Abstract
The hydroxylamine derivative bimoclomol (BM) has been shown to activate natural cytoprotective homeostatic responses by enhancing the capability of cells to cope with various pathophysiological conditions. It exerts its effect in synergy with low levels of stress to induce the synthesis of members of major stress protein families. We show here that the presence of BM does not influence protein denaturation in the cells. BM and its derivatives selectively interact with acidic lipids and modulate their thermal and dynamic properties. BM acts as a membrane fluidizer at normal temperature, but it is a highly efficient membrane stabilizer, inhibiting the bilayer–nonbilayer phase transitions during severe heat shock. We suggest that BM and the related compounds modify those domains of membrane lipids where the thermally or chemically induced perturbation of lipid phase is sensed and transduced into a cellular signal, leading to enhanced activation of heat shock genes. BM may be a prototype for clinically safe membrane-interacting drug candidates that rebalance the level and composition of heat shock proteins.
A large number of studies now provide convincing evidence that the increase in the cellular levels of cytoprotective stress (or heat shock) proteins (HSPs) is a powerful means to decrease or neutralize the deleterious effects of acute or chronic stresses. Genetic and biochemical evidence suggests a physiological role of HSPs in a number of human diseases. Thus, identification of nontoxic agents that allow pharmacological manipulation of HSP expression, together with evaluation of their efficacy and safety, is a stimulating challenge with prospects for clinical applications.
Whereas it is well established that enhanced heat shock gene expression in response to various stimuli is regulated by heat shock factors (HSFs), essential elements of the perception of the stress and the signal cascade leading to the elevated formation of HSPs are still unknown. According to the classical model, the common primary signal is an increase in the amount of denatured proteins within the cell during stress and their competition with HSFs, forming a regulatory loop (1). However, overexpression of SSA2, a member of the HSP70 family and a prominent candidate for the feedback regulation of HSFs, does not inhibit the heat shock response in yeast (2). Thus, the concept that a general relief of the suppression of HSF activity by HSPs alone can account for the acute heat shock response is not completely consistent with the available data. Furthermore, HSPs are present in abnormal levels in a variety of human diseases (and during aging), but there is no evidence for concomitant modification of the kinetics of accumulation of denatured protein that could justify the changes observed in the expression of HSPs (see reviews in refs. 3 and 4). A number of observations indicate that HSP expression in response to various stressors is regulated by differential control mechanisms, rather than by uniform mechanisms (5). On the basis of earlier experimental evidence, we proposed that specific membrane domains may act as such alternative sensors, or “cellular thermometers,” where stress-induced membrane perturbations are converted into signal(s) leading to activation of heat shock genes (3, 4, 6, 7). Changes in membrane composition and/or fluidity in bacteria, yeast, and mammalian cells alter the set point for HSP expression, with expression initiated at lower temperatures in cells with more fluid membranes (7–9). We also suggested that a lipid-selective association of a subpopulation of HSPs with membranes, leading to increased molecular order, may in turn lead to down-regulation of the heat shock gene expression (10–12).
A beneficial effect of HSP induction has been suggested by the well-documented cytoprotective activity of a hydroxylamine derivative, bimoclomol (N-[2-hydroxy-3-(1-piperidinyl)propoxy]-3-pyridine carboximidoylchloride maleate; BM) (13), and other related compounds. It has been shown that these nontoxic compounds are coinducers of HSPs, i.e., they cannot amplify the HSP gene expression without a concomitant stress, but the stress-induced increase in HSP levels is further elevated by their presence. The precise mechanism of HSP coinduction by these new drugs is still unknown, but they have been documented not to interact with a number of known conventional pharmacological targets. Coinducing effects of BM on HSP expression were shown to be mediated by means of HSF-1, because its effect was abolished in mouse cells lacking HSF-1. In addition, BM enhanced the nuclear transport of HSF1 and prolonged HSF DNA binding (J.H., H. Lewis, I. Boros, T. Rácz, A. Fiser, I. Kurucz, I. Benjamin, L.V., Z.P., P.C., and D. S. Latchman, unpublished data).
In this study, we provide evidence that the presence of BM does not affect protein denaturation in the cells. The drug, however, specifically interacts with and significantly increases the fluidity of negatively charged membrane lipids. On the other hand, BM is an efficient inhibitor of bilayer–nonbilayer lipid phase transitions. To our knowledge, this is the first evidence that nontoxic compounds possess the capability of HSP coinduction in the absence of the production of unfolded proteins. We suggest that BM and the related compounds modify those domains of membrane-lipid phase where the thermally or chemically induced perturbation of lipid phase is sensed and transduced into a cellular signal, leading to enhanced activation of heat shock genes.
Materials and Methods
Materials.
Synthetic dielaidoyl phosphatidylethanolamine (DEPE), dimyristoyl phosphatidylcholine (DMPC), dimyristoyl phosphatidylserine (DMPS), and bovine heart cardiolipin (BHCL) were purchased from Avanti Polar Lipids; dioleoyl phosphatidylglycerol (DOPG), dioleoyl phosphatidylethanolamine (DOPE), dipalmitoyl phospatidylcholine (DPPC), bovine brain phosphatidylserine (BBPS), and bovine brain sphingomyelin (BBSM) were purchased from Sigma and used without further purification. BM, BRX-345, and BRX-1237 were synthesized at Biorex R & D (Veszprém, Hungary).
Sample Preparation.
Liposomes were prepared by drying aliquots of the lipid under a stream of nitrogen gas, followed by at least 2 h on a lyophilizer. Lipids were dispersed in 10 mM N-[tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid (Tes), pH 7.2, and heated to a temperature above the phase transition (40, 45, and 20°C for DEPE, DMPC/DMPS, and BHCL, respectively) followed by shaking. Multilamellar vesicles with a lipid concentration of 100 or 30 mg/ml were used for the differential scanning calorimetry (DSC) and Fourier transform infrared (FTIR) studies, respectively. HSP coinducers were incubated with the liposomes above the temperature of the phase transition for 30 min before the measurements.
HSP70 Coinducing Effect of BRX Compounds.
HSP70 content was measured by the competitive enzyme immunoassay developed at Biorex by using an affinity-purified HSP70-specific polyclonal antibody (14). Protein content of the cell extracts was measured by the Bradford method (15).
Heat Inactivation Studies.
Luciferase tests in HeLa cells transfected with plasmid pRSVLL/V (kindly provided by S. Subrami, University of California at San Diego, La Jolla) encoding a cytoplasmic firefly luciferase and testing the heat-induced insolubilization of HSC70 were conducted as in ref. 16.
Monolayer Studies.
Monolayer experiments at constant surface area were carried out as described (10) by using a subphase of 10 mM sodium phosphate (pH 7.0). Experiments at constant surface pressure were done in a thermostated Teflon trough. The surface area and the subphase temperature were kept constant until the surface pressure reached an equilibrium, after which the subphase temperature was increased by 0.7°C/min. Meanwhile, the surface pressure was kept constant (25 mN/m) by changing the monolayer area.
10-N-Nonylacridine Orange (NAO) Binding Assay to Assess BM–Cardiolipin Interaction.
Mitochondria were isolated as in ref. 17 and incubated at 1 mg of protein per ml concentration in 0.03 M Mops (pH 7.4) containing 0.25 M sucrose and 0.02 mM EDTA with 100 μM [14C]BM (419 MBq/mmol for 30 min at 25°C). An increasing concentration of NAO was added to the samples and the mitochondria were then centrifuged. Radioactivity was measured and the cardiolipin-bound BM was quantified.
DSC.
DSC measurements were carried out on a high-sensitivity differential scanning calorimeter from Calorimetry Sciences (Provo, UT) purged with nitrogen gas at a scanning rate of 12°C/h.
FTIR Spectroscopy.
Spectra were obtained with a Perkin–Elmer 2000 infrared spectrometer (Norwalk, CT) essentially as in ref. 12.
Results
HSP Coinducing Effect of BM and Its Structural Analogs.
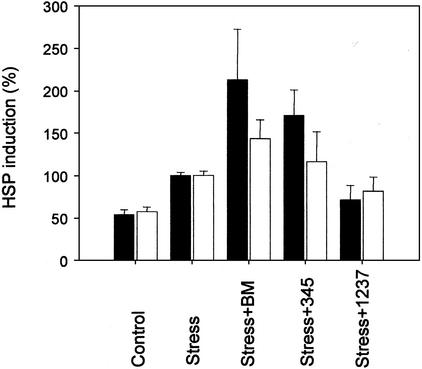
BM and two other nontoxic hydroxylamine-based compounds, BRX-345 and BRX-1237, were examined for their HSP70 coinducing effect on NIH 3T3 cells. Heat shock significantly elevated HSP70 formation when tested after a 5-h recovery at 37°C (Fig. 1), whereas treatment with any of these compounds had no measurable effect (data not shown).
Figure 1.
Effect of BM and its structural analogues on the stress-induced HSP70 level. HSP70 formation in heat-treated (42°C, 10 min) NIH 3T3 (filled bars) or serum-deprived (2% FCS for 20 h) H9c2 (open bars) cells, was followed by ELISA analysis. Reported data are averages of three measurements. Error bars, ±SD.
HSP coinducing activity is well demonstrated for BM and, to a lesser extent, for its structural analog, BRX-345. It is noted that, whereas both of these hydroxylamine derivatives act synergistically with sublethal heat shock when added 30 min before the thermal stress (Fig. 1), such an effect on HSP70 levels is not observed on the addition of BRX-1237, a structurally distantly related third compound. As is well established, serum (growth factor) deprivation also induces stress response. Both BM and (to a lesser extent) BRX-345 enhance the expression of HSP70 when tested on partially serum-deprived H9c2 cells. Under the same conditions, the additive effect on potentiation of the expression of HSP70 is not measurable for the distantly related BRX-1237.
Effect of BM on Heat Denaturation of Intracellular Proteins.
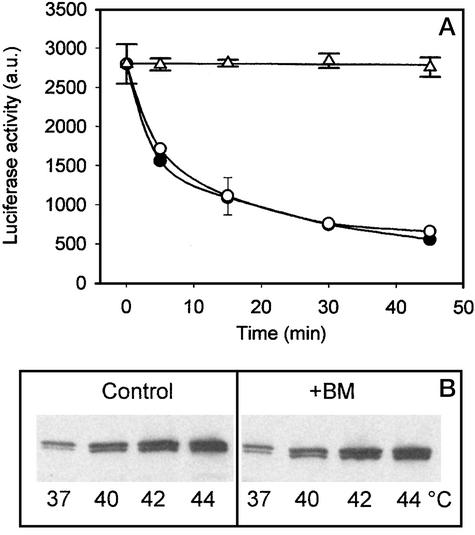
Firefly luciferase is inactivated by a mild heat shock when it is expressed in mammalian cells. The loss of enzymatic activity correlates with the loss of solubility and can be taken as direct evidence for protein denaturation (16). We used HeLa cells expressing cytoplasmic firefly luciferase as a tool for examining whether BM affects protein denaturation and exerts its HSP coinducing effect by generating unfolded proteins. The presence of BM (10−5M) had no effect on luciferase activity when tested on cells at their growth temperature (Fig. 2A). We observed an enhancement of protein denaturation in cells exposed to 42°C (Fig. 2A). In the presence of BM, luciferase activity declined at a precisely identical rate. The heat-enhanced aggregation of the 70-kDa constitutive HSP (HSC70) is an additional sensitive indicator of protein denaturation (16). On incubation of HeLa cells at 40, 42, or 44°C for 30 min, a considerable increase in the amount of HSC70 was observed in the pellet fraction compared with that of control cells (37°C, 0 mM BM; Fig. 2B). There was no significant difference in the amount of insoluble HSC70, however, when cells were simultaneously treated with 10−5 M BM. Similar results were obtained with NIH 3T3 cells (data not shown). Based on these results, we conclude that it is unlikely that the generation of an extra amount of unfolded proteins resulting from the combination of heat treatment and the presence of BM are the underlying mechanisms of the HSP coinduction phenomenon.
Figure 2.
BM exerts no effect on protein denaturation in HeLa cells. (A) Heat-induced (42°C, 30 min) inactivation of heterogeneously expressed cytoplasmic firefly luciferase was followed in the absence (●) or in the presence (○) of 10−5 M BM by luminometry. ▵, the effect of BM at 37°C. (B) Heat-induced (40, 42, or 44°C, 30 min) insolubilization of HSC70 in control and BM-treated (10−5 M) cells (Western blots).
BM Interacts Specifically with Negatively Charged Monomolecular Lipid Layers.
In addition to unfolding proteins, heat shock increases the fluidity of membranes and remodels their microdomain organization. It has been proposed that the physical state of the membrane-lipid matrix is directly involved in the perception of temperature stress (3, 4). We speculate, therefore, that the signal activating elevated heat shock response on the addition of BM and related compounds may be generated as a consequence of a specific drug–lipid interaction at sublethal heat and, if so, may be attributable to the heat-analogous hyperfluidization of certain membrane domains.
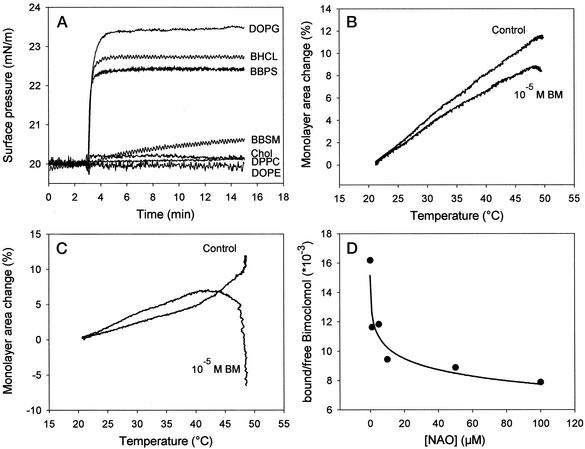
To test our hypothesis, the monolayer technique, an effective tool for studying membrane/drug interactions (18), was used at a constant temperature and surface area. Monolayers were formed from cholesterol, from zwitterionic lipids such as BBSM, DOPE, and DPPC, or from acidic lipids such as DOPG, BHCL, and BBPS. BM was added in a physiologically relevant concentration (10−5 M) to the subphase. We obtained a prominent difference in the surface-pressure profile of different lipid monolayers, representing the major lipid classes of mammalian cell membranes. As shown in Fig. 3A, by using monolayers of negatively charged lipids, the surface-pressure profile displayed typical insertion kinetics, reflected an increase of pressure for ≈2 min after an equilibrium level was reached. The steady-state levels followed a slightly different, but distinct, order with the acidic lipids, i.e., DOPG > BHCL > BBPS, suggesting electrostatic interactions between the drug and the membranes (Fig. 3A). In contrast, BM caused only a small or no change in the surface pressure in monolayers composed of cholesterol or zwitterionic lipids. We also monitored temperature-dependent changes of monolayer surface areas at constant surface pressure in the presence of BM (Fig. 3B). Surprisingly, even in the case of lipid molecular species like DOPE, a noninteracting lipid with BM at 25°C, the drug reduced the monolayer surface area at elevated temperatures (Fig. 3B). BM inclusion in the monolayer made of well-interacting BHCL caused a slightly higher surface area between 20 and 42°C (Fig. 3C). BHCL monolayers revealed a strong increase in the surface area above 45°C, presumably as a result of heat-induced destabilization. In contrast, possibly by inserting between the lipid headgroups, at that temperature range BM affects the monolayer area–temperature curves in a completely opposite way.
Figure 3.
Interaction of BM and its structural analogues with lipids. (A) Drug-induced surface-pressure increase of different lipid monolayers was followed as a function of time. BM was injected underneath the monolayer at t = 3 min to a final concentration of 10−5 M. (B and C) Temperature-dependent surface area change of DOPE (B) or BHCL (C) in the absence or presence of 10−5 M BM in the subphase at constant surface pressure. (D) Displacement of [C14]BM from mitochondria by the cardiolipin-specific dye NAO.
BM Binding to Mitochondrial Membranes.
Further evidence that BM interacts with natural lipids in physiologically active concentrations is provided by using the fluorescent dye NAO, which binds specifically to cardiolipin (19), which is found primarily in mitochondrial membranes (20). Isolated rat heart mitochondrial membranes were preincubated with [14C]BM and increasing concentrations of NAO were added to the suspensions and incubated for 30 min at 25°C. After pelleting mitochondria, the amounts of membrane-associated and free BM were estimated by counting the radioactivity. A significant portion of the drug was membrane associated in the absence of the dye, but about half of this membrane-bound pool was displaced during incubations of mitochondria with NAO (Fig. 3D). We obtained no significant reduction in the size of the membrane-bound BM pool in control preparations not treated with the dye.
Hydroxylamine Derivatives Have Different Affinity for Acidic Lipids.
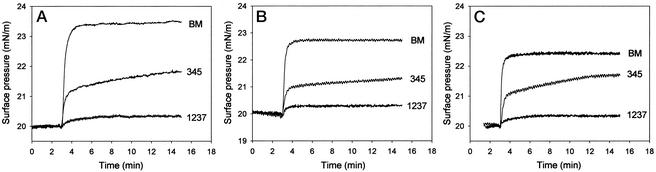
Anionic phospholipids play a major role in the cell-function regulation as a result of promoting binding of specific signaling proteins (like phospholipase A2 or protein kinase C) to the membrane interface (21). The purpose of this study was to investigate the existence of a potential difference in the level of the interaction of the three drugs at a concentration of 10−5 M with monolayers of negatively charged lipids. By monitoring the kinetics of insertion of hydroxylamine derivatives at room temperature, it was revealed that irrespective of the lipid species (see Fig. 4 A, B, and C, for DOPG, BHCL, and BBPS, respectively), BM, the most active HSP coinducer, caused the highest increase in interfacial pressure. It was followed by BRX-345, the compound with intermediate coinducing activity. The derivative BRX-1237 does not act as an HSP coinducer and did not affect the surface pressure of monolayers composed of any of the acidic lipids.
Figure 4.
Comparison of different BM analogues on the basis of their interaction with lipids. Drug-induced surface-pressure increases were followed with monolayers of DOPG (A), BHCL (B), or BBPS (C). Test substances were injected underneath the monolayer at t = 3 min to a final concentration of 10−5 M.
Hydroxylamine Derivatives Increase the Fluidity of Anionic Lipids.
Molecular interactions between foreign molecules and lipid membranes can be studied by following discrete frequency ranges in the FTIR spectrum of lipids. In the present work, we observed changes in the vibrational frequency of the methylene symmetric stretching mode at 2,850 cm−1, which reflect alteration of the membrane fluidity. The thermal profile of the CH2 band of BHCL shows multiple thermal events between 7 and 30°C, with a gradual increase in the vibrational frequency, typical for most naturally derived lipids (Fig. 5A). Increasing concentrations of BM produced a fluidizing effect on BHCL membranes, as illustrated by the increase in the CH2 vibrational frequency (Fig. 5A). BRX-345 also caused membrane disordering, although the fluidizing effect was lower than that of BM (Fig. 5B). On the other hand, BRX-1237 showed no effect on the membrane fluidity. Further, we studied the effect of BM on phosphatidylserine (PS), one of the most abundant anionic phospholipids in mammalian cells (21). In liposomes composed of DMPC/DMPS (9:1), BM induced a downward shift in the transition temperatures, as shown by the DSC thermograms (Fig. 5C). This decrease is more pronounced for the pretransitions (from 14.2°C in the control to 10.7°C in the presence of BM) than for the main transitions (24.3°C for the control and 23.0°C for lipid/BM). On the basis of these data, we suggest that the drug not only stabilizes the liquid-crystalline (or disordered) state in PS-containing membranes, but also affects the lipid packing in the gel phase by inhibiting the formation of the metastable ripple structure. These results also demonstrate that, unlike BRX-1237, both BM and BRX-345 stabilize the liquid-crystalline phase and have a fluidizing effect on anionic phospholipids.
Figure 5.
(A and B) Thermal profiles of the CH2 stretching band in BHCL liposomes in the presence of BM at the indicated molar ratios (A) and in BHCL liposomes in the presence of various BM analogues (B). The molar ratios of lipid to drug in B were 10:4 for BM and 10:3 for BRX-345 and BRX-1237. (C) DSC heating thermograms of DMPC/DMPS (9:1) in the presence of BM (lipid to BM ratios are indicated in the key at the upper left).
Influence of BM on Membrane Lipid Polymorphism.
It is assumed that under physiological conditions, the presence of lipids with nonbilayer-phase-forming propensity leads to packing constraints and optimal matching of protein–lipid interface in the hydrophobic region, and therefore is an important feature of membrane functionality (22). On the other hand, massive formation of nonbilayer structures in heat-exposed cell membranes (23) could interfere with the membrane-barrier function.
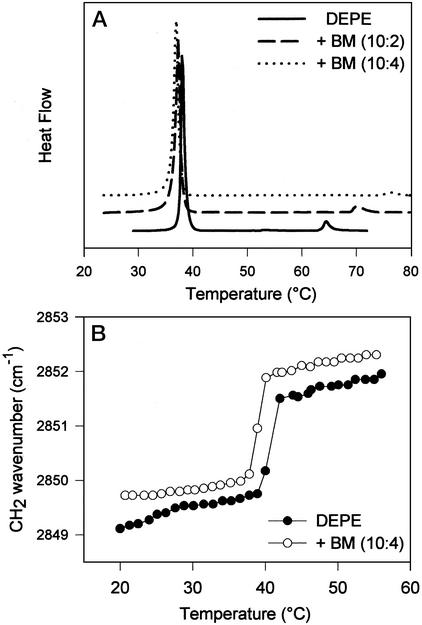
We showed earlier that BM exerts a condensing effect on the surface area of phosphatidylethanolamine monolayers at elevated temperature (Fig. 3B). We hypothesize, therefore, that BM may also affect the critical temperature of the lamellar-to-nonlamellar lipid transition. DEPE membranes are known to display a phase sequence of gel → liquid-crystalline → inverted hexagonal phase (Lβ → Lα → HII) during heating (24). A large endotherm at 38°C, corresponding to the main-phase transition, is followed by a much smaller endotherm (Lα → HII) at 64°C (see Fig. 6A). Thermograms of DEPE membranes containing increasing amounts (DEPE/BM = 10:2 and DEPE/BM = 10:4) of BM clearly showed that the compound shifted the transition of the nonlamellar phase to higher temperatures (to ≈72°C at lower and to ≈77°C at higher BM levels). In addition, there is a slight decrease in the Tm values of the main transition of DEPE liposomes in the presence of BM (from 38.15 to 37.31 to 37.04°C). This latter finding was confirmed by examining the thermal profiles of the CH2 band in DEPE/BM liposomes (Fig. 6B). They showed an increase in the vibrational frequency through the main transition, consistent with conformational disorder of the lipid acyl chains in the presence of the drug. Thus, both DSC and FTIR data suggest that BM has a fluidizing effect on the membranes and stabilize the lamellar liquid-crystalline phase relative to the inverted hexagonal one. However, we obtained no evidence for direct interactions between the lipid head groups and the drugs analyzing the thermal profiles of the phosphate stretching band both in gel and in liquid-crystalline state (data not shown).
Figure 6.
(A) DSC heating thermograms of DEPE in the presence of BM. (B) Thermal profiles of the CH2 stretching band in DEPE liposomes in the absence (●) or presence (○) of BM (lipid to BM ratios are indicated in the keys).
Discussion
BM and its derivatives have potential therapeutic value in the treatment of diabetic complications (25, 26), protected rat neonatal cardiomyocytes under experimental stress conditions and reduced myocardial infarct size in a rat model of ischemia and reperfusion at clinically relevant concentrations (27). The compound was suggested to act by means of the up-regulation of HSPs (13). BM and specific derivatives (BRX compounds) elevated the level of HSPs synergistically with high-temperature stress, serum deprivation, and various pathophysiological conditions, and are designated therefore as HSP coinducers.
The major objective of the present study was to identify likely sites of action of these compounds and to explore the primary cellular event(s) sufficient to evoke an up-regulated stress response on their administration. We further substantiated the HSP coinducing activity of BM by documenting HSP70 augmentation when added to mammalian cells in combination with either sublethal heat shock or serum deprivation as stressors. Two additional hydroxylamine derivatives have been investigated. BRX-345 acts as HSP coinducer, but is less effective than BM, whereas BRX-1237 is inactive in HSP coinduction.
According to a classical model, the major trigger for HSP synthesis is the accumulation of denatured proteins in the cytoplasm (1, 28). Our findings provide support for the direct involvement of an important key element, the membrane status in stress detection and signaling. Whereas BM acted in a synergistic manner with stress concerning HSP70 formation, we were unable to detect a simultaneous synergistic accumulation of denatured proteins. We proposed therefore, that other signal(s) might also be involved in the primary activation of heat shock response. Our alternative, but not necessarily exclusive, approach is based on the concept that the initial stress-sensing events are associated with the physical state and lipid composition of cellular membranes, i.e., the subtle alteration(s) of membrane fluidity, phase state, and/or microheterogeneity may operate as a cellular thermometer (3, 4). This model also implies that the wide variations of membrane lipids in cells and tissues may provide cell-specific response to combinations of intra- and extracellular stress signals. Close correlations between changes in composition and physical order of membranes and cellular stress response have been widely documented (8, 9, 29, 30). Combining these observations with our results, we conclude that BM and its derivatives may partially elicit their effect by interacting with specific lipid components in the cellular membranes and may activate membrane-associated signaling cascade(s) linked to the heat shock response. This initiating effect is further enhanced by the BM-induced persistence of HSF binding to DNA (J.H., H. Lewis, I. Boros, T. Rácz, A. Fiser, I. Kurucz, I. Benjamin, L.V., Z.P., P.C., and D. S. Latchman, unpublished data). The inability of BM and its derivatives to alter protein denaturation has also been demonstrated in various in vitro model systems (G. Nardai and P.C., unpublished data).
The monolayer technique was used to test whether interactions exist between different lipid classes and BRX compounds. BM inserts preferentially into monolayers composed of negatively charged lipids. The selective interaction of BM with the typical mitochondrial inner membrane lipid, cardiolipin, was also demonstrated in vitro by showing that the cardiolipin-specific dye displaces BM from mitochondria. Interestingly, DOPE, a lipid class virtually unable to interact with the drugs at physiological temperatures, showed a condensed monolayer surface in the presence of BM at higher temperatures. Each hydroxylamine derivative caused similar changes in the surface pressure–time profiles of monolayers composed of acidic lipids. Whereas BM appeared to be the most interactive, followed by BRX-345, BRX-1237 was nearly ineffective. Evidence from FTIR indicated that BM stabilizes the liquid-crystalline state and elevates membrane fluidity in multilamellar vesicles made of BHCL. Again, the systematic comparison of the three derivatives on the same model membrane revealed that the order of the increase in membrane fluidity corresponds to their activities found in HSP-coinduction assays. The lipid-condensing effect documented for BM at elevated temperatures appears to be consistent with its ability to stabilize the lamellar phase in the presence of nonbilayer-forming lipids during heat stress. It is reasonable to hypothesize that BM acts as a molecular spacer and may induce a less tight packing of lipids at normal or medium-high temperatures, thus producing a more fluid bilayer, as seen for acidic lipids. We suggest that BM shifts the critical temperatures of HII phase formation upward during heat stress, by inserting between headgroups of lipids with nonbilayer propensity and, presumably, by alleviating the curvature stress (22) on the membrane.
One might expect that perturbation of the organization and phase state of acidic glycerophospholipids, the major determinants of membrane topology (31) by BM and related compounds should influence the structure/activity of membrane-bound proteins without direct drug–protein interaction. Protein function influenced by membrane fluidity and/or heterogeneity has been suggested for phospholipase A2, the enzymatic activity of which is known to be stimulated by heat shock (30). Elevated activity of membrane-bound phospholipases and the resultant release of lipid mediators could also enhance the subsequent membrane association and activation of protein kinase C, found to drive the phosphorylation of HSFs (32). A transmembrane protein essential for the heat-activation of MAPK Mpk1 is thought to act as a thermosensor by detecting changes in membrane fluidity induced by thermal stress (33). Recently, a member of the BRX family, BRX-235, was shown to induce phosphorylation of p38 SAPK (34). It is noted that both this derivative and BM caused a remarkable translocation of the calcium-dependent protein kinase C isoform to membranes (P. Nánási, T. Bíró, and T. Bányász, unpublished data; and K. Bíró and L.V., unpublished data).
If pharmacological compounds such as the hydroxylamine derivatives are interacting with membranes, the interaction may result in higher heterogeneity with more interfacial regions. Interfacial regions are domains with special packing properties that may attract proteins, and possibly induce conformational changes within them. Accumulation of drug molecules in such regions provides direct access to many proteins and receptors, which also have tendency to accumulate along membrane defects (35). Hence, the actual local concentration of BM and its derivatives in certain membrane regions can be more relevant in relation to defining the clinical concentrations than the global drug concentration.
In conclusion, we have shown that BM and its derivatives selectively interact with acidic lipids and induce modifications in their dynamics, molecular packing, and phase behavior. Their ability to elevate fluidity in specific membrane domains, similar to the effect of high temperature, may be of paramount importance to perceive heat shock and to generate the primary stress signal culminating in the activation of heat shock genes. We suggest that it may be of considerable therapeutic benefit to further exploit the above principles in the systematic development of other membrane-interacting drug candidates that are clinically safe and yet able to induce or attenuate the level and composition of HSPs.
Acknowledgments
This work was financially supported by Grants T029883 and T038334 from the Országos Tudományos Kutatási Alapprogramok (Hungarian National Scientific Research Foundation), from the Hungarian National Research and Development Program (1/040/2001), by the Ministry of University and Scientific Research 2000/2003, and BraneTech, Naples.
Abbreviations
- HSP
heat shock protein
- HSF
heat shock factor
- DSC
differential scanning calorimetry
- FTIR
Fourier transform infrared
- DOPG
1,2-dioleoyl-sn-glycero-3-phosphoglycerol
- BHCL
bovine heart cardiolipin
- DMPS
1,2-dimiristoyl-sn-glycero-3-phosphoserine
- DMPC
1,2-dimiristoyl-sn-glycero-3-phosphocholine
- DEPE
1,2-dielaidoyl-sn-glycero-3-phosphoethanolamine
- DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- BM
bimoclomol
- NAO
10-N-nonylacridine orange
References
- 1.Morimoto R I. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 2.Hjorth-Sorensen B, Hoffmann E R, Lissin N M, Sewell A K, Jakobsen B K. Mol Microbiol. 2001;39:914–923. doi: 10.1046/j.1365-2958.2001.02279.x. [DOI] [PubMed] [Google Scholar]
- 3.Vígh L, Maresca B, Harwood J. Trends Biochem Sci. 1998;23:369–374. doi: 10.1016/s0968-0004(98)01279-1. [DOI] [PubMed] [Google Scholar]
- 4.Vígh L, Maresca B. In: Cell and Molecular Responses to Stress. Storey K B, Storey J M, editors. Amsterdam: Elsevier; 2002. pp. 173–188. [Google Scholar]
- 5.Khar A, Ali A M, Pardhasaradhi B V, Varalakshmi C H, Anjum R, Kumar A L. Cell Stress Chaperones. 2001;6:368–376. doi: 10.1379/1466-1268(2001)006<0368:iosrrh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vígh L, Los D A, Horváth I, Murata N. Proc Natl Acad Sci USA. 1993;90:9090–9094. doi: 10.1073/pnas.90.19.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carratù L, Franceschelli S, Pardini C L, Kobayashi G S, Horváth I, Vígh L, Maresca B. Proc Natl Acad Sci USA. 1996;93:3870–3875. doi: 10.1073/pnas.93.9.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horváth I, Glatz A, Varvasovszki V, Török Z, Páli T, Balogh G, Kovács E, Nádasdi L, Benkő S, Joó F, Vígh L. Proc Natl Acad Sci USA. 1998;95:3513–3518. doi: 10.1073/pnas.95.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee M T, Khalawan S A, Curran B P G. Microbiology. 2000;146:877–884. doi: 10.1099/00221287-146-4-877. [DOI] [PubMed] [Google Scholar]
- 10.Török Z, Horváth I, Goloubinoff P, Kovács E, Glatz A, Balogh G, Vígh L. Proc Natl Acad Sci USA. 1997;94:2192–2197. doi: 10.1073/pnas.94.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Török Z, Goloubinoff P, Horváth I, Tsvetkova N M, Glatz A, Balogh G, Varvasovszki V, Los D A, Vierling E, Crowe J, Vígh L. Proc Natl Acad Sci USA. 2001;98:3098–3103. doi: 10.1073/pnas.051619498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsvetkova N M, Horváth I, Török Z, Wolkers W F, Balogi Z, Shigapova N, Crowe L M, Tablin F, Vierling E, Crowe J H, Vígh L. Proc Natl Acad Sci USA. 2002;99:13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vígh L, Literáti P N, Horváth I, Török Z, Balogh G, Glatz A, Kovács E, Boros I, Ferdinándy P, Farkas B, et al. Nat Med. 1997;3:1150–1154. doi: 10.1038/nm1097-1150. [DOI] [PubMed] [Google Scholar]
- 14.Kurucz I, Tombor B, Prechl J, Erdo F, Hegedus E, Nagy Z, Vitai M, Koranyi L, Laszlo L. Cell Stress Chaperones. 1999;4:139–152. doi: 10.1379/1466-1268(1999)004<0139:ulohew>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen V T, Bensaude O. Eur J Biochem. 1994;220:239–246. doi: 10.1111/j.1432-1033.1994.tb18619.x. [DOI] [PubMed] [Google Scholar]
- 17.Schlame M, Horváth L I, Vígh L. Biochem J. 1990;265:79–85. doi: 10.1042/bj2650079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demel R A. Subcell Biochem. 1994;23:83–120. doi: 10.1007/978-1-4615-1863-1_3. [DOI] [PubMed] [Google Scholar]
- 19.Petit J-M, Maftah A, Ratinaud M-H, Julien R. Eur J Biochem. 1992;209:267–273. doi: 10.1111/j.1432-1033.1992.tb17285.x. [DOI] [PubMed] [Google Scholar]
- 20.Schlame M, Rua D, Greenberg M L. Prog Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- 21.Buckland A G, Wilton D C. Biochim Biophys Acta. 2000;1483:199–216. doi: 10.1016/s1388-1981(99)00188-2. [DOI] [PubMed] [Google Scholar]
- 22.de Kruijff B. Nature. 1997;386:129–130. doi: 10.1038/386129a0. [DOI] [PubMed] [Google Scholar]
- 23.Quinn P J, Joó F, Vígh L. Prog Biophys Mol Biol. 1989;53:71–103. doi: 10.1016/0079-6107(89)90015-1. [DOI] [PubMed] [Google Scholar]
- 24.Koynova R, Caffrey M. Chem Phys Lipids. 1994;69:1–34. doi: 10.1016/0009-3084(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 25.Biro K, Jednakovits A, Kukorelli T, Hegedus E, Koranyi L. Brain Res Bull. 1997;44:259–263. doi: 10.1016/s0361-9230(97)00118-4. [DOI] [PubMed] [Google Scholar]
- 26.Erdo F, Erdo S L. Brain Res Bull. 1998;45:163–166. doi: 10.1016/s0361-9230(97)00333-x. [DOI] [PubMed] [Google Scholar]
- 27.Lubbers N L, Polakowski J S, Wegner C D, Burke S E, Diaz G J, Daniell K M, Cox B F. Eur J Pharmacol. 2002;435:79–83. doi: 10.1016/s0014-2999(01)01552-7. [DOI] [PubMed] [Google Scholar]
- 28.Ananthan J, Goldberg A L, Voellmy R. Science. 1986;232:522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- 29.Moskvina E, Imre E-M, Ruis H. Mol Microbiol. 1999;32:1263–1272. doi: 10.1046/j.1365-2958.1999.01438.x. [DOI] [PubMed] [Google Scholar]
- 30.Samples B L, Pool G L, Lumb R H. Comp Biochem Physiol B Biochem Mol Biol. 1999;123:389–397. doi: 10.1016/s0305-0491(99)00083-8. [DOI] [PubMed] [Google Scholar]
- 31.van Klompenburg W, Nilsson I M, von Heijne G, de Kruijff B. EMBO J. 1997;16:4261–4266. doi: 10.1093/emboj/16.14.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmberg C I, Leppa S, Eriksson J E, Sistonen L. J Biol Chem. 1997;272:6792–6798. doi: 10.1074/jbc.272.10.6792. [DOI] [PubMed] [Google Scholar]
- 33.Philip B, Levin D E. Mol Cell Biol. 2001;21:271–280. doi: 10.1128/MCB.21.1.271-280.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dénes L, Jednákovits A, Hargitai J, Pénzes Z, Balla A, Tálosi L, Krajcsi P, Csermely P. Br J Pharmacol. 2002;136:597–603. doi: 10.1038/sj.bjp.0704738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraser D M, Louro S R W, Horváth L I, Miller K W, Watts A. Biochemistry. 1990;29:2664–2669. doi: 10.1021/bi00463a007. [DOI] [PubMed] [Google Scholar]