Abstract
Human hematopoietic stem cells are defined by their ability to repopulate multiple hematopoietic lineages in the bone marrow of transplanted recipients and therefore are functionally distinct from hematopoietic progenitors detected in vitro. Although factors capable of regulating progenitors are well established, in vivo regulators of hematopoietic repopulating function are unknown. By using a member of the vertebrate Wnt family, Wnt-5A, the proliferation and differentiation of progenitors cocultured on stromal cells transduced with Wnt-5A or treated with Wnt-5A conditioned medium (CM) was unaffected. However, i.p. injection of Wnt-5A CM into mice engrafted with human repopulating cells increased multilineage reconstitution by >3-fold compared with controls. Furthermore, in vivo treatment of human repopulating cells with Wnt-5A CM produced a greater proportion of phenotypically primitive hematopoietic progeny that could be isolated and shown to possess enhanced progenitor function independent of continued Wnt-5A treatment. Our study demonstrates that Wnt-5A augments primitive hematopoietic development in vivo and represents an in vivo regulator of hematopoietic stem cell function in the human. Based on these findings, we suggest a potential role for activation of Wnt signaling in managing patients exhibiting poor hematopoietic recovery shortly after stem cell transplantation.
Keywords: stem cell regulators
Hematopoiesis is sustained by uncommitted stem cells that give rise to progenitors capable of producing mature blood cells (1, 2). This process is governed by numerous factors that regulate the differentiation of these progenitors into specified lineages (3, 4). Distinct subclasses of primitive cells defined by functional assays have lead to the identification and hierarchical arrangement of stem cells comprising the human hematopoietic system (5). Although the developmental relationship among these subclasses is well characterized, factors capable of acting specifically on stem cells with in vivo repopulating ability are unknown.
Because hematopoietic stem cells can only be assayed by transplantation in vivo, our understanding of factors acting on human repopulation and hematopoietic development has been limited. Xenotransplantation of human hematopoietic cells into nonobese diabetic (NOD)/severe combined immunodeficient (SCID) mice has proven to be a reliable model for the detection of primitive human blood cells with repopulation capacity (6). These primitive human blood cells have been operationally defined as SCID-repopulating cells (SRCs). Phenotypically, human SRCs are highly enriched among extremely rare CD34+CD38− cells of lineage-depleted (Lin−) cells, whereas hematopoietic progenitors devoid of repopulating function are detected among more mature CD34+CD38+ cells (5, 7).
Recent evidence suggests that proliferation and differentiation of the most primitive subclasses of hematopoietic cells capable of repopulation are regulated by factors first identified for their instructive role during embryonic development. For example, regulators of Notch, bone morphogenetic protein (BMP), and Sonic hedgehog (Shh) signaling pathways, known to orchestrate patterning, organogenesis, and cell fate in lower organisms, have been shown to control the function of ex vivo-cultured adult hematopoietic stem cells (8–10). Characterization of these factors in nonhematopoietic systems indicates that considerable cross talk exists among these signaling pathways (11). The Wnt signaling pathway has been shown to synergize with Notch, BMP, and Shh function (11, 12). Wnt genes encode secreted glycoproteins that act as paracrine or autocrine factors and are highly conserved from hydra to mammals. However, similar to the Notch, BMP, and Shh pathways, members of the Wnt family confer distinct cellular functions depending on the nature of the target cell and expression of the Frizzled (Frz) family of Wnt receptors (13).
Evidence for the role of Wnt signaling in mesodermal cell fate, from which hematopoiesis is initiated in the developing embryo (14), combined with the expression of Wnt-5A in primitive hematopoietic cells (15), suggests a potential role for Wnt-5A in hematopoietic regulation. Here, overexpression of Wnt-5A by bone marrow (BM) stromal cells was unable to modulate the proliferation or differentiation of human hematopoietic progenitors by either direct coculture or treatment with Wnt-5A conditioned medium (CM). However, Wnt-5A CM treatment of NOD/SCID mice engrafted with human SRCs increased human hematopoietic repopulation by 3-fold compared with mice treated with control CM. Moreover, although lymphoid and myeloid maturation was unaffected, larger pools of phenotypically primitive progeny were present in mice treated with Wnt-5A CM, which possessed enhanced progenitor function after isolation. Our results identify Wnt-5A as a positive regulator of in vivo hematopoietic repopulating function, thereby providing evidence for a role of Wnts in the human hematopoietic stem cell function.
Materials and Methods
Retroviral-Producing Cell Lines.
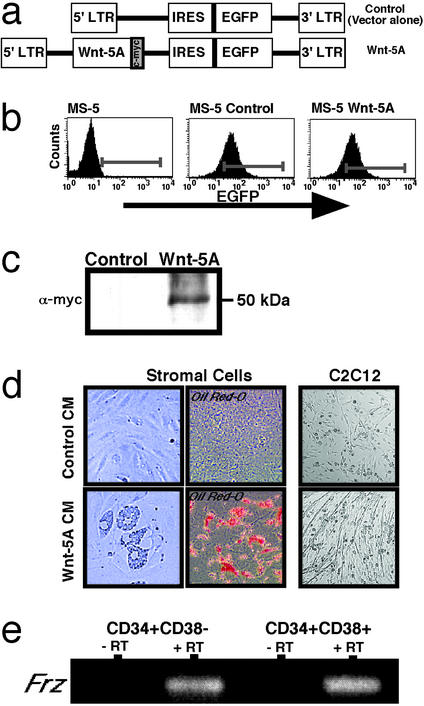
A 1.2-kb BglII Wnt-5A fragment from Xenopus laevis and tagged with c-Myc was subcloned into a control MIEV-based retroviral vector (16, 17) upstream of an enhanced GFP (EGFP) reporter (Fig. 1a). Wnt-5A and control MIEV vectors were transfected into PA317 packaging lines, and transient virus was harvested to transduce GP+E-86 cells. Stable retroviral-producing cell lines were isolated based on EGFP expression by using fluorescence-activated cell sorting (FACS) and confirmed by using viral RNA slot blots (18).
Figure 1.
Establishment of transgenic murine stromal cells producing Wnt-5A. (a) Retroviral vectors: control (MIEV vector) and Wnt-5A. IRES, internal ribosome entry site. (b) Histograms showing flow-cytometric analyses of EGFP fluorescence from parent murine stromal cells (Left), transduced MS-5 transduced with either control MIEV vector alone (Center), or Wnt-5A (Right). (c) Western analysis of immunoprecipitated protein isolated from MS-5 control or MS-5 Wnt-5A cells by using c-Myc antibody. As expected, c-Myc-tagged Wnt-5A protein reveals a positive signal at ≈50 kDa. (d) Functional assessment of control and Wnt-5A CM collected from transgenic stromal cell lines. CM from parent stromal cells was removed and replaced with control or Wnt-5A CM derived from 80% confluent stromal cell cultures and monitored for up to 6 days. An increased frequency of adipocytes was morphologically and phenotypically observed in cultures treated with Wnt-5A CM compared with cultures treated with control CM. C2C12 myogenic cells did not respond to control CM treatment but showed marked increases in myotube formation in response to Wnt-5A. (e) Purified CD34+CD38−Lin− and CD34+CD38+ Lin cells derived from human CB were analyzed by RT-PCR for Frz transcript expression using degenerate primers. Both populations showed the presence of amplified products that were sequenced-verified and showed the expression of human Frz in these subsets. No products were detected in preparations devoid of reverse transcription (−RT) during the first-strand cDNA synthesis reaction.
Production of Transgenic Stromal Cells.
The stromal cell line MS-5 (19, 20) was transduced with control or Wnt-5A ecotropic retrovirus from GP+E-86 producers. Cells expressing EGFP were isolated by using FACS to yield stable MS-5 control- and MS-5 Wnt-5A-transduced populations.
Analysis of Frz Expression.
Degenerate primers were used for the PCR amplification of known members of the frz gene family of Wnt-5A receptors, from cDNA generated from human CB CD34+CD38−Lin−, and from CD34+CD38+Lin− cells as shown previously (8) by using forward primer (5′-nnngaattctayccngarmgnccnat-3′) and reverse primer (5′-nnnaagcttngcngcnarraacca-3′), amplification for one cycle at 94°C for 12 min, 40 cycles at 94°C for 1 min, 55°C for 1.5 min, and 72°C for 1 min, and one cycle at 72°C for 10 min. Amplified products were sequenced, verifying Frz-3, -5 and -7 expression.
Western Blots.
Total protein from lysed MS-5 control and MS-5 Wnt-5A cells was used for immunoprecipitation with a c-Myc antibody, clone 9E10 (Roche, Indianapolis). Immunoprecipitates were subjected to Western analysis, and Wnt-5A was detected on poly(vinylidene difluoride) with a c-Myc antibody and enhanced chemiluminescence substrate (Amersham Pharmacia).
Human Cells.
Samples of full-term human umbilical cord blood (CB) were obtained in conjunction with local ethical and biohazard authorities of the University of Western Ontario and London Health Sciences Centre (London, ON, Canada). CB mononuclear cells (MNCs), CD34+CD38−Lin−, and CD34+CD38+Lin− cells were isolated as described (21).
Colony-Forming Unit (CFU) Assays.
Human clonogenic progenitor assays were performed by plating CB cells into methylcellulose with human hematopoietic growth factors as described (22). Cell inputs for each assay were 250 de novo CD34+CD38−Lin− cells, entire wells for the 5-week stromal cultures, and 5,000 human (CD45+) CD34+ cells isolated from the BM of transplanted mice (CFU-SRCs).
Stromal Cocultures.
One hundred purified CD34+CD38−Lin− CB cells (7) cocultured with control- or Wnt-5A-transduced MS-5 stromal layers. Cells were grown in Myelocult H5100 (StemCell Technologies, Vancouver) supplemented with 10−4 M hydrocortisone 21-hemisuccinate (Sigma) with weekly half-media changes. After 5 weeks, the contents of individual wells were harvested and plated into CFU assays with six-well replicates (23).
CM and Immunodepletion of Wnt-5A.
CM was collected from 70–80% confluent MS-5 cells transduced with either control MIEV vector or MIEV-Wnt-5A for injections into engrafted NOD/SCID mice as described below. A total of 5 ml of CM was freshly harvested from Wnt-5A-transduced MS-5 cells and divided equally into two conical tubes for incubation with or without AF645-IgG antibody against mouse Wnt-5A (R & D Systems, lot no. BVY02) at a concentration of 1 μg of antibody/ml of CM. Wnt-5A CM was incubated for 1 h at room temperature on a platform shaker, and 500 μl was used for injection into each engrafted NOD/SCID mouse as scheduled over 2 weeks, every other day.
Transplantation of Human Hematopoietic Cells and Analysis of NOD/SCID Mice.
Sublethally irradiated NOD/SCID mice (6) were i.v. transplanted with 5 × 106 CB MNCs or purified CD34+CD38−Lin− cells enriched for primitive hematopoietic cells. Mice transplanted with human SRCs that have established human BM chimerism after 2–3 weeks (24, 25) were injected i.p. every other day with 500 μl of freshly harvested CM from control- or Wnt-5A-transduced MS-5 cells. Murine BM was analyzed 4–5 weeks after transplant of human CB MNCs or purified SRCs. Analysis of human hematopoietic engraftment was performed by flow cytometry using the human specific pan-leukocyte marker CD45. Gated human cells were analyzed for populations of primitive hematopoietic cells expressing CD34 and CD38, myeloid cells expressing CD33, and lymphoid cells expressing CD19 (7). Cells were stained with mouse IgG1 as isotype controls to determine quadrants. Engraftment analysis was performed by using a FACSCalibur and CELLQUEST software (Becton Dickinson Immunocytometry Systems). For CFU-SRCs, CD45+CD34+ cells were isolated by using a FACSVantage SE (Becton Dickinson Immunocytometry Systems).
Statistical Analyses.
Analyses were performed by using the two-tailed unpaired student t test with levels of significance reported according to P < 0.05.
Results
Generation of BM-Derived Stromal Cells Capable of Expressing Functional Wnt-5A Protein.
To evaluate whether soluble Wnt-5A protein is capable of regulating human hematopoiesis, Wnt-5A cDNA tagged with a c-Myc epitope was subcloned into a bicistronic retroviral vector (MIEV) (16, 26) to allow for concurrent expression of EGFP and Wnt-5A (Fig. 1a). Retroviral-producing lines were used to transduce a BM stromal cell line, MS-5, selected for its ability to support primitive hematopoietic cells (19, 20). Stably transduced MS-5 cells expressing control and Wnt-5A retroviral vectors were isolated by FACS through selection of EGFP-positive cells (Fig. 1b). To confirm expression of Wnt-5A, we performed Western analysis of immunoprecipitates from control- or Wnt-5A-transduced MS-5 cell lysates. Blots probed with a c-Myc antibody demonstrated the expression of a Wnt-5A-c-Myc protein of the anticipated size (50 kDa) from MS-5 Wnt-5A cells, whereas MS-5 controls were negative (Fig. 1c).
Although low levels of soluble Wnts are sufficient to induce signaling (27), quantitative detection of bioactive secreted Wnt protein has been notoriously difficult (28–30). For nontransforming Wnt proteins such as Wnt-5A, no reporter assays are available for the demonstration of biological activity. Wnt-5A can activate signaling through the canonical Wnt/β-catenin pathway or through a noncanonical Wnt pathway (31, 32). Accordingly, we used a cell-differentiation response to investigate whether medium conditioned with factors secreted from the transduced Wnt-5A stroma was active in eliciting any known Wnt responses distinct from the effect of CM from control-transduced MS-5 cells. Inhibition of the canonical Wnt/β-catenin signaling pathway promotes adipogenesis (33). Interestingly, in some contexts Wnt-5A antagonizes Wnt/β-catenin signaling both at the level of signaling and function (32, 34, 35). Therefore, if our culture medium contained active Wnt-5A protein one might expect Wnt-5A to induce adipogenesis. To test this, naive stromal cells were treated for 6 days with control CM harvested from either control- or Wnt-5A-transduced MS-5 cells (Fig. 1d). Stromal cells treated with control CM did not differ from untreated cells. In contrast and consistent with the presence of active Wnt-5A protein, the addition of MS-5 Wnt-5A CM caused an increase in adipogenesis as detected by distinct morphological characteristics (Fig. 1d Left, n = 4) and oil red-O staining of lipid-filled droplets (Fig. 1d Center, n = 4). Because Wnt-5A is known to activate myogenesis (36, 37), we then tested the ability of Wnt-5A CM to promote myogenesis of the myoblastic cell line C2C12. C2C12 cells exposed to Wnt-5A CM for 5 days differentiated into myotubes as indicated by fusion and elongation of cells (Fig. 1d Right, n = 4). In contrast, cells treated with control CM demonstrated no myotube formation under identical conditions (Fig. 1d, n = 4). Collectively, the expression of Wnt-5A protein in transduced MS-5 cells (Fig. 1c) combined with the ability of Wnt-5A CM to induce adipogenesis and myotube formation (Fig. 1d) suggests that functional Wnt-5A is produced by transduced MS-5 cells.
Functional Analysis of Wnt-5A on Purified Human Hematopoietic Progenitors Detected in Vitro.
Human hematopoiesis is organized as a hierarchical arrangement of primitive cells with distinct capacities to proliferate and differentiate (38). These subclasses of primitive cells can be discriminated by functional assays that detect progenitors capable of proliferating in CFU assays, cells capable of survival and subsequent progenitor function after coculture on BM stroma, or rarer cells with the ability to reconstitute hematopoiesis after transplantation into immune deficient mice (39–41). Using these assays, we evaluated the potential effect of Wnt-5A CM on progenitors at various developmental stages of human hematopoiesis.
Primary human CD34+CD38−Lin− cells enriched for both hematopoietic progenitor and SRC function (7, 42) and CD34+CD38+Lin− cells enriched for progenitors but devoid of SRC function were isolated from umbilical CB. Degenerate RT-PCR analysis demonstrated that both CD34+CD38−Lin− and CD34+CD38+ cells express members of the Frz family of putative Wnt receptors, suggesting that these populations have the capacity to respond to Wnt signaling (Fig. 1e). In preliminary experiments, sequencing of subcloned products indicate expression of Frz-1 and -5 in CD34+CD38−Lin− cells, whereas Frz-7 was found to be expressed in CD34+CD38+Lin− (data not shown).
Based on the expression of Frz in both SRC- and progenitor cell-enriched populations, we initially examined the functional capacity of Wnt-5A CM to affect the proliferation and differentiation of hematopoietic progenitors detected in vitro. From four independent CB samples, 250 CD34+CD38−Lin− cells were seeded into clonogenic progenitor assays containing methylcellulose supplemented with human hematopoietic growth factors (6, 40) together with either control or Wnt-5A CM. Approximately 89.1 ± 10.1 and 90.3 ± 15.5 CFU were generated in control- and Wnt-5A-treated progenitors, respectively. To determine the effect of Wnt-5A on more primitive cells capable of survival and subsequent progenitor capacity after stromal coculture (40, 43), we compared changes in progenitor capacity after 5 weeks of coculture of purified CD34+CD38− cells with either control- or Wnt-5A-transduced MS-5 cells. Similar to CM treatment in CFU assays, cocultured hematopoietic cells displayed no differences in progenitor frequency (control MS-5 formed 57.6 ± 29.3 colonies, whereas MS-5 Wnt-5A produced 93.6 ± 52.1 colonies in six replicates), although there was a trend toward enhanced progenitor capacity in the presence of Wnt-5A. In addition, retroviral transduction of primary human SRCs using the Wnt-5A retroviral-producer line showed no difference in Wnt-5A- vs. vector control-transduced cells that repopulated NOD/SCID mice in vivo (data not shown) and may be due to the inability of the vector to express adequate amounts of Wnt-5A in human hematopoietic cells repopulating this xenograft. Nevertheless, these combined data demonstrate that Wnt-5A has no significant effect on the proliferative and differentiative capacity of hematopoietic progenitors after coculture with transgenic stromal cells or direct expression of Wnt-5A in human SRCs.
Human SRC Repopulating Function and Primitive Hematopoiesis Is Increased by in Vivo Treatment of Wnt-5A.
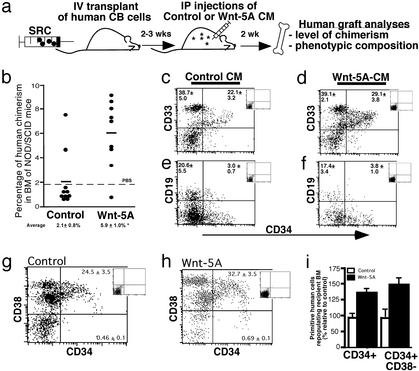
Rare human SRCs capable of multilineage in vivo repopulation of immune-deficient NOD/SCID mice have been shown to be biologically distinct from other subclasses of progenitors detected in surrogate stem cell assays (5, 6, 44). To examine the role of Wnt-5A on candidate repopulating stem cells represented by human SRCs (Fig. 2a), NOD/SCID mice were i.v. transplanted with 5 × 106 CB MNCs. For direct treatment of human SRCs actively sustaining multilineage hematopoiesis, recipient mice were administered i.p. injections of control or Wnt-5A CM 2–3 weeks posttransplantation to allow establishment of human SRC engraftment (25). After 2 weeks of CM treatment, the BM of transplanted mice was analyzed by flow cytometry for the level of human hematopoietic chimerism with an antibody for the human-specific pan-leukocyte marker CD45 (7, 24). A summary of the level of human engraftment in individually treated mice transplanted with human SRCs is shown in Fig. 2b. SRC-repopulated mice treated with Wnt-5A CM demonstrated an average of 3-fold higher levels of human engraftment (5.9 ± 1.0%, n = 9) compared with SRCs treated with control CM (2.1 ± 0.8%, n = 10). Mice treated with control CM demonstrated similar levels of chimerism as mice treated with PBS dotted line (Fig. 2b, dotted line), indicating that the control CM had no detrimental affect on human SRC capacity.
Figure 2.
Analysis of human SRCs treated with Wnt-5A. (a) Schematic diagram illustrating the experimental approach used to examine the in vivo effect of Wnt-5A on human repopulating cells. CB MNCs or purified populations of CD34+CD38− cells enriched for SRCs were transplanted into irradiated NOD/SCID mice. Between 2 and 3 weeks posttransplantation, mice were injected i.p. every other day with either control or Wnt-5A CM collected from transgenic stromal producers for 2 weeks. After these treatments, murine BM cells were analyzed for the level and composition of the human graft arising from treated SRCs. (b) Summary of the level of human chimerism in the BM of mice transplanted with CB MNCs treated with control or Wnt-5A CM, where each symbol represents a single transplanted mouse (n = 19) from five independent CB samples. The horizontal bar indicates the average level of human engraftment in each group of treated mice and is shown including SEM (Lower). The dotted line represents the average level of human engraftment when PBS was injected i.p. into recipient mice (n = 5) and shows similar levels of human chimerism to mice treated with control CM. *, Statistically significant difference between the average levels of engraftment in SRC-engrafted mice treated with control CM compared with those treated with Wnt-5A CM, P < 0.05. (c–h) Phenotypic analyses of comparing human hematopoietic grafts of control- and Wnt-5A-treated SRCs. Human cells from mice treated with either control (c, e, and g) or Wnt-5A CM (d, f, and h) were gated by using the human-specific cell-surface marker CD45 and further analyzed for cell-surface phenotypes of primitive CD34, myeloid (CD33), lymphoid (CD19), and primitive (CD38) cell contents based on isotype controls (Insets). Average percentages of the respective phenotypes for six and nine mice from control and Wnt-5A CM treatments are shown in each quadrant. (i) Summary of the frequency of human CD34+ and CD34+CD38− cells from the BM of SRC-engrafted mice treated with control or Wnt-5A CM as a percentage relative to controls.
We next evaluated the ability of the Wnt-5A CM to affect cell fate of hematopoietic lineages established by repopulating human SRCs. The multilineage composition of human hematopoietic cells (CD45+) was compared between both groups of treated SRCs by using phenotypic analyses for human myeloid (CD33) and lymphoid (CD19) lineages (ref. 7; Fig. 2 c–f Insets corresponding to isotype control). Both immature (CD34+) and mature (CD34−) populations of myelopoietic (CD33+) and lymphopoietic (CD19+) function were comparable in SRCs treated with control or Wnt-5A CM. We further characterized the human graft of these mice using a combination of CD34 and CD38 cell-surface markers to identify primitive subsets of human hematopoietic cells (7). SRCs treated with Wnt-5A CM gave rise to an increase in the frequency of CD34+ cells compared with control-treated recipients and a further increase among the rarer subfraction of more primitive CD34+CD38− cells (Fig. 2 g and h). This effect is summarized in Fig. 2i, where the levels of primitive human cells relative to control-treated SRCs revealed that Wnt-5A-treated SRCs gave rise to 50% more primitive human cells in vivo (Fig. 2i).
To determine whether the effect of Wnt-5A on human SRCs repopulating the NOD/SCID BM could be due to an indirect alteration of the recipient murine microenvironment by the Wnt-5A CM, we performed similar in vivo repopulation studies using murine GFP+ BM MNCs as donor cells. As indicated in Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org, there was no effect of Wnt-5A CM on the repopulating function of mouse hematopoietic stem cells. The absence of a Wnt-5A effect on murine stem cells supports that the role of Wnt-5A on human SRC is not due to indirect regulation of Wnt-5A CM on the recipient murine microenvironment. Alternatively, the lack of a Wnt-5A effect on murine stem cells may suggest that the action of Wnts on hematopoietic stem cells is species-specific or represents differences in quantity or quality of Wnts required to elicit a response from mouse-repopulating cells.
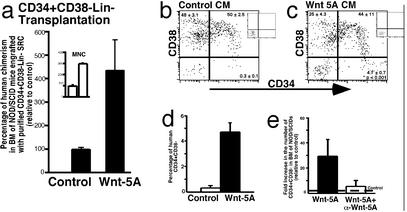
Cotransplantation of mature populations of hematopoietic cells together with purified SRCs has been shown to facilitate the repopulating capacity in NOD/SCID recipients (24). To further characterize the effects of Wnt-5A CM treatment on human SRCs directly, as opposed to potential actions of Wnt-5A on mature hematopoietic cells present in transplanted CB MNCs that may indirectly enhance SRC function, mice engrafted with purified SRCs were treated with control and Wnt-5A CM. Because human SRCs are highly enriched within CB CD34+CD38−Lin− cells (5, 45), we evaluated the effect of Wnt-5A CM on this more-purified source of transplanted human-repopulating cells. CD34+CD38−Lin− cells were isolated from CB MNCs by using FACS at >98% purity (data not shown). Mice were i.v. transplanted with purified CD34+CD38−Lin− cells and subsequently treated with control or Wnt-5A CM (n = 10). A summary of the human engraftment levels relative to control CM is shown in Fig. 3a. Similar to CB MNCs (Inset), treatment of purified CD34+CD38−Lin−-repopulating cells with Wnt-5A CM increased the level of human hematopoietic repopulation by 4-fold compared with control CM.
Figure 3.
Analysis of the effect of in vivo Wnt-5A treatment on NOD/SCID mice engrafted with highly purified human SRCs. (a) Summary of the level of human chimerism in NOD/SCID mice engrafted with purified CD34+CD38−Lin− SRCs treated with control or Wnt-5A CM. (Inset) Compared levels of chimerism in similarly treated mice transplanted with unpurified SRCs from CB MNCs. (b and c) Comparison of the frequency of human CD34+ and CD34+CD38− cells engrafting the BM of NOD/SCID mice treated with control vs. Wnt-5A CM. Average frequency ± SEM is shown in each quadrant (n = 4 for each treatment). (d) Summary of the level of primitive human CD34+CD38− progeny from control- vs. Wnt-5A-treated purified SRCs. (e) Summary of the fold increase (compared with PBS or Iscove's modified Dulbecco's medium i.p.-injected controls, dotted line) in the frequency of CD34+CD38− cells in the BM of NOD/SCID mice injected with Wnt-5A CM incubated with or without α-Wnt-5A antibody as indicated (n = 9).
Purified CD34+CD38−Lin− SRCs generated similar lymphoid and myeloid compositions compared with unpurified SRCs (Fig. 2 c and d) in mice treated with control and Wnt-5A CM (data not shown). Consistent with unpurified SRCs, a higher frequency of primitive CD34+CD38− cells and CD34+ cells (summary of collective data not shown) were present in the BM of Wnt-5A-treated mice transplanted with SRCs from CD34+CD38−Lin− cells as shown in representative examples in Fig. 3 b and c. The values indicated in quadrants represent the mean ± SEM from a total of eight mice from four independent CBs. Unlike CB MNCs, where only modest increases in CD34+CD38− SRC progeny were demonstrated with Wnt-5A CM treatment, transplantation of purified SRCs treated with Wnt-5A CM demonstrated a 15-fold increase compared with control CM as summarized in Fig. 3d. These results demonstrate the ability of Wnt-5A CM to preferentially enhance primitive hematopoietic development of human SRCs in vivo.
To examine the specificity of Wnt-5A in MS-5 Wnt-5A CM to augment primitive developmental capacity of human SRCs, we immunodepleted Wnt-5A from freshly harvested CM using a Wnt-5A-specific antibody. Because treatment of engrafted NOD/SCID mice with Wnt-5A CM had a profound effect on primitive CD34+CD38− human cells engrafting the BM of transplanted mice (Figs. 2i and 3d), we sought to evaluate the specificity of Wnt-5A using this endpoint measurement. By using the AF645 antibody against Wnt-5A (α-Wnt-5A), CM was freshly harvested from Wnt-5A MS-5 cells and incubated with or without Wnt-5A AF645 antibody for 1 h at room temperature before i.p. injection. Engrafted NOD/SCID mice injected with PBS or Iscove's modified Dulbecco's medium alone (control, Fig. 3e, dotted line) possessed the expected lower levels of primitive human CD34+CD38− cells in the BM, whereas parallel mice transplanted with the same number of human SRCs contained nearly 30-fold greater numbers of CD34+CD38− cells (Fig. 3e). However, mice injected with Wnt-5A-CM incubated with α-Wnt-5A reduced the enhanced number of CD34+CD38− cells observed in mice injected with Wnt-5A CM. These results suggest that soluble Wnt-5A produced in the CM of Wnt-5A-transduced MS-5 cells accounts for the effects of augmenting primitive SRC capacity in vivo.
Functional Analysis of Primitive Subfractions Arising from Human SRCs Treated with Wnt-5A.
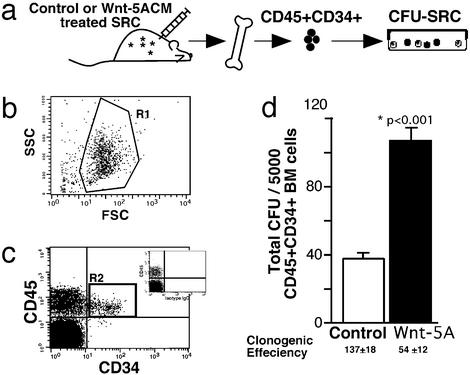
To determine whether the increased frequency of primitive CD34+ cells from Wnt-5A-treated SRCs was indicative of enhanced progenitor function arising from engrafted SRCs, human CD45+CD34+ cells were isolated from the BM of repopulated NOD/SCID mice by using FACS and assayed for progenitor function in CFU assays (CFU-SRCs; Fig. 4a). Forward/side-scatter characteristics of murine BM (Fig. 4b) and stained isotype controls (Fig. 4c Inset) were used to define quadrants for the isolation of human CD45+CD34+ cells (gated as R2; Fig. 3c). Human CD34+ cells from the BM of SRC-engrafted mice treated with both control and Wnt-5A CM gave rise to daughter hematopoietic progenitors detectable in vitro (25, 46). The multilineage progenitors from the resulting CFU-SRCs demonstrated typical morphologies and similar colony subtypes from both control and Wnt-5A treatments (data not shown). However, engrafted mice treated with Wnt-5A CM gave rise to significantly more CFU-SRCs than did mice treated with control CM, resulting in 104 ± 15 colonies vs. 38 ± 5 colonies per 5,000 cells, respectively (P < 0.001, n = 8; Fig. 2d). Because the CFU capacity of de novo isolated and cocultured progenitors under the direct influence of Wnt-5A had no effect, whereas primitive cells derived from Wnt-5A CM-treated SRCs possess enhanced progenitor function in the absence of continued Wnt-5A treatment, we suggest that Wnt-5A-secreted factors are capable of intrinsically affecting the functional program of human-repopulating stem cells and their progeny.
Figure 4.
Isolation and clonogenic CFU capacity of primitive CD34+ cells arising from Wnt-5A-treated SRCs. (a) Progenitors of human SRCs (CFU-SRCs): schematic diagram illustrating the experimental design used to examine the functional capacity of primitive CD34+ derived from control- vs. Wnt-5A-treated SRCs. Primitive CD45+CD34+ progeny arising from treated SRCs were isolated from the BM of repopulated NOD/SCID mice by using forward and side-scatter (SSC) properties of cells (R1) (b) and further gated on CD45+ human cells expressing CD34 (R2) (c). R2 gating was based on stringent isotype controls for human CD45+ cells shown in Inset. (d) Summary of the total number of CFU-SRCs per 5,000 CD45+CD34+ cells isolated from mice repopulated with control- or Wnt-5A-treated SRCs (n = 10). The frequency of CFU-SRCs was calculated by dividing the number of input cells by the total number of CFU produced. *, Statistical significance of P < 0.05.
Discussion
Our results indicate that Wnt-5A plays a regulatory role in the development of human hematopoietic-repopulating cells but not more mature progenitors detected in vitro. In vivo treatment of active human SRCs with Wnt-5A CM increased both the level of human chimerism in the BM of NOD/SCID recipients and the frequency of primitive CD34+ and CD34+CD38− cells. Additionally, the clonogenic capacity of CD34+ cells derived from Wnt-5A-treated SRCs was enhanced in the absence of continued Wnt-5A treatment. Previous studies of avian, murine, and human hematopoietic cells have reported the in vitro regulation of hematopoietic progenitors by Wnt-5A protein (15, 28, 47). The absence of Wnt-5A effects on purified human hematopoietic progenitors shown in the present study is contradictory to these previous reports, potentially illustrating the diversity of Wnt-5A signaling effects, where even within the same studies coculture and Wnt CM have demonstrated distinct responses (15, 28). In the absence of common functional assays for Wnt expression, it is unknown whether some of these observed differences are not simply due to the use of Wnts from different species. Although previous studies evaluating the role of Wnt-5A have assessed its effect on progenitors from various hematopoietic sources (15, 28, 47), none of these studies examined the effects of Wnt-5A in vivo and its functional role on rare human stem cells capable of repopulation. These seemingly inconsistent observations among studies support that notion that effects of Wnt signaling are species- and cell type-specific. Autocrine and paracrine utilization of Wnt proteins, in concert with other unique cell-specific factors, together with Frz expression profiles, will ultimately determine the cellular response.
Recent evidence indicates that proliferation and differentiation of the most primitive subclasses of progenitors are regulated in vitro by factors known for their instructive role during embryonic development including mammalian homologs of Sonic hedgehog (8), bone morphogenetic proteins (9), and Notch ligands (10). However, factors capable of regulating the capacity of repopulating stem cells during in vivo reconstitution are largely unknown (39) and would serve as the most feasible manner to manage patients presenting with poor hematopoietic reconstitution shortly after autologous or allogenic transplantation of BM or mobilized peripheral blood (39, 48, 49). In contrast to the conventional hematopoietic cytokines classically used to modulate mature progenitors in vitro (9), analysis of the effects of Wnt-5A reveals that Wnt-5A acts on human-repopulating stem cells, whereas more mature human progenitors are not responsive. This study represents the first demonstration of the ability to modulate the repopulating function of candidate human blood stem cells in vivo. Our study serves as the foundation to investigate the utility of Wnts, their peptide derivatives, or small-molecule Wnt pathway agonists to augment in vivo hematopoietic reconstitution in patients receiving stem cell transplants.
Supplementary Material
Abbreviations
- NOD
nonobese diabetic
- SCID
severe combined immunodeficient
- SRC
SCID-repopulating cell
- Frz
Frizzled
- BM
bone marrow
- EGFP
enhanced GFP
- FACS
fluorescence-activated cell sorting
- CB
cord blood
- MNC
mononuclear cell
- CFU
colony-forming unit(s)
References
- 1.Dieterlen-Lievre F, Godin I, Pardanaud L. Int Arch Allergy Immunol. 1997;112:3–8. doi: 10.1159/000237423. [DOI] [PubMed] [Google Scholar]
- 2.Morrison S J, Wandycz A M, Hemmati H D, Wright D E, Weissman I L. Development (Cambridge, UK) 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- 3.Domen J, Weissman I L. Mol Med Today. 1999;5:201–208. doi: 10.1016/S1357-4310(99)01464-1. [DOI] [PubMed] [Google Scholar]
- 4.Dieterlen-Lievre F. Curr Biol. 1998;8:R727–R730. doi: 10.1016/s0960-9822(98)70460-9. [DOI] [PubMed] [Google Scholar]
- 5.Larochelle A, Vormoor J, Hanenberg H, Wang J C, Bhatia M, Lapidot T, Moritz T, Murdoch B, Xiao X L, Kato I, et al. Nat Med. 1996;2:1329–1339. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 6.Dick J E, Bhatia M, Gan O, Kapp U, Wang J C. Stem Cells (Dayton) 1997;15, Suppl. 1:199–207. doi: 10.1002/stem.5530150826. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia M, Wang J C Y, Kapp U, Bonnet D, Dick J E. Proc Natl Acad Sci USA. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhardwaj G, Murdoch B, Wu D, Baker D P, Williams K P, Chadwick K, Ling L E, Karanu F N, Bhatia M. Nat Immunol. 2001;2:172–180. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia M, Bonnet D, Wu D, Murdoch B, Wrana J, Gallacher L, Dick J E. J Exp Med. 1999;189:1139–1148. doi: 10.1084/jem.189.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, Pear W S, Bernstein I D. Nat Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 11.Polakis P. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 12.Kuhl M, Sheldahl L C, Park M, Miller J R, Moon R T. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 13.Huelsken J, Birchmeier W. Curr Opin Genet Dev. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 14.Mead P E, Zon L I. Curr Opin Hematol. 1998;5:156–160. doi: 10.1097/00062752-199803000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Van Den Berg D J, Sharma A K, Bruno E, Hoffman R. Blood. 1998;92:3189–3202. [PubMed] [Google Scholar]
- 16.Leung B L, Haughn L, Veillette A, Hawley R G, Rottapel R, Julius M. J Immunol. 1999;163:1334–1341. [PubMed] [Google Scholar]
- 17.Murdoch B, Gallacher L, Awaraji C, Hess D A, Keeney M, Jay K, Chadwick K, Fowley S R, Howson-Jan K, Chin Yee I, et al. FASEB J. 2001;15:1628–1630. doi: 10.1096/fj.00-0654fje. [DOI] [PubMed] [Google Scholar]
- 18.Murdoch B, Pereira D S, Wu X, Dick J E, Ellis J. Gene Ther. 1997;4:744–749. doi: 10.1038/sj.gt.3300448. [DOI] [PubMed] [Google Scholar]
- 19.Kobari L, Dubart A, Le Pesteur F, Vainchenker W, Sainteny F. J Cell Physiol. 1995;163:295–304. doi: 10.1002/jcp.1041630210. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki J, Fujita J, Taniguchi S, Sugimoto K, Mori K J. Leukemia. 1992;6:452–458. [PubMed] [Google Scholar]
- 21.Gallacher L, Murdoch B, Wu D, Karanu F, Fellows F, Bhatia M. Blood. 2000;96:1740–1747. [PubMed] [Google Scholar]
- 22.Rosu-Myles M, Gallacher L, Murdoch B, Hess D A, Keeney M, Kelvin D, Dale L, Ferguson S S, Wu D, Fellows F, Bhatia M. Proc Natl Acad Sci USA. 2000;97:14626–14631. doi: 10.1073/pnas.97.26.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallacher L, Murdoch B, Wu D M, Karanu F N, Keeney M, Bhatia M. Blood. 2000;95:2813–2820. [PubMed] [Google Scholar]
- 24.Bonnet D, Bhatia M, Wang J C, Kapp U, Dick J E. Bone Marrow Transplant. 1999;23:203–209. doi: 10.1038/sj.bmt.1701564. [DOI] [PubMed] [Google Scholar]
- 25.Cashman J D, Lapidot T, Wang J C, Doedens M, Shultz L D, Lansdorp P, Dick J E, Eaves C J. Blood. 1997;89:4307–4316. [PubMed] [Google Scholar]
- 26.De Sepulveda P, Okkenhaug K, Rose J L, Hawley R G, Dubreuil P, Rottapel R. EMBO J. 1999;18:904–915. doi: 10.1093/emboj/18.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christian J L. Curr Opin Cell Biol. 2000;12:244–249. doi: 10.1016/s0955-0674(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 28.Brandon C, Eisenberg L M, Eisenberg C A. Blood. 2000;96:4132–4141. [PubMed] [Google Scholar]
- 29.Bradley R S, Brown A M. EMBO J. 1990;9:1569–1575. doi: 10.1002/j.1460-2075.1990.tb08276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papkoff J, Schryver B. Mol Cell Biol. 1990;10:2723–2730. doi: 10.1128/mcb.10.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moon R T, Brown J D, Torres M. Trends Genet. 1997;13:157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- 32.Torres M A, Yang-Snyder J A, Purcell S M, DeMarais A A, McGrew L L, Moon R T. J Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross S E, Hemati N, Longo K A, Bennett C N, Lucas P C, Erickson R L, MacDougald O A. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 34. Ishitani, T., Kishida, S., Hyodo, J., Ueno, N., Yasuda, J., Waterman, M., Shibuya, H., Moon, R. T., Ninomiya-Tsuji, J. & Matsumoto, K. (2003). Mol. Cell Biol., in press. [DOI] [PMC free article] [PubMed]
- 35.Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K. Nature. 2002;417:295–299. doi: 10.1038/417295a. [DOI] [PubMed] [Google Scholar]
- 36.Tajbakhsh S, Borello U, Vivarelli E, Kelly R, Papkoff J, Duprez D, Buckingham M, Cossu G. Development (Cambridge, UK) 1998;125:4155–4162. doi: 10.1242/dev.125.21.4155. [DOI] [PubMed] [Google Scholar]
- 37.Borello U, Coletta M, Tajbakhsh S, Leyns L, De Robertis E M, Buckingham M, Cossu G. Development (Cambridge, UK) 1999;126:4247–4255. doi: 10.1242/dev.126.19.4247. [DOI] [PubMed] [Google Scholar]
- 38.Weissman I L. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 39.Weissman I L. Science. 2000;287:1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- 40.Eaves C, Miller C, Conneally E, Audet J, Oostendorp R, Cashman J, Zandstra P, Rose-John S, Piret J, Eaves A. Ann NY Acad Sci. 1999;872:1–8. doi: 10.1111/j.1749-6632.1999.tb08447.x. [DOI] [PubMed] [Google Scholar]
- 41.Dick J E. Nat Med. 2000;6:624–626. doi: 10.1038/76188. [DOI] [PubMed] [Google Scholar]
- 42.Petzer A L, Zandstra P W, Piret J M, Eaves C J. J Exp Med. 1996;183:2551–2558. doi: 10.1084/jem.183.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bock T A. Stem Cells (Dayton) 1997;15:185–195. doi: 10.1002/stem.5530150824. [DOI] [PubMed] [Google Scholar]
- 44.Bhatia M, Bonnet D, Murdoch B, Gan O I, Dick J E. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 45.Bhatia M, Bonnet D, Kapp U, Wang J C, Murdoch B, Dick J E. J Exp Med. 1997;186:619–624. doi: 10.1084/jem.186.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guenechea G, Gan O I, Dorrell C, Dick J E. Nat Immunol. 2001;2:75–82. doi: 10.1038/83199. [DOI] [PubMed] [Google Scholar]
- 47.Austin T W, Solar G P, Ziegler F C, Liem L, Matthews W. Blood. 1997;89:3624–3635. [PubMed] [Google Scholar]
- 48.Stiff P J. Bone Marrow Transplant. 1999;23:S29–S33. doi: 10.1038/sj.bmt.1701671. [DOI] [PubMed] [Google Scholar]
- 49.Siena S, Schiavo R, Pedrazzoli P, Carlo-Stella C. J Clin Oncol. 2000;18:1360–1377. doi: 10.1200/JCO.2000.18.6.1360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.