Abstract
Excess lipid accumulation in non-adipose tissues is associated with insulin resistance, pancreatic β-cell apoptosis and heart failure. Here, we demonstrate in cultured cells that the relative toxicity of two common dietary long chain fatty acids is related to channeling of these lipids to distinct cellular metabolic fates. Oleic acid supplementation leads to triglyceride accumulation and is well tolerated, whereas excess palmitic acid is poorly incorporated into triglyceride and causes apoptosis. Unsaturated fatty acids rescue palmitate-induced apoptosis by channeling palmitate into triglyceride pools and away from pathways leading to apoptosis. Moreover, in the setting of impaired triglyceride synthesis, oleate induces lipotoxicity. Our findings support a model of cellular lipid metabolism in which unsaturated fatty acids serve a protective function against lipotoxicity though promotion of triglyceride accumulation.
The striking prevalence of obesity world-wide is a significant health problem due to serious medical complications that include hypertension, insulin resistance, diabetes, coronary artery disease, and heart failure (1). Evidence is emerging that elevated serum free fatty acid (FA) levels contribute to the pathogenesis of the metabolic syndrome and heart disease. Whereas adipocytes have a unique capacity to store excess FAs in the form of triglyceride in lipid droplets, non-adipose tissues such as cardiac myocytes and pancreatic β-cells have a limited capacity for storage of lipids. In hyperlipidemic states, accumulation of excess lipid in non-adipose tissues leads to cell dysfunction and/or cell death, a phenomenon known as lipotoxicity. Excess lipid accumulation in skeletal muscle is associated with the development of insulin resistance (2). Lipid overload in pancreatic β-cells leads to dysregulated insulin secretion (3, 4) and apoptotic cell death (5), both of which may contribute to the genesis of diabetic states. Lipoapoptosis is also observed in the heart, in which it leads to the development of heart failure (6, 7).
In a variety of experimental systems, saturated and unsaturated FAs differ significantly in their contributions to lipotoxicity. Previous studies in Chinese hamster ovary (CHO) cells (8), cardiac myocytes (9), pancreatic β-cells (10, 11), breast cancer cell lines (12), and hematopoietic precursor cell lines (13) all suggest that lipotoxicity from accumulation of long chain FAs is specific for saturated FAs. This selectivity has been attributed to the generation of specific proapoptotic lipid species or signaling molecules in response to saturated but not unsaturated FAs. The nature of such signals may differ across cell types, but includes reactive oxygen species generation (8), de novo ceramide synthesis (14), nitric oxide generation (15), decreases in phosphatidylinositol-3-kinase (12), and primary effects on mitochondrial structure or function (16). Long chain FAs may also suppress anti-apoptotic factors such as BclII (17). Cosupplementation with unsaturated FAs (9, 10, 12) has been shown to rescue saturated FA-induced lipoapoptosis by an unknown mechanism.
The present study was designed to characterize the fundamental cellular mechanisms though which the common saturated and unsaturated dietary lipids, palmitic and oleic acids, exert their differential effects on survival of cultured cells. We provide evidence that the differential toxicity of these FAs is directly related to their ability to promote triglyceride accumulation. We show that exogenous or endogenously generated unsaturated FAs rescue palmitate-induced apoptosis by promoting palmitate incorporation into triglyceride. Moreover, oleic acid, as well as palmitic acid, is toxic in cells with impaired triglyceride synthetic capacity. In vivo, triglyceride accumulation in non-adipose tissues occurs in the setting of mismatch between cellular lipid influx and lipid utilization. Our study suggests that triglyceride accumulation in non-adipose cells in response to acute lipid overload represents an initial cellular defense against lipotoxicity.
Methods
Materials.
Unlabeled lipids were obtained from Nu Chek Prep (Elysian, MN); radiolabeled lipids from Perkin–Elmer and American Radiolabeled Chemicals (St. Louis); Nile red and C-2938 from Molecular Probes; propidium iodide and solvents from Sigma–Aldrich; and cell culture reagents from Invitrogen.
Cell Culture.
CHO cells (American Type Culture Collection) and 25RA cells (gift from T. Y. Chang, Dartmouth College) were cultured as described (8). Primary mouse embryonic fibroblasts from wild-type and Dgat1 null mice (18) were isolated and cultured in knockout Dulbecco's modified Eagle's medium supplemented with 10% FBS, 1 mM l-glutamine, 50 units/ml penicillin G sodium, and 50 units/ml streptomycin sulfate. Where indicated, cell culture media were supplemented with FA (complexed to BSA at a 6.6:1 molar ratio; ref. 8).
Detection of Apoptotic Markers.
DNA laddering, caspase 3 activity, and reactive intermediate generation were detected as described (8). For DNA laddering, 10 μg of isolated genomic DNA was loaded for each sample on a 1.4% agarose gel.
Uptake and Accumulation of Palmitate.
Cells were supplemented with FA media for 6 h including 0.2 μCi (1 Ci = 37 GBq) of [14C]palmitate in each sample; washed with PBS containing 0.5 mM MgCl2, 0.92 mM CaCl2, 500 μM phloretin, and 0.1% BSA; and lysed with 1% SDS. [14C]Palmitate in the cell lysate was determined by scintillation counting and normalized for cell number. Results are reported as the average ± SE of six samples from two independent experiments.
Nile Red Staining.
Cells were supplemented with FA for 6 h, fixed with 3.7% paraformaldehyde, incubated for 5 min with 0.1 μg/ml Nile red in PBS, and examined by fluorescence microscopy (Zeiss Axiovert epifluorescence microscope or Bio-Rad MRC 1024 laser scanning confocal microscope).
Incorporation of [14C]Palmitate into Triglyceride.
Cells were supplemented with FA media and 0.2 μCi of [14C]palmitate for 6 h. Lipids were extracted including a recovery standard in each sample (0.02 μCi of [3H]cholesterol) and analyzed by thin layer chromatography by using hexane:ethyl ether:acetic acid (80:20:1) as described (19). Incorporation of [14C]palmitate into triglyceride or cholesteryl ester is normalized for lipid recovery and protein concentration.
Mass Spectrometry for Detection of Ceramide and Triglyceride.
Fatty acid-supplemented cells were homogenized in water, protein concentration was determined [bicinchoninic acid (BCA), Pierce], and lipids were extracted by a modified Bligh and Dyer technique (20, 21). Triheptadecenoin (5 mmol/mg protein) and C17 ceramide (2 nmol/mg protein) were used as internal standards for triglyceride and ceramide quantification, respectively. Total ceramide and triglyceride levels, as well as their molecular species, were determined by electrospray ionization mass spectrometry (21, 22).
Generation of Stearoyl-CoA Desaturase 1 (SCD1)-Overexpressing CHO Cells.
The murine SCD1 cDNA with a 3′myc-epitope tag (provided by J. Ntambi, University of Wisconsin, Madison) was subcloned into the ΔU3 retroviral vector (23) by PCR. ΔU3SCD1 retrovirus was generated and used to transduce CHO cells (23).
SCD Enzyme Activity Assays.
Microsomes were isolated and assayed for desaturase activity toward palmitic acid substrate (C16:0) as described (24). Reaction products were analyzed by thin layer chromatography and autoradiography and quantified by densitometry.
Propidium Iodide (PI) Staining.
After 46 h of supplementation with media alone or media containing 1 mM palmitate or 1 mM oleate, cells were collected, washed with PBS, and resuspended in media containing 1 μM PI and analyzed by flow cytometry.
Results
Oleate Prevents Palmitate-Induced Apoptosis.
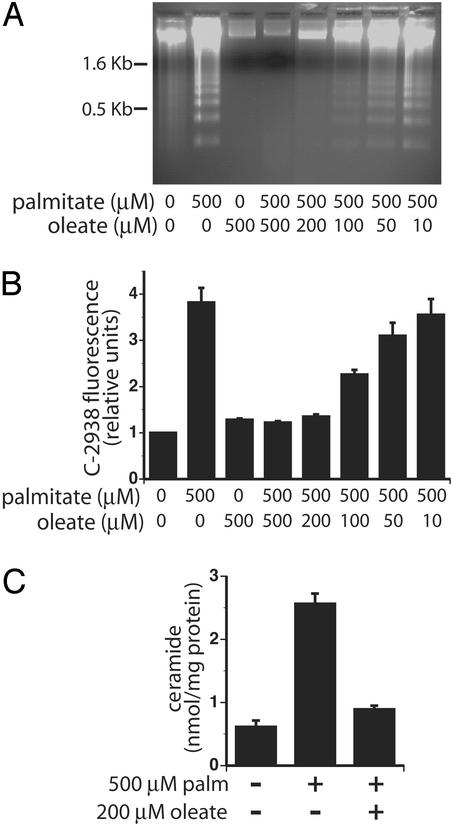
Previous studies have suggested that cosupplementation with the monounsaturated FA oleate inhibits the toxicity of the saturated FA palmitate in cardiomyocytes and pancreatic β-cells (9, 10). To extend these findings to a cell line that is easily cultured and genetically manipulated, we cultured CHO cells in media supplemented with 500 μM palmitate and increasing concentrations of oleate. In a dose-dependent manner, cosupplementation of CHO cells with oleate prevents the morphological changes associated with palmitate-induced cell death (data not shown). Moreover, cosupplementation with 200 μM oleate prevents palmitate-induced DNA laddering, a marker of apoptotic cell death (Fig. 1A). These experiments were performed by using a total FA:BSA ratio of 6.6:1, conditions that model pathophysiologic states in which unbound free FA concentrations are high (25, 26). In experiments designed to mimic normal physiologic states by using a FA:BSA ratio of 2:1 (low unbound free FA concentrations), we also observe palmitate-, but not oleate-, induced apoptosis and oleate rescue of palmitate-induced apoptosis (data not shown). These findings suggest that lipotoxic cell death is highly dependent on the molecular species of FA to which cells are exposed.
Figure 1.
Cosupplementation with oleate prevents palmitate-induced apoptosis. (A) DNA laddering was assessed in CHO cells after supplementation with the indicated FAs for 26 h. Each sample is representative of three independent experiments. (B) Cells were supplemented with the indicated concentrations of palmitate and oleate for 14 h followed by C-2938 loading. C-2938 fluorescence was determined by flow cytometry and is indicative of the oxidation of C-2938 by intracellular reactive intermediates. The bar graph displays median C-2938 fluorescence of 104 cells normalized to unsupplemented CHO cells. Each bar represents the average median fluorescence of three or four independent experiments ± SE. (C) Ceramide levels were determined by mass spectrometry after 6 h of FA supplementation. The bar graph displays mean ± SE ceramide content per mg of cellular protein, measured in three independent experiments.
Palmitate-induced apoptosis in CHO cells occurs though a mechanism involving increases in reactive intermediates (8). Palmitate is also a precursor for de novo ceramide synthesis, and palmitate supplementation of CHO cells increases ceramide levels though this pathway. Ceramide likely serves to amplify signals for palmitate-induced apoptosis; however, synthesis of ceramide is not essential for induction of programmed cell death (8). To determine whether oleate prevents palmitate-induced apoptosis before or after reactive intermediate and ceramide generation, we measured reactive intermediate and ceramide production after cosupplementation with palmitate and oleate. Cosupplementation with 200 μM oleate prevents palmitate-induced increases in both reactive intermediates (Fig. 1B) and ceramide (Fig. 1C). Thus, cosupplementation with oleate either prevents reactive intermediate generation and ceramide synthesis or results in scavenging of both these molecular species.
Notably, the protective effect of oleate cosupplementation is not due to a decrease in palmitate uptake in the presence of oleate. The accumulation of [14C]palmitate was determined after supplementation for 6 h with 500 μM palmitate or with 500 μM palmitate and 200 μM oleate. Total intracellular accumulation of [14C]palmitate does not significantly differ between cells supplemented with palmitate alone (0.683 ± 0.025 pmol of [14C]palmitate per 1,000 cells, n = 6) or cells supplemented with palmitate and oleate together (0.672 ± 0.056 pmol of [14C]palmitate per 1,000 cells, n = 6). A 6-h time point was chosen for these experiments, because it is the earliest point at which we detect morphological differences between palmitate- and oleate-supplemented cells, and it is before detection of markers of cell death, which could bias measurements in palmitate-treated cells.
Oleate Increases Neutral Lipid Storage.
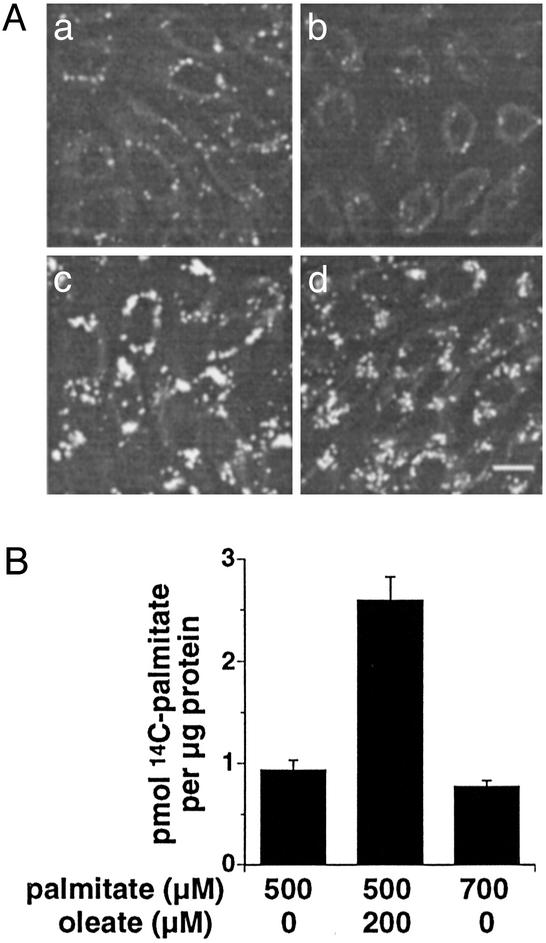
Because initial palmitate uptake and accumulation is not affected by cosupplementation with oleate, we sought to determine whether the metabolic fate of intracellular palmitate differs in the presence of oleate cosupplementation. We examined neutral lipid accumulation after 6 h of FA supplementation (a time point before detection of markers of cell death). Neutral lipid was detected by fluorescence microscopy of cells stained with Nile red, a hydrophobic dye that accumulates in lipid droplets. Neutral lipid accumulation is apparent after supplementation with oleate alone or oleate and palmitate together, but not with palmitate alone (Fig. 2A). Moreover, lipid accumulation is not due to increases in the total amount of supplemental FA, because 700 μM palmitate alone does not increase Nile red staining (data not shown). Thus, the resistance to lipoapoptosis observed with oleate cosupplementation correlates with increased capacity for accumulation of neutral lipid.
Figure 2.
Oleate supplementation promotes neutral lipid storage. (A) CHO cells were supplemented with media alone (Aa) or media containing 500 μM palmitate (Ab), 200 μM oleate (Ac), or 500 μM palmitate + 200 μM oleate (Ad) for 6 h. Cells were fixed with paraformaldehyde and stained with Nile red as a marker for neutral lipid. Confocal images are of a single section. Scale bar = 10 μm. (B) Incorporation of exogenous [14C]palmitate into triglyceride was measured 6 h after FA supplementation as indicated. Bar graph displays palmitate incorporation per μg of cellular protein. Data are the average ± SE for 6–12 samples from three independent experiments.
To test whether this increased neutral lipid accumulation reflects channeling of exogenous palmitate to triglyceride stores and away from the pathways leading to apoptosis, we performed metabolic labeling experiments. CHO cells were supplemented with palmitate alone or palmitate plus oleate for 6 h in the presence of 0.2 μCi of [14C]palmitate. After FA supplementation, total lipids were extracted and separated by thin layer chromatography, and radio-label incorporation into the triglyceride fraction was determined. Cosupplementation with oleate increases the incorporation of the 14C label into triglyceride ≈2.8-fold (Fig. 2B). The increase in [14C]palmitate incorporated into the triglyceride fraction is not due simply to the increased total lipid in the media, because supplementation with 700 μM palmitate does not lead to increased labeling (Fig. 2B). Whereas cosupplementation increases incorporation of [14C]palmitate into the triglyceride fraction, incorporation into cholesteryl ester is not significantly affected (0.078 ± 0.005 pmol of [14C]palmitate per μg of protein for palmitate-supplemented cells, n = 12; vs. 0.081 ± 0.008 pmol of [14C]palmitate per μg of protein for cosupplemented cells, n = 9). These results show that, in the presence of exogenous oleate, palmitate is channeled toward pathways leading to triglyceride accumulation.
Increased Desaturase Activity in 25RA and SCD Cells.
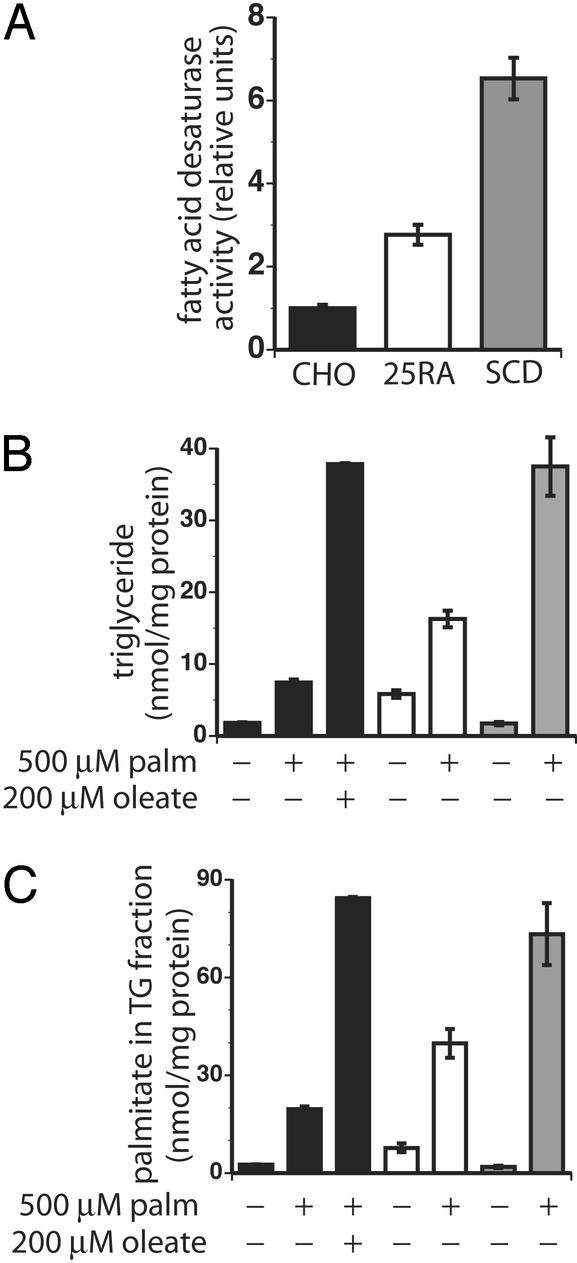
Previous studies of asebia mice, which harbor a mutation in the gene encoding SCD1, indicate that endogenously generated unsaturated FAs are critical for triglyceride synthesis in the liver (27, 28). To determine whether endogenously generated unsaturated FAs promote triglyceride synthesis in CHO cells, we examined two CHO cell-derived lines with increased FA desaturase activity. The 25RA cell line harbors an activating mutation in sterol regulatory element binding protein (SREBP) cleavage activating protein that results in constitutive activation of the SREBP pathway that promotes lipogenesis (29). SREBP up-regulates transcription of SCD1 (30), an enzyme that catalyzes Δ9-cis desaturation of palmitoyl- and stearoyl-CoA (31). We confirmed that 25RA cells have increased expression of SCD1 compared with wild-type CHO cells by Western blot analysis (data not shown). We also generated CHO cells that stably overexpress myc-tagged murine SCD1 (SCD cells) and confirmed expression of the myc-tagged SCD1 by Western blot analysis (data not shown). We compared FA desaturase activity in microsomes prepared from CHO, 25RA, and SCD-overexpressing cells, by using [14C]palmitate as a substrate. Whereas parental CHO cells have low levels of FA desaturase activity, 25RA and SCD cells have 3-fold and 6.5-fold increased activity, respectively (Fig. 3A).
Figure 3.
Increased FA desaturase activity correlates with triglyceride storage. Data are represented by filled bars are for CHO cells, open bars for 25RA cells, and shaded bars for SCD cells. Desaturase activity (A) of equivalent quantities of microsomal proteins from CHO, 25RA, and SCD-overexpressing cells was determined (mean ± SE, CHO n = 8, 25RA n = 8; SCD n = 10). Total triglyceride (nmol triglyceride per mg of protein, B) and accumulation of palmitate into triglyceride (nmol palmitate per mg of protein, C) were measured by mass spectrometry after 6 h of FA supplementation. Data displayed in bar graphs are the average of three separate experiments ± SE.
Desaturase Activity Correlates with Triglyceride Storage.
To determine whether increased FA desaturase activity promotes triglyceride synthesis, we compared neutral lipid accumulation in 25RA, SCD, and parental CHO cells. Nile red staining of palmitate-supplemented cells shows increased neutral lipid accumulation in 25RA and SCD cells (data not shown). Moreover, analysis of lipid extracts from palmitate-supplemented cells demonstrates that, compared with parental CHO cells, 25RA and SCD cells have 2.2- and 5-fold more triglyceride, respectively (Fig. 3B). This finding is similar to the 5.1-fold increase in cellular triglyceride levels observed when CHO cells are cosupplemented with palmitate and oleate. Incorporation of 16:0 acyl chains in the triglyceride pool is increased 2- and 3.7-fold in palmitate-supplemented 25RA and SCD cells, respectively, compared with palmitate-supplemented CHO cells (Fig. 3C). Importantly, the ability of CHO, 25RA, and SCD cells to channel palmitate to triglyceride pools correlates with the level of FA desaturase activity, suggesting that endogenously produced unsaturated FAs promote neutral lipid storage. Analysis of the molecular species in the triglyceride pool also suggests that 25RA and SCD cells have greater proportions of unsaturated acyl chains than parental CHO cells (Table 1). An increase in the percentage of unsaturated acyl chains is consistent with the increased FA desaturase activity in these cells.
Table 1.
Fatty acid species in triglyceride
| Cells | FA supplement | FA, nmol/mg protein
|
||||
|---|---|---|---|---|---|---|
| 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | ||
| CHO | None | 2.59 ± 0.04 | 0.33 ± 0.02 | 0.69 ± 0.03 | 1.59 ± 0.23 | 0.26 ± 0.08 |
| CHO | Palm | 19.56 ± 0.92 | 0.26 ± 0.04 | 1.02 ± 0.14 | 1.31 ± 0.14 | 0.17 ± 0.05 |
| CHO | Palm/oleate | 84.39 ± 0.36 | 3.74 ± 0.79 | 4.13 ± 0.74 | 20.77 ± 1.64 | 0.43 ± 0.18 |
| 25RA | None | 7.72 ± 1.34 | 0.81 ± 0.00 | 1.53 ± 0.11 | 6.87 ± 0.69 | 0.58 ± 0.16 |
| 25RA | Palm | 39.79 ± 4.44 | 1.94 ± 0.06 | 2.14 ± 0.10 | 4.74 ± 0.83 | 0.29 ± 0.04 |
| SCD | None | 1.88 ± 0.37 | 0.41 ± 0.02 | 0.30 ± 0.05 | 2.40 ± 0.29 | 0.20 ± 0.30 |
| SCD | Palm | 73.31 ± 9.50 | 13.10 ± 2.33 | 2.35 ± 0.41 | 22.75 ± 3.17 | 1.05 ± 0.24 |
CHO, 25RA, and SCD-overexpressing CHO cells (SCD) were incubated in media supplemented where indicated with 500 μM palmitate (palm) or 500 μM palmitate plus 200 μM oleate (palm/oleate) for 6 h. The amount of palmitate (16:0), palmitoleate (16:1), stearate (18:0), oleate (18:1), and linoleate (18:2) present in triglyceride pool is reported and represents the mean of three independent experiments ± SE.
The number of 16:0 acyl chains in the triglyceride fraction of 25RA and SCD cells suggests that much of the exogenous palmitate is being incorporated into triglyceride without being desaturated. To directly assess the molecular fate of exogenous palmitate in SCD cells, we supplemented CHO and SCD cells with deuterated palmitate for 6 h and analyzed cellular triglyceride composition by mass spectrometry (Table 2). In both CHO and SCD cells, exogenously provided deuterated FA is incorporated into triglyceride; however, the total amount of deuterated C16 or C18 FA in the triglyceride pool of SCD cells is increased 4.6-fold over the amount detected in CHO cells. In SCD cells, ≈25% (18.8 nmol/mg protein) of the deuterated label in triglyceride is associated with 16:1 or 18:1 unsaturated acyl chains, whereas CHO cells have only a small amount (1.10 nmol/mg protein) of deuterated C16:1 and C18:1 FAs. This increase is consistent with the increase in cellular desaturase activity in the SCD cells. However, the majority of the deuterated label (71%) in the triglyceride pool of SCD cells remains associated with palmitate (C16:0). These findings suggest that the presence of increased quantities of unsaturated FA in SCD cells facilitates channeling of exogenous saturated species to triglyceride stores and/or promotes its retention there.
Table 2.
Deuterated fatty acids in triglyceride fractions of SCD and CHO cells
| Cell type | Deuterated FA, nmol/mg protein
|
|||
|---|---|---|---|---|
| d16:0 | d16:1 | d18:0 | d18:1 | |
| CHO | 14.07 ± 1.66 | 0.52 ± 0.13 | 1.37 ± 0.07 | 0.58 ± 0.06 |
| SCD | 54.63 ± 2.63 | 7.79 ± 0.35 | 3.06 ± 0.31 | 11.01 ± 0.58 |
CHO and SCD-overexpressing cells (SCD) were incubated in media supplemented with 500 μM deuterated palmitate for 6 h. Incorporation of deuterated palmitate (d16:0), deuterated palmitoleate (d16:1), deuterated stearate (d18:0), and deuterated oleate (d18:1) in the triglyceride fraction is reported and represents the mean of three independent experiments ± SE.
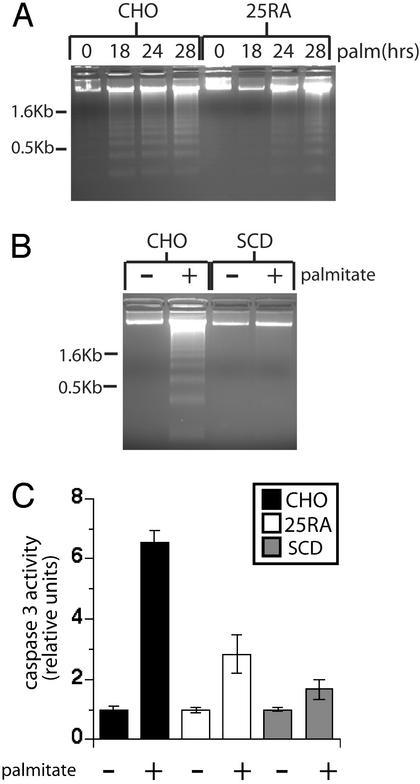
25RA and SCD Cells Are Palmitate-Resistant.
To determine whether increased triglyceride accumulation correlates with resistance to palmitate-induced cell death, we assessed the induction of apoptosis in 25RA cells. Morphological changes associated with cell death (data not shown) and DNA laddering (Fig. 4A) in response to palmitate are significantly reduced and delayed in 25RA cells, compared with wild-type CHO cells, and prevented in SCD cells (Fig. 4B). Caspase 3 activity, another marker of apoptosis, is also reduced in response to palmitate supplementation in both cell types (Fig. 4C). This resistance to palmitate-induced apoptosis in 25RA cells (partially resistant) and SCD-overexpressing cells (completely resistant) correlates with the level of triglyceride synthesis and extent of palmitate incorporation into triglyceride. The 25RA cells, which have intermediate levels of desaturase activity and triglyceride synthesis, display a more modest resistance to apoptosis than the SCD cells.
Figure 4.
25RA and SCD cells are resistant to palmitate-induced apoptosis. (A and B) DNA laddering was assessed in CHO and 25RA (A) or SCD (B) cells after supplementation with 500 μM palmitate (palm) for 0, 18, 24, and 28 h (A) or 28 h (B). The data are representative of three independent experiments. (C) Caspase 3 activity was measured in cell lysates collected after 24 h of FA supplementation. Data are expressed as the average fold increase over untreated cells ± SE. For each sample, n = 9 from three independent experiments.
Decreased Capacity for Triglyceride Synthesis Promotes Lipotoxicity.
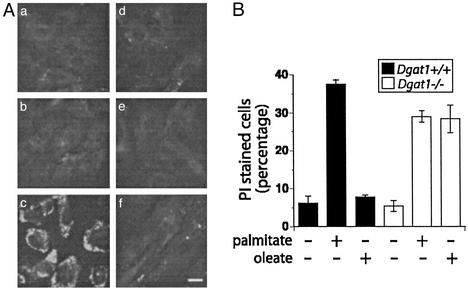
To test whether triglyceride synthesis is essential to prevent lipotoxicity, we evaluated the effects of FA supplementation on mouse embryonic fibroblasts derived from mice in which the gene for acyl CoA:diacylglycerol transferase 1 (DGAT1) is disrupted (18). DGAT catalyzes the final step in mammalian triglyceride synthesis, and Dgat1−/− mice show reduced levels of tissue triglycerides (32). Fibroblasts from wild-type mice demonstrate robust triglyceride synthesis in response to 6 h of oleate but not palmitate supplementation (Fig. 5A). By contrast, Dgat1−/− fibroblasts accumulate little triglyceride in response to either FA. To determine whether the inability to accumulate triglyceride affects lipotoxicity, we supplemented wild-type and Dgat1−/− fibroblasts with palmitate or oleate and assessed cell death by PI staining (Fig. 5B). Fibroblasts of both genotypes undergo cell death in response to palmitate supplementation. However, Dgat1−/− fibroblasts are also sensitive to oleate-induced cell death. Therefore, the failure to accumulate triglyceride in response to FA supplementation is associated with cell death. These results suggest that the ability to synthesize triglyceride plays a critical role in protection from lipotoxicity.
Figure 5.
Lipotoxicity in Dgat1−/− cells. (A) Wild-type (Aa–Ac) and Dgat1−/− (Ad–Af) mouse embryonic fibroblasts were incubated with media alone (Aa and Ad) or media supplemented with 1 mM palmitate (Ab and Ae) or 1 mM oleate (Ac and Af) for 12 h. Triglyceride accumulation was detected by Nile red staining. Bar = 20 μm. (B) Wild-type and Dgat1−/− fibroblasts were supplemented with 1 mM palmitate or 1 mM oleate for 46 h. Cell death was assessed by PI staining and flow cytometry. For each sample, fluorescence of 104 cells was assessed, and the percentage of cells with PI fluorescence was determined. The bar graph displays the median percentage of PI-positive cells from three independent measurements ± SE.
Discussion
Lipotoxicity has been implicated in the pathogenesis of clinically important human diseases. However, the cellular mechanisms that determine whether excess lipid accumulation is well tolerated or cytotoxic remain largely unknown. In the present study, we correlate the channeling of common dietary long chain FAs to distinct metabolic fates with their propensity for inducing apoptosis. Monounsaturated oleic acid is readily incorporated into triglyceride and is well tolerated by CHO cells. Alternatively, saturated palmitic acid does not lead to triglyceride accumulation and induces CHO cell apoptosis. Our studies show that the presence of unsaturated FAs, from endogenous or exogenous sources, promotes triglyceride accumulation with channeling of excess saturated FA to triglyceride stores and consequent rescue of palmitate-induced apoptosis. We demonstrate that impaired triglyceride synthesis in cells from Dgat1 null mice leads to lipotoxicity from oleate as well as palmitate. This is direct evidence that accumulation of excess FA in cellular triglyceride stores may be protective against lipotoxicity.
Triglyceride accumulation in response to increased cellular levels of unsaturated FAs may be a general metabolic phenomenon. Exogenous oleic acid supplied in the media leads to triglyceride synthesis in CHO cells and mouse embryonic fibroblasts (the present study), cardiac myocytes (9), pancreatic β-cells (11), and human skin fibroblasts (33). Additionally, endogenously generated unsaturated FAs play an important role in hepatic triglyceride accumulation, as demonstrated by lack of hepatic triglyceride accumulation in SCD1-deficient mice on a lipogenic diet (27, 28) and by absence of hepatic steatosis in ob/ob mice that also have mutations in SCD1 (34). Our studies show that increases in SCD1 activity alone are sufficient to increase triglyceride accumulation in CHO cells supplemented with exogenous palmitic acid. Moreover, we extend previous observations to show that increased triglyceride synthesis associated with SCD1 overexpression can protect against lipoapoptosis.
There are several mechanisms through which unsaturated FAs may promote triglyceride accumulation. Unsaturated FAs can serve as ligands for transcription factors such as peroxisome proliferator-activated receptor γ (35). However, the increased triglyceride accumulation we observe during the 6-h incubations with oleate or oleate plus palmitate is not associated with increased expression of enzymes for triglyceride synthesis, nor do we observe increases in expression of proteins known to be induced during adipogenesis (unpublished data). Whereas it is possible that unsaturated FAs activate signaling pathways that promote triglyceride storage (or inhibit triglyceride hydrolysis), we find that inhibitors of protein kinase C or phosphatidylinositol-3-kinase have no effect on unsaturated FA-induced triglyceride storage in CHO cells (unpublished data). Alternatively, it is likely that increased triglyceride accumulation in CHO cells in response to oleate supplementation may reflect a preference of some enzymes in triglyceride synthetic pathways for unsaturated FA substrates (36) or increased solubility/stability of lipid droplets containing a higher percentage of unsaturated acyl chains.
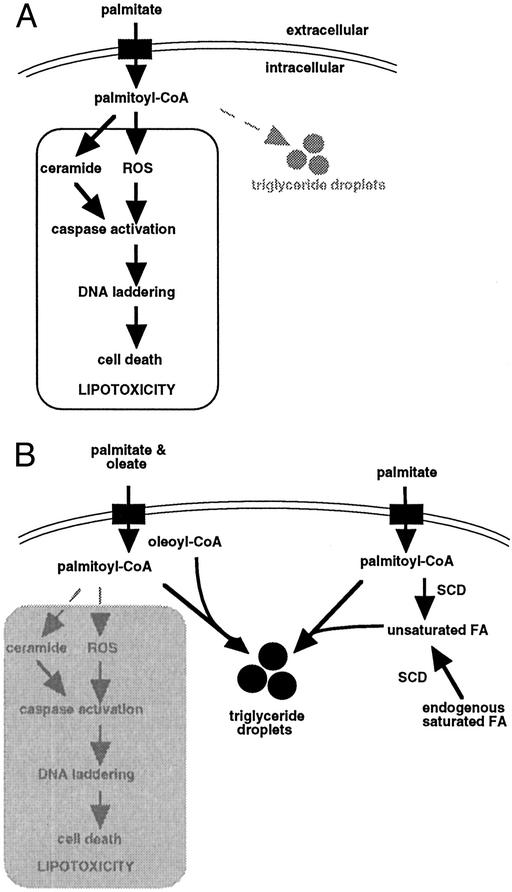
Several of our findings suggest that, when cultured cells are exposed to high concentrations of exogenous palmitic acid for up to 28 h, triglyceride synthesis prevents lipotoxicity. First, the magnitude of triglyceride accumulation correlates with cell survival. Second, we observe a correlation between cell survival and sequestration of palmitate in the triglyceride fraction. We hypothesize that palmitate channeled toward triglyceride storage is unavailable for pathways leading to cell death, such as the generation of reactive intermediates and ceramide (Fig. 6). In this scenario, triglyceride is inert with respect to induction of apoptosis, whereas other FA metabolites or free FAs themselves initiate apoptotic signaling pathways. Third, whereas previous studies have demonstrated that FA-induced apoptosis is specific for saturated FAs, we demonstrate that unsaturated FAs are also toxic when triglyceride synthesis is impaired. Dgat1−/− fibroblasts, which are unable to increase triglyceride storage in response to unsaturated FAs, show toxicity to oleate as well as palmitate.
Figure 6.
Mechanism for preventing lipotoxicity though triglyceride storage. (A) The long chain saturated FA palmitate induces apoptosis in CHO cells though a mechanism involving reactive intermediate (ROS) and ceramide generation. In the absence of additional signals, palmitate is poorly incorporated into cellular triglyceride pools. (B) The presence of unsaturated FAs promotes channeling of palmitate toward triglyceride storage, thus sequestering palmitate away from pathways leading to cell death. Unsaturated FAs provided as media supplements (though cosupplementation with oleate) or generated though the action of cellular desaturase enzymes (e.g., SCD) show this effect.
Accumulation of triglyceride in non-adipose tissues likely serves as a barometer of the lipid overload state in human disorders such as hyperlipidemia and lipodystrophies and in animal models such as the Zucker diabetic fatty rat or the ob/ob mouse. However, our experiments suggest that cellular triglyceride accumulation itself is not initially toxic. Rather, accumulation of excess FAs in triglyceride pools likely diverts these molecules from pathways that lead to cytotoxicity and may thus serve as a buffer against lipotoxicity. In pathologic states, lipotoxicity may occur over time, despite triglyceride accumulation, when either the cellular capacity for triglyceride storage is exceeded or when triglyceride pools are hydrolyzed, resulting in increased cellular free FA levels. Thus, the duration and extent of lipid overload may determine whether a cell is protected or damaged. Further characterization of cellular signals that channel FAs to triglyceride pools may identify additional mechanisms that prevent lipotoxicity. Future studies using animal models of lipid overload states will be useful to determine whether triglyceride accumulation in non-adipose tissues prevents lipotoxicity in vivo.
Acknowledgments
We thank R. O. L. Wong for providing access to the confocal microscopy resources in the Bakewell NeuroImaging Laboratory, and the Washington University Mass Spectrometer Facility Center for allowing use of the electrospray ionization mass spectrometer. This work was supported by grants from the American Heart Association (EIA 0040040N), the National Institutes of Health (T32 HL07275, P01HL57278), and the Washington University Pharmacia Biomedical Research Program.
Abbreviations
- CHO
Chinese hamster ovary
- SCD
stearoyl-CoA desaturase
- DGAT1
acyl CoA:diacylglycerol transferase 1
- FA
fatty acid
- PI
propidium iodide
References
- 1.Kopelman P G. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 2.Shulman G I. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y P, Grill V. J Clin Endocrinol Metab. 1995;80:1584–1590. doi: 10.1210/jcem.80.5.7745004. [DOI] [PubMed] [Google Scholar]
- 4.Prentki M, Vischer S, Glennon M C, Regazzi R, Deeney J T, Corkey B E. J Biol Chem. 1992;267:5802–5810. [PubMed] [Google Scholar]
- 5.Shimabukuro M, Zhou Y T, Levi M, Unger R H. Proc Natl Acad Sci USA. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu H C, Kovacs A, Ford D A, Hsu F F, Garcia R, Herrero P, Saffitz J E, Schaffer J E. J Clin Invest. 2000;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y-T, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger R H. Proc Natl Acad Sci USA. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Listenberger L L, Ory D S, Schaffer J E. J Biol Chem. 2001;276:14890–14895. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 9.deVries J E, Vork M M, Roemen T H M, deJong Y F, Cleutjens J P M, van der Vusse G J, van Bilsen M. J Lipid Res. 1997;38:1384–1394. [PubMed] [Google Scholar]
- 10.Maedler K, Spinas G A, Dyntar D, Moritz W, Kaiser N, Donath M Y. Diabetes. 2001;50:69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 11.Cnop M, Hannaert J C, Hoorens A, Eizirik D L, Pipeleers D G. Diabetes. 2001;50:1771–1777. doi: 10.2337/diabetes.50.8.1771. [DOI] [PubMed] [Google Scholar]
- 12.Hardy S, Langelier Y, Prentki M. Cancer Res. 2000;60:6353–6358. [PubMed] [Google Scholar]
- 13.Paumen M B, Ishida Y, Muramatsu M, Yamamoto M, Honjo T. J Biol Chem. 1997;272:3324–3329. doi: 10.1074/jbc.272.6.3324. [DOI] [PubMed] [Google Scholar]
- 14.Shimabukuro M, Higa M, Zhou Y T, Wang M Y, Newgard C B, Unger R H. J Biol Chem. 1998;273:32487–32490. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- 15.Shimabukuro M, Ohneda M, Lee Y, Unger R H. J Clin Invest. 1997;100:290–295. doi: 10.1172/JCI119534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrander D B, Sparagna G C, Amoscato A A, McMillin J B, Dowhan W. J Biol Chem. 2001;276:38061–38067. doi: 10.1074/jbc.M107067200. [DOI] [PubMed] [Google Scholar]
- 17.Shimabukuro M, Wang M Y, Zhou Y T, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1998;95:9558–9561. doi: 10.1073/pnas.95.16.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith S J, Cases S, Jensen D R, Chen H C, Sande E, Tow B, Sanan D A, Raber J, Eckel R H, Farese Jr R V. Nat Genet. 2000;25:87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- 19.Millard E E, Srivastava K, Traub L M, Schaffer J E, Ory D S. J Biol Chem. 2000;275:38445–38451. doi: 10.1074/jbc.M003180200. [DOI] [PubMed] [Google Scholar]
- 20.Bligh E G, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 21.Han X, Gross R W. Anal Biochem. 2001;295:88–100. doi: 10.1006/abio.2001.5178. [DOI] [PubMed] [Google Scholar]
- 22.Han X. Anal Biochem. 2002;302:199–212. doi: 10.1006/abio.2001.5536. [DOI] [PubMed] [Google Scholar]
- 23.Ory D S, Neugeborn B A, Mulligan R C. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obukowics M G, Welsch D J, Salsgiver W J, Martin-Berger C L, Chinn K S, Duffin K L, Raz A, Needleman P. J Pharmacol Exp Ther. 1998;287:157–166. [PubMed] [Google Scholar]
- 25.Kurien V A, Oliver M F. Lancet. 1966;2:122–127. doi: 10.1016/s0140-6736(66)92420-2. [DOI] [PubMed] [Google Scholar]
- 26.Kleinfeld A M, Protho D, Brown D L, Davis R C, Richieri G V, DeMaria A. Am J Cardiol. 1996;78:1350–1354. doi: 10.1016/s0002-9149(96)00651-0. [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki M, Kim Y C, Gray-Keller M P, Attie A D, Ntambi J M. J Biol Chem. 2000;275:30132–30138. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki M, Kim Y C, Ntambi J M. J Lipid Res. 2001;42:1018–1024. [PubMed] [Google Scholar]
- 29.Hua X, Nohturfft A, Goldstein J L, Brown M S. Cell. 1996;87:415–426. doi: 10.1016/s0092-8674(00)81362-8. [DOI] [PubMed] [Google Scholar]
- 30.Shimano H, Horton J D, Hammer R E, Shimomura I, Brown M S, Goldstein J L. J Clin Invest. 1996;98:1575–1584. doi: 10.1172/JCI118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enoch H G, Catala A, Strittmatter P. J Biol Chem. 1976;251:5095–5103. [PubMed] [Google Scholar]
- 32.Chen H C, Smith S J, Ladha Z, Jensen D R, Ferreira L D, Pulawa L K, McGuire J G, Pitas R E, Eckel R H, Farese R V. J Clin Invest. 2002;109:1049–1055. doi: 10.1172/JCI14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenthal M D. Lipids. 1981;16:173–182. doi: 10.1007/BF02535435. [DOI] [PubMed] [Google Scholar]
- 34.Cohen P, Miyazaki M, Socci N D, Hagge-Greenberg A, Liedtke W, Soukas A A, Sharma R, Hudgins L C, Ntambi J M, Friedman J M. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 35.Kliewer S A, Sundseth S S, Jones S A, Brown P J, Wisely G B, Koble C S, Devchand P, Wahli W, Wilson T M, Lenhard J M, Lehmann J M. Proc Natl Acad Sci USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell R M, Coleman R A. Annu Rev Biochem. 1980;1980:459–487. doi: 10.1146/annurev.bi.49.070180.002331. [DOI] [PubMed] [Google Scholar]