Abstract
The antigen-presenting cells that initiate and maintain MHC class II-associated organ-specific autoimmune diseases are poorly defined. We now describe a new T cell antigen receptor (TCR) transgenic (Tg) model of inflammatory skin disease in which keratinocytes activate and are the primary target of autoreactive CD4+ T cells. We previously generated keratin 14 (K14)-Aβb mice expressing MHC class II only on thymic cortical epithelium. CD4+ T cells from K14-Aβb mice fail to undergo negative selection and thus have significant autoreactivity. The TCR genes from an autoreactive K14-Aβb CD4 hybridoma were cloned to produce a TCR Tg mouse, 2-2-3. 2-2-3 TCR Tg cells are negatively selected in WT C57BL/6 mice but not in 2-2-3/K14-Aβb mice. Interestingly, a significant number of mice that express both the K14-Aβb transgene and the autoreactive 2-2-3 TCR spontaneously develop inflammatory skin disease with mononuclear infiltrates, induction of MHC class II expression on keratinocytes, and T helper 1 cytokines. Disease can be induced by skin inflammation but not solely by activation of T cells. Thus, cutaneous immunopathology can be directed through antigen presentation by tissue-resident keratinocytes to autoreactive TCR Tg CD4+ cells.
The antigen-presenting cells (APCs) that drive the various phases of MHC class II-dependent organ-specific autoimmune diseases, such as insulin-dependent diabetes mellitus, experimental allergic encephalitis, and thyroiditis, are not fully identified. Professional APCs, including dendritic cells, macrophages, and B cells, constitutively express MHC class II molecules; however, MHC class II molecules also can be induced on epithelial and endothelial tissues by inflammatory signals. Lymphocytes traffic through the secondary lymphoid organs and interact in lymph nodes (LNs) with dendritic cells transporting tissue antigens to initiate immune responses to model antigens, microbial antigens, and apoptotic cells (1). This pathway has been presumed true for autoimmune responses as well. Höglund et al. (2) demonstrated that the activation of islet-specific CD4+ cells that precedes diabetes occurs in the draining pancreatic LN, rather than in the pancreatic islet; similar results have been obtained in primate experimental allergic encephalitis and human multiple sclerosis (3). However, the APCs required for invasion and destruction of the tissue itself have not been identified. Indeed, recent work in transplantation suggests that endothelial expression of MHC class II molecules is sufficient to mediate rejection of a solid organ transplant (4). Thus, tissue expression of class II molecules on nonprofessional APCs can direct pathogenic immune responses.
The APCs that mediate cutaneous immunologic diseases, such as psoriasis, atopic dermatitis, cutaneous T cell lymphoma, and cutaneous graft-versus-host disease (GVHD), are similarly poorly defined. Epidermal Langerhans cells and dermal dendritic cells transport cutaneous antigens to draining LNs; keratinocytes are nonfunctional, tolerizing APCs in normal skin. However, activated keratinocytes produce inflammatory cytokines, including tumor necrosis factor α (TNF-α) and IL-1 (5–7) and clearly express both MHC class II molecules and the intercellular adhesion molecule-1 (ICAM-1). They also express the costimulatory molecule CD80 (B7–1) after exposure to chemical sensitizers and irritants (8). These characteristics suggest that keratinocytes may directly participate in cutaneous immunologic diseases.
We have developed a T cell antigen receptor (TCR) transgenic (Tg) model in which CD4+ cells are positively and negatively selected by endogenous peptides. Interestingly, the mice spontaneously develop autoimmune skin disease in which keratinocytes activate and are the primary targets of autoreactive CD4+ T cells.
Materials and Methods
Mice.
C57BL/6 (B6) and invariant chain (Ii)-deficient mice (9) were purchased from The Jackson Laboratory. Aβb-deficient (10) and keratin 14 (K14)-Aβb (11) mice have been described. H2-DMα-deficient mice were the kind gift of Luc van Kaer (Vanderbilt University, Nashville, TN) (12). All mice were maintained under specific pathogen-free conditions at the University of Pennsylvania.
Generation of 2-2-3 TCR Tg Mice.
The 2-2-3 TCR cDNAs, a previously undescribed Vα1 paired with Vβ5.1, were cloned from an I-Ab-reactive K14-Aβb hybridoma (Hybrid no. 14) (13). The α chain uses a Vα1 segment identical to a previously submitted sequence (GenBank accession no. AF034160) and Jα16. The β chain uses Vβ5.1 and Jβ1.5. The rearranged variable regions were obtained by RT-PCR from the 2-2-3 hybridoma and subcloned into 2B4 TCRα (14) or 3A9 TCRβ shuttle vectors (15). Transgenes were injected into B6 × BALB/c F1 fertilized oocytes, and transgene-positive founders were backcrossed to B6, K14-Aβb, and Aβb−/− mice. Transgene-expressing animals were identified by flow cytometric analysis.
Generation of the 2-2-3 Clonotypic Ab.
The 2-2-3 clonotypic mAb was made by following published procedures (16, 17). Supernatants were screened by flow cytometry for staining of 2-2-3 cells. A clone was isolated that stained 2-2-3 (Vα1, Vβ5) but not another CD4+ T cell hybridoma, 4-2-2 (Vα11, Vβ8) (Hybrid no. 5), derived from the same T cell fusion (13). The 2-2-3 clonotypic Ab also did not stain a Vβ5-expressing hybridoma, B3H (Vα2, Vβ5), a gift of P. Fink (University of Washington, Seattle), or the Vα1-expressing hybridoma, 2.5/BW1-31.3 (Vα1, Vβ4), a gift of K. Haskins (University of Colorado, Denver) (18). The 2-2-3 clonotypic Ab was purified from tissue culture supernatants by using protein A agarose (Bio-Rad) and subsequently biotinylated.
Flow Cytometry.
The following antibodies for flow cytometry were purchased from PharMingen: CD4 (RM4-5), CD8 (Ly-2), streptavidin–phycoerythrin, Vβ5 (MR9-4), and CD3 (2C11). Stained cells were collected on a Becton Dickinson FACSCalibur and analyzed by using either cellquest (Becton Dickinson) or flojo (Tree Star, San Carlos, CA) software.
Proliferation Assays and T Hybridoma Stimulation.
CD4+ T cells were purified from splenic and LN single-cell suspensions by negative selection. Cells were incubated with anti-CD8 (2.43), anti-B220 (RA3), anti-Mac-1 (M1/70.15), F4/80, and anti-I-A (M5/114), followed by incubation with magnetic microbead-conjugated goat anti-rat IgG (Polysciences). Hybridoma stimulations (13) and mixed lymphocyte reactions (11) were performed as described.
Carboxyl Fluorescein Succinimide Ester (CFSE) Labeling and Analysis.
CD4+ T cells were purified and enriched from spleen and LN by negative selection as above. Single-cell suspensions were labeled with CFSE (Molecular Probes) as described (19, 20). CD4+ T cells (5 × 107) labeled with CFSE were injected intravenously into the indicated mice in a total volume of 0.2 ml of sterile PBS. Recipients were killed at 72 h; total spleen and LN cells were purified, labeled with biotinylated clonotypic mAb and streptavidin–allophycocyanin, and analyzed by flow cytometry as above.
Acute GVHD.
Acute GVHD was induced as described (13), by using 2 × 106 donor CD4+ T cells.
Histopathology.
Cryostat sections (5 μm) of skin were fixed in cold acetone, then incubated with primary rat Ab to MHC class II I-A (M5/114), CD4 (GK1.5), or F4/80, followed by biotinylated mouse anti-rat IgG or by biotinylated 2-2-3 TCR clonotypic Ab and streptavidin–horseradish peroxidase with 3-amino-9-ethylcarbazole as the chromagen. ICAM-1 expression was identified with primary Ab 3E2 followed by secondary biotinylated goat anti-hamster Ab and was developed with the ABC kit (Vector Laboratories). Sections were counterstained with hematoxylin.
Cytokine and Chemokine Analysis.
Total RNA was obtained from skin by homogenization in Trizol (Life Technologies, Gaithersburg, MD) according to the manufacturer's instructions. First-strand cDNA was synthesized with M-MLV reverse transcriptase (Superscript, Life Technologies), and expression of cDNAs was assessed by PCR using published primers (21).
Croton Oil Treatment.
We applied 0.8% croton oil (Sigma) diluted in acetone under the chin of 10-day-old mice.
Results
Generation of 2-2-3 TCR Tg Mice.
We previously described K14-Aβb mice in which the Aβb cDNA is targeted to stratified squamous epithelia of Aβb (class II)-deficient mice by the human K14 promoter (22). Hematopoietic APCs are MHC class II-negative. Similarly, keratinocytes and other stratified squamous epithelia are I-A-negative in unmanipulated mice despite the expression of Aβb mRNA.
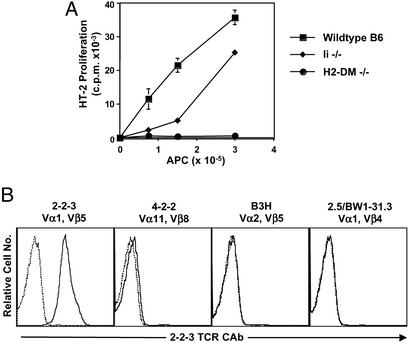
CD4+ cells from K14-Aβb mice have significant autoreactivity; they proliferate in vitro to APCs and mediate both chronic and acute lethal GVHD when transferred into MHC-positive WT B6 mice (11, 13). To skew the peripheral repertoire toward a single autoreactive specificity, we have cloned the Vα1 and Vβ5 TCR cDNAs from an autoreactive K14 T cell hybridoma, 2-2-3 (Fig. 1A) and generated TCR Tg mice. The 2-2-3 hybridoma produced IL-2 in response to WT B6 and C57BL/6 Ii-deficient APCs but not to H2-DM-deficient APCs. This pattern of reactivity suggests that the 2-2-3 TCR is specific for a prevalent peptide presented by I-Ab. Two different TCR Tg founder lines, 3339 and 3341, were produced and backcrossed to WT, Aβb−/−, and K14-Aβb mice; the experiments presented here focus on one founder line, 3339.
Figure 1.
Characterization of the 2-2-3 hybridoma and 2-2-3 clonotypic Ab. (A) The 2-2-3 T cell hybridoma recognizes an I-Ab-associated peptide. 2-2-3 hybridoma T cells (1 × 105) were stimulated with increasing numbers of splenocytes from the indicated mice, and IL-2 in the supernatant was assayed by using the HT-2 cell line. (B) The 2-2-3 clonotypic Ab recognizes only 2-2-3 T cells. The T cell hybridomas shown were stained with 2-2-3 clonotypic Ab followed by FITC-conjugated goat anti-mouse IgG (solid line). The dashed line shows staining with secondary Ab alone.
To follow the fate of the TCR Tg cells, we also generated a clonotypic mAb specific for the 2-2-3 TCR (Fig. 1B); 2-2-3 hybridoma cells produce IL-2 in response to platebound clonotypic Ab (data not shown). The clonotypic Ab was used to determine the proportion of 2-2-3 cells in the K14-Aβb CD4+ cell repertoire. Clonotype-positive cells were undetectable among naive CD4+ cells from K14-Aβb mice (data not shown) and were not a major component of the polyclonal K14 anti-I-Ab response (Fig. 3B).
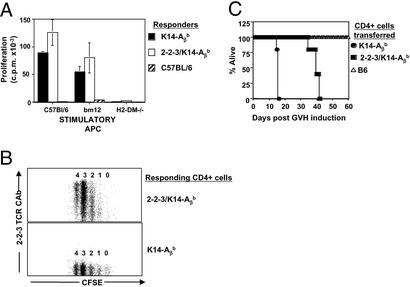
Figure 3.
2-2-3/K14-Aβb cells are not tolerant of I-Ab. (A) 2-2-3/K14-Aβb cells respond to syngeneic splenocytes in vitro. Responder splenocytes (4 × 105) were cultured with 4 × 105 irradiated splenocytes from the indicated mice for 72 h, pulsed with [3H]thymidine for 16 h, and harvested. (B) 2-2-3/K14-Aβb CD4+ cells proliferate in syngeneic hosts. CD4+ cells from 2-2-3/K14-Aβb (Upper) or K14-Aβb (Lower) mice were CFSE labeled and injected intravenously into WT C57BL/6 mice. After 72 h, host mice were killed, and splenocytes were analyzed by flow cytometry for expression of CD4 and 2-2-3 clonotypic Ab and CFSE levels. The division number is indicated above each CFSE peak. (C) B6 mice were lethally irradiated and reconstituted with 107 T-depleted B6 bone marrow cells and 2 × 106 CD4+ cells from B6, K14-Aβb, or 2-2-3/K14-Aβb mice. Survival analysis is shown.
T Cell Development in 2-2-3 TCR Tg Mice.
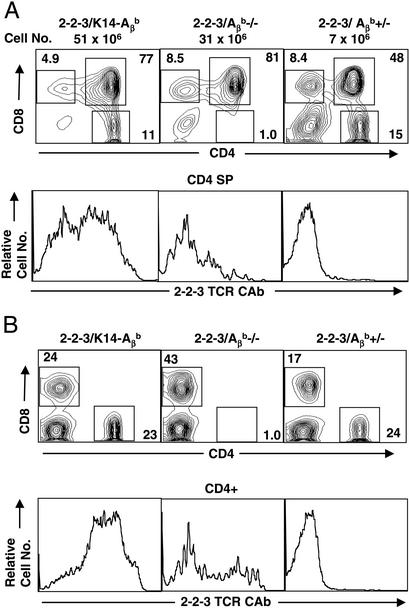
We first examined the thymic development of 2-2-3 TCR Tg cells. In 2-2-3/Aβb+/− (class II WT) mice, there were markedly fewer thymocytes and decreased numbers of CD4+ and CD8+ single positive (SP) cells in both the thymus and periphery (Fig. 2 and data not shown). Fewer than half of the small number of remaining CD4+ cells expressed the Tg Vβ5 chain (data not shown), but, as seen in Fig. 2B, all were clonotype-negative. Interestingly, CD8 SP cells expressed high levels of clonotypic receptor (data not shown), suggesting that deletion requires the CD4 coreceptor expression and is a relatively late intrathymic event. This deletion was not present in 2-2-3 Tg Aβb-deficient animals; however, only a small number of clonotype-positive cells were exported to the periphery as either CD8 SP or CD4−CD8− (Fig. 2B and data not shown). In contrast, a large population of clonotype-positive CD4+ SP cells are present in the 2-2-3/K14-Aβb thymus. The 2-2-3 Vβ5 β chain prevents rearrangement at the endogenous TCR β locus, and all of the CD4+ T cells in 2-2-3/K14-Aβb mice were Vβ5-positive (data not shown). In contrast, cells with low surface levels of clonotype TCR also express endogenous α chains (data not shown). Therefore, Tg clonotype-positive thymocytes require MHC class II expression for positive selection but are almost completely deleted through thymic negative selection in the presence of WT expression of I-Ab on bone marrow-derived APCs.
Figure 2.
2-2-3 CD4+ cells are positively selected in the K14 thymus. (A) The total number of thymocytes in a representative thymus from the indicated mice. Thymocytes were stained with CD4-phycoerythrin, CD8-FITC, and biotinylated 2-2-3 clonotypic Ab followed by streptavidin–allophycocyanin. The CD4/CD8 profiles are shown in Upper and 2-2-3 clonotypic Ab levels on CD4 SP cells are shown in Lower. (B) Splenocytes were stained as in A. Numbers indicate the percentage of cells in the gates shown.
To determine whether 2-2-3 TCR Tg cells that matured in a K14-Aβb thymus were tolerant to self, the function of peripheral CD4+ cells was examined. Similar to the parental hybridoma, CD4+ cells from 2-2-3/K14-Aβb mice proliferated in a mixed lymphocyte reaction in response to syngeneic B6 and allogeneic I-Abm12, but not H2-DM-deficient APCs (Fig. 3A). Secondly, 2-2-3/K14-Aβb CD4+ cells were CFSE-labeled and injected intravenously into sex-matched, syngeneic B6 recipients. CFSE-labeled 2-2-3/K14-Aβb CD4+ cells with all levels of clonotype TCR expression proliferated (Fig. 3B). Finally, acute GVHD was induced. WT B6 mice were lethally irradiated and reconstituted with T-depleted B6 bone marrow and 2 × 106 mature CD4+ cells. Mice treated with 2-2-3/K14-Aβb CD4+ cells succumbed to acute GVHD in 35–40 days (Fig. 3). Therefore, 2-2-3/K14-Aβb TCR Tg CD4+ cells are not tolerized to syngeneic I-Ab-expressing APCs. Thus, we have generated a TCR Tg animal in which I-Ab-restricted CD4+ cells are positively and negatively selected on endogenous peptides
Spontaneous Skin Disease in 2-2-3/K14-Aβb Mice.
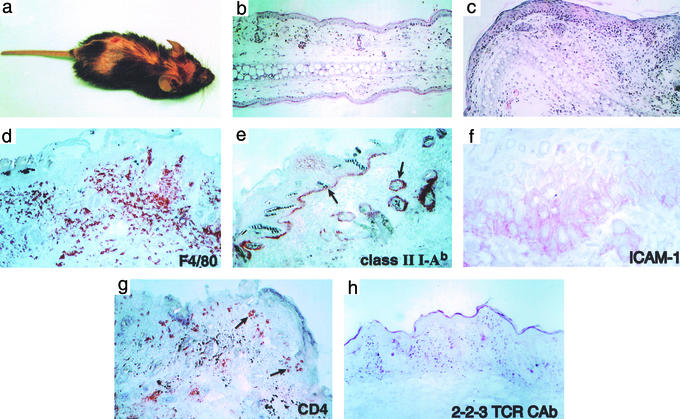
Approximately 20% of 2-2-3/K14-Aβb mice of the 3339 line spontaneously developed generalized skin inflammation at 3–5 weeks of age. The disease was characterized by flaky, scaly skin that progressed to alopecia and excoriations (Fig. 4a). Histologic examination of affected skin showed a patchy, lichenoid process with a striking mononuclear infiltration associated with marked hyperkeratosis, dermatitis, subepidermal inflammatory infiltrates, and apoptotic keratinocytes (Fig. 4). Skin disease persisted for 2–3 weeks and then resolved spontaneously; no residual pathology other than some minor alteration in fur pigmentation (not shown) was seen.
Figure 4.
Spontaneous skin disease in 2-2-3/K14-Aβb mice. (a) Gross skin lesions with alopecia, skin thickening, and ulcer formation. (b and c) Hematoxylin/eosin staining of ear skin from polyclonal K14-Aβb (b) and 2-2-3/K14-Aβb (c) mice shows marked mononuclear dermal infiltrates with epidermal thickening of the involved skin. (d–h) Immunohistochemistry of involved trunk skin reveals I-Ab expression of basal keratinocytes and cells at the base of hair follicles. Basal keratinocytes are also ICAM-1-positive. The predominant dermal infiltrating cells are F4/80+ mature macrophages. CD4+ cells cluster around hair follicles and the basal epidermis but also are present among the dermal infiltrate. 2-2-3 clonotype-positive cells also are identified in the infiltrate. All histology is shown at ×100 except ICAM-1 (×400).
Under normal conditions, epithelial tissues in K14 mice are class II-negative (ref. 11 and data not shown), and we have found that this also is true in healthy double Tg 2-2-3/K14-Aβb mice (data not shown). Autoreactive 2-2-3 CD4+ cells exported to the periphery from the K14 thymus would not typically encounter class II antigens. However, the basal epithelial keratinocytes in diseased skin, as well as cells around the base of hair follicles in affected areas, expressed both MHC class II I-Ab and the costimulatory molecule ICAM-1 (Fig. 4). Thus, whereas the predominant skin-infiltrating cells were F4/80+ mature macrophages (Fig. 4), CD4+ and CD8+ T cells also were present; interestingly, the CD4+ T cells seemed to associate with class II-positive epithelial cells (note arrows in Fig. 4g). Clonotype-positive cells also were identified among the infiltrating cells in the dermis.
Microscopic examination also revealed mononuclear infiltration of the tongue of 2-2-3/K14-Aβb mice [another site of K14-driven transgene expression (23)] (data not shown); however, no other epithelial or nonepithelial tissues, including kidney and thymus, had evidence of inflammation or other pathology either grossly or microscopically. Additionally, no antinuclear antibodies were detected in the serum of sick mice.
The skin disease was variably penetrant between the two founder lines. Approximately 40% of mice of the 3341 line developed spontaneous disease, whereas fewer (≈20%) 3339 mice became ill. However, once it developed, disease appeared grossly and microscopically similar between the two lines of mice. Importantly, no skin disease has been observed in single Tg K14-Aβb mice, even though these mice contain polyclonal autoreactive CD4+ T cells. Thus, autoimmune skin disease requires either the 2-2-3 TCR transgene specifically, or skewing of the entire population toward autoreactivity. Together, these findings suggest that, in some 2-2-3/K14-Aβb mice, keratinocytes spontaneously express MHC class II, which leads to the activation of autoreactive 2-2-3 T cells and the development of cutaneous GVHD.
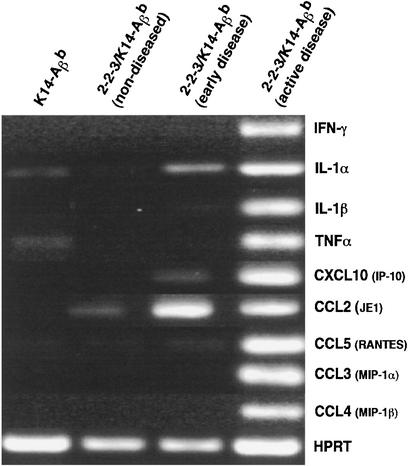
To further characterize the dermal immune response, skin samples were taken from mice at various stages of disease, and cytokine and chemokine expression was analyzed by RT-PCR. Samples were analyzed from young unaffected mice, mice with active skin lesions, and mice that had spontaneously recovered from skin disease. Skin from unaffected K14-Aβb mice lacking the 2-2-3 TCR transgene was examined in parallel. Interestingly, small amounts of IL-1 and TNF-α could be detected in the skin of unaffected K14-Aβb mice; however, chemokine expression was not present. The earliest skin changes detectable in 2-2-3/K14-Aβb mice, with erythema but no alopecia, were accompanied by production of IL-1 and CCL-2 (JE/MCP-1). This progressed to a T helper 1 (Th1)-skewed immune response with prominent expression of IFN-γ and TNF-α in the skin of diseased animals (Fig. 5); no IL-4 was detected (data not shown). In this setting, the IFN-γ-inducible inflammatory chemokines, IP-10 and Mig, also were produced. Thus, prominent production of Th1 cytokines and IFN-γ-inducible chemokines correlated with cellular infiltrates and MHC class II expression.
Figure 5.
Skin disease in 2-2-3/K14-Aβb mice is a type 1 inflammatory response. Total RNA was prepared from skin specimens from the indicated mice and reverse-transcribed into cDNA, and RT-PCR was performed for the messages indicated.
Induction of Skin Disease by Skin Inflammation.
To examine the role of CD4+ cells in disease induction, we evaluated the effect of manipulating the activation of the CD4+ cells. These experiments all were performed with asymptomatic mice from the 3339 line. We first asked whether injection of I-Ab-positive APCs into 2-2-3/K14-Aβb mice would activate T cells and initiate disease, because Tg T cells generated strong proliferative responses to C57BL/6 spleen cells in vitro. Therefore, three 2-2-3/K14-Aβb mice received s.c. injections of syngeneic WT C57BL/6 (I-Ab-positive) T-depleted splenocytes. These mice lost no weight, and no skin lesions developed. Secondly, naive K14-Aβb mice received a single-cell suspension of LN and spleen cells from one 2-2-3/K14-Aβb mouse to determine whether the transfer of the autoreactive T cells would cause disease in the recipients. Intravenous transfer of 2-2-3/K14-Aβb T cells had no effect on the overall appearance, weight, or skin of either sublethally irradiated (500 rad, 1 rad = 0.01 Gy, n = 4) or nonirradiated (n = 4) mice. Finally, because Th1-mediated diseases such as diabetes are accelerated by injection with the alkylating agent cyclophosphamide, 2-2-3/K14-Aβb mice were given a standard course of cyclophosphamide (200 mg/kg, twice, 2 weeks apart). This treatment has been shown to increase the production of IFN-γ by T cells (24, 25). However, as in the first two situations, asymptomatic mice (n = 4) did not develop weight loss, skin inflammation, or any other manifestation of GVHD. These results suggest that primary activation of autoreactive 2-2-3 CD4+ T cells is not sufficient to induce inflammatory skin disease.
Because primary activation of T cells did not induce cutaneous inflammation, we next asked whether the disease could be triggered by skin irritation. Inflammation was induced by painting the skin of 3339 mice with croton oil. Croton oil causes local irritation and erythema, which peaks within 2–3 days after application and thereafter resolves. When croton oil was used to paint the skin of adult (4- to 8-week-old) 2-2-3/K14-Aβb mice, transient inflammation was evident after 2 days, but it resolved spontaneously with no spread to other areas. Mice lacking either the 2-2-3 TCR transgene or the K14-Aβb transgene only developed a transient erythema over the painted site. However, when a small patch of skin was painted in 10-day-old 2-2-3/K14-Aβb animals, a scaly plaque formed over the painted site within 1 week. In these 2-2-3/K14-Aβb mice, this plaque also quickly resolved, but within 3 weeks, a high percentage of these mice developed generalized disease. These experiments are shown in Table 1. To date, 7 of 18 mice treated with croton oil (40%) have developed skin disease. Although spontaneous disease does not show a gender bias, 71% (5/7) of female mice versus 18% (2/11) of male mice developed disease with croton oil. Histologic analysis showed that the disease that developed after croton oil in both sexes was indistinguishable from spontaneous disease. Thus, autoimmunity can be initiated in female mice by dermal irritation but not by primary T cell activation.
Table 1.
Croton oil initiates skin disease in female mice
| Spontaneous disease | 0.8% croton oil | Fisher's exact test | |
|---|---|---|---|
| Male | 9/47 (19%) | 2 /11 (18%) | P = 1.0 |
| Female | 9/43 (21%) | 5/7 (71%) | P = 0.014 |
| Total | 18/90 (20%) | 7/18 (39%) | P = 0.122 |
The number of either unmanipulated mice or mice treated at age 10 days with 0.8% croton oil that developed clinically apparent cutaneous GVHD. All mice tallied here were from the 3339 line of 2-2-3/K14-Aβ
mice.
Discussion
The development of cutaneous pathology in 2-2-3/K14-Aβb mice has three components: an autoreactive CD4+ cell pool, antigen presentation by keratinocytes, and an initiating inflammatory event. In this context, we would characterize the processes leading to skin disease in 2-2-3/K14-Aβb mice as follows: defective thymic selection leads to a repertoire of autoreactive CD4+ cells that respond to keratinocytes, keratinocytes are activated by local inflammatory signals to express MHC class II and costimulatory molecules, and these keratinocytes stimulate autoreactive CD4+ cells to produce Th1 cytokines, which drive further keratinocyte proliferation and macrophage recruitment.
The Autoreactive T Cell Repertoire.
An autoreactive repertoire of CD4+ cells arises in K14-Aβb mice because the thymus lacks expression of I-Ab in anatomic compartments, such as medullary epithelium and bone marrow-derived cells, which effectively mediate clonal deletion. Despite this anatomic limitation, skin disease does not develop under any conditions in K14-Aβb mice with a polyclonal CD4+ cell repertoire, so the 2-2-3 Tg CD4+ cells are required for disease. The 2-2-3 TCR may recognize an I-Ab-associated peptide specifically expressed in keratinocytes. Many proteins (including K14) are expressed by both thymic and skin epithelial cells, and it is possible that positive selection of CD4+ cells by thymic cortical epithelium actually skews the repertoire toward recognition of epithelial antigens without tolerizing those autoreactive cells. In this scenario, the 2-2-3 TCR transgene increases the frequency of autoreactive CD4+ cells specific for keratinocytes past a “tipping point” required for the induction of autoimmunity.
A second possibility is that the presence of the 2-2-3 TCR inhibits tolerance mechanisms that normally function in the K14-Aβb mouse to prevent CD4+ cell-mediated autoimmunity. Approximately 50% of 2-2-3/K14-Aβb CD4+ cells express second endogenous TCR α chains (Fig. 2). In other autoreactive TCR Tg systems, the presence in TCR Tg thymocytes of additional endogenous TCR α chains can inhibit the thymic deletion of autoreactive CD4+ thymocytes. These “escaped” autoreactive cells retain expression of the pathogenic TCR, and animals develop accelerated organ-specific autoimmunity (26, 27). Using this model, we would propose that the pathogenic CD4+ cells in 2-2-3/K14-Aβb mice are those expressing endogenous α chains. Expression of the 2-2-3 TCR transgene would decrease the levels of these autoreactive TCRs to disrupt mechanisms mediated by thymic cortical epithelium [either deletional or anergizing (19)] or MHC class II-positive skin (28, 29) in K14-Aβb mice. We currently are mating nonautoreactive TCR Tg mice to K14-Aβb mice to address this possibility.
Keratinocytes as APCs.
The keratinocytes in polyclonal K14 mice are I-A-negative and should be incapable of activating autoreactive CD4+ cells. We propose that an inflammatory stimulus leads to production of IL-1, and perhaps TNF-α, by keratinocytes and causes expression of the endogenous MHC class II Aαb chain and surface I-Ab expression on keratinocytes, which function as the primary APCs.
Several candidate host APCs previously have been proposed to cause tissue-specific autoimmunity. In a GVHD model of class II-induced tissue destruction, Teshima et al. (30) demonstrated that class II-positive hematopoietic APCs were sufficient to induce disease. This model contrasts with our suggestion that class II-positive keratinocytes present antigens and stimulate disease in the absence of antigen presentation by class II-positive Langerhans cells or other hematopoietic APCs. However, Teshima et al. (30) addressed the sufficiency of antigen presentation by hematopoietic APCs to mediate GVHD, rather than the necessity. The candidate disease-mediating peptides in the current system could be restricted to keratinocytes. In this regard, it is interesting that Hogquist and colleagues (31) have observed similar skin disease when class I-restricted peptides are expressed in keratinocytes via the K14 promoter. In any event, our model would suggest that, in the presence of significant tissue-specific inflammation, tissue parenchymal cells function as primary APCs.
The I-Ab-positive keratinocytes must express costimulatory molecules if they are to function as APCs to naive T cells. Many cutaneous diseases with epidermal T cell infiltration, including psoriasis (32, 33), allergic contact dermatitis, delayed hypersensitivity reactions (34), and cutaneous GVHD, are associated with increased expression of ICAM-1 on keratinocytes. ICAM-1 can be induced on keratinocytes by a variety of stimuli: proinflammatory cytokines such as TNF-α, IL-1, and IFN-γ (reviewed in ref. 35); UV irradiation (36); and histamine (37). Furthermore, we have shown that ICAM-1 is diffusely expressed throughout the dermal and epidermal layers of the diseased skin of 2-2-3/K14-Aβb mice. Conversely, costimulation could be supplied in trans by professional APCs, specifically Langerhans cells or dermal dendritic cells. Costimulation by B7 family members can be provided in vitro by bystander APCs (38), and Mandelbrot et al. (39) have demonstrated elegantly that B7 costimulation provided in trans can mediate solid organ transplantation rejection. In this setting, experiments to compare the development of skin disease in the presence and absence of Langerhans cells and dermal dendritic cells are ongoing.
The inflammatory signal that induces keratinocyte activation could be initiated through multiple pathways that converge in the production of proinflammatory cytokines. Skin irritation induced by croton oil, a plant derivative which contains multiple fatty acids and phorbol esters, stimulates keratinocytes to produce IL-1 and TNF-α. The signal that induces spontaneous disease is less clear. A likely explanation is that routine grooming among littermates leads to minor skin breaks and an inflammatory reaction. The requirement for skin irritation or trauma, rather than T cell activation, in the onset of disease is reminiscent of Koebner's phenomenon, the observation that skin trauma induces psoriasis in susceptible patients. Thus, one potential value of the present mouse model is that the specific events associated with local inflammation that lead to autoimmunity can be studied in detail.
Is this proposed mechanism consistent with the penetrance (20–50% in the two lines) of spontaneous skin disease in genetically identical mice? This low rate suggests there must be a stochastic component to disease induction; we can identify two. One alternative, discussed above, is that disease depends on the endogenous nonclonotypic CD4+ cell repertoire and that the self-reactivity of this repertoire varies between mice. Secondly, the limited time in which mice are susceptible to disease induction by croton oil suggests that skin inflammation must occur within a narrow window before the development of complete self-tolerance. Thus, skin inflammation or trauma sufficient to induce class II expression must occur while the CD4+ repertoire is intolerant; these events may be rare in specific pathogen-free facilities. Indeed, five of five 2-2-3/K14-Aβb mice painted at 10 days of age with oxazalone, a harsher irritant than croton oil, have progressed to cutaneous inflammation. Thus, the rate of disease reflects the stochastic development of the CD4+ cell repertoire and random induction of skin inflammation.
In conclusion, we have developed a model of autoimmune skin disease that requires a repertoire of CD4+ T cells not tolerant of that tissue, initiating inflammatory events that must occur in the tissue, and antigen presentation by keratinocytes. These mice should prove valuable in continuing studies on the pathogenic mechanisms of skin inflammation mediated by T cells.
Acknowledgments
We thank C. Reilly and B. Adair for help with immunohistochemistry, Dr. J. Sekora for help with photography, and Drs. P. Cohen, S. Emerson, and S. Smiley for review of the manuscript. This work was supported by American Cancer Society Institutional Grant IRG 78-002-24 (to the University of Pennsylvania) and by National Institutes of Health Grant RO1 AI48117 (to T.M.L.) and Training Grant 5T32 EY07131 (to B.W.B.).
Abbreviations
- APC
antigen-presenting cell
- LN
lymph node
- TNF-α
tumor necrosis factor α
- ICAM
intercellular adhesion molecule
- K14
keratin 14
- TCR
T cell antigen receptor
- Tg
transgenic
- CFSE
carboxyl fluorescein succinimide ester
- GVHD
graft-versus-host disease
Footnotes
References
- 1.Gallucci S, Lolkema M, Matzinger P. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 2.Höglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Vos A F, Van Meurs M, Brok H P, Boven L A, Hintzen R Q, Van Der Valk P, Ravid R, Rensing S, Boon L, Hart B A, Laman J D. J Immunol. 2002;169:5415–5423. doi: 10.4049/jimmunol.169.10.5415. [DOI] [PubMed] [Google Scholar]
- 4.Kreisel D, Krupnick A S, Gelman A E, Engels F H, Popma S H, Krasinskas A M, Balsara K R, Szeto W Y, Turka L A, Rosengard B R. Nat Med. 2002;8:233–239. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 5.Kupper T S. J Clin Invest. 1990;86:1783–1789. doi: 10.1172/JCI114907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker J N, Mitra R S, Griffiths C E, Dixit V M, Nickoloff B J. Lancet. 1991;337:211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- 7.Lee R T, Briggs W H, Cheng G C, Rossiter H B, Libby P, Kupper T. J Immunol. 1997;159:5084–5088. [PubMed] [Google Scholar]
- 8.Wakem P, Burns R P, Jr, Ramirez F, Zlotnick D, Ferbel B, Haidaris C G, Gaspari A A. J Invest Dermatol. 2000;114:1085–1092. doi: 10.1046/j.1523-1747.2000.00997.x. [DOI] [PubMed] [Google Scholar]
- 9.Bikoff E K, Huang L Y, Episkopou V, van Meerwijk J, Germain R N, Robertson E J. J Exp Med. 1993;177:1699–1712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grusby M J, Johnson R S, Papaioannou V E, Glimcher L H. Science. 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 11.Laufer T M, DeKoning J, Markowitz J S, Lo D, Glimcher L H. Nature. 1996;383:81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 12.Martin W D, Hicks G G, Mendiratta S K, Leva H I, Ruley H E, Van Kaer L. Cell. 1996;84:543–550. doi: 10.1016/s0092-8674(00)81030-2. [DOI] [PubMed] [Google Scholar]
- 13.Laufer T M, Fan L, Glimcher L H. J Immunol. 1999;162:5078–5084. [PubMed] [Google Scholar]
- 14.Berg L J, Fazekas de St. Groth B, Ivars F, Goodnow C C, Gilfillan S, Garchon H J, Davis M M. Mol Cell Biol. 1988;8:5459–5469. doi: 10.1128/mcb.8.12.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho W Y, Cooke M P, Goodnow C C, Davis M M. J Exp Med. 1994;179:1539–1549. doi: 10.1084/jem.179.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rojo J M, Janeway C A., Jr J Immunol. 1988;140:1081–1088. [PubMed] [Google Scholar]
- 17.Kersh G J, Donermeyer D L, Frederick K E, White J M, Hsu B L, Allen P M. J Immunol. 1998;161:585–593. [PubMed] [Google Scholar]
- 18.Haskins K, Portas M, Bradley B, Wegmann D, Lafferty K. Diabetes. 1988;37:1444–1448. doi: 10.2337/diab.37.10.1444. [DOI] [PubMed] [Google Scholar]
- 19.Bensinger S J, Bandeira A, Jordan M S, Caton A J, Laufer T M. J Exp Med. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells A D, Gudmundsdottir H, Turka L A. J Clin Invest. 1997;100:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James S P. In: Current Protocols in Immunology. Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. New York: Wiley; 1994. , Chap. 10.23. [Google Scholar]
- 22.Vassar R, Fuchs E. Genes Dev. 1991;5:714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- 23.Guo L, Yu Q C, Fuchs E. EMBO J. 1993;12:973–986. doi: 10.1002/j.1460-2075.1993.tb05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell I L, Kay T W, Oxbrow L, Harrison L C. J Clin Invest. 1991;87:739–742. doi: 10.1172/JCI115055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ablamunits V, Quintana F, Reshef T, Elias D, Cohen I R. J Autoimmun. 1999;13:383–392. doi: 10.1006/jaut.1999.0331. [DOI] [PubMed] [Google Scholar]
- 26.Sarukhan A, Garcia C, Lanoue A, von Boehmer H. Immunity. 1998;8:563–570. doi: 10.1016/s1074-7613(00)80561-0. [DOI] [PubMed] [Google Scholar]
- 27.Fossati G, Cooke A, Papafio R Q, Haskins K, Stockinger B. J Exp Med. 1999;190:577–583. doi: 10.1084/jem.190.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doan T, Chambers M, Street M, Fernando G J, Herd K, Lambert P, Tindle R. Virology. 1998;244:352–364. doi: 10.1006/viro.1998.9128. [DOI] [PubMed] [Google Scholar]
- 29.Frazer I H, Fernando G J, Fowler N, Leggatt G R, Lambert P F, Liem A, Malcolm K, Tindle R W. Eur J Immunol. 1998;28:2791–2800. doi: 10.1002/(SICI)1521-4141(199809)28:09<2791::AID-IMMU2791>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 30.Teshima T, Ordemann R, Reddy P, Gagin S, Liu C, Cooke K R, Ferrara J L. Nat Med. 2002;8:575–581. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 31.McGargill M A, Mayerova D, Stefanski H E, Koehn B, Parke E A, Jameson S C, Panoskaltsis-Mortari A, Hogquist K A. J Immunol. 2002;169:2141–2147. doi: 10.4049/jimmunol.169.4.2141. [DOI] [PubMed] [Google Scholar]
- 32.Horrocks C, Duncan J I, Oliver A M, Thomson A W. Clin Exp Immunol. 1991;84:157–162. doi: 10.1111/j.1365-2249.1991.tb08140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terajima S, Higaki M, Igarashi Y, Nogita T, Kawashima M. Arch Dermatol Res. 1998;290:246–252. doi: 10.1007/s004030050299. [DOI] [PubMed] [Google Scholar]
- 34.Lewis R E, Buchsbaum M, Whitaker D, Murphy G F. J Invest Dermatol. 1989;93:672–677. doi: 10.1111/1523-1747.ep12319838. [DOI] [PubMed] [Google Scholar]
- 35.Murphy J E, Robert C, Kupper T S. J Invest Dermatol. 2000;114:602–608. doi: 10.1046/j.1523-1747.2000.00917.x. [DOI] [PubMed] [Google Scholar]
- 36.Krutmann J, Grewe M. J Invest Dermatol. 1995;105:67S–70S. doi: 10.1111/1523-1747.ep12316095. [DOI] [PubMed] [Google Scholar]
- 37.Mitra R S, Shimizu Y, Nickoloff B J. J Cell Physiol. 1993;156:348–357. doi: 10.1002/jcp.1041560218. [DOI] [PubMed] [Google Scholar]
- 38.Ding L, Shevach E M. Eur J Immunol. 1994;24:859–866. doi: 10.1002/eji.1830240413. [DOI] [PubMed] [Google Scholar]
- 39.Mandelbrot D A, Kishimoto K, Auchincloss H, Jr, Sharpe A H, Sayegh M H. J Immunol. 2001;167:1174–1178. doi: 10.4049/jimmunol.167.3.1174. [DOI] [PubMed] [Google Scholar]