Abstract
Recent studies have suggested that antibodies can catalyze the generation of previously unknown oxidants including dihydrogen trioxide (H2O3) and ozone (O3) from singlet oxygen (1O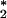 ) and water. Given that neutrophils have the potential both to produce 1O
) and water. Given that neutrophils have the potential both to produce 1O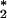 and to bind antibodies, we considered that these cells could be a biological source of O3. We report here further analytical evidence that antibody-coated neutrophils, after activation, produce an oxidant with the chemical signature of O3. This process is independent of surface antibody concentration down to 50% of the resting concentration, suggesting that surface IgG is highly efficient at intercepting the neutrophil-generated 1O
and to bind antibodies, we considered that these cells could be a biological source of O3. We report here further analytical evidence that antibody-coated neutrophils, after activation, produce an oxidant with the chemical signature of O3. This process is independent of surface antibody concentration down to 50% of the resting concentration, suggesting that surface IgG is highly efficient at intercepting the neutrophil-generated 1O . Vinylbenzoic acid, an orthogonal probe for ozone detection, is oxidized by activated neutrophils to 4-carboxybenzaldehyde in a manner analogous to that obtained for its oxidation by ozone in solution. This discovery of the production of such a powerful oxidant in a biological context raises questions about not only the capacity of O3 to kill invading microorganisms but also its role in amplification of the inflammatory response by signaling and gene activation.
. Vinylbenzoic acid, an orthogonal probe for ozone detection, is oxidized by activated neutrophils to 4-carboxybenzaldehyde in a manner analogous to that obtained for its oxidation by ozone in solution. This discovery of the production of such a powerful oxidant in a biological context raises questions about not only the capacity of O3 to kill invading microorganisms but also its role in amplification of the inflammatory response by signaling and gene activation.
Neutrophils (PMNs) are the most abundant leukocytes in the bloodstream. Their function is the killing of bacteria and fungi, in part by the triggering of an oxidative burst that is composed of a set of enzymatic and chemical reactions ultimately leading to the formation of hypohalous acid, 1O , and hydroxyl radical (HO•) (1, 2). The first step in this cascade, the reduction of dioxygen, is initiated by the enzyme NAD(P)H oxidase. This oxidase is a complex enzyme composed of five components: gp91phox (with phox being phagocyte oxidase), a heavily glycosylated 56-kDa protein that contains the electron-carrying components of the oxidase; p67phox, p47phox, and p22phox, which are proteins named according to their approximate molecular weights; and rac2, a low molecular weight GTPase. In the resting cell, p47phox and p67phox form a complex in the cytosol (which also contains p40phox, a protein whose effect on oxidase activity is unclear), whereas gp91phox and p22phox are in the membrane. When the PMN is activated by antibody-coated bacteria, p47phox is phosphorylated on particular serines and moves to the membrane to assemble the active oxidase, carrying with it its cargo of p67phox and the enigmatic p40phox. Rac 2, also necessary for oxidase activity, picks up a GTP and moves into the oxidase assembly. The NAD(P)H oxidase then produces superoxide anion (O
, and hydroxyl radical (HO•) (1, 2). The first step in this cascade, the reduction of dioxygen, is initiated by the enzyme NAD(P)H oxidase. This oxidase is a complex enzyme composed of five components: gp91phox (with phox being phagocyte oxidase), a heavily glycosylated 56-kDa protein that contains the electron-carrying components of the oxidase; p67phox, p47phox, and p22phox, which are proteins named according to their approximate molecular weights; and rac2, a low molecular weight GTPase. In the resting cell, p47phox and p67phox form a complex in the cytosol (which also contains p40phox, a protein whose effect on oxidase activity is unclear), whereas gp91phox and p22phox are in the membrane. When the PMN is activated by antibody-coated bacteria, p47phox is phosphorylated on particular serines and moves to the membrane to assemble the active oxidase, carrying with it its cargo of p67phox and the enigmatic p40phox. Rac 2, also necessary for oxidase activity, picks up a GTP and moves into the oxidase assembly. The NAD(P)H oxidase then produces superoxide anion (O ) (Eq. 1).
) (Eq. 1).
 |
1 |
The reactivity of O with cellular components is relatively low, although it is known to oxidize iron-sulfur clusters. Its function, it seems, is to give rise to a large variety of more powerful reactive oxygen species. The key reactions of O
with cellular components is relatively low, although it is known to oxidize iron-sulfur clusters. Its function, it seems, is to give rise to a large variety of more powerful reactive oxygen species. The key reactions of O within the phagosome are either protonation to its conjugate acid, the hydroperoxyl radical (HO
within the phagosome are either protonation to its conjugate acid, the hydroperoxyl radical (HO , pKa = 4.9), followed by a fast (kbi = 1.0 × 108 M−1⋅s−1) dismutation into H2O2 and 3O2 (3), or action as a substrate for superoxide dismutase-catalyzed bimolecular dismutation into H2O2 and 3O2 (refs. 4 and 5; Fig. 1). The H2O2 then is used to oxidize Cl− to hypochlorite (OCl−), a reaction catalyzed by myeloperoxidase. Hypochlorite itself is highly bactericidal but is also used to form chloramines, some of which are even more microbicidal than OCl− (e.g., NH2Cl). An alternative fate of H2O2 is conversion to a hydroxyl radical (HO•) via Fenton chemistry.
, pKa = 4.9), followed by a fast (kbi = 1.0 × 108 M−1⋅s−1) dismutation into H2O2 and 3O2 (3), or action as a substrate for superoxide dismutase-catalyzed bimolecular dismutation into H2O2 and 3O2 (refs. 4 and 5; Fig. 1). The H2O2 then is used to oxidize Cl− to hypochlorite (OCl−), a reaction catalyzed by myeloperoxidase. Hypochlorite itself is highly bactericidal but is also used to form chloramines, some of which are even more microbicidal than OCl− (e.g., NH2Cl). An alternative fate of H2O2 is conversion to a hydroxyl radical (HO•) via Fenton chemistry.
Figure 1.
Oxygen-dependent microbicidal action of PMNs with the new antibody-catalyzed water-oxidation pathway shown. MPO, myeloperoxidase.
Given that H2O2 and HOCl are present in such high concentrations within the phagosome, there exists the prospect for a chemical production of high levels of 1O (Eq. 2). Recent evidence points to such a reaction taking place as evidenced by detection of 1O
(Eq. 2). Recent evidence points to such a reaction taking place as evidenced by detection of 1O within PMNs (6, 7).
within PMNs (6, 7).
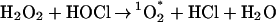 |
2 |
Recently we discovered that all antibodies can catalyze the formation of H2O2 from 1O and H2O (8) via the postulated intermediacy of dihydrogen trioxide (H2O3) (9). An oxidative component of the cascade of reactive intermediates generated during this process possesses the chemical signature of ozone (10). This oxidant is a component of the overall antibody-catalyzed cascade that is capable of effectively killing bacteria by a process that involves the generation of holes in the bacterial membrane.
and H2O (8) via the postulated intermediacy of dihydrogen trioxide (H2O3) (9). An oxidative component of the cascade of reactive intermediates generated during this process possesses the chemical signature of ozone (10). This oxidant is a component of the overall antibody-catalyzed cascade that is capable of effectively killing bacteria by a process that involves the generation of holes in the bacterial membrane.
With the prevailing evidence that PMNs generate 1O (6, 7) and that antibody molecules are present during PMN activation, the possibility arose that a component of the function of these cells may also be linked to ozone production. We recently showed preliminary experimental evidence to support this notion (10). We now report the effect of modifying both surface antibody concentration and the presence of catalase on the production of ozone by PMNs. Furthermore we add further weight to our original observation that a powerful oxidant with the chemical signature of ozone is generated by human PMNs with the use of a second ozone probe, vinylbenzoic acid.
(6, 7) and that antibody molecules are present during PMN activation, the possibility arose that a component of the function of these cells may also be linked to ozone production. We recently showed preliminary experimental evidence to support this notion (10). We now report the effect of modifying both surface antibody concentration and the presence of catalase on the production of ozone by PMNs. Furthermore we add further weight to our original observation that a powerful oxidant with the chemical signature of ozone is generated by human PMNs with the use of a second ozone probe, vinylbenzoic acid.
Materials and Methods
Whole antibodies 31154 (human IgG) and 31127 (horse IgG) were obtained from PharMingen. Bovine catalase was obtained from Sigma. All assays were carried out in PBS (10 mM phosphate/160 mM sodium chloride, pH 7.4). Commercial protein solution samples were dialyzed into PBS as necessary. Indigo carmine, isatin sulfonic acid, HOCl, H2O2, vinylbenzoic acid, and 4-carboxybenzaldehyde were obtained from Aldrich.
Human PMN Isolation.
PMNs were isolated from human peripheral blood according to a published procedure (11). In brief, a 6% dextran 70 solution was added to citrate-treated human whole blood. After inversion of the cell suspension and incubation at room temperature, total leukocytes were removed by a series of centrifugation steps followed by hypotonic lysis of residual erythrocytes. Cells were subjected to centrifugation in a Ficoll Hypaque density gradient (Amersham Pharmacia) to further purify PMNs. Prepared PMNs were resuspended in Hanks' balanced salt solution or PBS (pH 7.4).
Fluorescence-Activated Cell Sorter Analysis of Human PMNs and Other Cell Lines.
In a typical experiment, ≈105 low-level quantum simply cellular microbeads (Bangs Laboratories, Carmel, IN; low-level quantum simply cellular microbeads are typically appropriate for quantification of ≈1,000–80,000 surface-bound particles per cell) were diluted with PBS (pH 7.4) containing 1% FCS and 0.05% sodium azide. Saturating amounts of primary mouse (isotype IgG1) anti-human IgG (heavy and light chain-specific) FITC-conjugated antibodies (Jackson ImmunoResearch) were added, vortexed, and incubated for 1 h at 25°C. Isolated PMNs from human peripheral blood were incubated with the same FITC-conjugated antibody for 2 h at 0°C. Goat F(ab′)2 fragment and whole IgG, anti-human IgG FITC-conjugates were used to control for binding of the FITC-conjugated anti-human IgG H+L antibodies by the PMN's Fc receptor. Residual fluorescence associated with the cells and antibody-FITC conjugates were determined by using an FITC-conjugated mouse anti-goat F(ab′)2 Fab-specific mAb. Labeled microbeads and cells were washed three times with PBS/FCS/0.05% sodium azide to remove antibody bound nonspecifically. Samples were analyzed by a FACSCalibur flow cytometer (Becton Dickinson) equipped with CELLQUEST software. The PMN/lymphocyte gates were set according to cell size and forward-scatter properties.
Analytical Studies of Indigo Carmine Oxidation During PMN Activation.
Isolated human PMNs were diluted to 1.5 × 107 cells per ml in PBS containing 100 units/ml bovine catalase. PMNs were activated for 10 min at 37°C by the addition of a solution of phorbol 12-myristate 13-acetate (PMA, 1 μg/ml) in DMSO. A solution of the ozone probe, indigo carmine, in PBS (pH 7.4) was added to the activated cells to give a final concentration of 30 μM. At time intervals (3 min), aliquots (1 ml) were removed from the cell suspension and filtered through a 0.22-μm syringe. The indigo carmine content at each time point was determined by a spectrophotometric assay in a 96-well microtiter plate reading at 610 nm. Isatin sulfonic acid formation was quantified by HPLC (see below).
Modification of Surface Antibody Concentrations.
Total white cells were isolated and either washed in PBS (pH 7.4, three changes of buffer) with shaking at 37°C for 30 min or resuspended in 100 mM citrate buffer (pH 4.0) and incubated on ice for 5, 10, 20, or 30 min. Cells then were centrifuged and resuspended in PBS (pH 7.4) followed by Ficoll density-gradient centrifugation for purification of PMNs. Oxidant production of the PMNs was then monitored, and cells were analyzed by flow cytometry as detailed above to quantitate surface IgG.
Investigating the Effects of Catalase on Ozone Production by Human PMNs.
Cells were subjected to centrifugation in a Ficoll/Paque density gradient (Amersham Pharmacia) to purify PMNs. PMNs were washed and resuspended at 1.5 × 107 cells per ml in PBS (pH 7.4) containing PMA. Indigo carmine (30 μM) was subsequently added. Reactions were performed in the presence of 0 or 100 units/ml bovine catalase. Cell filtrates (0.2-μm filter) were collected at various time intervals during the reaction. Oxidation of indigo carmine was monitored at 610 nm and by HPLC for isatin sulfonic acid formation (see below). Cell suspensions contained no more than 0.2% DMSO as a cosolvent.
HPLC Assay for Detection of Isatin Sulfonic Acid.
Samples were analyzed on a reversed-phase C18 HPLC column eluting with 70:30 acetonitrile/H2O (0.1% trifluoroacetic acid) with an L 6000 Hitachi HPLC system (isatin sulfonic acid, RT = 9.4 min; indigo carmine, RT = 30.5 min). Peak areas were converted to concentrations by comparison to standard curves.
Activated PMN-Mediated Oxidation of Vinylbenzoic Acid.
Vinylbenzoic acid (0.1 mM) was incubated with PMNs (1.5 × 107 cells per ml), catalase (100 units/ml), and PMA (0.1 μg/ml) in PBS (0.2 ml). Reactions were carried out for 10 min at 37°C. The PMNs then were removed by filtration, and the supernatants were analyzed by HPLC analysis. The HPLC assay consisted of a reversed-phase C18 HPLC column using an isocratic elution of 80:20 acetonitrile/H2O (0.1% trifluoroacetic acid) with an L 6000 Hitachi HPLC system (vinyl benzoic acid, RT = 8.7 min; 4-carboxybenzaldehyde, RT = 5.7 min). Peak areas were converted to concentrations by comparison to standard curves.
Results and Discussion
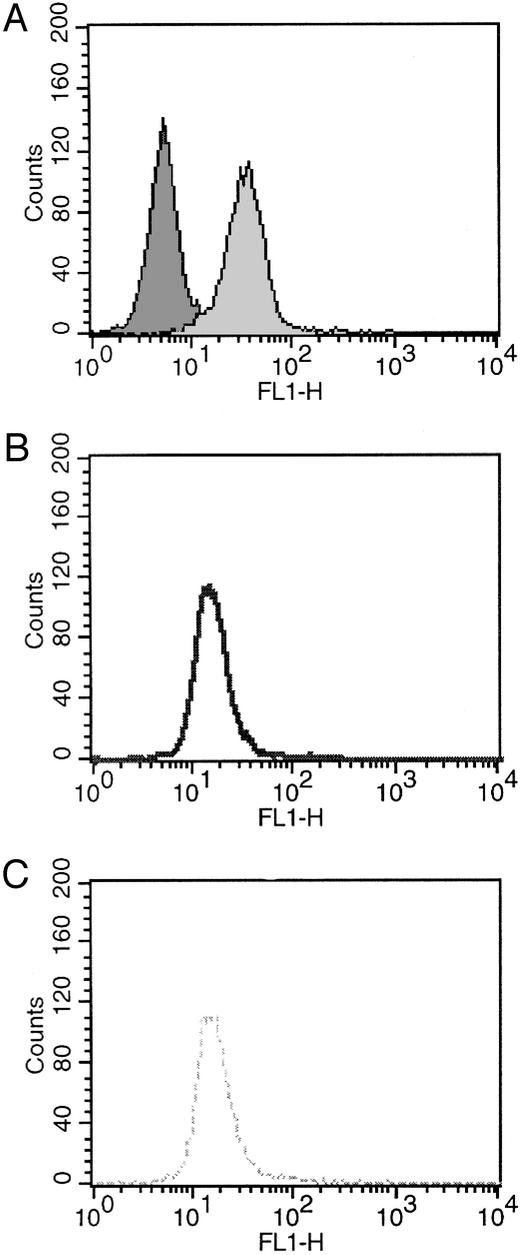
Our original fluorescence-activated cell sorter analysis, repeated in this study, reveals that human PMNs are coated with ≈5 × 104 antibody molecules per cell (n = 3) (10). To determine the potential of these bound antibodies to use the 1O generated by activated cells to produce ozone, we attempted to remove the surface IgG by various methods. The two most successful approaches thus far have involved acidification of the cells to pH 4.0 or warming the cells to 37°C before activation. These approaches removed ≈50% of the surface IgG (Fig. 2). Under these conditions, analytical experiments using indigo carmine as the chemical probe for ozone (refs. 12 and 13; Fig. 3) revealed no significant impairment of the ability of the PMNs to generate ozone (Fig. 4A).
generated by activated cells to produce ozone, we attempted to remove the surface IgG by various methods. The two most successful approaches thus far have involved acidification of the cells to pH 4.0 or warming the cells to 37°C before activation. These approaches removed ≈50% of the surface IgG (Fig. 2). Under these conditions, analytical experiments using indigo carmine as the chemical probe for ozone (refs. 12 and 13; Fig. 3) revealed no significant impairment of the ability of the PMNs to generate ozone (Fig. 4A).
Figure 2.
Fluorescence-activated cell sorter analysis of human PMNs. (A) Human PMNs ± secondary goat anti-human FITC-labeled antibody (FITC-labeled antibody, mean fluorescence 31.75 arbitrary units). (B) Human PMNs after acid treatment (pH 4.0) + secondary anti-human FITC-labeled antibody (mean fluorescence 14.61 arbitrary units). (C) Human PMNs after heating to 37°C + secondary anti-human FITC-labeled antibody (mean fluorescence 16.45 arbitrary units).
Figure 3.
Oxidation of indigo carmine 1 to isatin sulfonic acid 2.
Figure 4.
Oxidation of indigo carmine by activated human PMNs. (A) Effect of surface IgG concentration. ♦, unactivated PMNs; ▴, PMNs activated with PMA after acid treatment (pH 4.0); □, PMA-activated PMNs. (B) Effect of catalase on the time course of indigo carmine bleaching. ♦, unactivated PMNs; ■, PMNs activated with PMA and no catalase; ▴, PMNs activated with PMA and 100 units/ml catalase. (C) Effect of catalase on isatin sulfonic acid 2 (ISA) formation. ♦, PMA-activated PMNs and 100 units/ml catalase; □, PMA-activated PMNs and no catalase.
Thus, when a solution of indigo carmine is incubated with PMNs (1.5 × 107 cells per ml, coated with between 2 and 5 × 104 antibody molecules per cell, equivalent to an antibody concentration of ≈0.5–1 nM) that have been activated with PMA (10 μg/ml) in the presence of bovine catalase (100 units/ml), oxidation of indigo carmine occurs in equivalent amounts (Fig. 4A).
This observation hints that there may be an alternative chemical source of ozone within the PMN other than the antibody-catalyzed water-oxidation pathway, or it may be a result of a sufficient concentration of antibody catalyst still on the PMN surface reacting with a limiting amount of 1O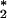 being generated.
being generated.
Catalase is used as an additive in these experiments to prolong the lifetime of any O3 generated in this system, because it is known that H2O2 catalyzes the decomposition of O3 via the peroxone process (14, 15). We now have studied the effect of removing catalase from the cell assay and showed that the oxidation of indigo carmine (Fig. 4B) and formation of isatin sulfonic acid (Fig. 4C) is reduced, especially between 6 and 12 min after the initial activation, supporting the notion that the O3 being generated by the antibody-coated PMNs is destroyed by H2O2.
It is important to reiterate at this juncture that indigo carmine, although a sensitive probe, is not selective. We have shown that 1O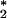 , HOCl, H2O3, and O3 but not H2O2 or superoxide anion bleach indigo carmine. A three-step analysis allows us to distinguish between the oxidants that do oxidize indigo carmine. However, we have sought additional probes that are more selective for ozone. This led us to the use of vinylbenzoic acid to trap ozone. As we have shown previously, conventional ozonolysis of vinylbenzoic acid (1 mM) in PBS (pH 7.4) at room temperature leads to the production of the 4-carboxybenzaldehyde with minor production of the 4-oxiranylbenzoic acid at a ratio of ≈10:1 (10). When human PMNs are activated with PMA (10 μg/ml), an oxidant is generated that oxidizes vinylbenzoic acid into 4-carboxybenzaldehyde in a yield of 5–7% over the 10-min activation time frame. This result offers strong evidence that O3 is indeed the oxidant that is generated during the oxidative burst of PMNs.
, HOCl, H2O3, and O3 but not H2O2 or superoxide anion bleach indigo carmine. A three-step analysis allows us to distinguish between the oxidants that do oxidize indigo carmine. However, we have sought additional probes that are more selective for ozone. This led us to the use of vinylbenzoic acid to trap ozone. As we have shown previously, conventional ozonolysis of vinylbenzoic acid (1 mM) in PBS (pH 7.4) at room temperature leads to the production of the 4-carboxybenzaldehyde with minor production of the 4-oxiranylbenzoic acid at a ratio of ≈10:1 (10). When human PMNs are activated with PMA (10 μg/ml), an oxidant is generated that oxidizes vinylbenzoic acid into 4-carboxybenzaldehyde in a yield of 5–7% over the 10-min activation time frame. This result offers strong evidence that O3 is indeed the oxidant that is generated during the oxidative burst of PMNs.
An analysis of the significance of the observation that ozone is generated in a cellular system has to begin with a consideration of the beneficial as well as the detrimental consequences associated with the production of this powerful chemical. Because ozone is highly reactive against double bonds, it could participate in the killing of invading microorganisms by disrupting their cellular membranes. Ozone also elicits the production of cytokines, including tumor necrosis factor-α and IL-8, thereby allowing amplification of the inflammatory cascade. In this sense it behaves in a fashion that is similar to the classic mediators of inflammation such as the complement cascade in that it has both effector and amplifier functions. Although inflammation is a necessary component of host defense, it of course is not without its detrimental consequences. Here the production of ozone could play a major role in a wide variety of pathologies ranging from arthritis to atherosclerosis. Unlike 1O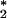 , ozone has a relatively long half-life and is known to react with H2O2 to generate even more toxic chemicals, thereby extending its potential to be harmful. Finally, by analogy to the way the organism deals with relatively long- and short-lived oxidants such as H2O2 and O
, ozone has a relatively long half-life and is known to react with H2O2 to generate even more toxic chemicals, thereby extending its potential to be harmful. Finally, by analogy to the way the organism deals with relatively long- and short-lived oxidants such as H2O2 and O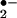 , both to minimize collateral damage and control signaling, it would seem reasonable that a similar enzyme exists to detoxify O3.
, both to minimize collateral damage and control signaling, it would seem reasonable that a similar enzyme exists to detoxify O3.
Acknowledgments
We thank Profs. Richard A. Lerner and Albert Eschenmoser for valuable discussions during manuscript preparation. This work was supported by The Skaggs Institute for Chemical Biology. C.T. is supported in part by National Institutes of Health Training Grant 5T32AI07606.
Abbreviations
- PMN
neutrophil
- phox
phagocyte oxidase
- PMA
phorbol 12-myristate 13-acetate
Footnotes
See commentary on page 3013.
References
- 1.Klebanoff S. Encyclopedia of Immunology. 1998;3:1713–1718. [Google Scholar]
- 2.Klebanoff S. Inflammation: Basic Principles and Clinical Correlates. Williams & Wilkins, Philadelphia: Lippincott; 1999. [Google Scholar]
- 3.Bielski B H J, Allen A O. J Phys Chem. 1977;81:1048–1050. [Google Scholar]
- 4.Hassan H M, Fridovich I. J Bacteriol. 1977;129:1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCord J M, Fridovich I. J Biol Chem. 1968;243:5753–5760. [PubMed] [Google Scholar]
- 6.Steinbeck M J, Khan A U, Karnovsky M J. J Biol Chem. 1992;267:13425–13433. [PubMed] [Google Scholar]
- 7.Steinbeck M J, Khan A U, Karnovsky M J. J Biol Chem. 1993;268:15649–15654. [PubMed] [Google Scholar]
- 8.Wentworth A D, Jones L H, Wentworth P J, Janda K D, Lerner R A. Proc Natl Acad Sci USA. 2000;97:10930–10935. doi: 10.1073/pnas.97.20.10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wentworth P, Jr, Jones L H, Wentworth A D, Zhu X, Larsen N A, Wilson I A, Xu X, Goddard W A, III, Janda K D, Eschenmoser A, Lerner R A. Science. 2001;293:1806–1809. doi: 10.1126/science.1062722. [DOI] [PubMed] [Google Scholar]
- 10.Wentworth P, Jr, McDunn J, Wentworth A D, Takeuchi C, Nieva J, Janda K D, Eschenmoser A, Lerner R A. Science. 2002;298:2195–2199. doi: 10.1126/science.1077642. [DOI] [PubMed] [Google Scholar]
- 11.Markert M, Andrews P C, Babior B M. Methods Enzymol. 1984;105:358–365. doi: 10.1016/s0076-6879(84)05048-5. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi K, Takeuchi I. Anal Chem. 1989;61:619–623. doi: 10.1021/ac00181a025. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi K, Kutsuna S, Ibusuki T. Anal Chim Acta. 1990;230:183–187. [Google Scholar]
- 14.Glaze W H, Kang J W, Chapin D H. Ozone Sci Eng. 1987;8:335. [Google Scholar]
- 15.Kuo C-H, Zhong L, Zappi M E, Hong A P. Can J Chem Eng. 1999;77:473–482. [Google Scholar]