Abstract
Type III secretion systems and bacterial flagella are broadly compared at the level of their genetic structure, morphology, regulation, and function, integrating structural information, to provide an overview of how they might function at a molecular level.
Type III secretion systems (TTSS or secretons), essential determinants of the interaction of many Gram-negative bacteria with animal or plant hosts, serve to translocate bacterial proteins into eukaryotic host cells to manipulate them during infection. These devices perform regulated posttranslational and cotranslational protein translocation across three biological membranes, involving novel pathways of protein targeting. Sequence similarities exist between components of TTSSs and those of the flagellar assembly machineries in prokaryotes (1). We explore functional parallels between these systems to gain mechanistic insights about TTSSs and flagella alike.
TTS is characterized by (i) host contact-mediated TTSS induction, (ii) energy requirement for protein secretion and translocation into host cells, (iii) secretion-regulated expression of genes encoding proteins secreted downstream in the pathway, and (iv) dedicated cytoplasmic chaperones for some secreted proteins. It differs from other secretion pathways in Gram-negative bacteria by the absence of (i) primary sequence conservation in regions of secreted proteins involved in targeting except within some species (2), (ii) a cleaved signal sequence in secreted polypeptides, and (iii) a periplasmic secretion intermediate (3). TTSSs and flagella share all but the first characteristic and the ability to translocate proteins into eukaryotic cells.
Structure
Old and New Sequence Homologies.
TTS apparatuses are encoded by ≈25 genes (4), nearly all essential for function. About 10 TTSS proteins are similar in sequence or membrane topology to cytoplasmic or inner membrane proteins of flagellar hook-basal bodies (HBBs; refs. 5 and 6). Others show no significant sequence homology. However, they show “functional conservation” because when knocked out, they lead to similar phenotypes in assembly or function of the apparatuses. By matching biochemical characteristics and biological information about each protein (see Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org), we propose the functional homologs shown in Fig. 1.
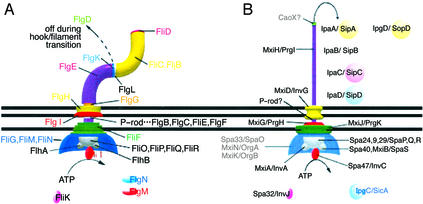
Figure 1.
Diagrams of known positions of major flagellar components (A) and established and hypothetical TTSS functional homologs (B). Functions of proteins conserved in both systems are marked by similar position, shading, and coloring whether they share sequence homologies or not. Those TTSS components for which no sequence homology or experimental evidence exists to establish their relation to the similarly positioned and colored flagellar components in A are shown transparently in B.
Morphological Similarities.
A part of the TTS machinery, the “needle complex” (NC) resembles HBBs (6, 7). NCs comprise a 10 × 60-nm external needle inserted within a 30-nm (in diameter) cylinder traversing both bacterial membranes and the peptidoglycan. The Shigella secreton has an additional “bulb,” 45 × 25 nm, on the cytoplasmic side of the inner membrane, similar to the flagellar C-ring (refs. 8 and 9; Fig. 1). NCs are traversed by a 2- to 3-nm channel (10), which exists also within the entire bacterial flagellum (11). Flagellin may transit partially unfolded (12) through this channel to its tip, where it refolds and inserts into the growing filament (13, 14). Effectors from plant pathogen TTSSs are also secreted from the distal tip of their TTS machineries (15). During assembly of flagella in vivo, a cap is added before each transition to a new part of the flagellum so new subunits, which would otherwise diffuse away, can be inserted directly under the cap (16). NC components, including the needle component MxiH/PrgI, have been identified (17, 18). No cap has been identified in any TTSS. Morphological divergence between TTSSs is discussed in Supporting Text.
Regulation
Timing of Assembly and Maintenance.
The NC assembly pathway shares organizing principles with that of HBBs (19, 20). The flagellar assembly pathway takes at least one bacterial generation to complete from initiation of transcription (21), a long time for a bacterium entering a well defended host. When Salmonella enter a phagocytic vacuole, a transition from SPI1 to SPI2 expression is observed (22). Are there storage/recycling mechanisms at work when the bacterium enters/leaves the environment in which a particular apparatus is required? For species possessing two TTSSs and a flagellum, such as Salmonella and Yersinia, some degree of coordination is required (23). Studies of flagellar shedding during the Caulobacter crescentus differentiation cycle (24) provide some clues.
Regulation of Expression.
In flagellar assembly, only master regulator genes (with class I promoters; ref. 25) are basally expressed constitutively. They are required for expression of genes encoding HBB components (with class II promoters), genes encoding regulators of hook/filament transition and filament components (with both class II and III promoters), and genes encoding motor and chemotaxis components (with class III promoters; ref. 26). “Early” TTSS effector genes (class II+III-like?) are often located adjacent to those encoding the machinery (class II-like?), and their basal transcription seems regulated by master regulators analogous to those found in the flagellum-encoding operons acting on a similar transcriptional hierarchy (see Supporting Text). To ensure appropriate flagellar and TTSS-encoding gene up-regulation, many bacteria seem to have “intensity” switches that sense environmental queues (22, 27, 28). These signals often act together to obtain high expression levels and feed into the master two-component regulatory systems (29).
Posttranslational Secretion and Chaperones.
Some effectors are synthesized before the machine is ready to secrete them. Posttranslational secretion was demonstrated by pulse–chase experiments (30). Effector storage involves specialized chaperones, often encoded near effectors. Chaperones are small proteins with an acidic pI and a C-terminal amphiphilic α-helix (31), often required for stability and partitioning of effector(s) before secretion (32). They bind between amino acids 50 and 100 of their substrate (33, 34). Some flagellar chaperones bind to the C terminus of their substrate (35). The chaperone-binding domain seems essential only for posttranslational secretion (36) and solely when the other effectors are present, suggesting that the chaperones help control a hierarchy of secretion (37). Another region, the first 15 aa of the protein, is independently required for secretion (33, 34, 38). It shows a bias toward amphipathic amino acids (39, 40), reflecting a poorly ordered helix, found also in the N termini of flagellar proteins (41). The chaperone-binding domain may localize the protein at the C-ring, and the N-terminal region could serve to initiate entry into the secreton.
Cotranslational Secretion.
However, some secreted proteins are used repeatedly during infection, for instance, the Shigella translocators, required for lysis of both the initial single membrane phagocytic vacuole and the second, two-membraned vacuole upon entry into the neighboring cell (42). They are secreted posttranslationally upon activation of secretion and continue to be secreted, presumably cotranslationally, if the TTS apparatus is artificially left open (43). In contrast, some genes encoding proteins secreted by the Shigella TTSS are transcribed only after activation of secretion (44, 45). These putative “late” effectors (class III-like?) do not have nearby-encoded chaperone candidates, nor do most of the described effectors of plant pathogens (46, 47). The hypothetical “secretion signal” for these proteins lies in the first few codons of their mRNA, making it rather a probable “antidiffusion signal” serving to localize the mRNA near the secreton, ready for cotranslational secretion (48). Yet, secretion of proteins using the cotranslational route is rendered more efficient if the N-terminal secretion motif is also preserved (49).
Chaperones as a Bridge Between Posttranslational and Cotranslational Secretion.
The flagellar chaperone FlgN.
How is the choice made between signals operating at different levels for proteins that have both export routes? The chaperones may regulate the transcription and translation of two different mRNAs encoding such effectors. Flagellar FlgN binds two proteins, FlgK and FlgL, exported upon hook completion (50). FlgN is also required for translation of the anti-σ factor-encoding flgM class III transcript but not its earlier transcribed class II transcript (51). FlgM translated from the class II transcript is retained in the cytoplasm, perhaps because FlgN is then binding FlgK and FlgL. However, when it is translated from the class III transcript, FlgM is secreted cotranslationally. Upon secretion of FlgM, transcription from class III promoters initiates via a specific σ factor.
Evidence for regulatory chaperones from virulence TTSSs.
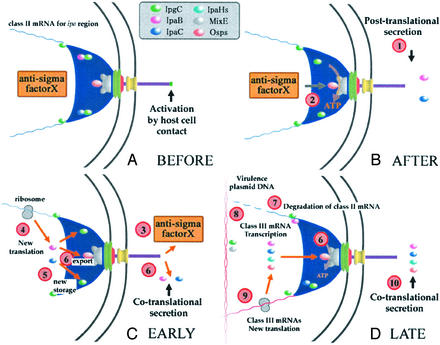
We propose that the equivalent of FlgN in TTSSs is the chaperone of the translocator complex IpgC (binds IpaC and IpaB of Shigella, Fig. 2A; see Supporting Text). Increasing the level of free IpgC (Fig. 2B) leads to premature expression of genes encoding putative late effectors and even to secretion of their products (IpaHs and Ops, Fig. 2D, point 10). Free IpgC interacts with the transcription factor MxiE to activate transcription from these late gene promoters (ref. 45; Fig. 2D, point 8). Mutants in Shigella ipaD or ipaB and flagellar flgK and flgL demonstrate constitutive secretion of class III products (43, 52). Thus, cotranslational secretion of effectors produced from class III mRNAs is contact-independent (Fig. 2, point 10). However, contact-dependent secretion of effectors may remain possible because some return to storage before secretion (Fig. 2, points 5 and 6). Termination of secretion may occur by mRNA degradation when the effector becomes abundant enough to compete for chaperone binding with the mRNA encoding it or proteins secreted downstream in the pathway (ref. 53; Fig. 2D, point 7).
Figure 2.
Model for switching from posttranslational to cotranslational secretion. (A and B) Brief TTSS activation. (C and D) Contact-independent secretion by the Shigella TTSS.
Hence, cotranslational secretion (Fig. 2 C and D) may involve a chaperone/transcription factor complex activating the relevant DNA region(s) and later binding the mRNAs and/or any proteins derived from them to the secreton. If and how chaperone-protein/mRNA complexes become localized at the C-ring is unknown (54). No TTSS equivalent of FlgM has been identified (Fig. 2 B and C, points 2 and 3). In assembly of flagella and TTSSs, the regulatory circuit involves a morphogenic switch from exporting hook/needle to filament/effector components (refs. 55 and 56 and Supporting Text). Flagellum assembly is a continuum, whereas TTSSs arrest at needle completion.
Function
Activation of Secretion.
Yet, a signal is required to allow either the hook/filament transition (57) or induction of secretion by TTSSs. Induction of secretion is the instantaneous, minimum 50% posttranslational release of proteins with translocator or effector functions. Cotranslational secretion probably occurs at a slower rate (58). Assembling TTSSs secrete proteins that are not effectors, but external parts of the machine or regulators of assembly. Beyond this stage, bacteria continue to secrete these proteins and also early effectors at low levels (43), much as flagella do before the hook/filament transition (52). The natural signal for secretion may be the lipid membrane of animal cells or the cell wall of plant cells (30). Presumably, the signal is transmitted from the distal needle tip to the cytoplasmic ATPase powering secretion. The needle of the NC reaches to the periplasmic leaflet of the inner membrane (10). Flagellar filament switching from right- to left-handed supercoiled helical conformations (59) is thought to occur by a 0.8-Å increase in the distance between flagellin molecules within some protofilament(s), leading to discreet alterations of helical parameters (12). If needle subunits also pack helically, similar switch(es) in their straight superstructure might transmit tactile signal(s). How the flagellar hook endogenously signals its completion, leading to FlgK, FlgL, and FliD secretion, is unclear. The hook cap FlgD is displaced by FlgK, allowing filament growth (Fig. 1A). The NC cap may sense host cell proximity, an external signal, via a related mechanism and become lost at TTSS activation (Fig. 1B).
The ATPase: Energy Requirements and Transport Intermediates.
Are effector motifs recognized by the secreton?
Recently determined structures of effectors alone or in complex with their chaperone suggest that (i) the binding of the chaperone partially unfolds the underlying effector region, preventing the N-terminal amphipathic helix from folding against the rest of the protein, and (ii) the part of the effector beyond the chaperonebinding domain is folded during storage (see Supporting Text). If and how part(s) of the secreton recognize the opened helix is unknown. How a motif as common as a loose amphipathic helix serves as a selective secretion signal is unclear. The hypothetical localization provided by chaperones to the C-ring may be key to specificity.
In what state do the substrate proteins travel down the central channel?
The channels through flagella and TTSSs could accommodate several α-helices or a β-sheet domain, short or unfolded into hairpins. The structure of flagellin (11, 12) shows that it could readily unfold into subdomains <30 Å in diameter, connected by linearized regions. The mode of unfolding of tightly folded TTSS effector domains (60–63) is less obvious. Some proteins have high rates of conformational breathing, which is key for their import into mitochondria. Experiments with chimeric proteins show that the ability to readily unfold is required for TTSS export (64) and used to exclude cytoplasmic proteins from export.
What are the energizers of posttranslational and cotranslational secretion?
The flagellar ATPase FliI is required for export of all flagellar proteins except the outer membrane components (5). Without it only the inner membrane and cytoplasmic components are assembled. Mutants in homologous TTSS ATPases display analogous phenotypes (65). Does cotranslational secretion occur by docking of the ribosome to the cytoplasmic part of the TTS machine like cotranslational export across the membrane of the endoplasmic reticulum? An empty flagellar C-ring could only accommodate two ribosomes, the protein channels of which could not directly dock to the HBB without a gap being left. Therefore, cotranslational secretion is probably also driven by the ATPase and hence indirect.
How is energy transduced by the export motor during secretion?
The ATPases interact with cytoplasmic components of TTSSs or flagella but the function(s) of these interactions are mostly unidentified (66, 67). The biological cycle of the enzyme is unknown and its localization is debated [cytosolic or membrane-bound (69)?]. How might these ATPases catalyze processive protein export? Spa47 (the Shigella FliI homolog) shares 33% amino acid identity with the β-subunit of F1-ATPase. Proteins with >30% sequence identity have a high probability of sharing similar structures (69). Active F1-ATPase is a heterohexamer consisting of alternating α- and β-subunits with a γ-subunit inserted in a central channel where it rotates during the catalytic cycle (70). No equivalent of the α-subunit of F1-ATPases is found within flagellar or TTSS-encoding operons, so we assume that the type III export motor is a homohexamer. When modeled on the F1 structure, Spa47 fits at the inner membrane base of our NC structure (Fig. 3). It would contain a central channel aligned with the one found within the NC and of similar diameter to it, through which the proteins could be secreted (see Supporting Text).
Figure 3.
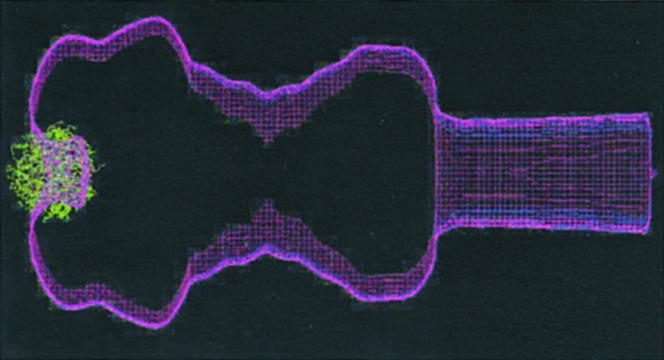
Docking of a Spa47 ATPase F1-like model (green) to the outline of the Shigella NC (purple). Spa47 was aligned to the sequences of α- and β- subunits of the F1-ATPase. Six different Spa47 monomer 3D models were generated and assembled by using whatif and the F1-coordinates (70). An outline of our NC reconstruction (10) was made by using spider and empirically scaled to the ATPase model by using the molecular dimensions of the complex measured in electron microscopy images (±15% accuracy). The ATPase was docked to the NC outline with ATP-binding sites facing the bacterial cytoplasm by using whatif.
Translocation.
Evidence for a pore.
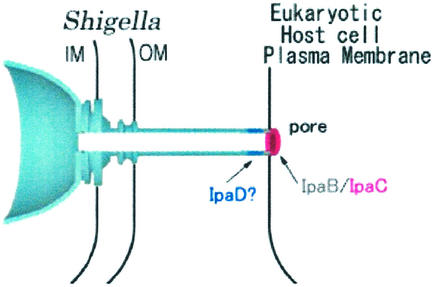
The insertion of a bacterial pore into the host cell membrane (Fig. 4) is central to translocation of effectors. Yersinia, Shigella, and enteropathogenic Escherichia coli TTSSs form 1.5- to 3-nm (in diameter) pores in eukaryotic cells (73) by using at least two bacterial proteins, YopB/IpaB/EspD and YopD/IpaC/EspB, sharing disposition of hydrophobic α-helical regions. IpaB/IpaC and EspD/EspB are inserted into eukaroytic cell membranes during this process (8, 72, 73). Association of YopB, IpaB, IpaC, and SipB individually with artificial membranes does not generate pores. Incubating secreting Yersinia with liposomes leads to association of YopB and YopD with liposomes. This is necessary and sufficient for detection of ionically aselective channels with 105 pS of conductance (74). Pore protein insertion is mysterious. Whether eukaryotic cell receptors are used by TTSSs to initiate activation and/or pore insertion is also unclear (details and species-specific modifications are discussed in Supporting Text).
Figure 4.
Model of inserted putative translocation pore after activation of the S. flexneri TTS apparatus.
A model for pore function.
Might the pore “passively” translocate effectors like the flagellar cap FliD allows insertion of flagellin at the flagellar tip (14)? FliD is a pentamer, whereas the flagellar filament contains 5.5 subunits per helical turn. There is a symmetry mismatch that may cause FliD to rotate by 6.5° per step as it catalyzes insertion of new flagellin subunits by using the energy provided by the cytoplasmic ATPase during substrate unfolding and stored in the extended flagellin during export. IpaC may be equivalent to FliD, whereas IpaD and IpaB may be analogous to FlgK and FlgL (Fig. 1). This would explain why pore formation by most TTSSs does not cause host cell lysis: the pore is closed from the outside and inside (Fig. 4).
Continuity between the secreton needle and the translocation pore is necessary for probable energetic coupling of secretion, needle growth, pore insertion, and effector translocation. Continuity is provided in the flagellum by packing of the components into a hollow helical superstructure. In TTSSs, evidence for continuity is still circumstantial. For instance, continuity between needle and pore is suggested by the similarity of the inner diameters of the pore and of the NC channel (refs. 8 and 10; Supporting Text).
Conclusion
Our understanding of TTSSs was applied to obtain an MHC class I response against a heterologous translocated protein (75). TTSSs are targets for new antimicrobial drugs. Work on type IV secretion systems in other Gram-negative pathogens shows that they, too, can perform host cell contact-mediated protein translocation (76). Type IV secretion apparatuses resemble bacterial conjugation systems, which function differently from TTSSs. The sec-dependent secretion pathway of Gram-positive bacteria also seems capable of polarized protein translocation into host cells (77). These may be examples of convergent evolution.
Supplementary Material
Acknowledgments
A.B. thanks Eric Larquet (Institute Pasteur, Paris) for the NC outline and Gert Vriend (Centre for Molecular and Biomolecular Informatics, Nijmegen, The Netherlands) for making and docking the ATPase model in Fig. 3. Shoutaro Ishii (Teikyo University) drew Figs. 1, 2, and 4. A.B.'s laboratory is supported by the National Institute of Allergy and Infectious Diseases (Grant K22 AI01847), the G. G. F. Newton Trust Research Fellowship, and the Sasakawa Fund (Oriental Institute, Oxford). K.K. is the recipient of an E. P. Abrahams Trust studentship.
References
- 1.Galan J E, Ginocchio C, Costeas P. J Bacteriol. 1992;174:4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miao E A, Miller S I. Proc Natl Acad Sci USA. 2000;97:7539–7544. doi: 10.1073/pnas.97.13.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charkowski A O, Huang H-C, Collmer A. J Bacteriol. 1997;179:3866–3874. doi: 10.1128/jb.179.12.3866-3874.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hueck C J. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macnab R M. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt F C, Curtis R III, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Vol. 1. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 123–145. [Google Scholar]
- 6.Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, Galan J E, Aizawa S-I. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 7.Thomas D, Morgan D G, DeRosier D J. J Bacteriol. 2001;183:6404–6412. doi: 10.1128/JB.183.21.6404-6412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blocker A, Gounon P, Larquet E, Niebuhr K, Cabiaux V, Parsot C, Sansonetti P. J Cell Biol. 1999;147:683–693. doi: 10.1083/jcb.147.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan I H, Reese T S, Khan S. Proc Natl Acad Sci USA. 1992;89:5956–5960. doi: 10.1073/pnas.89.13.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blocker A, Jouihri N, Larquet E, Gounon P, Ebel F, Parsot C, Sansonetti P, Allaoui A. Mol Microbiol. 2001;39:652–663. doi: 10.1046/j.1365-2958.2001.02200.x. [DOI] [PubMed] [Google Scholar]
- 11.Morgan D G, Owen C, Melanson L A, DeRosier D J. J Mol Biol. 1995;249:88–110. doi: 10.1006/jmbi.1995.0282. [DOI] [PubMed] [Google Scholar]
- 12.Samatey F A, Imada K, Nagashima S, Vonderviszt F, Kumasaka T, Yamamoto M, Namba K. Nature. 2001;410:331–337. doi: 10.1038/35066504. [DOI] [PubMed] [Google Scholar]
- 13.Emerson S U, Tokuyasu K, Simon M I. Science. 1970;169:190–192. doi: 10.1126/science.169.3941.190. [DOI] [PubMed] [Google Scholar]
- 14.Yonekura K, Maki S, Morgan D G, DeRosier D J, Vonderviszt F, Imada K, Namba K. Science. 2000;290:2148–2152. doi: 10.1126/science.290.5499.2148. [DOI] [PubMed] [Google Scholar]
- 15.Jin Q, He S Y. Science. 2001;294:2556–2558. doi: 10.1126/science.1066397. [DOI] [PubMed] [Google Scholar]
- 16.Ohnishi K, Ohto Y, Aizawa S-I, Macnab R M, Iino T. J Bacteriol. 1994;176:2272–2281. doi: 10.1128/jb.176.8.2272-2281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamano K, Aizawa S-I, Katayama E, Nonaka T, Imajoh-Ohmi S, Kuwae A, Nagai S, Sasakawa C. EMBO J. 2000;19:3876–3887. doi: 10.1093/emboj/19.15.3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubori T, Sukhan A, Aizawa S-I, Galan J E. Proc Natl Acad Sci USA. 2000;97:10225–10230. doi: 10.1073/pnas.170128997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubori T, Shimamoto N, Yamaguchi S, Namba K, Aizawa S-I. J Mol Biol. 1992;266:433–446. doi: 10.1016/0022-2836(92)90958-m. [DOI] [PubMed] [Google Scholar]
- 20.Sukhan A, Kubori T, Wilson J, Galan J E. J Bacteriol. 2001;183:1159–1167. doi: 10.1128/JB.183.4.1159-1167.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlinsey J E, Tanaka S, Bettenworth V, Yamaguchi S, Boos W, Aizawa S I, Hughes K T. Mol Microbiol. 2000;37:1220–1231. doi: 10.1046/j.1365-2958.2000.02081.x. [DOI] [PubMed] [Google Scholar]
- 22.Deiwick J, Nikolaus T, Erdogan S, Hensel M. Mol Microbiol. 1999;31:1759–1773. doi: 10.1046/j.1365-2958.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- 23.Deiwick J, Nikolaus T, Shea J E, Gleeson C, Holden D W, Hensel M. J Bacteriol. 1998;180:4775–4780. doi: 10.1128/jb.180.18.4775-4780.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenal U, Shapiro L. EMBO J. 1996;15:2393–2406. [PMC free article] [PubMed] [Google Scholar]
- 25.Claret L, Hughes C. J Mol Biol. 2000;303:467–478. doi: 10.1006/jmbi.2000.4149. [DOI] [PubMed] [Google Scholar]
- 26.Kutsukake K, Ohya Y, Iino T. J Bacteriol. 1990;172:741–747. doi: 10.1128/jb.172.2.741-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutsukake K. Mol Gen Genet. 1997;254:440–448. doi: 10.1007/s004380050437. [DOI] [PubMed] [Google Scholar]
- 28.Aldon D, Brito B, Boucher C, Genin S. EMBO J. 2000;19:2304–2314. doi: 10.1093/emboj/19.10.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hensel M, Shea J E, Waterman S R, Mundy R, Nikolaus T, Banks G, Vazquez-Torres A, Gleeson C, Fang F C, Holden D W. Mol Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- 30.Ménard R, Sansonetti P, Parsot C. EMBO J. 1994;15:5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wattiau P, Bernier B, Deslee P, Michiels T, Cornelis G R. Proc Natl Acad Sci USA. 1994;91:10493–10497. doi: 10.1073/pnas.91.22.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ménard R, Sansonetti P, Parsot C, Vasselon T. Cell. 1994;79:515–525. doi: 10.1016/0092-8674(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 33.Sory M P, Boland A, Lambermont I, Cornelis G R. Proc Natl Acad Sci USA. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woestyn S, Sory M P, Boland A, Lequenne O, Cornelis G R. Mol Microbiol. 1996;20:1261–1271. doi: 10.1111/j.1365-2958.1996.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 35.Kornacker M G, Newton A. Mol Microbiol. 1994;14:73–85. doi: 10.1111/j.1365-2958.1994.tb01268.x. [DOI] [PubMed] [Google Scholar]
- 36.Page A-L, Fromont-Racine M, Sansonetti P, Legrain P, Parsot C. Mol Microbiol. 2001;42:1133–1145. doi: 10.1046/j.1365-2958.2001.02715.x. [DOI] [PubMed] [Google Scholar]
- 37.Boyd A, Lambermont I, Cornelis G R. J Bacteriol. 2000;182:4811–4821. doi: 10.1128/jb.182.17.4811-4821.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michiels T, Cornelis G R. J Bacteriol. 1991;173:1677–1685. doi: 10.1128/jb.173.5.1677-1685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lloyd S A, Sjostrom M, Andersson S, Wolf-Watz H. Mol Microbiol. 2002;43:51–59. doi: 10.1046/j.1365-2958.2002.02738.x. [DOI] [PubMed] [Google Scholar]
- 40.Guttman D S, Vinatzer B A, Sarkar S F, Ranall M V, Kettler G, Greenberg J T. Science. 2002;295:1722–1726. doi: 10.1126/science.295.5560.1722. [DOI] [PubMed] [Google Scholar]
- 41.Vonderviszt F, Kanto S, Aizawa S, Namba K. J Mol Biol. 1989;209:127–133. doi: 10.1016/0022-2836(89)90176-9. [DOI] [PubMed] [Google Scholar]
- 42.Schuch R, Sandlin R C, Maurelli A T. Mol Microbiol. 1999;34:675–689. doi: 10.1046/j.1365-2958.1999.01627.x. [DOI] [PubMed] [Google Scholar]
- 43.Parsot C, Ménard R, Gounon P, Sansonetti P J. Mol Microbiol. 1995;16:291–300. doi: 10.1111/j.1365-2958.1995.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 44.Demers B, Sansonetti P J, Parsot C. EMBO J. 1998;17:2894–2903. doi: 10.1093/emboj/17.10.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mavris M, Page A-L, Tournebize R, Demers B, Sansonetti P, Parsot C. Mol Microbiol. 2002;43:1543–1553. doi: 10.1046/j.1365-2958.2002.02836.x. [DOI] [PubMed] [Google Scholar]
- 46.Anderson D M, Fouts D E, Collmer A, Schneewind O. Proc Natl Acad Sci USA. 1999;96:12839–12843. doi: 10.1073/pnas.96.22.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mudgett M B, Chesnokova O, Dahlbeck D, Clark E T, Rossier O, Bonas U, Staskawicz B J. Proc Natl Acad Sci USA. 2000;97:13324–13329. doi: 10.1073/pnas.230450797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson D M, Schneewind O. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- 49.Ramamurthi K S, Schneewind O. J Bacteriol. 2002;184:3321–3328. doi: 10.1128/JB.184.12.3321-3328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fraser G M, Bennett J C, Hughes C. Mol Microbiol. 1999;32:569–580. doi: 10.1046/j.1365-2958.1999.01372.x. [DOI] [PubMed] [Google Scholar]
- 51.Karlinsey J E, Lonner J, Brown K L, Hughes K T. Cell. 2000;102:487–497. doi: 10.1016/s0092-8674(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 52.Komoriya K, Shibano N, Higano T, Azuma N, Yamaguchi S, Aizawa S I. Mol Microbiol. 1999;34:767–779. doi: 10.1046/j.1365-2958.1999.01639.x. [DOI] [PubMed] [Google Scholar]
- 53.Anderson D M, Ramamurthi K S, Tam C, Schneewind O. J Bacteriol. 2002;184:1287–1295. doi: 10.1128/JB.184.5.1287-1295.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Makishima S, Komoriya K, Yamaguchi S, Aizawa S-I. Science. 2001;291:2411–2413. doi: 10.1126/science.1058366. [DOI] [PubMed] [Google Scholar]
- 55.Bonifield H R, Yamaguchi S, Hughes K T. J Bacteriol. 2000;182:4044–4050. doi: 10.1128/jb.182.14.4044-4050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karlinsey J E, Tsui H C, Winkler M E, Hughes K T. J Bacteriol. 1998;180:5384–5397. doi: 10.1128/jb.180.20.5384-5397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kutsukake K. J Bacteriol. 1997;179:1268–1273. doi: 10.1128/jb.179.4.1268-1273.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koroyasu S, Yamazato M, Hirano T, Aizawa S I. Biophys J. 1998;74:436–443. doi: 10.1016/S0006-3495(98)77801-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calladine C R. Nature. 1975;255:121–124. doi: 10.1038/255121a0. [DOI] [PubMed] [Google Scholar]
- 60.Stuckey J A, Schubert H L, Fauman E B, Zhang Z Y, Dixon J E, Saper M A. Nature. 1994;370:571–575. doi: 10.1038/370571a0. [DOI] [PubMed] [Google Scholar]
- 61.Luo Y, Frey E A, Pfuetzner R A, Creagh A L, Knoechel D G, Haynes C A, Finlay B B, Strynadka N C. Nature. 2000;405:1073–1077. doi: 10.1038/35016618. [DOI] [PubMed] [Google Scholar]
- 62.Evdokimov A G, Anderson D E, Routzahn K M, Waugh D S. J Mol Biol. 2001;312:807–821. doi: 10.1006/jmbi.2001.4973. [DOI] [PubMed] [Google Scholar]
- 63.Evdokimov A G, Tropea J E, Routzahn K M, Waugh D S. Protein Sci. 2002;11:401–408. doi: 10.1110/ps.34102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feldman M F, Muller S, Wuest E, Cornelis G R. Mol Microbiol. 2002;46:1183–1197. doi: 10.1046/j.1365-2958.2002.03241.x. [DOI] [PubMed] [Google Scholar]
- 65.Eichelberg K, Ginocchio C C, Galan J E. J Bacteriol. 1994;176:4501–4510. doi: 10.1128/jb.176.15.4501-4510.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jackson M W, Plano G V. FEMS Microbiol Lett. 2000;186:85–90. doi: 10.1111/j.1574-6968.2000.tb09086.x. [DOI] [PubMed] [Google Scholar]
- 67.Minamino T, Macnab R M. Mol Microbiol. 2000;37:1494–1503. doi: 10.1046/j.1365-2958.2000.02106.x. [DOI] [PubMed] [Google Scholar]
- 68.Auvray F, Ozin A J, Claret L, Hughes C. J Mol Biol. 2002;318:941–950. doi: 10.1016/S0022-2836(02)00172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sander C, Schneider R. Proteins. 1991;9:56–68. doi: 10.1002/prot.340090107. [DOI] [PubMed] [Google Scholar]
- 70.Abrahams J P, Leslie A G W, Lutter R, Walker J E. Nature. 1994;370:621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 71.Håkansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homble F, Wolf-Watz H. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 72.Wachter C, Beinke C, Mattes M, Schmidt M A. Mol Microbiol. 1999;31:1695–1707. doi: 10.1046/j.1365-2958.1999.01303.x. [DOI] [PubMed] [Google Scholar]
- 73.Ide T, Laarmann S, Greune L, Schillers H, Oberleithner H, Schmidt M A. Cell Microbiol. 2001;3:669–679. doi: 10.1046/j.1462-5822.2001.00146.x. [DOI] [PubMed] [Google Scholar]
- 74.Tardy F, Homble F, Neyt C, Wattiez R, Cornelis G R, Ruysschaert J M, Cabiaux V. EMBO J. 1999;18:6793–6799. doi: 10.1093/emboj/18.23.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Russmann H, Shams H, Poblete F, Fu Y, Galan J E, Donis R O. Science. 1998;281:565–568. doi: 10.1126/science.281.5376.565. [DOI] [PubMed] [Google Scholar]
- 76.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 77.Madden J C, Ruiz N, Caparon M. Cell. 2001;104:143–152. doi: 10.1016/s0092-8674(01)00198-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.