Abstract
Vaccine efficacy is determined largely by cellular and humoral immunity as well as long-lasting immunological memory. IL-2 and IL-15 were evaluated in vaccinia vectors expressing HIV gp160 for the establishment of an effective vaccine strategy. Both IL-2 and IL-15 in the vaccinia vector induced strong and long-lasting antibody-mediated immunity as well as a short-term cytotoxic T cell response against HIV gp120. In addition, IL-15 also supported robust CD8+ T cell-mediated long-term immunity, whereas the CD8+ T cell-mediated immunity induced by IL-2 was short-lived. Moreover, we found that the cytokine milieu at the time of priming had surprisingly persistent effects on the character of the memory CD8 T cells long afterward with respect to their fate, functional activities, cytokine receptor expression, and antigen-independent proliferation.
One of the major factors that determines the efficacy of vaccines is the long-term maintenance of immunological memory for both humoral and cellular responses. Mounting evidence from recent HIV vaccine studies suggests that both CD8+ and antibody-mediated immunity are important for host defense by curtailing viral replication and neutralizing virus particles, respectively. Therefore, optimal strategies in the development of an effective HIV vaccine should elicit both arms of immunity. In addition, both types of immunity must be of long duration. In this study we compared IL-2 and IL-15 for their efficiency in accomplishing these goals and for the qualitative differences in CD8+ T cells primed in the presence of these cytokines.
Both IL-2 and IL-15 have similar biological characteristics such as activation, proliferation, and cytokine release by various subsets of T, natural killer (NK), and B cells (1–3) and share IL-2Rβ and -γ chains for signal transduction (4, 5). IL-2 is produced by T cells, and IL-15 mRNA is expressed by a broad range of cell types including activated monocytes, dendritic cells, and fibroblasts (3, 6) but not T cells (4). IL-15 also inhibits IL-2-mediated activation-induced cell death (7) and is pivotal in antigen-independent memory CD8+ T cell proliferation (8–11). Although, the role of IL-15 in initiation of the memory phenotype is less well understood, there is evidence to suggest that IL-15 induces CD8+ T cell-dependent protective immunity (12–14). IL-2 is a potent adjuvant for T cell-mediated immunity, and it has been used in many different vaccine studies including HIV vaccines (15–18). Although IL-2 and IL-15 have been found to have different effects on memory T cells (19), the effects of IL-2 on long-term CD8+ T cell-mediated immunity as well as memory B cell immunity remain obscure. IL-15 is known to promote B cell proliferation (2), but its role in antigen-specific B cell immunity and memory is less clear. Here we asked whether the cytokine milieu at the time of first exposure to antigen would alter the character and longevity of CD8+ memory T cells many months later. Study of the impact of IL-2 and IL-15 at the time of immunization on long-term immunity for both cellular and humoral responses may provide important clues for new vaccine strategies against HIV or other infections.
Methods
Viruses.
Recombinant vaccinia viruses (VVs) expressing human IL-15 (VV-IL-15) and IL-2 (VV-IL-2) were generated by standard procedures using pSC11 (20) as the transfer vector. The recombinant VV expressing the full-length HIV-1IIIB gp160 (vPE16) was described previously (21). We also made dual recombinant vaccinia vectors that express both gp160 and human IL-15 or IL-2 by using vPE16.
Animal Immunization.
Female BALB/c mice were used at 6–8 weeks of age. Mice were immunized s.c. in the base of the tail with different combinations of recombinant VVs and boosted after 3 weeks. Control mice received vPE16 and vSC-11. All animals were immunized with 6–7 × 106 plaque-forming units of each virus at priming and boost.
Peptides, Media, and Cells.
Both β-galactosidase (β-gal) peptide (TPHPARIGL) and P18-I10 (RGPGRAFVTI) peptides were commercially synthesized. For in vitro restimulation, partially purified CD8+ T cells (4 × 106) from animal spleens were cultured with γ-irradiated (3,000 rad) syngeneic splenocytes, 2 × 106 per well in 24-well plates. RPMI medium 1640 supplemented with 10% rat T-Stim (Collaborative Biomedical Products, Bedford, MA) was used, and peptides were added to the wells in a soluble form. For the proliferation assay, spleen CD8+ T cells from animals were purified positively by using antibody-coated magnetic beads (Miltenyi Biotec, Auburn, CA) following manufacturer instructions. Cells were labeled with 5-(and -6)-carboxyfluorescein diacetate-succinimidyl ester (CFSE) (22, 23). For an in vivo proliferation assay, 6 × 106 CFSE-labeled CD8+ T cells were transferred into naive animals by i.v. injection. For an in vitro assay, cells were resuspended into RPMI medium 1640 and plated at 1 × 105 per well in 24-well culture plates. Ten units/ml IL-2 or 50 ng per well IL-15 were added into the culture. For another set of in vitro proliferation assays, 2 × 104 P18-I10-specific CD8+ T cells purified by Epics Elite ESP sorter (Beckman Coulter) were labeled with CFSE and then plated in 96-well flat-bottomed plates. Cells were pulsed with 1 μCi of [3H]thymidine (1 Ci = 37 GBq) 18 h before harvesting and then counted by using a Microbeta plate counter (Wallac, Gaithersburg, MD).
51Cr-Release Assay.
Antigen-specific cytolytic activity of CD8+ T cells was measured with a 5-h 51Cr-release assay. Target cells, P815, were pulsed with β-gal peptide and P18-I10 peptide, respectively. Effector cells were restimulated with 0.005 μM β-gal peptide and 0.001 μM P18-I10 peptide for 1 week. To measure NK cell activity, Yac-1 cells were used as target cells. NK cells were activated by a 5-day incubation in RPMI medium 1640 containing 10% T-Stim. The percentage of specific lysis was calculated as 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release). Maximum release was determined from supernatants of cells that were lysed by the addition of 2% Triton X-100.
Antibodies, Tetramer, and Flow Cytometry.
Fluorochrome-labeled anti-mouse CD8 (53-6.7), CD44 (IM-7), CD62L (MEL-14), CD69 (H1.2F3), and anti-goat IgG were purchased from PharMingen. Goat anti-human IL15Rα was from R & D Systems, and crossreactivity with mouse IL-15Rα was tested by using IL-15Rα (−/−) mice from The Jackson Laboratory. For intracellular IFN-γ staining, all reagents were purchased from PharMingen, and staining was carried out by following the manufacturer's protocol. Phycoerythrin-labeled H-2Dd–P18-I10 tetramer was provided by the National Institutes of Health Tetramer Core Facility (Atlanta). For flow-cytometric analysis of cell surface, cells were washed and resuspended in PBS containing 0.2% BSA and 0.1% sodium azide. Cells were incubated on ice with the appropriate antibody or tetramer for 20–30 min and then washed. Samples were analyzed on a FACScan (Becton Dickinson). Background staining was assessed by use of an isotype control antibody (PharMingen).
Antibody ELISA.
Antibody titers against HIV gp120 in the serum of immunized mice were determined by an ELISA. Briefly, plates were first coated with goat anti-HIV gp120 polyclonal antibodies overnight followed by the addition of recombinant gp120 at a concentration of 1.0 μg/ml in blocking buffer containing 20 mM Tris, 150 mM NaCl, 0.1% Tween 20, and 0.1%BSA. After a 2-h incubation at room temperature, mouse serum was added, with appropriate dilutions being made in the blocking buffer. After a 2-h incubation at room temperature and extensive washing, HIV gp120-bound mouse antibodies then were detected with a goat anti-mouse horseradish peroxidase-conjugate. Antibodies and recombinant gp120 were from the National Institute of Allergy and Infectious Diseases AIDS Research and Reference Reagent Program.
Results
In Vivo Persistence of Antigen-Specific CD8+ Cytotoxic T Lymphocytes (CTLs).
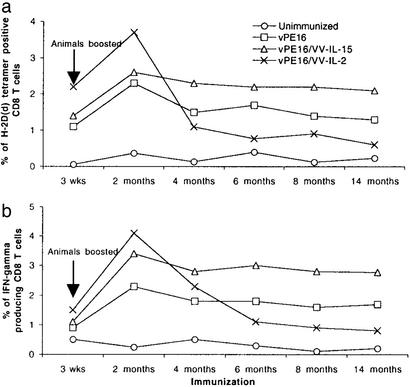
The number of antigen-specific memory CD8+ T cells after viral infection or vaccination does not seem to depend on a single factor. Nonetheless, in terms of the maintenance of memory CD8+ T cells, IL-15 plays an important role in antigen-independent proliferation. We initially examined the role of IL-15 as a vaccine adjuvant in the induction as well as the maintenance of memory CD8+ T cells over a period of 14 months. To determine the frequency of antigen-specific CD8+ T cells in the spleens of animals immunized with recombinant vaccinia expressing gp160, we measured the number of H-2Dd–P18-I10 tetramer-positive CD8+ T cells. P18-I10 is an immunodominant epitope of HIV-1 gp160 presented by H-2Dd, thus the tetramers of this complex will stain the major population of antigen-specific CTLs induced by immunization with HIV-1 gp160-expressing VVs (24, 25). As shown in Fig. 1a, mice coimmunized with VV-IL-2 showed the highest frequency of antigen-specific CD8+ T cells during the first 2 months after the boost. In this period, mice coimmunized with VV-IL-15 showed a slightly increased number of antigen-specific CD8+ T cells compared with control mice, suggesting that the role of IL-15 in enhancing the early phase of immune response was not substantial. Between 2 and 4 months after the boost, however, the number of antigen-specific CD8+ T cells in the mice coimmunized with VV-IL-2 declined remarkably. Control mice immunized with vPE16 virus that expresses HIV gp160 alone showed a steadily decreasing number of antigen-specific CD8+ T cells by 4 months after the boost as well. Importantly, mice coimmunized with VV-IL-15 maintained relatively higher numbers of antigen-specific CD8+ CTL from 4 months until at least 14 months after the boost compared with the two other groups of animals. In contrast, at this latter time point mice coimmunized with VV-IL-2 had almost no detectable level of antigen-specific CD8+T cells.
Figure 1.
The fate of long-term memory CD8+ T cells is determined largely by the cytokine present during the early phase of the immune response. Animals were immunized with viruses s.c. in the base of the tail and boosted once after 3–4 weeks. (a) The number of P18-I10-specific CD8+ T cells in the spleen was measured by H-2Dd–P18-I10 tetramer staining. (b) The number of CD8+ T cells that were producing IFN-γ in response to specific antigen. CD8+ T cells from the spleens of vaccinated animals were restimulated with P18-I10 and stained for intracellular IFN-γ.
As shown in Fig. 1b, the percentages of IFN-γ-producing CD8+ T cells were relatively consistent with the data in Fig. 1a. By 2 months after the boost, mice coimmunized with VV-IL-2 showed the greatest number of IFN-γ-producing cells, but this number steadily decreased by 6 months after the boost. From 2 months after the boost onward, more IFN-γ-producing CD8+ T cells were counted in the mice coimmunized with VV-IL-15 than control mice, in parallel with the observations made by tetramer staining (Fig. 1a). Compared with the control mice, however, the overall numbers of P18-I10-specific CD8+ CTLs in both groups were higher when determined by IFN-γ staining than when determined by tetramer staining, suggesting that functional activities of CD8+ T cells in each group of mice may not be the same even though they all are specific for the same peptide antigen, P18-I10. This difference may reflect the fact that the IFN-γ staining may be more sensitive.
Cytolytic Activity of Antigen-Specific CD8+ CTLs and NK Cells.
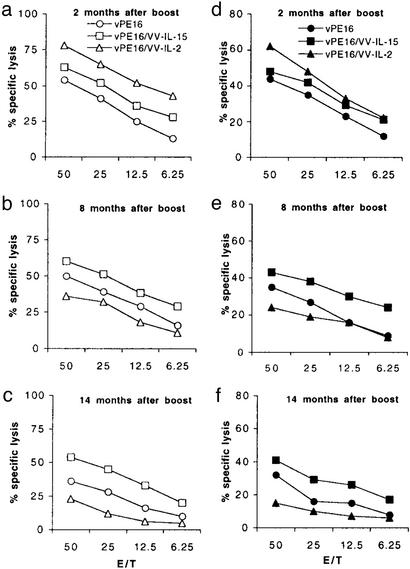
Cytolytic activity of CD8+ CTLs was tested by using P18-I10 or β-gal peptide-pulsed target cells (because these recombinant viruses express both β-gal and HIV-1 gp160). Consistent with the number of tetramer-positive CD8+ T cells, mice coimmunized with VV-IL-2 showed the greatest lytic activity to both P18-I10 and β-gal-pulsed target cells until 2 months after the boost (Fig. 2 a and d). However, from 4 months after the boost onward, animals that received VV-IL-2 showed remarkably decreased lytic activity (data not shown) such that the levels of cytolytic activity at 8 months after the boost showed a permuted rank order (IL-15 > no cytokine > IL-2) (Fig. 2 b and e). At 14 months, the activity had almost disappeared in the mice coimmunized with VV-IL-2 (Fig. 2 c and f). Compared with VV-IL-2, however, animals that received VV-IL-15 showed a largely undiminished level of CTL activity to both P18-I10 and β-gal peptide-pulsed target cells even at 14 months after the boost. In addition, CD8 T cells reactive to P18-I10 were more persistent in vivo than CD8+ T cells to β-gal peptide, an observation that may be because the persistence of memory CD8+ T cells is also dependent on the characteristics of the antigen itself.
Figure 2.
Cytolytic activity of CD8+ CTLs after 1-week restimulation with antigen in vitro. Spleen CD8+ T cells from each group of animals were stimulated in vitro with 1.0 nM P18-I10 (a–c) or 5 nM β-gal peptide (d–f) for 1 week. Peptide-pulsed P815 cells were used as target cells in a 5-h 51Cr-release assay.
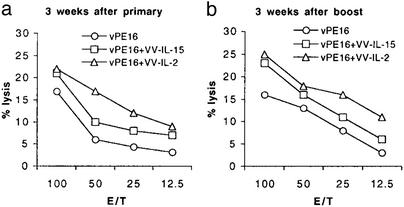
In comparing the NK activity after primary immunization, animals coimmunized with VV-IL-2 showed a greater lytic activity than the two other groups of animals (Fig. 3a). Similarly, 3 weeks after booster immunization, mice coimmunized with VV-IL-2 consistently showed a greater lytic activity than the other two groups of animals, although mice coimmunized with VV-IL-15 showed slightly increased activity, when compared with the control group (Fig. 3b).
Figure 3.
Lytic activity of NK cells in the mice immunized with recombinant vaccinia expressing IL-2 or IL-15. Spleen cells from each group of animals were cultured in medium containing 10% rat T-Stim for 5 days, and then lytic activity was measured by using YAC-1 as target cells.
In Vitro Proliferation of CD8+ T Cells.
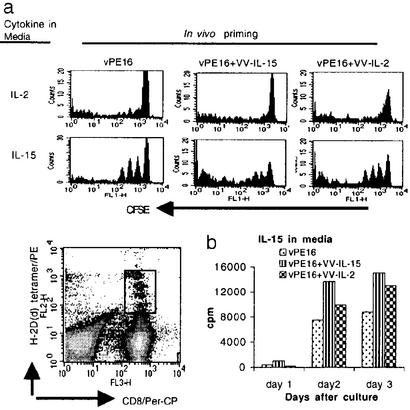
We have shown above that the life span and functional activity of CD8+ T cells induced in different cytokine environments are not the same. Because VV is cleared within ≈1 week, vaccinia-expressed cytokine production does not persist. In fact, even during the acute phase of the vaccinial infection after immunization, no detectable levels of either IL-2 or IL-15 were present in the sera of immunized mice (data not shown). To address the possible mechanism by which mice coimmunized with VV-IL-15 but not VV-IL-2 can maintain memory CD8+ T cells for an extended period, we examined the responsiveness of CD8+ T cells to IL-2 and IL-15 in vitro. Although it is known that memory CD8+ T cells can proliferate in response to IL-15, we show that the responsiveness of CD8+ T cells to IL-15 depends on the cytokine milieu present during priming such that CD8+ T cells from each group of animals show different degrees of proliferation even several months after the priming. Antigen-specific CD8+ T cells from all three groups showed no significant proliferation in the medium supplemented with IL-2 alone (Fig. 4a Upper). In striking contrast, IL-15-supplemented medium supported CD8+ T cells from all three groups. Furthermore, CD8+ T cells from the mice coimmunized with VV-IL-15 showed greater proliferation in response to IL-15 than did the CD8+ T cells from the other two groups. Although CFSE-staining data (Fig. 4a) on day 3 showed that CD8+ T cells from the mice coimmunized with VV-IL-2 proliferated better than CD8+ T cells from control mice, the early responsiveness to IL15 was not greater as measured by [3H]thymidine incorporation (Fig. 4b). CD8+ T cells in the mice coimmunized with VV-IL-2 proliferated mostly late in the incubation period, suggesting that the expression levels of receptors for IL-15 or some other biochemical characteristics involved in cell proliferation are not the same in the cells from the different groups of animals.
Figure 4.
CD8+ T cells induced with IL-15 showed better responsiveness to exogenous IL-15. (a) Positively purified CD8+ T cells from each group of animals were labeled with CFSE and then cultured for 3 days in the medium containing IL-2 or IL-15. Proliferation was measured by gating on anti-CD8α and P18-I10–H-2Dd tetramer-positive cells. (b) P18-I10-specific CD8+ T cells were purified by sorting anti-CD8α and P18-I10–H-2Dd tetramer-positive cells. Cells were cultured and proliferation was measured by determining [3H]thymidine uptake.
Proliferation of Memory CD8+ T Cells in Naive Animals.
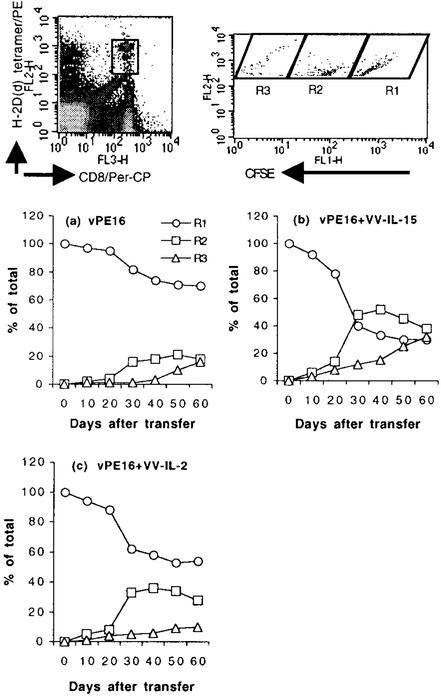
To assess the natural behavior of antigen-specific memory CD8+ T cells in vivo, we transferred CD8+ T cells from each group of mice to naive mice and monitored their proliferation for 2 months. Concordant with the in vitro proliferative response to IL-15, the greatest CD8+ T cell proliferation was observed in the mice that received CD8+ T cells induced with VV-IL-15 (Fig. 5). On day 30 after transferring the cells from VV-IL-15-immunized mice, >60% of the P18-I10-specific CD8+ T cells had already proliferated at least once, and the percentage of the sum of gates R2 and R3 (proliferating cells) was augmented continuously. On day 60, the percentage of CD8+ T cells in gate R3 was >30%, suggesting that these cells continuously proliferate, albeit slowly. Some CD8+ T cells from control mice also proliferated in naive animals, but the rate of proliferation was minimal. Even after 60 days, >65% of the cells survived without proliferation. Although almost 50% of the CD8+ T cells induced with VV-IL-2 proliferated at least once during the 60 days, there was no significant increase in gate R3 (cells dividing multiple times), suggesting that either these cells proliferate much more slowly than did VV-IL-15-induced cells or that the majority of the proliferating antigen-specific CD8 T cells may disappear by an apoptotic process. The data in Fig. 5 also indicated that only some subpopulations of the CD8+ T cells from each group of animals proliferate in vivo. Most of the proliferating CD8+ T cells in all three groups of animal responded by 30 days posttransfer, and there was no meaningful proliferation after 30 days of transfer, suggesting that only some of the CD8+ T cells may respond to IL-15, possibly related to different levels of IL-15Rα receptor expression. It is conceivable that cells expressing IL-15Rα are more sensitive to the low levels of endogenous IL-15 found in normal mice. It is important to note that some of the memory CD8+ T cells may not proliferate or that there are at least two different types of memory CD8+ T cells based on our in vitro and in vivo proliferation data. Thus, we conclude that cytokines supplemented during CD8+ T cell priming can influence the fate of CD8+ T cells as well as the quality of CD8+ T cells months later.
Figure 5.
In vivo proliferation of antigen-specific CD8+ memory T cells does not depend merely on the cytokine in the host but on the property of CD8+ T cells. CD8+ T cells from each group of animals were positively purified at 3 months after boost, and CFSE-labeled cells were transferred to naive mice. Spleen cells were stained with anti-CD8α and P18-I10–H-2Dd tetramer, and double-positive cells were analyzed by FACScan to determine antigen-specific CD8+ T cell proliferation. Based on the intensity of CFSE, cells were gated. R1, R2, and R3 reflect cells with no, one, or more than one cell division in vivo, respectively.
Expression of Cell-Surface Molecules in CD8+ T Cells.
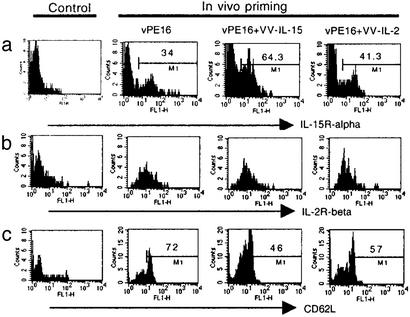
We have shown that CD8+ T cells induced with IL-15 proliferated better than CD8+ T cells induced with IL-2 or vPE16 alone even without T cell antigen receptor recognition. One possible explanation we hypothesized was the up-regulation of receptors for IL-15 by exposure to IL-15. To address this question, we examined the expression levels of IL-15Rα by staining CD8+ T cells from fresh spleens with anti-IL-15Rα. Τhe results showed that the diverse groups of mice had different percentages of CD8+ T cells that express IL-15Rα (Fig. 6), with 34% in control mice and 64% and 41% for the mice that received IL-15 and IL-2, respectively. We also stained antigen-specific CD8+ T cells with anti-IL-2Rα and -IL-2Rβ, and all memory CD8+ T cells expressed IL-2Rβ. A small percentage (30–35%) of CD8+ T cells was positive for IL-2Rα, but there was no significant difference in the expression levels of IL-2Rα among the groups of animals. It has been reported that IL-2Rβ-expressing CD8+ T cells can proliferate in response to exogenous IL-15 (11) albeit at supraphysiological 10−9 M concentrations of IL-15, but our data suggested that differences in IL-15-dependent CD8+ T cell proliferation are more dependent on IL-15Rα, which requires only 10−11 M IL-15. Taken together, our data indicate that administration of a vaccine expressing IL-15 can yield a higher percentage of IL-15Rα-expressing CD8+ memory T cells, and those cells can proliferate better in response to IL-15 in vivo and in vitro.
Figure 6.
IL-15 induces increased numbers of CD8+ T cells that express IL-15Rα and CD62Llow. Antigen-specific CD8+ T cells from the spleens of each group of animals were stained with anti-IL-15Rα (a), anti IL-2Rβ (b), or anti-CD62L (c). Anti-CD8α and P18-I10–H-2Dd tetramer double-positive cells were gated for the analysis of both IL-15Rα and CD62L expression level.
It is clear that CD44highCD62Llow CD8+ T cells, effector memory T cells, respond better than CD44highCD62Lhigh CD8+ T cells, central memory T cells (26) in response to T cell antigen receptor recognition. During the experiment, therefore, we monitored the expression levels of the cell-surface molecules CD44, CD69, and CD62L. Although animals that received VV-IL-15 showed slightly increased numbers (5%) of CD44high CD8+ T cells compared with the animals in the two other groups in the early phase of priming and boosting, the percentage of CD44high CD8+ T cells in the different groups was similar, and >90% of the P18-I10-specific CD8+ T cells in all three groups were CD44high 2 months after the boost (data not shown). However, the mice that received either VV-IL-2 or VV-IL-15 had greater numbers of CD62Llow CD8+ T cells (Fig. 6) 3 months after the boost than mice that received VPE16. Compared with these two groups, the majority of P18-I10-specific CD8+ T cells (>70%) in the mice immunized with vPE16 alone were CD62Lhigh, suggesting that a higher percentage of CD8+ T cells induced with either IL-2 or IL-15 were probably effector memory CD8+ T cells, and CD8+ T cells induced by vPE16 alone were probably central memory T cells. It is also interesting to note that the percentage of CD62Llow CD8+ T cells in each group is consistent with the percentage of CD8+ T cells that proliferated during the 60 days after transferring as shown in Fig. 5. Thus, IL-15 seems to enhance the frequency of effector memory CD8+ T cells.
Maintenance of Memory B Cells.
Numerous studies have addressed the role of IL-2 as a potent vaccine adjuvant, particularly for both CD4+ and CD8+ T cell responses. Both VV-IL-2 (27) and VV-IL-15 (28) are also known to enhance natural immunity in a short period. However, the effects of IL-2 and IL-15 in antigen-specific humoral immune responses remain less clear. In this study we tested the role of IL-15 in the humoral immune response and compared it with that of IL-2.
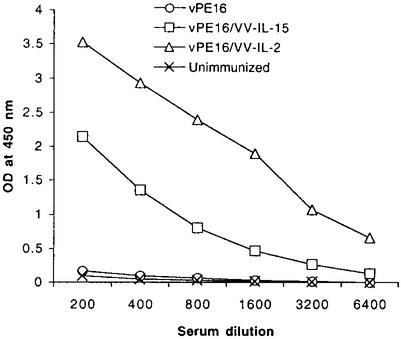
After primary immunization with or without cytokine, there was no significant difference in the antibody titer against gp120 (data not shown). However, animals coimmunized with VV-IL-2 or VV-IL-15 showed significantly increased titers of antibody after the boost. Fig. 7 shows the anti-gp120 titer at 8 months after the boost. Although we could detect a measurable amount of anti-gp120 from the animals immunized with vPE16 alone at 6 months after the boost (data not shown), those animals no longer had a significant concentration of antibody by 8 months. Compared with titers in the control animals, antibody titers in animals coimmunized with IL-2 or IL-15 did not wane even by 8 months after the boost, illustrating the ability of these two cytokines to invoke a sustainable humoral response.
Figure 7.
Both IL-2 and IL-15 coexpressing vaccines resulted in a strong memory B cell response. Sera from five mice in each group were collected at 8 months after the boost and analyzed for antibody titer against gp120 by using sandwich ELISA as described in Methods.
Discussion
Memory for both CD8+ T cell and antibody-mediated immunity is crucial for host defense against many viral infections. Recent evidence suggests that the number and longevity of memory CD8+ T cells are determined by the types and strength of signals that T cells receive (29), clonal expansion and burst size (30, 31), and the differentiation mechanism for antigen-specific CD8+ T cells (29). We show here that IL-2 and IL-15 substantially affect immune memory for both humoral and cellular responses, and that the quality and phenotype of memory CD8+ T cells acquired during priming in the presence of IL-15 persist for a long time.
In this study we compared the utility of IL-2 and IL-15 as vaccine components for inducing both long-lasting cellular and humoral immunity. In the early phase of immune responses, animals that received IL-2 had the highest frequency of antigen-specific CD8+ T cells. This finding was not surprising in that IL-2 is known to affect both CD4+ and CD8+ T cell activation and clonal expansion. Although IL-2 can induce activation-induced cell death (32, 33), this is not overwhelming in the total immune response during priming of naive and memory CD8+ T cells. Animals that received IL-15 showed only a slightly increased frequency of antigen-specific CD8+ T cells compared with control mice in the early phase, supporting the view that IL-15 is not necessary for the induction of antigen-specific memory CD8+ T cells in normal mice (34). Schluns et al. (35) reported that IL-15 is required for both primary and memory CD8+ T cell response, but it is not clear whether IL-15 is required for the induction or just the expansion of memory CD8+ T cells.
In contrast to the early phase of the immune response, the number of antigen-specific CD8+ T cells induced with supplemental IL-2 was remarkably reduced at 4 months after the boost. However, the frequency of CD8+ T cells induced with IL-15, on the other hand, unlike the immunizations with IL-2 or no cytokine, was very persistent until at least 14 months after the boost, suggesting that IL-15 during priming may alter the quality rather than just the quantity of CD8+ T cells during the immune-induction phase. Our data also indicated that the number of long-lived memory CD8+ T cells depends more on the quality of CD8+ T cells than the burst size of effector cells (30, 36). In addition, the cytokine milieu during the priming may be one of the major factors that determine the life and quality of memory CD8+ T cells. Although IL-15 inhibits activation-induced cell death (7, 37), this cannot explain the long-term effects, because animals that received either VV-IL-2 or VV-IL-15 cleared virus within 1 or 2 weeks, and therefore long-term expression of cytokines by vaccinia cannot be involved.
One unexpected qualitative difference seems to be a higher expression of IL-15Rα, induced by priming in the presence of IL-15, that persists for many months and results in greater sensitivity to IL-15 in vivo. It is believed that memory CD8+ T cells persist by a homeostatic proliferation in an antigen-independent manner (8, 11, 31, 38). As part of the evidence, several studies showed that IL-15 alone can support memory CD8+ T cell proliferation (8–11). However, it is unclear that all memory CD8+ T cells respond to IL-15 in the same way. We have shown that the quality, functional activity and expression of cell-surface molecules (CD62L), of antigen-specific memory CD8+ T cells in different group of animals are not the same, suggesting that the responsiveness of all CD8+ T cells to IL-15 may not be the same. In support of this hypothesis, CD8+ memory T cells in animals that received IL-15 proliferated better than CD8+ T cells in the other two groups in response to exogenous IL-15 in vitro. However, this does not prove that CD8+ T cells from IL-15-treated animals would proliferate better under physiological conditions in vivo. Data from the adoptive-transfer experiments clearly show that antigen-specific memory CD8+ T cells from each group of animals do not proliferate in the same manner, and in particular, CD8+ T cells from animals that received IL-15 proliferated more in vivo than CD8+ T cells from the other groups. One possible explanation for this observation is the increased number of CD8+ T cells that express IL-15Rα in the animals receiving IL-15 during priming. Notably, each group of animals had a similar percentage of CD8+ T cells that express IL-2Rβ, thus suggesting that the proliferation of CD8+ T cells depends more on variations in IL-15Rα than in IL-2Rβ.
Although the biological mechanism by which both IL-2 and IL-15 result in the long-lasting humoral immunity remains to be determined, we report here that both IL-2 and IL-15 delivered with antigen significantly enhance B cell memory. We showed previously that IL-2 incorporated in the adjuvant increased the magnitude of the antibody response in low responder mice, but B cell memory was not assessed in that study. In many studies (18, 39–41), IL-2 was tested as a vaccine adjuvant for enhancing CD8+ T cell-mediated immunity and/or CD4+ helper T cells. It can be suggested that IL-2 induces enhanced CD4+ helper T cell responses that act on B cells through the interaction of CD40-CD40L, resulting in enhanced B cell responses. Recent data showed that IL-15 supports human B cell proliferation (42) in combination with CD40L (2) and regulates differentiation of B-1 cells into IgA-producing cells. However, the precise mechanism by which IL-15 induces enhanced memory B cell-mediated immunity remains to be elucidated.
It is important to note that the findings reported herein are from experiments where animals have been coimmunized with two recombinant viruses, i.e., one expressing the HIV gp160 and the other expressing cytokine adjuvant separately. In improving the versatility of our vaccine vectors we subsequently generated dual recombinant VVs that express both HIV gp160 and IL-2 or IL-15. Animals immunized with dual recombinants displayed immunological profiles concordant with those of animals immunized with two separate viruses.
More importantly, our data collectively support the notion that IL-2 directly or more likely indirectly affects the longevity of CD8 T cell-mediated immune responses adversely despite its short-term stimulatory effects. Thus, we propose that IL-15, with its capacity to invoke sustainable cellular and humoral responses, is a superior cytokine adjuvant that can be used in developing an effective vaccine against HIV.
Abbreviations
- NK
natural killer, VV, vaccinia virus
- β-gal
β-galactosidase
- CFSE
carboxyfluorescein diacetate-succinimidyl ester
- CTL
cytotoxic T lymphocyte
References
- 1.Carson W E, Giri J G, Lindemann M J, Linett M L, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri M A. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitage R J, Macduff B M, Eisenman J, Paxton R, Grabstein K H. J Immunol. 1995;154:483–490. [PubMed] [Google Scholar]
- 3.Grabstein K H, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn M A, Ahdieh M, et al. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 4.Waldmann T A, Tagaya Y. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 5.Tagaya Y, Bamford R N, DeFilippis A P, Waldmann T A. Immunity. 1996;4:329–336. doi: 10.1016/s1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- 6.Bamford R N, Grant A J, Burton J D, Peters C, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. Proc Natl Acad Sci USA. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marks-Konczalik J, Dubois S, Losi J M, Sabzevari H, Yamada N, Feigenbaum L, Waldmann T A, Tagaya Y. Proc Natl Acad Sci USA. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 9.Murali-Krishna K, Lau L L, Sambhara S, Lemonnier F, Altman J, Ahmed R. Science. 1999;286:1377–1380. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 10.Swain S L, Hu H, Huston G. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Sun S, Hwang I, Tough D F, Sprent J. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 12.Yajima T, Nishimura H, Ishimitsu R, Yamamura K, Watase T, Busch D H, Pamer E G, Kuwano H, Yoshikai Y. Eur J Immunol. 2001;31:757–766. doi: 10.1002/1521-4141(200103)31:3<757::aid-immu757>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 13.Yajima T, Nishimura H, Ishimitsu R, Watase T, Busch D H, Pamer E G, Kuwano H, Yoshikai Y. J Immunol. 2002;168:1198–1203. doi: 10.4049/jimmunol.168.3.1198. [DOI] [PubMed] [Google Scholar]
- 14.Umemura M, Nishimura H, Hirose K, Matsuguchi T, Yoshikai Y. J Immunol. 2001;167:946–956. doi: 10.4049/jimmunol.167.2.946. [DOI] [PubMed] [Google Scholar]
- 15.Tryniszewska E, Nacsa J, Lewis M G, Silvera P, Montefiori D, Venzon D, Hel Z, Parks R W, Moniuszko M, Tartaglia, et al. J Immunol. 2002;169:5347–5357. doi: 10.4049/jimmunol.169.9.5347. [DOI] [PubMed] [Google Scholar]
- 16.Moore A C, Kong W P, Chakrabarti B K, Nabel G J. J Virol. 2002;76:243–250. doi: 10.1128/JVI.76.1.243-250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sin J I, Kim J J, Zhang D, Weiner D B. Hum Gene Ther. 2001;12:1091–1102. doi: 10.1089/104303401750214302. [DOI] [PubMed] [Google Scholar]
- 18.Barouch D H, Craiu A, Kuroda M J, Schmitz J E, Zheng X X, Santra S, Frost J D, Krivulka G R, Lifton M A, Crabbs C L, et al. Proc Natl Acad Sci USA. 2000;97:4192–4197. doi: 10.1073/pnas.050417697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ku C C, Murakami M, Sakamoto A, Kappler J, Marrack P. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 20.Chakrabarti S, Brechling K, Moss B. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Earl P L, Hugin A W, Moss B. J Virol. 1990;64:2448–2451. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gudmundsdottir H, Wells A D, Turka L A. J Immunol. 1999;162:5212–5223. [PubMed] [Google Scholar]
- 23.Lyons A B, Parish C R. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi H, Cohen J, Hosmalin A, Cease K B, Houghten R, Cornette J, DeLisi C, Moss B, Germain R N, Berzofsky J A. Proc Natl Acad Sci USA. 1988;85:3105–3109. doi: 10.1073/pnas.85.9.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeshita T, Takahashi H, Kozlowski S, Ahlers J D, Pendleton C D, Moore R L, Nakagawa Y, Yokomuro K, Fox B S, Margulies D H, et al. J Immunol. 1995;154:1973–1986. [PubMed] [Google Scholar]
- 26.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Nature. 1999;401:708–712. [PubMed] [Google Scholar]
- 27.Hugin A W, Flexner C, Moss B. Cell Immunol. 1993;152:499–509. doi: 10.1006/cimm.1993.1307. [DOI] [PubMed] [Google Scholar]
- 28.Perera L P, Goldman C K, Waldmann T A. Proc Natl Acad Sci USA. 2001;98:5146–5151. doi: 10.1073/pnas.081080298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaech S M, Wherry E J, Ahmed R. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 30.Hou S, Hyland L, Ryan K W, Portner A, Doherty P C. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 31.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 32.Lenardo M J. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 33.Dai Z, Arakelov A, Wagener M, Konieczny B T, Lakkis F G. J Immunol. 1999;163:3131–3137. [PubMed] [Google Scholar]
- 34.Becker T C, Wherry E J, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schluns K S, Williams K, Ma A, Zheng X X, Lefrancois L. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 36.Doherty P C, Hou S, Tripp R A. Curr Opin Immunol. 1994;6:545–552. doi: 10.1016/0952-7915(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 37.Ohta N, Hiroi T, Kweon M N, Kinoshita N, Jang M H, Mashimo T, Miyazaki J, Kiyono H. J Immunol. 2002;169:460–468. doi: 10.4049/jimmunol.169.1.460. [DOI] [PubMed] [Google Scholar]
- 38.Gasser S, Corthesy P, Beerman F, MacDonald H R, Nabholz M. J Immunol. 2000;164:5659–5667. doi: 10.4049/jimmunol.164.11.5659. [DOI] [PubMed] [Google Scholar]
- 39.Chow Y H, Huang W L, Chi W K, Chu Y D, Tao M H. J Virol. 1997;71:169–178. doi: 10.1128/jvi.71.1.169-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barouch D H, Santra S, Steenbeke T D, Zheng X X, Perry H C, Davies M E, Freed D C, Craiu A, Strom T B, Shiver J W, et al. J Immunol. 1998;161:1875–1882. [PubMed] [Google Scholar]
- 41.Barouch D H, Kunstman J, Kuroda M J, Schmitz J E, Santra S, Peyerl F W, Krivulka G R, Beaudry K, Lifton M A, Gorgone D A, et al. Nature. 2002;415:335–339. doi: 10.1038/415335a. [DOI] [PubMed] [Google Scholar]
- 42.Seder R A, Grabstein K H, Berzofsky J A, McDyer J F. J Exp Med. 1995;182:1067–1078. doi: 10.1084/jem.182.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]