly toxic gas is produced in biological systems.
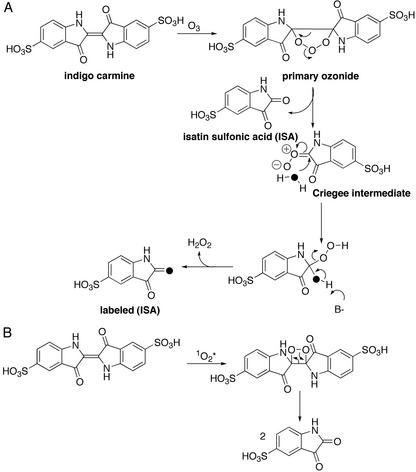
A proper study of ozone formation in biological systems is difficult, not only because of its high reactivity and short half-life (t1/2 in water at room temperature is ≈1 min), but also because its spectral properties (λmax = 260 nm) overlap with many biological molecules such as proteins and nucleic acids, as well as small molecules generated in inflammation (H2O2 and HOCl). However, its unique chemical signature can be found when a series of analytical studies involving the oxidative cleavage of molecules with activated double bonds such as indigo carmine is coupled with analysis of the participation of solvent in this reaction (Fig. 1). Of all of the likely oxidants produced by either antibody catalysis or white cells (1O2, H2O2, HOCl, H2O3, ⋅O2−) only ozone both cleaves the double bond of indigo carmine and incorporates a solvent water molecule in an asymmetric fashion, into the two molecules of isatin sulfonic acid formed (Fig. 1).
Figure 1.
(A) Based on the Criegee mechanism (9), the postulated pathway by which ozonolysis of indigo carmine in H218O leads to incorporation of the isotopic label into the isatin sulfonic acid product. (B) The postulated mechanism by which oxidation of indigo carmine by 1O2* in H218O does not lead to incorporation of the isotopic label into the isatin sulfonic acid product.
Given the weight of the claim that ozone is produced by biological systems, seeking additional evidence for its presence is important. Such evidence is provided by Babior et al. (1), who show that 4-vinyl benzoic acid is oxidized to give the same products as its chemical ozonolysis. Unlike the C,C-double bond of indigo carmine, that of vinyl benzoic acid is not activated by adjacent heteroatoms and does not have allylic hydrogen atoms that offer the opportunity for ene reactions with 1O2. Although not reported here, those authors and their colleagues have observed the chemical signature of ozone in a biological system involving a carbon–carbon double bond that is neither activated by heteroatoms nor conjugated to an aromatic system (P. Wentworth, Jr., personal communication).
Babior et al. (1) also study the effect of catalase on the oxidative cleavage of indigo carmine to produce isatin sulfonic acid. They show that the oxidation is actually enhanced in the presence of catalase. These studies are in line with the known destruction of ozone by H2O2 and also support the authors' consideration that the bactericidal effect of antibodies may be linked to the peroxone process, i.e., a reaction between H2O2 and ozone. Those results, together with previous studies, leave little doubt that antibodies catalyze the production of a molecule that possesses the chemical signature of ozone.
Killing occurs by a highly reactive oxidant formed when H2O2 reacts with another oxidant.
The efficiency of ozone production by white cells is worth comment beyond that contained in ref. 1. In their introduction, Babior et al. introduce us to the chemical riches of the granulocyte oxidase. One is struck by two features. First, nature has gone to extreme lengths to impose a “fail safe” system that keeps the oxidase inactive until the white cell is called on during infection. Only then do a series of activation steps lead to the assembly of the membrane bound holo-enzyme that now is competent to catalyze a one-electron reduction of triplet molecular oxygen. Such control over activation is necessary for a system whose output is the production of highly toxic materials designed to kill organisms whose chemical components are, in the main, not very different from the host. But, no biological control system is perfect and one can only speculate as to the consequences of leakage, especially when one of the leaked products could be ozone with its high reactivity and diffusion radius. The second feature of the granulocyte oxidase that strikes one is its similarity to other membrane assemblies such as those that carry out oxidative phosphorylation. If a broad view is taken, one can begin to envision pathways in which the electron transport that ultimately leads to the reduction of oxygen and proton shuttling are coupled to ATP-ases in the phagolysosome (8). But, toward what end? This issue deserves experimental study. In this sense, the initial discovery that antibodies can catalyze the oxidation of water by 1O2 showed us the potential for a reaction that may only operate at maximum efficiency when it is part of a larger complex. The fact that the pure antibody and the cellular complex carry out redundant reactions has evolutionary implications that we will return to below.
Babior et al. (1) hint at the possibility that white cells may make ozone on their own, by the interaction between molecules generated during the respiratory burst. One such possibility is that the hypochlorite anion undergoes a nucleophilic addition to 1O2, generating an intermediate that is expected to fragment into ozone and chloride ion. This hypothesis is currently under investigation (P. Wentworth, Jr., personal communication). If the reaction between 1O2 and hypochlorite anion indeed were to generate ozone it would constitute one of the rare chemical modes of ozone formation found in a century (10, 11).
From an evolutionary point of view, it is striking that cells and free antibodies in the absence of cells should be able to make similar oxidants such as H2O2 and ozone. For free antibodies, the only requirement is a source of singlet oxygen. There is much appeal in a concept according to which 1O2 may have been the first “antigen” of an evolutionary forerunner of antibodies. In a period when the major challenge to living organisms became infection it may have become important to make use of the highly reactive but short-lived 1O2 for microbial killing by converting 1O2 into longer-lived and therefore more efficient killing agents. In essence, this would amount to the storage of the energy of 1O2 in molecules that are more compatible with the purpose of defense. With the evolution of antibody diversity, killing could become linked with recognition.
Wentworth et al. (5) show that when the antibody-catalyzed water oxidation pathway is operative bound antigens and the antibody itself can be hydroxylated by processes of the type usually considered to be associated with the chemistry of hydroxyl radicals. As mentioned in the present report, one of the possible pathways in which the generation of hydroxyl radicals can be expected is the reaction of ozone with H2O2 (Eq. 2). Another possibility is the oxidation or disproportionation of H2O3 (5).
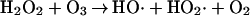 |
2 |
These processes are expected to proceed via the hydrogen trioxide radical (HO3⋅), a species that in biological terms would have a number of remarkable features. First, it is a masked form of the hydroxyl radical and may explain the long-standing problem of how a molecule with diffusion controlled reactivity can act at a distance. Perhaps of greater significance is the feature that that the HO3⋅ could be the link between the dioxygen and newly discovered trioxygen worlds in biology (5). Once one entertains the possibility of trioxygen chemistry in biology, a vast array of new reaction paths, both spontaneous and enzyme catalyzed, must be considered. For instance, Babior et al. (1) speculate that an ozonase may yet be discovered. Such an enzyme could be considered to be analogous to superoxide dismutase, which has evolved as a universal enzyme, even though the dismutation reaction of its substrate is so rapid (kbi = 1.0 × 108 M−1⋅s−1).
In biological systems the generation of a gas with the reactivity of ozone has consequences for the pathogenesis of any disease that has an inflammatory component. Nowadays inflammation is thought to play a role in an ever-widening network of problems including autoimmunity, aging, and atherosclerosis. There are a variety of ways that ozone could play a role in these processes. The most obvious stems from its chemical capacity, as a highly reactive gaseous allotrope of oxygen, to oxidatively cleave virtually any compound that contains an olefin, such as unsaturated lipids, or oxidize the sulfur and nitrogen atoms of proteins. Also, ozone can react with other chemicals to generate even more toxic materials. These downstream processes include the generation of the hydrotrioxy and hydroxyl radicals. In addition to the toxicity of these species, products derived from ozonolysis may have toxicity that were not present in the parental molecules, either directly or by modification of other molecules such as proteins. Proteins that are modified by reaction with the products of ozonolysis may be seen as foreign, leading to autoimmune reactivity. Also, like other oxidants, ozone may function as a signaling agent to amplify the inflammatory cascade. Indeed, ozone is known to be a potent inducer of the NF-κB pathway leading to the induction of IL-8 and tumor necrosis factor-α among other lymphokines (ref. 4 and references therein).
Finally, there is a certain irony in the finding that all antibodies are catalysts. One of the authors of ref. 1 is part of the community of scientists that realized that the programmable binding energy of the antibody molecule could be used to generate a wide range of catalysts. It seems that evolution stumbled on the same concept and antibodies were catalysts all along. Yet again we see that during evolution any chemistry that can happen will as long as it is useful.
Footnotes
See companion article on page 3031.
References
- 1.Babior B M, Takeuchi C, Ruedi J, Gutierrez A, Wentworth P., Jr Proc Natl Acad Sci USA. 2003;100:3031–3034. doi: 10.1073/pnas.0530251100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wentworth A D, Jones L H, Wentworth P, Jr, Janda K D, Lerner R A. Proc Natl Acad Sci USA. 2000;97:10930–10935. doi: 10.1073/pnas.97.20.10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wentworth P, Jr, Jones L H, Wentworth A D, Zhu X, Larsen N A, Wilson I A, Xu X, Goddard W A, III, Janda K D, Eschenmoser A, Lerner R A. Science. 2001;293:1806–1809. doi: 10.1126/science.1062722. [DOI] [PubMed] [Google Scholar]
- 4.Wentworth P, Jr, McDunn J E, Wentworth A D, Takeuchi C, Nieva J, Jones T, Bautista C, Ruedi J M, Gutierrez A, Janda K D, et al. Science. 2002;298:2195–2199. doi: 10.1126/science.1077642. [DOI] [PubMed] [Google Scholar]
- 5.Wentworth P, Jr, Wentworth A D, Zhu X, Wilson I A, Janda K D, Eschenmoser A, Lerner R A. Proc Natl Acad Sci USA. 2003;100:1490–1493. doi: 10.1073/pnas.0437831100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinbeck M J, Khan A U, Karnovsky M J. J Biol Chem. 1992;267:13425–13433. [PubMed] [Google Scholar]
- 7.Xu X, Muller P R, Goddard W A., III Proc Natl Acad Sci USA. 2002;99:3376–3381. doi: 10.1073/pnas.052710099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Finlay, B. B. & Falkow, S. (1997) Microbiol. Mol. Biol. Rev. 136–169. [DOI] [PMC free article] [PubMed]
- 9.Criegee R. Argewandte Chem Int Ed Engl. 1975;14:765–771. [Google Scholar]
- 10.Castro C E. J Am Chem Soc. 1996;118:3984–3985. [Google Scholar]
- 11.Dimitrou A, Seppelt K, Scheffler D, Willner H. J Am Chem Soc. 1998;120:8711–8714. [Google Scholar]