Abstract
Natriuretic peptides (NPs), which consist of atrial, brain, and C-type natriuretic peptides (ANP, BNP, and CNP, respectively), are characterized as cardiac or vascular hormones that elicit their biological effects by activation of the cGMP/cGMP-dependent protein kinase (cGK) pathway. We recently reported that adenoviral gene transfer of CNP into rabbit blood vessels not only suppressed neointimal formation but also accelerated reendothelialization, a required step for endothelium-dependent vasorelaxation and antithrombogenicity. Accordingly, we investigated the therapeutic potential of the NPs/cGMP/cGK pathway for vascular regeneration. In transgenic (Tg) mice that overexpress BNP in response to hindlimb ischemia, neovascularization with appropriate mural cell coating was accelerated without edema or bleeding, and impaired angiogenesis by the suppression of nitric oxide production was effectively rescued. Furthermore, in BNP-Tg mice, inflammatory cell infiltration in ischemic tissue and vascular superoxide production were suppressed compared with control mice. Ischemia-induced angiogenesis was also significantly potentiated in cGK type I Tg mice, but attenuated in cGK type I knockout mice. NPs significantly stimulated capillary network formation of cultured endothelial cells by cGK stimulation and subsequent Erk1/2 activation. Furthermore, gene transfer of CNP into ischemic muscles effectively accelerated angiogenesis. These findings reveal an action of the NPs/cGMP/cGK pathway to exert multiple vasculoprotective and regenerative actions in the absence of apparent adverse effects, and therefore suggest that NPs as the endogenous cardiovascular hormone can be used as a strategy of therapeutic angiogenesis in patients with tissue ischemia.
Natriuretic peptides (NPs) consist of atrial NP (ANP), brain NP (BNP), and C-type NP (CNP). They share the same intracellular signal transduction pathway for cGMP/cGMP-dependent protein kinase (cGK) as nitric oxide (NO). We have demonstrated that ANP and BNP are cardiac hormones that are produced mainly in the atrium and ventricle, respectively (1, 2). CNP, in contrast, is produced in and secreted from endothelial cells (ECs) to act as a local regulator of vascular tone and growth (3).
We recently reported that in both rabbit balloon injury and vein graft models, overexpression of the CNP gene by adenoviral vector accelerated reendothelialization and inhibited vascular smooth muscle proliferation (4, 5). This finding indicates the complex responses to NPs in different types of vascular cells, both ECs and smooth muscle cells (SMCs).
A large body of literature indicates an essential role of endothelial NO for angiogenesis. Previous studies demonstrated that vascular endothelial growth factor (VEGF) stimulates Akt/protein kinase B (6, 7), which has been shown to phosphorylate endothelial NO synthase, leading to its activation (8, 9). VEGF-stimulated proliferation of cultured ECs, triggered by endothelial NO synthase activation (10), was also shown to require intracellular signaling through cGK, Raf-1 kinase, and Erk1/2 (11, 12). Based on these findings, we hypothesized that NPs could promote vascular regeneration. To examine this hypothesis, we used a mouse model of operatively induced hindlimb ischemia (13) to investigate the effects of NPs on angiogenesis by using transgenic (Tg) mice that overexpress BNP (14) with or without Nω-nitro-l-arginine methyl ester (l-NAME), an inhibitor of NO synthase. We also applied this model to both cGK type I (cGKI)-knockout (15) and Tg mice to investigate the impact of cGKI, one of the cGK isoforms, which is present in ECs and SMCs. Finally, we examined the effect of CNP on angiogenesis by a gene-transfer approach to seek the therapeutic potentials of NPs in vascular regeneration. The present study elucidates the action of the NPs/cGMP/cGKI pathway on angiogenesis and provides a strategy for therapeutic angiogenesis by using an endogenous cardiovascular hormone to exert vasculoprotective and vasculoregenerative actions in the absence of apparent adverse effects.
Materials and Methods
BNP-Tg Mice.
Generation of BNP-Tg mice (line 55) was reported (14). BNP-Tg showed a marked increase in plasma BNP levels (1.8 ± 1.1 × 10−9 M) compared with their control littermates (non-Tg) (<0.06 × 10−9 M; refs. 14 and 16). These mice (10–15 wk) were randomly allotted to four treatment groups: BNP-Tg and non-Tg, with or without l-NAME (Nacalai Tesque, Kyoto) administration (200 mg/liter in drinking water; ref. 17).
cGKI-Knockout Mice.
We developed mice with targeted disruption of the cGKI gene (15). We used homozygous cGKI mutant mice (cGKI−/−), heterozygous mutant mice (cGKI+/−), and their control littermates (cGK+/+) (10–15 wk).
cGKI-Tg Mice.
We generated cGKI-Tg mice (T.-H.C. and H.I., unpublished data). Briefly, the cDNA coding for human cGKIα, which we cloned (18), was subcloned into the expression vector pCXN2 (19), driven by the CAG promoter (pCXN2-hcGKIα). The fragment of pCXN2-hcGKIα was microinjected into a C57BL/6 mouse. We used mice with 15 copies of the transgene and their control littermates at the age of 10–15 wk. To confirm cGKI expression in cGKI-Tg mice, Northern blot analysis was performed with a human cGKIα-specific probe, a 311-bp-long fragment at the 5′ end sequence released by pCXN2-hcGKIα.
Ligation Model.
After being anesthetized with pentobarbital (80 mg/kg, i.p.), the right femoral artery and vein were exposed, dissected free, and excised (13). Experimental procedures were performed according to Kyoto University standards for animal care.
Hindlimb blood flow was assessed with a laser Doppler perfusion image (LDPI) analyzer (Moor Instruments, Devon, U.K.) as described (13).
Immunohistochemistry.
After fixation with 4% paraformaldehyde, ischemic lower legs were embedded in OCT compound (Sakura Finetechnical, Tokyo) and frozen at −80°C. Cryostat sections (4–8 μm thick) of the tissues were stained with rat anti-mouse platelet EC adhesion molecule-1 (PECAM-1) (PharMingen), mouse anti-α smooth muscle actin (SMA) (Sigma), rat anti-mouse CD45 (PharMingen), rabbit anti-cGKI (Calbiochem), rabbit anti-guanylyl cyclase A (GC-A) and B (GC-B) antibody that we developed (20), rabbit anti-Erk1/2 and phospho-Erk1/2 (Thr-202/Tyr-204) antibody (Cell Signaling Technology, Beverly, MA), or mouse anti-CNP antibody that we developed (KY-CNP-1; ref. 21). As the negative control, rabbit preimmune serum or normal Ig fraction (DAKO) was used to show antibody specificity.
Analysis of Capillary Density and Inflammation.
Four random fields on two different sections (≈3 mm apart) from each mouse were photographed with a digital camera (Olympus, Tokyo). By computer-assisted analysis using nih image, capillary density was calculated as the mean number of capillaries stained with PECAM-1 (endothelial marker) or α SMA (vascular smooth muscle marker), and the mean number of infiltrating CD45-positive leukocytes was counted as the assessment of inflammation.
Evaluation of in Situ Reactive Oxygen Production.
The oxidative fluorescent dye dihydroethidium (2 × 10−6 M) was used to evaluate the in situ concentration of superoxide in ischemic hindlimb tissue, as described (22). We also stained 4-hydroxy-2-nonenal (4-HNE) (Nippon Oil & Fats, Tokyo), an unsaturated aldehyde that can be formed by the peroxidation of unsaturated fatty acids, such as linoleic and arachidonic acids (23).
Capillary Network Formation Assay.
Human umbilical vein ECs (Clonetics, Walkersville, MD) were grown in basic medium (EBM2) (Clonetics) containing growth supplements (EGM2) (Clonetics). They (two to three passages, 4 × 104 cells per well) were seeded at Matrigel-coated Cellware 24-well plates (Becton Dickinson) and incubated for 1 h in 100 μl of EBM2 containing 10% FBS. Serum-free medium (400 μl) containing human ANP, BNP, and CNP (Peptide Institute, Osaka) with or without Rp-8-pCPT-cGMP/PD98059 (Calbiochem) were added. After a 10-h incubation, they were fixed with 10% buffered formalin. Two random fields of view in three or four replicate wells were visualized, and images were captured by using the Olympus digital camera. Network formation was assessed by calculating the total area covered by capillaries in each field of view using nih image.
Construction of CNP Plasmid and Its Administration to Ligation Model.
The full length of rat CNP cDNA (384 bp) was inserted into the pAC-CMVpLpA vector (4). The plasmid DNA was prepared from cultures of pAC-CMVpLpA-transformed Escherichia coli by the EndoFree Plasmid Kit (Qiagen, Valencia, CA). After mouse femoral artery ligation, a local injection of plasmid carrying the CNP cDNA (pAC.CMV/CNP) or none (pAC.CMV) was performed (500 μg per mouse in 200 μl of PBS in 10 injection sites). Plasma CNP level was confirmed by an EIA (Phoenix Pharmaceuticals, St. Joseph, MO).
Statistical Analysis.
Results are presented as means ± SEM. The statistical significance of differences in the studies was evaluated by ANOVA. A P value < 0.05 was considered significant.
Results
Ischemia-Induced Angiogenesis Was Accelerated in BNP-Tg Mice.
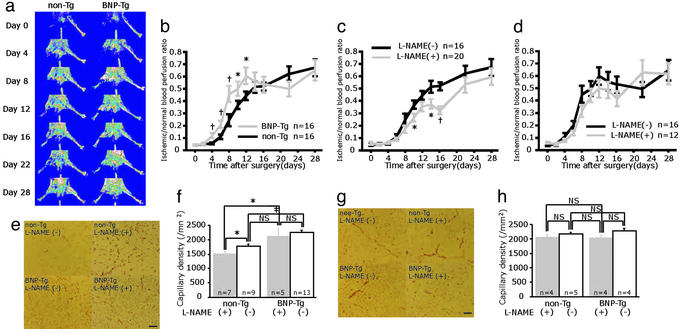
Serial blood flow measurements by LDPI revealed that accelerated limb perfusion improvement was observed for up to 12 days in BNP-Tg mice compared with non-Tg mice (Fig. 1 a and b). The calculated perfusion ratio of ischemic to nonischemic hindlimb was 0.12 ± 0.02 for BNP-Tg vs. 0.06 ± 0.01 for non-Tg at day 4 (P = 0.002), 0.46 ± 0.06 vs. 0.24 ± 0.05 at day 8 (P = 0.006), and 0.61 ± 0.07 vs. 0.45 ± 0.04 at day 12 (P = 0.04), respectively. After 14 days, restoration of perfusion in BNP-Tg mice was close to non-Tg mice, and no significant difference was seen.
Figure 1.
Ischemia-induced angiogenesis was accelerated in BNP-Tg mice, and overexpression of BNP restored delayed angiogenesis induced by NO blockade. (a) Serial LDPI analysis of hindlimb ischemia in BNP-Tg and non-Tg mice. (b) Quantitative analysis of ischemic/normal hindlimb perfusion ratio in BNP-Tg and non-Tg mice. (c and d) Serial LDPI measurements in non-Tg (c) and BNP-Tg (d) mice with and without l-NAME treatment. (e and g) Immunostaining of the ischemic hindlimb tissues with anti-PECAM-1 antibody (bright red) at day 10 (e) and day 28 (g). (f and h) Quantitative analysis of capillary density at day 10 (f) and day 28 (h). *, P < 0.05; †, P < 0.01; ‡, P < 0.001; NS, not significant. (Scale bar, 100 μm.)
Compatible with the result of blood flow measurement, capillary density in the BNP-Tg group (2,265 ± 62 per mm2) at 10 days was significantly higher than that of the non-Tg group (1,778 ± 74 per mm2; P < 0.0001; Fig. 1 e and f). At day 28, capillary density was equivalent in both groups (Fig. 1 g and h).
Overexpression of BNP Restored Delayed Angiogenesis Induced by NO Blockade.
l-NAME administration to non-Tg mice disclosed impaired recovery in hindlimb perfusion compared with the non-Tg without l-NAME group (Fig. 1c). The ratio of ischemic/normal blood flow measured at 14 days was 0.37 ± 0.04 for the non-Tg (+l-NAME) and significantly lower compared with 0.43 ± 0.03 for the non-Tg alone (P = 0.015). After 14 days, the ratio of both groups became similar.
In contrast to non-Tg, l-NAME administration had no significant effect on the ratio of blood perfusion in BNP-Tg at any time point (Fig. 1d).
On day 10, capillary density of l-NAME-treated non-Tg mice (1,516 ± 62 per mm2) was lower than that of untreated non-Tg mice (P = 0.014; Fig. 1 e and f). In contrast, no difference was seen in BNP-Tg with (2,113 ± 27 per mm2) or without l-NAME administration (day 10). Capillary density in the l-NAME-treated BNP-Tg group was significantly higher than that of the l-NAME-treated non-Tg group (P = 0.023) (day 10). On day 28, capillary density was equivalent in these four groups (Fig. 1 g and h).
Maturity of Newly Formed Blood Vessels in BNP-Tg Mice.
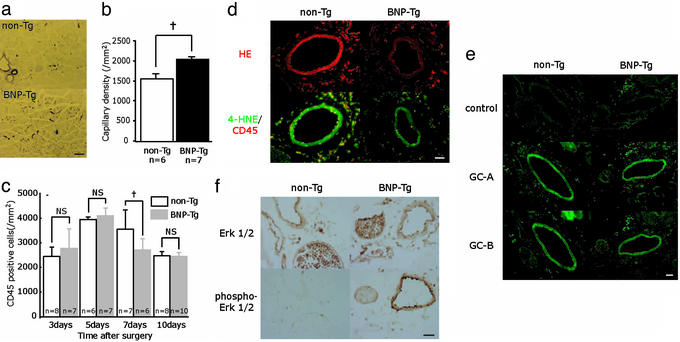
From double immunostaining of ischemic hindlimb tissue with PECAM-1 and α SMA, the structure of capillaries (ECs with adhering mural cells) showed no apparent difference between BNP-Tg and non-Tg mice (data not shown). Accordingly, immunostaining of the ischemic hindlimb tissues at day 10 with anti-αSMA antibody revealed significantly increased α SMA-positive capillary density in BNP-Tg mice (2,061 ± 65 per mm2) compared with non-Tg mice (1,578 ± 79 per mm2; P = 0.0001; Fig. 2 a and b). In addition, edema or bleeding in the ischemic hindlimb tissues was not observed in BNP-Tg mice.
Figure 2.
Evaluation of ischemic hindlimb tissue of BNP-Tg mice. (a and b) α SMA staining (purple) of the ischemic hindlimb tissues at day 10 (a) and quantitative analysis of capillary density (b). (c) Time course of focal inflammation of ischemic hindlimb obtained from immunostaining with anti-CD45 antibody. (d) Dihydroethidium (HE) staining (Upper; red) and 4-hydroxy-2-nonenal (4-HNE)/CD45 staining (Lower; green fluorescence/red) of the ischemic hindlimb tissue at day 7. (e) Expression of GC-A and GC-B (green fluorescence) in the ischemic hindlimb at day 7. Negative controls for these antibodies are also shown. (f) Immunostaining of the ischemic hindlimb tissues at day 7 with anti-Erk1/2 or phosphor-Erk1/2 antibody (brown). †, P < 0.01. (Scale bars: a, 100 μm; d–f, 25 μm.)
Focal Inflammation in BNP-Tg Mice.
In both BNP-Tg and non-Tg mice, the number of CD45-positive infiltrating leukocytes of the ischemic hindlimb tissues increased until day 5, then gradually decreased (Fig. 2c). At day 7, infiltrating leukocytes in BNP-Tg mice were significantly lower than those of non-Tg mice (P = 0.002), and no significant difference was seen at days 3, 5, and 10.
Reactive Oxygen Production in Blood Vessels of Ischemic Hindlimb Tissue.
By dihydroethidium staining of ischemic hindlimb tissue at day 7, in situ concentration of superoxide in the blood vessels of BNP-Tg mice was obviously lower than that of non-Tg mice (Fig. 2d). Furthermore, immunostaining of 4-HNE demonstrated that reactive oxygen production was also suppressed in BNP-Tg. From double immunostaining with CD45 in non-Tg mice, reactive oxygen production was prominent in infiltrating leukocytes around and within the blood vessels, as well as SMCs. In BNP-Tg mice, the number of reactive oxygen-positive inflammatory cells was decreased and reactive oxygen production in SMCs was diminished.
Expression of GC-A and GC-B in Ischemic Hindlimb Tissue.
Immunostaining (after antigen retrieval) of these receptors in ischemic hindlimb tissue at day 7 revealed that GC-A and GC-B were similarly expressed in the blood vessels of both BNP-Tg and non-Tg mice (Fig. 2e). Negative controls showed virtually no significant staining in these serial sections.
Expression of Erk1/2 and Phospho-Erk1/2 in Ischemic Hindlimb Tissue.
By immunostaining of Erk1/2 in ischemic hindlimb tissue at day 7, Erk1/2 was equally expressed in ECs and SMCs of both BNP-Tg and non-Tg mice. On the other hand, the expression of phospho-Erk was obviously enhanced in BNP-Tg mice, compared with non-Tg mice (Fig. 2f).
Angiogenesis Was Blunted in cGKI-Knockout Mice and Accelerated in cGKI-Tg Mice.
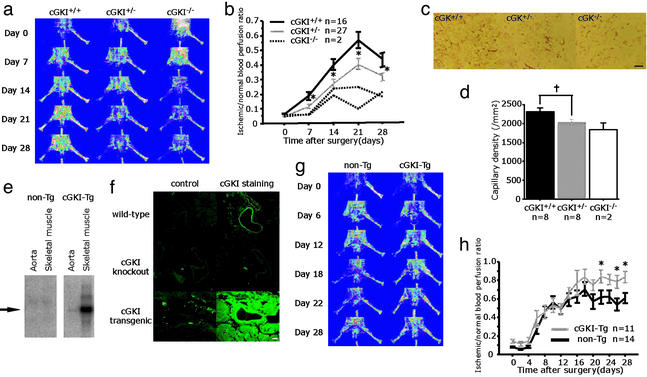
The constitution of the homozygous mice gradually deteriorated and most of them died before 10 wk (15). Because nutrition may influence angiogenesis, we mainly compared heterozygous mutant mice (cGKI+/−) with their control littermates (cGK+/+). By LDPI analysis, limb perfusion among cGKI+/− mice remained significantly impaired throughout the 28-day follow-up period in comparison with cGK+/+ (Fig. 3 a and b). In two cGKI-knockout mice (cGKI−/−), recovery from limb ischemia was remarkably reduced, compared with cGKI+/− or cGK+/+. Immunostaining of ischemic hindlimb tissue at day 28 with anti-PECAM-1 antibody revealed decreased capillary density in cGKI+/− (2,025 ± 51 per mm2) compared with cGK+/+ (2,302 ± 87 per mm2; P = 0.010; Fig. 3 c and d). Two cGKI−/− mice had decreased capillary density (1,845 ± 163 per mm2) compared with cGKI+/− or cGKI+/+.
Figure 3.
Angiogenesis was blunted in cGKI-knockout mice and accelerated in cGKI-Tg mice. (a and b) Serial LDPI measurements in cGKI-knockout mice. (c and d) PECAM-1 staining (bright red) of the ischemic hindlimb tissues at day 28 in cGKI-Tg mice (c) and quantitative analysis of capillary density (d). (e) The expression of cGKI mRNA in non-Tg and cGKI-Tg mice. (f) Immunostaining of cGKI (green fluorescence) with their negative control in WT, cGKI-knockout, and cGKI-Tg mice. (g and h) Serial LDPI measurements in cGKI-Tg mice. *, P < 0.05; †, P < 0.01. (Scale bars: c, 100 μm; f, 25 μm.)
By Northern blotting of cGKI-Tg mice, we confirmed overexpression of cGKI observed in the aorta and skeletal muscle compared with non-Tg mice (Fig. 3e). By immunostaining of cGKI, after antigen retrieval, high expression was also seen in the skeletal muscle and blood vessels (Fig. 3f). By LDPI analysis, cGKI-Tg mice showed significantly higher perfusion improvement at the end of the study compared with non-Tg mice (Fig. 3 g and h).
NPs Potentiated Capillary Network Formation of Cultured ECs.
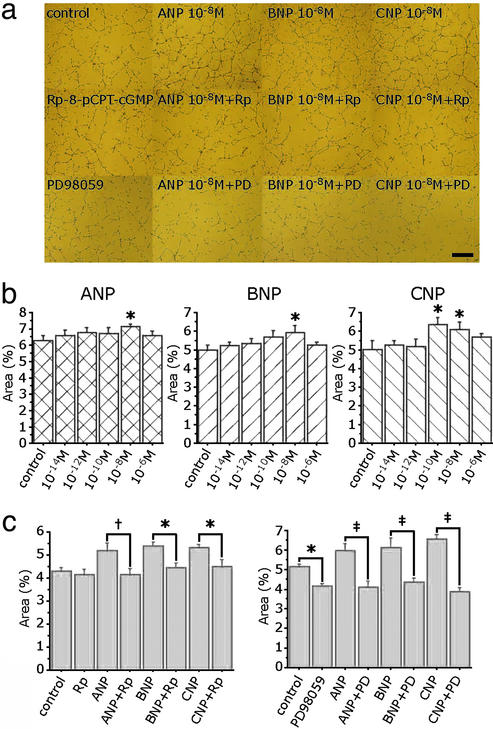
NPs significantly potentiated capillary network formation of human umbilical vein ECs in a bell-shaped fashion (Fig. 4 a and b). Network formation was prominent at 10−8 M ANP, 10−8 M BNP, and 10−10 M to 10−8 M CNP. The increase of network formation induced by NPs was completely blocked by Rp-8-pCPT-cGMP, a cGK inhibitor, at a concentration of 5 × 10−6 M (Fig. 4 a and c). Furthermore, treatment with 10−5 M PD 98059, an Erk1/2 inhibitor, significantly suppressed the increase of network formation induced by NPs (Fig. 4 a and d).
Figure 4.
NPs potentiated capillary network formation of cultured human umbilical vein ECs. (a) Capillary network formation by Matrigel assay in the presence of 10−8 M ANP, BNP, and CNP with or without Rp-8-pCPT-cGMP (Rp: 5 × 10−6 M), a cGK inhibitor, and PD 98059 (PD: 10−5 M), an Erk1/2 inhibitor. (b) Mean area of tube formation in the presence of various concentrations of ANP, BNP, and CNP. (c and d) Effects of Rp-8-pCPT-cGMP (c) and PD 98059 (d) in network formation induced by NPs. *, P < 0.05; †, P < 0.01; ‡, P < 0.001. (Scale bar, 500 μm.)
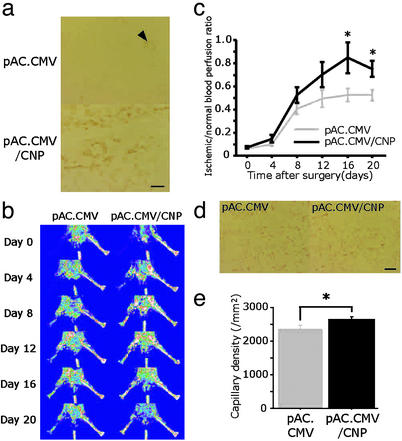
CNP Gene Transfer Enhanced Angiogenesis in Ischemic Hindlimb.
CNP immunostaining was detected in skeletal muscle from the ischemic hindlimb of mice that received pAC.CMV/CNP at day 20 (Fig. 5a). Endogenous CNP was also detected in blood vessels of mice that received control vector, pAC.CMV (Fig. 5a; arrowhead). Plasma CNP level was similarly below sensitivity threshold value (<1.32 × 10−10 M) in mice injected with pAC.CMV/CNP or pAC.CMV. By LDPI analysis, mice receiving pAC.CMV/CNP showed a significant increase in blood flow at the end of the study compared with mice receiving pAC.CMV (Fig. 5 b and c). Immunostaining of ischemic hindlimb tissues at day 20 with anti-PECAM-1 antibody revealed an increased capillary density in mice injected with pAC.CMV/CNP (2,643 ± 88 per mm2) compared with pAC.CMV (2,364 ± 104 per mm2; P = 0.048; Fig. 5 d and e).
Figure 5.
Effect of CNP gene transfer in the murine ischemic hindlimb model. (a) CNP immunostaining (brown) after local injection of control vector (pAC.CMV) or pAC.CMV/CNP. (b and c) Serial LDPI measurements in mice receiving pAC.CMV or pAC.CMV/CNP (n = 16 per group). (d and e) PECAM-1 staining (bright red) of the ischemic hindlimb tissues at day 20 in mice injected with pAC.CMV or pAC.CMV/CNP (d), and quantitative analysis of capillary density (e) (n = 10 per group). *, P < 0.05. (Scale bars, 100 μm.)
Discussion
Experiments performed in this study reveal actions of NPs on vascular regeneration in response to ischemia. BNP overproduced systemically in mice accelerated angiogenesis in the setting of tissue ischemia with activation of Erk1/2 in blood vessels. This evidence was confirmed by a combination of LDPI analysis and capillary density measurement. In addition, overproduction of BNP compensated for impaired neovascularization because of l-NAME treatment. We also succeeded in demonstrating that ischemia-induced angiogenesis is significantly potentiated in cGKI-Tg mice, but attenuated in cGK-knockout mice. Furthermore, CNP gene delivery in the ischemic hindlimb could significantly enhance angiogenesis. These results indicate that the NPs/NO/cGMP/cGK pathway is critical for neovascularization in vivo.
NPs stimulate two biologically active receptors, GC-A and GC-B. We and others have demonstrated that ANP and BNP show high affinity for GC-A, whereas CNP selectively binds to GC-B (24). We have already reported that BNP-Tg mice show skeletal phenotypes through activation of the CNP/GC-B pathway (16, 24). It is important to clarify, therefore, whether the effects of BNP in the hindlimb ischemia model of BNP-Tg mice are mediated through GC-A, GC-B, or both. By immunostaining of these receptors in the ischemic hindlimb of BNP-Tg mice, we confirmed that both GCs expressed in ECs and SMCs. Considering the finding that CNP also enhanced angiogenesis in our model, activation of GC-B is more likely in BNP-Tg mice; however, the analyses of GC-A-knockout mice (25), which we recently developed, would provide answers to that question.
To supplement these in vivo findings with genetically engineered mouse models, we performed several in vitro experiments. In cultured ECs, NPs significantly increased capillary network formation at the concentrations of 10−10 to 10−8 M, which were the same as the plasma BNP level in BNP-Tg mice (14, 16). Early studies, including our own report, have shown that NPs inhibit EC proliferation (26) and migration (27). The concentrations of NPs used in these reports are much higher than physiological (1, 2) and, thus, the EC growth inhibition by NPs seems to be a result of a pharmacological effect. We also confirmed that NP-induced capillary network formation was significantly blocked by Rp-8-pCPT-cGMP and PD98059, indicating the involvement of cGK and Erk1/2 in this phenomenon. Furthermore, we recently observed that ANP increases cultured EC proliferation and migration in vitro by activating the cGK and subsequent Akt/PKB and Erk1/2 pathways (28). These results indicate that NPs can act directly on ECs and potentiate endothelial regeneration.
To achieve recovery from tissue ischemia, not only ECs but also SMCs must migrate and proliferate to produce functionally mature vessels (29). Early studies, including our own report, have shown that NPs inhibit cell growth of vascular SMCs (3). From these reports, it is anticipated that newly formed vessels of BNP-Tg mice in our model might represent immature capillaries without adequate mural cell coating. However, we confirmed that ECs possessed adhering mural cells, and the capillary structures were not different in BNP-Tg or non-Tg mice. Furthermore, the α SMA-positive capillary density in BNP-Tg mice was significantly increased compared with non-Tg mice. These results suggest that antiproliferative effects of NPs on SMCs may not play a role in angiogenesis.
Recently, the participation of inflammation in angiogenesis has been an area of focus. Arras et al. (30) reported monocyte activation with the production of cytokines and vessel proliferation might associate with angiogenesis in a rabbit hindlimb ischemia model. Izumi et al. (31) reported that infarct size after myocardial ischemia/reperfusion injury was smaller in mice lacking GC-A, accompanied with decreases in neutrophil infiltration. From these results, angiogenesis in our model could be explained in part by potential proinflammatory effects of NPs. However, we confirmed that the number of infiltrating leukocytes in the ischemic limb of BNP-Tg mice was similar or even lower than that of non-Tg mice. This finding indicates that possible proinflammatory effects of NPs do not play a role in angiogenesis.
Nitrates, which are clinically used to relieve coronary vasoconstriction, might be useful for the management of vascular obstructions because NPs and NO share the same intracellular signal-transduction pathway. However, there is growing evidence that nitroglycerin-induced production of oxygen-derived free radicals such as superoxide plays an important role in mediating the tolerance and endothelial dysfunction in response to long-term treatment (32). However, the present study revealed that NPs suppress reactive oxygen production in inflammatory cells and blood vessels. Therefore, our findings suggest that NPs possess clinical advantages over nitrates.
The time course of vascular regeneration was different between BNP-Tg mice and cGKI-Tg/knockout mice. The changes in vascular regeneration in cGKI-Tg and cGKI-knockout mice were clearly opposite (“mirror image”) and the continuous activation or inactivation of cGKI might result in the final augmentation or suppression of vascular regeneration, which indicates the significance of the cGKI activation level to determine the extent of vascular regeneration. On the other hand, no significant difference in the blood flow or capillary density was seen between BNP-Tg and non-Tg mice at the end of our study. Attenuation of overactivation of the cGMP/cGKI pathway can be one of the possible explanations; however, the expression of GC-A/GC-B showed no apparent difference between BNP-Tg and non-Tg mice at day 7, and also before ischemia (data not shown). Therefore, even if the attenuation of overactivation of cGKI might be the cause, it must not have occurred at the level of regulation of receptor expression.
Clinical applications of cardiovascular gene therapy have been launched during the last several years. However, we know very little about either the therapeutic or toxic effects of overexpressing angiogenic proteins, including VEGF. VEGF overexpression could accelerate atherosclerosis (33), promote pathological angiogenesis (34), or develop limb-threatening peripheral edema. Less adverse effects, such as edema, are seen in patients treated with ANP (35). We have demonstrated that NPs are the vasculoprotective factor against atherosclerotic lesion (4, 5). Furthermore, in the present study, we confirmed that angiogenesis was enhanced in hindlimb-ischemic mice after CNP gene transfer. From these results, NPs in clinical use seem to possess multiple coordinate actions that result in vascular protection and regeneration. In addition, inhibition of NPs can be a potential target of antineoplastic drugs for suppression of angiogenesis.
In conclusion, we have revealed an activity of the NPs/cGMP/cGKI pathway and suggest that NPs, as endogenous cardiovascular hormones, have significant advantages for the treatment of tissue ischemia. Thus, NPs can be used as a promising strategy for therapeutic angiogenesis in patients with tissue ischemia.
Abbreviations
- NP
natriuretic peptide
- ANP
atrial NP
- BNP
brain NP
- CNP
C-type NP
- cGK
cGMP-dependent protein kinase
- EC
endothelial cell
- SMC
smooth muscle cell
- Tg
transgenic
- l-NAME
Nω-nitro-l-arginine methyl ester
- LDPI
laser Doppler perfusion image
- PECAM-1
platelet EC adhesion molecule-1
- SMA
smooth muscle actin, GC, guanylyl cyclase
- VEGF
vascular endothelial growth factor
References
- 1.Sugawara A, Nakao K, Morii N, Yamada T, Itoh H, Shiono S, Saito Y, Mukoyama M, Arai H, Nishimura K, et al. J Clin Invest. 1988;81:1962–1970. doi: 10.1172/JCI113544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H, et al. J Clin Invest. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komatsu Y, Itoh H, Suga S, Ogawa Y, Hama N, Kishimoto I, Nakagawa O, Igaki T, Doi K, Yoshimasa T, Nakao K. Circ Res. 1996;78:606–614. doi: 10.1161/01.res.78.4.606. [DOI] [PubMed] [Google Scholar]
- 4.Doi K, Ikeda T, Itoh H, Ueyama K, Hosoda K, Ogawa Y, Yamashita J, Chun T H, Inoue M, Masatsugu K, et al. Arterioscler Thromb Vasc Biol. 2001;21:930–936. doi: 10.1161/01.atv.21.6.930. [DOI] [PubMed] [Google Scholar]
- 5.Ohno N, Itoh H, Ikeda T, Ueyama K, Yamahara K, Doi K, Yamashita J, Inoue M, Masatsugu K, Sawada N, et al. Circulation. 2002;105:1623–1626. doi: 10.1161/01.cir.0000014985.50017.6e. [DOI] [PubMed] [Google Scholar]
- 6.Gerber H P, McMurtrey A, Kowalski J, Yan M, Keyt B A, Dixit V, Ferrara N. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 7.Fujio Y, Walsh K. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fulton D, Gratton J P, McCabe T J, Fontana J, Fujio Y, Walsh K, Franke T F, Papapetropoulos A, Sessa W C. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher A M. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 10.Ziche M, Morbidelli L, Choudhuri R, Zhang H T, Donnini S, Granger H J, Bicknell R. J Clin Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hood J, Granger H J. J Biol Chem. 1998;273:23504–23508. doi: 10.1074/jbc.273.36.23504. [DOI] [PubMed] [Google Scholar]
- 12.Parenti A, Morbidelli L, Cui X L, Douglas J G, Hood J D, Granger H J, Ledda F, Ziche M. J Biol Chem. 1998;273:4220–4226. doi: 10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- 13.Couffinhal T, Silver M, Zheng L P, Kearney M, Witzenbichler B, Isner J M. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa Y, Itoh H, Tamura N, Suga S, Yoshimasa T, Uehira M, Matsuda S, Shiono S, Nishimoto H, Nakao K. J Clin Invest. 1994;93:1911–1921. doi: 10.1172/JCI117182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang G X, Korth M, Aszodi A, Andersson K E, et al. EMBO J. 1998;17:3045–3051. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suda M, Ogawa Y, Tanaka K, Tamura N, Yasoda A, Takigawa T, Uehira M, Nishimoto H, Itoh H, Saito Y, et al. Proc Natl Acad Sci USA. 1998;95:2337–2342. doi: 10.1073/pnas.95.5.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elhage R, Bayard F, Richard V, Holvoet P, Duverger N, Fievet C, Arnal J F. Circulation. 1997;96:3048–3052. doi: 10.1161/01.cir.96.9.3048. [DOI] [PubMed] [Google Scholar]
- 18.Tamura N, Itoh H, Ogawa Y, Nakagawa O, Harada M, Chun T H, Suga S, Yoshimasa T, Nakao K. Hypertension. 1996;27:552–557. doi: 10.1161/01.hyp.27.3.552. [DOI] [PubMed] [Google Scholar]
- 19.Niwa H, Yamamura K, Miyazaki J. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 20.Potter L R, Garbers D L. J Biol Chem. 1992;267:14531–14534. [PubMed] [Google Scholar]
- 21.Naruko T, Ueda M, van der Wal A C, van der Loos C M, Itoh H, Nakao K, Becker A E. Circulation. 1996;94:3103–3108. doi: 10.1161/01.cir.94.12.3103. [DOI] [PubMed] [Google Scholar]
- 22.Miller F J, Jr, Gutterman D D, Rios C D, Heistad D D, Davidson B L. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 23.Toyokuni S, Miyake N, Hiai H, Hagiwara M, Kawakishi S, Osawa T, Uchida K. FEBS Lett. 1995;359:189–191. doi: 10.1016/0014-5793(95)00033-6. [DOI] [PubMed] [Google Scholar]
- 24.Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, et al. Proc Natl Acad Sci USA. 2001;98:4016–4021. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez M J, Wong S K, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers D L, Beuve A. Nature. 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 26.Itoh H, Pratt R E, Ohno M, Dzau V J. Hypertension. 1992;19:758–761. doi: 10.1161/01.hyp.19.6.758. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda M, Kohno M, Takeda T. Hypertension. 1995;26:401–405. doi: 10.1161/01.hyp.26.3.401. [DOI] [PubMed] [Google Scholar]
- 28. Kook, H., Itoh, H., Choi, B. S., Sawada, N., Doi, K., Hwang, T. J., Kim, K. K., Arai, H., Baik, Y. H. & Nakao, K. (2003) Am. J. Physiol., in press. [DOI] [PubMed]
- 29.Folkman J. Ann NY Acad Sci. 1982;401:212–227. doi: 10.1111/j.1749-6632.1982.tb25720.x. [DOI] [PubMed] [Google Scholar]
- 30.Arras M, Ito W D, Scholz D, Winkler B, Schaper J, Schaper W. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izumi T, Saito Y, Kishimoto I, Harada M, Kuwahara K, Hamanaka I, Takahashi N, Kawakami R, Li Y, Takemura G, et al. J Clin Invest. 2001;108:203–213. doi: 10.1172/JCI12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sage P R, de la Lande I S, Stafford I, Bennett C L, Phillipov G, Stubberfield J, Horowitz J D. Circulation. 2000;102:2810–2815. doi: 10.1161/01.cir.102.23.2810. [DOI] [PubMed] [Google Scholar]
- 33.Inoue M, Itoh H, Ueda M, Naruko T, Kojima A, Komatsu R, Doi K, Ogawa Y, Tamura N, Takaya K, et al. Circulation. 1998;98:2108–2116. doi: 10.1161/01.cir.98.20.2108. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka Y, Katoh S, Hori S, Miura M, Yamashita H. Lancet. 1997;349:1520. doi: 10.1016/S0140-6736(05)62099-5. (lett.). [DOI] [PubMed] [Google Scholar]
- 35.Saito Y, Nakao K, Nishimura K, Sugawara A, Okumura K, Obata K, Sonoda R, Ban T, Yasue H, Imura H. Circulation. 1987;76:115–124. doi: 10.1161/01.cir.76.1.115. [DOI] [PubMed] [Google Scholar]