Abstract
CD8+ cells from healthy HIV-infected individuals can suppress HIV replication in infected CD4+ cells without killing the cells. This CD8+ cell noncytotoxic antiviral response (CNAR), observed by coculture of CD8+ cells with infected CD4+ cells, is associated with secretion of a CD8+ cell antiviral factor (CAF). In attempts to identify CAF, we discovered that certain protease inhibitors, particularly leupeptin, can block, by up to 95%, the anti-HIV activity in CD8+ cell culture fluids as well as inhibit CNAR. The effect is dose-dependent and is observed in up to 70% of the CAF and CNAR assays by using fluids and cells from several different subjects. Pretreatment of CD8+ cells with leupeptin reduces CNAR, further supporting an inhibitory effect on a CD8+ cell product. This inhibitory activity of protease inhibitors does not affect cell growth, expression of activation antigens, or viability of either CD8+ cells or the infected CD4+ cells. The results suggest that a part of the CD8+ cell noncytotoxic response involves the activity of a protease or a protein that interacts with protease inhibitors. Proteolysis of a CD8+ cell product(s) may be involved. This observation offers a promising approach for identifying the mechanism of CNAR/CAF activity.
Keywords: CD8+ cell antiviral responses‖CD8+ cell antiviral factor‖HIV replication
Most individuals infected with HIV do not immediately develop symptoms of the disease (1). A small percentage can live more than 10 years, some over 24 years, without showing clinical signs of the infection (1–4). Our laboratory has demonstrated that CD8+ cells from these asymptomatic individuals are able to suppress replication of all HIV-1 and -2 strains tested in target CD4+ lymphocytes and macrophages through a noncytotoxic mechanism (4–6). This CD8+ noncytotoxic antiviral response (CNAR), measured by coculture of CD8+ cells with acutely infected CD4+ cells, is present at very early stages in acute infection (7) and is clinically relevant; the response can show complete suppression of HIV production, strongest in healthy individuals but lost with progression to disease (8–11).
CNAR appears to be mediated by the secretion of a soluble CD8+ cell antiviral factor (CAF), which, as observed with CNAR, blocks HIV transcription (8, 12, 13). CAF in culture fluids can be measured by culturing acutely infected CD4+ cells in the continual presence of the CD8+ cell supernatant. Virus replication is reduced by 30–80% compared with control-infected CD4+ cells. This antiviral activity was found to be heat and pH stable (86°C, 10 min; pH 2–8) and is resistant to trypsin but sensitive to staph V8 protease (4). CAF activity lacks identity to other known cytokines, including interferons, interleukins, growth factors, chemokines, granulysin, and granzymes (4, 14–23). Most recently, we have shown that CAF activity is not found in exocytic granules of CD8+ cells (41).
In our studies evaluating the sensitivity of CAF to proteases, we observed that the addition of the protease inhibitor, leupeptin, to CAF-containing culture fluids resulted in reduced CAF activity. We subsequently determined that anti-HIV activity in both CNAR and CAF assays was sensitive (in a dose-dependent manner) to several protease inhibitors. Although not all of the studies showed this sensitivity, the extent of our observations suggested that a component of CNAR/CAF activity could involve the function of a protease produced by the antiviral CD8+ cells. This article reports our observations on this potential mechanism of CAF activity.
Materials and Methods
Subjects.
Heparinized blood samples were obtained by venipuncture from clinically healthy HIV seropositive donors who had been infected for >8 yr. Some of them were on antiretroviral therapy, but all subjects were selected because their CD8+ cells exhibited CNAR. Buffy coats from HIV seronegative donors were provided by the Blood Centers of the Pacific (San Francisco). The study received approval from the Committee on Human Research, University of California, San Francisco.
Protease Inhibitors.
Leupeptin hemisulfate (acetyl-leucine-leucine-arginine aldehyde), antipain hydrochloride (N-[Na-carbonyl-arginine-valine-arginine aldhyde]-phenylalanine), and Pefabloc [p-aminoethylbenzenesulfonyl fluoride-HCl (AEBSF)] were purchased from Boehringer Mannheim or Sigma. Calpain inhibitor II (acetyl-leucine-leucine-methionine aldhyde) was purchased from Peninsula Laboratories. The protease inhibitor mixture, which included AEBSF (500 μM), aprotinin (150 nM), E-64 (1 μM), EDTA (0.5 mM), and leupeptin (1 μM), was purchased from Calbiochem. Benzamidine, aprotinin, α2-macroglobulin, and the two forms of ecotin were provided by C.S.C.'s laboratory. All of the inhibitors were reconstituted in PBS to 1 mg/ml (except for calpain inhibitor II, which was dissolved in either methanol or dimethyl sulfoxide before dilution in PBS). Each inhibitor was immediately aliquoted and stored frozen for <3 mo at −20°C before use.
Flow Cytometric Analysis.
T cell subset separation was evaluated by standard flow cytometry (24). In all studies, the cell population purity was always ≥95%. The expressions of CD25 and CD71 were analyzed by a double-staining procedure by using a Becton Dickinson (BD) FACScan or FACSort machine as described (25). All antibodies were purchased from BD except for anti-CD71, which was obtained from Immunotech–Coulter (Miami).
Production of CAF by CD8+ Cells.
CD8+ cells were purified from Ficoll/Hypaque-separated peripheral blood mononuclear cells using immunomagnetic (IM) beads (8, 15). They were stimulated with anti-CD3-coupled IM beads at a bead/cell ratio of ≈4:1 (15, 26). The cells were initially cultured for 3 d in complete RPMI medium 1640 containing 10% heat-inactivated (56°C, 30 min) FCS (GIBCO/BRL) and then transferred to serum-free AIM V medium (GIBCO/BRL). Both media contained 200 units/ml recombinant IL-2 (generously provided by Glaxo Wellcome). Culture fluids were collected every 2 d and the cell density adjusted to 2 × 106 cells per ml in fresh medium. The collected culture fluid was filtered (0.45 μm) and stored (−70°C) until tested.
Assays for Measuring CAF Activity.
Acutely infected CD4+ cells were prepared as described (15). In brief, mitogen-stimulated CD4+ cells from HIV seronegative control subjects were acutely infected with 4,000 TCID50/107 cells of a molecular clone of HIV-1SF2, a β-chemokine insensitive virus (27). After 1 h incubation, the cells were washed and plated at 105 infected CD4+ cells per well in 96-well culture plates in the complete RPMI medium 1640 supplemented with 100 units/ml recombinant IL-2. In all cases, the acutely infected CD4+ cells were cultured in the presence of a 50% dilution of a CD8+ cell culture fluid (or control medium) in a final volume of 200 μl. The cultures, in triplicate, were passed every 2 d and monitored for viral reverse transcriptase (RT) activity (28) by removing 100 μl of culture fluid for the assay. An equal volume of CD8+ cell supernatant and control medium was added back. This acute infection method routinely yields 15–35% HIV antigen-positive cells as detected by immunofluorescence at the peak of HIV replication (7–9 d) (29). The amount of CAF activity is determined by dividing the level of reduction in RT activity in fluids from the CD8+ cell culture fluid-treated cells by that found in fluids from infected CD4+ cells receiving control medium. We consider a decrease in RT activity by ≥50% to be strong CAF activity.
Treatment of CAF with a protease inhibitor involved the incubation of a CD8+ cell culture fluid (with or without antiviral activity) and a medium control with dilutions of a protease inhibitor for 30–60 min at room temperature. The mixture was then added to the HIV-infected CD4+ cell cultures as described above. For these studies, the positive fluids gave 40–80% suppression of HIV replication, whereas the negative fluids showed ≤10%.
Assay for Measuring CNAR.
To measure the extent of CNAR, purified phytohemagglutinin (PHA)-stimulated CD4+ cells from HIV seronegative individuals were acutely infected with the β-chemokine-insensitive, highly cytopathic HIV-1SF33 virus isolate (27, 30). After 1 h, the cells were washed and 1 × 105 or 2 × 105 acutely infected CD4+ cells were plated per well in 96- or 48-well culture plates, respectively, in the complete RPMI medium 1640 supplemented with 100 units/ml rIL-2. These target cells were immediately cocultured, in duplicate or triplicate, with various 2-fold input amounts of purified CD8+ cells previously isolated from an HIV-infected subject's PHA-stimulated or unstimulated peripheral blood mononuclear cells (8, 27, 29). With autologous cells, the CD8+ cell/CD4+ cell input was generally 0.125:1 and 0.25:1; with heterologous cells, 0.5:1 and 1:1 input cell ratios were used. HIV replication was measured by the RT assay in culture fluids removed every 3 d. The extent of CNAR was determined by dividing the amount of RT activity in fluids from the CD8+ cell/CD4+ cell cocultures with that present in fluids from infected CD4+ cells cultured alone. Based on several studies in our laboratory, we consider a decrease in RT activity by ≥80% to be strong CNAR activity.
Protease inhibitors were added immediately upon initiation of the cocultures and replenished at each passage. In the experiments involving pretreatment of CD8+ and CD4+ cells, the respective cells were cultured in the presence of various concentrations of the protease inhibitor for 2 or 3 d (in some cases 2–3 h) before washing out the inhibitor and using the cells in the CNAR assay.
Results
Leupeptin Blocks the Antiviral Activity of CAF.
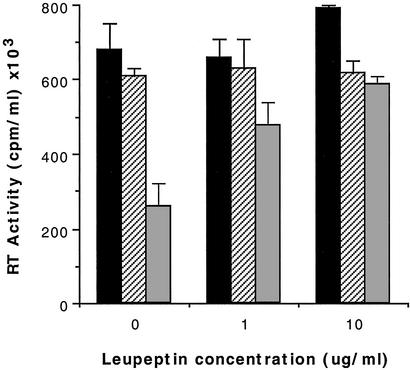
In studies examining the sensitivity of CAF to proteases, we observed in control cultures that the serine/thiol protease inhibitor, leupeptin, reduced the extent of CAF-mediated inhibition of HIV replication. Further analysis of this effect showed that treatment of CAF-active culture fluids with various concentrations of this tripeptide inhibitor, acetyl-leucine-leucine-arginine aldehyde, resulted in a dose-dependent inhibition of CAF activity (Fig. 1). The extent of inhibition of CAF activity varied considerably with the maximal amount being >90%. More commonly, it was between 40% and 70% at the highest inhibitor concentrations tested, or no effect was observed. Concentrations of leupeptin higher than 10–20 μg/ml (21–42 μM) usually did not result in further inhibition of antiviral activity. Leupeptin treatment of CD8+ cell culture fluids lacking CAF activity or of control media did not result in any substantial reduction in HIV replication relative to the respective untreated fluids. In some cases, the addition of leupeptin to the control medium enhanced HIV production in a dose-dependent fashion, but generally <1.5-fold (data not shown). However, doses >50 μg/ml (105 μM) usually reduced HIV replication in the CD4+ cells.
Figure 1.
Inhibition of CAF activity by the protease inhibitor leupeptin. CD4+ T cells, acutely infected with the β-chemokine-insensitive isolate HIV-1SF2C, were cultured in the presence of a 50% dilution of control medium (■), a CAF-negative CD8+ cell supernatant ( ), or a CAF-positive CD8+ cell supernatant (
), or a CAF-positive CD8+ cell supernatant ( ), each pretreated for 30 min with the indicated concentration of leupeptin. The data represent the peak RT activity in fluids from the respective cultures ± 1 SD (28). A representative example of six separate experiments is shown.
), each pretreated for 30 min with the indicated concentration of leupeptin. The data represent the peak RT activity in fluids from the respective cultures ± 1 SD (28). A representative example of six separate experiments is shown.
The consistency of this inhibitory effect was somewhat unusual. Typically the same antiviral culture fluid yielded similar results when retested. However, an appreciable number of different antiviral CD8+ cell culture fluids (≈30%) showed less than a 20% reduction of antiviral activity by leupeptin (Table 1). This finding appeared to be independent of the magnitude of CAF activity in the CD8+ cell supernatant. In total, of 13 different CAF-active fluids, obtained from stimulated primary CD8+ cells, ≈70% showed some sensitivity to leupeptin, whereas just over half (54%) showed strong sensitivity to this protease inhibitor (Table 1). The CAF activity in four of five culture fluids from HVS-transformed CD8+ cell lines (31) was also highly sensitive to the inhibitory effect of leupeptin (data not shown). These findings may reflect a proteolytic activity in CAF-containing fluids, which processes a protein to become antiviral. This protein in some culture fluids may already have been processed to some extent, and thus the antiviral activity in these fluids would be unaffected or only partially affected by a protease inhibitor.
Table 1.
Prevalence of leupeptin effect
| Antiviral* activity | Frequency of inhibition of antiviral activity†
|
||
|---|---|---|---|
| High | Moderate | Low | |
| CAF | 7/13 (54%) | 2/13 (15%) | 4/13 (31%) |
| CNAR | 18/39 (46%) | 8/39 (21%) | 13/39 (33%) |
| CNAR*‡ | 18/29 (62%) | 5/29 (17%) | 6/29 (20%) |
CAF and CNAR activities were measured as described in Materials and Methods. The results with CAF represent anti-HIV activity in CD8+ cell culture fluids evaluated with a β-chemokine-insensitive HIV isolate in primary CD4+ cells. In the untreated CD8+ cell culture fluids, the extent of CAF activity was 40–80% inhibition of HIV replication. The results from examination of CNAR represent the anti-HIV activity measured by coculture of CD8+ cells with primary CD4+ cells acutely infected with a β-chemokine-insensitive HIV isolate. These results from CNAR experiments were generated by using either PHA-stimulated (≈60% of the time) or “resting” CD8+ effector cells with either autologous (≈35% of the time) or heterologous CD4+ target cells (see text). The extent of virus replication inhibited by CNAR in the untreated cultures was 30–99%.
The frequency of inhibition of antiviral activity indicates the number of experiments showing high (>50%), moderate (>50% but <25%), or low/no (<20%) sensitivity to leupeptin (i.e., reduction of antiviral activity) divided by the total number of different experiments performed. The sensitivity of the antiviral activity to inhibition by leupeptin was tested over a range of 10-fold concentrations, 0.1–10 μg/ml (0.2–21 μM). CD8+ cell culture fluids from eight different subjects and CD8+ cells from 13 different subjects were evaluated for CAF and CNAR, respectively. CAF or CNAR was evaluated in some of the subjects more than once, but in these cases it was with samples taken from the subject at different points in time.
CNAR* represents a subset of the CNAR experiments, which excludes 10 cases where CNAR activity was high, showing ≥90% inhibition of HIV replication.
CAF Activity Is Sensitive to Various Protease Inhibitors.
To determine whether the antiviral activity of CAF was sensitive to protease inhibitors in general, a panel of protease inhibitors including small synthetic inhibitors (p-aminoethylbenzenesulfonyl fluoride-HCl (AEBSF) and benzamidine), tri- or tetrapeptide inhibitors, and larger protein inhibitors (aprotinin, α2-macroglobulin, and ecotin) were evaluated. The results indicated that many of the protease inhibitors could substantially and reproducibly reduce CAF activity, although to different extents (Table 2). Yet not all of the protease inhibitors affected CAF, suggesting some degree of specificity. Most notably, calpain inhibitor II and the wild-type ecotin exhibited very little inhibitory activity against CAF (Table 2).
Table 2.
The effect of various protease inhibitors on CAF
| Protease inhibitor | Maximal % reduction of CAF activity* | ||
|---|---|---|---|
| Antipain | 65 | 99 | 47 |
| Calpain inhibitor II | 0 | 8 | |
| Pefabloc (AEBSF) | 79 | 61 | 47 |
| Benzamidine | 40 | 0 | 24 |
| Ecotin (Arg-Met)† | 40 | 36 | 42 |
| Ecotin (wild type) | 5 | 11 | |
| Aprotinin | 42 | 43 | |
| α2-Macroglobulin | 58 | ||
The effect of each protease inhibitor on CAF activity was determined as described for leupeptin in the legend of Fig. 1. The maximal inhibitory effect was usually seen at 10–20 ug/ml of the protease inhibitor, with the exception of inhibition by α2-macroglobulin, which was maximal at 100 ug/ml. The range of molar concentrations (in 10-fold increments) tested for each protease inhibitor was as follows: antipain, 0.2–16 μM; calpain inhibitor II, 0.2–25 μM; AEBSF, 0.4–42 μM; benzamidine, 0.6–64 μM; ecotin, 2.6–260 nM; aprotinin, 0.01–1.5 μM; α2-macroglobulin, 1.4–138 nM. The numbers shown for each protease inhibitor were obtained from one to three separate experiments. The values for a given protease inhibitor were obtained by using different CD8+ cell fluids. See Table 1 for comparison with effect of leupeptin in several separate studies.
Ecotin (Arg-Met) represents a mutated form of wild-type ecotin with higher antitrypsin activity in which the methionine at position 84 was substituted with an arginine.
Protease Inhibitors Block CNAR and CAF to a Comparable Extent.
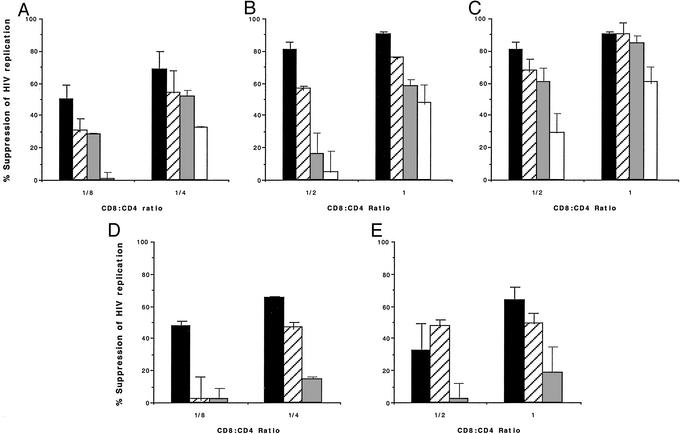
Because CNAR appears mediated by secretion of CAF, we expected that inhibition of HIV replication in the cell/cell contact setting would also be sensitive to leupeptin. We therefore tested the effect of leupeptin against CNAR using various procedures in which CNAR has been previously demonstrated (8, 9, 26). Under the classical conditions using PHA-stimulated CD8+ cells as effectors and heterologous HIV-infected CD4+ cells as targets (8, 26), continuous culture in the presence of leupeptin resulted in a dose-dependent reduction in antiviral activity similar to that seen with CAF (Fig. 2A; Table 1). At low effector/target cell ratios where the extent of HIV suppression is submaximal (i.e., 30–70%), 10 μg/ml leupeptin was able to block as much as 95% of CNAR activity. In general, the higher the effector/target cell ratio tested (hence the greater the suppression of HIV), the lower the effect of leupeptin seen. Usually, if >90% suppression of HIV replication occurred, leupeptin treatment resulted in a reduction in CNAR of only 10–50%. Treatment with a mixture of various types of protease inhibitors did not result in further inhibition of the antiviral activity.
Figure 2.
Leupeptin inhibition of the CD8+ cell-mediated suppression of HIV replication. The CNAR was measured against HIV-1SF33 acutely infected CD4+ T cells in the continued presence of the protease inhibitor leupeptin (A–E) or antipain (C) at the indicated CD8+ cell/CD4+ cell input ratios. The infected CD4+ target cells used were either heterologous (A–C) or autologous (D and E) with respect to the effector CD8+ cells, which were either PHA-stimulated (A and D) or nonstimulated (B, C, and E). The concentrations (in micrograms per milliliter) of the protease inhibitor used were 0 (■), 0.1 ( ), 1 (
), 1 ( ), and 10 (□) (see Tables 1 and 2 for molar equivalents). HIV replication was monitored every 3 d by measuring RT activity in culture fluid samples (28). The percent suppression of HIV replication was calculated by using RT values in the coculture fluids at the time of peak virus replication, typically on d 6 postinfection (see Materials and Methods). The level of RT activity in the fluids of untreated infected CD4+ cell control cultures always reached >200,000 cpm/ml at peak virus replication. The data are representative of three to eight separate experiments for each condition.
), and 10 (□) (see Tables 1 and 2 for molar equivalents). HIV replication was monitored every 3 d by measuring RT activity in culture fluid samples (28). The percent suppression of HIV replication was calculated by using RT values in the coculture fluids at the time of peak virus replication, typically on d 6 postinfection (see Materials and Methods). The level of RT activity in the fluids of untreated infected CD4+ cell control cultures always reached >200,000 cpm/ml at peak virus replication. The data are representative of three to eight separate experiments for each condition.
Analysis of this leupeptin effect on CNAR using freshly isolated, nonexogenously stimulated, “resting” CD8+ cells revealed a similar dose-dependent reduction of the suppressive activity exhibited by these effector cells (Fig. 2B). Coculturing autologous infected CD4+ target cells, which typically show increased sensitivity to CNAR (26), with either PHA-stimulated or resting CD8+ cells (9) also showed this inhibitory effect of leupeptin (Fig. 2 D and E). Moreover, just as with CAF activity, CNAR measured under these various conditions was also sensitive to other protease inhibitors such as antipain (Fig. 2C) and p-aminoethylbenzenesulfonyl fluoride-HCl (AEBSF) (not shown) but was insensitive to calpain inhibitor II (data not shown).
The reproducibility of this leupeptin inhibitory effect against CNAR was quite similar to that seen with CAF fluids. About one-third of the CNAR experiments conducted resulted in <20% inhibitory effect of leupeptin, whereas about one-half showed >50% reduction in antiviral activity (Table 1). These frequencies were obtained from all of the experiments regardless of the conditions by which they were tested. These included experiments with CD8+ cells from the same HIV-infected subjects collected over a 1- to 2-yr period. For example, in one untreated asymptomatic person studied over a 2-yr period, 9 of 12 of the CNAR assays showed a moderate to high sensitivity to leupeptin. As noted above, if the extent of CNAR activity examined was high (>80–90% suppression), the effect of leupeptin was greatly reduced. Thus, if we exclude those experiments in which the CNAR activity was very high (n = 10), then the efficacy of the leupeptin effect increases so that ≈80% of the experiments showed moderate to high sensitivity to leupeptin (see CNAR*, Table 1).
Analysis of the prevalence of sensitivity to leupeptin in 15 different HIV-infected asymptomatic subjects indicated that the CNAR of eight of the subjects had high sensitivity to this protease inhibitor, four showed moderate sensitivity, and the CNAR from three of the subjects showed low or no sensitivity to the protease inhibitor (data included in Table 1). Whether these latter three individuals are truly “nonresponders” to leupeptin remains to be determined.
Culture of the CD8+ effector cells (stimulated or not) or the infected CD4+ target cells in the continual presence of concentrations of leupeptin as high as 20 μg/ml (43 μM) did not affect the expression of the activation antigens, CD25 or CD71, by either T cell population, nor did this treatment affect the extent of cell proliferation or viability (data not shown).
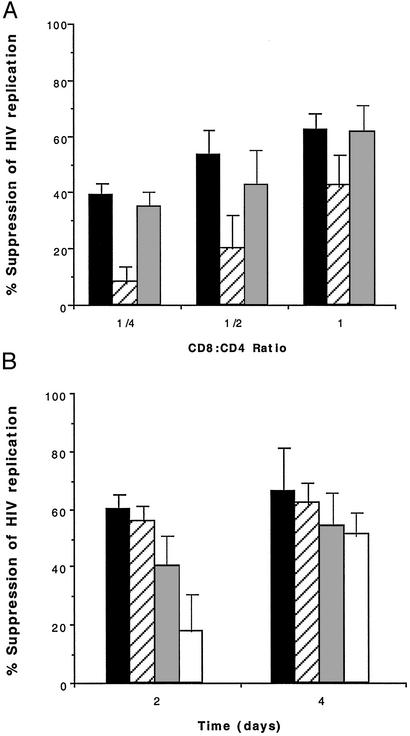
Leupeptin Mediates Its Effect on the CD8+ Effector Cells and Not the CD4+ Target Cells.
Because both the assay for CAF activity and the assay for CNAR measure effects on HIV replication in CD4+ cells, it was important to distinguish whether the presence of leupeptin in the cultures was affecting the target cell or the effector cell. To address this issue, the effector CD8+ cells and target CD4+ cells were separately cultured in the presence of leupeptin for 2–3 d before using these cell populations in CNAR assays. When the CD4+ target cells were pretreated with 20 μg/ml (43 μM) of leupeptin, followed by washing the cells free of the protease inhibitors, no difference was seen in the extent of HIV suppression at various effector/target cell ratios relative to the untreated control cultures (Fig. 3). However, pretreatment of the CD8+ cells with leupeptin resulted in a marked reduction in CNAR activity, again most prominently at effector/target cell ratios that yielded lower levels of HIV suppression. This effect was dose-dependent and seemed to be transient, waning after 4 d of coculture (Fig. 3B). Furthermore, pretreatment of CD8+ cells for 2–3 h with leupeptin was also sufficient to reduce CNAR activity, although to a lesser extent (data not shown).
Figure 3.
The effect of pretreatment of CD8+ and CD4+ T cells with leupeptin. (A) Nonexogenously stimulated antiviral CD8+ effector cells ( ) and infected heterologous CD4+ target cells (
) and infected heterologous CD4+ target cells ( ) were separately cultured in the absence (■) or presence of 20 μg/ml (43 μM) leupeptin for 2 d before washing and using in CNAR assays at the indicated CD8+ cell/CD4+ cell input ratios. The CD4+ cells cultured in the absence of leupeptin (■) served as cell targets for the control cocultures. The extent of suppression of HIV replication was determined by using RT levels in culture fluids at the peak of HIV replication, typically 4 d after the initiation of the coculture. The level of RT activity in the culture fluids of treated and untreated CD4+ target cells at this time point was 732,000 and 688,000 cpm/ml, respectively. The data are representative of two to three separate studies. (B) Nonexogenously stimulated antiviral CD8+ cells were cultured in the presence of various concentrations of leupeptin (micrograms per milliliter) for 2 d before washing and using in CNAR assays with autologous infected CD4+ T cells at a CD8+ cell/CD4+ cell input ratio of 0.5:1; 0 (■), 0.2 (
) were separately cultured in the absence (■) or presence of 20 μg/ml (43 μM) leupeptin for 2 d before washing and using in CNAR assays at the indicated CD8+ cell/CD4+ cell input ratios. The CD4+ cells cultured in the absence of leupeptin (■) served as cell targets for the control cocultures. The extent of suppression of HIV replication was determined by using RT levels in culture fluids at the peak of HIV replication, typically 4 d after the initiation of the coculture. The level of RT activity in the culture fluids of treated and untreated CD4+ target cells at this time point was 732,000 and 688,000 cpm/ml, respectively. The data are representative of two to three separate studies. (B) Nonexogenously stimulated antiviral CD8+ cells were cultured in the presence of various concentrations of leupeptin (micrograms per milliliter) for 2 d before washing and using in CNAR assays with autologous infected CD4+ T cells at a CD8+ cell/CD4+ cell input ratio of 0.5:1; 0 (■), 0.2 ( ), 2 (
), 2 ( ), 20 (□). The extent of suppression of HIV replication was determined at the indicated days after initiation of the CNAR coculture assays. After 2 d of culturing the infected CD4+ target cells alone (during the CD8+ cell pretreatment period), the level of RT activity in the culture fluid was 5,000. Two and 4 d after initiating the CD4+/CD8+ cocultures, the level of RT activity in the culture fluids of the CD4+ cell control wells was 67,000 and 228,000, respectively. The data are representative of two separate studies.
), 20 (□). The extent of suppression of HIV replication was determined at the indicated days after initiation of the CNAR coculture assays. After 2 d of culturing the infected CD4+ target cells alone (during the CD8+ cell pretreatment period), the level of RT activity in the culture fluid was 5,000. Two and 4 d after initiating the CD4+/CD8+ cocultures, the level of RT activity in the culture fluids of the CD4+ cell control wells was 67,000 and 228,000, respectively. The data are representative of two separate studies.
Discussion
HIV-infected individuals who remain asymptomatic have demonstrated high levels of CNAR that appear to protect them from progression to disease (1, 2, 4). CNAR is associated with secretion of a soluble factor, CAF, which acts on viral transcription (12–15, 29, 32). The present studies indicate that a major part of the anti-HIV activity measured by CNAR and CAF assays can be inhibited by protease inhibitors, particularly leupeptin (Tables 1 and 2; Figs. 1 and 2). Because the anti-HIV activity detected in assays of both cell-to-cell contact and of CD8+ cell fluids is affected, the same biologic process seems to be involved.
Inhibition of CNAR activity after pretreatment of CD8+ cells with leupeptin suggests this anti-HIV activity of CD8+ cells does not involve a component of the serum in the growth medium (see below). Most likely the activity of a CD8+ cell antiviral product is inhibited (Fig. 3). Because of the chemical nature (i.e., positive charge) of leupeptin, it should not enter the CD8+ cells to an appreciable level, thus suggesting it is affecting a membrane- associated protein.
Whether this observation indicates that CNAR represents the function of a protease or another type of protein that interacts with the protease inhibitor remains to be determined. Conceivably, CD8+ cells produce a protease that activates another protein produced by CD8+ cells to become antiviral. Alternatively, CAF could be a protein that cleaves a surface protein on CD4+ cells to induce signal transduction that results in the block of virus transcription. This type of mechanism has been described for thrombin and its receptor (33). This latter hypothesis seems less likely, because preliminary studies have shown that pretreatment of CD4+ cells for 2 h with CAF-containing fluid does not affect virus replication after subsequent HIV inoculation. The absence of this effect of leupeptin on CNAR/CAF in about one-third of the cases (Table 1) could indicate that the antiviral factor is already activated in some CD8+ cell fluids and thus does not depend on protease activity.
Previous studies in our laboratory have suggested that CAF is a stable protein of 10–30 kDa that lacks identity to other cytokines (4). It does not directly inactivate HIV but blocks virus transcription (12, 13, 29). The amount of CAF in CD8+ cell culture fluids is low (e.g., 4 units/ml in which 1 unit is 50% inhibition of HIV replication), and thus it is difficult to isolate CAF from the serum- or protein-containing media in which CD8+ cells must be grown (4). The present findings provide a potential method to identify one of the major components involved in CNAR/CAF activity. Leupeptin can be used as a probe or attached to a solid phase support to serve as an affinity ligand for CAF. The protein could then be labeled or captured with leupeptin (or other protease inhibitors). These studies are in progress. In preliminary experiments, we have linked leupeptin to Sepharose beads and were able to deplete CAF activity in CD8+ cell fluids (unpublished observations). We are currently trying to develop a method of recovering active CAF from the affinity matrix. With current advances in proteomics and mass spectrometry, it is hoped that these findings on the sensitivity of CNAR and CAF activity to leupeptin will make the objective of identifying CAF more feasible.
Some studies have suggested that part of the CAF activity can be associated with the activity of β-chemokines (34), IL-16 (35), or defensins (36), but we and others have not found a clinical relevance or correlation between the levels of these cellular products and the presence of CNAR or CAF activity (20–23, 27, 37–39) (unpublished observations). Recently, Geiben-Lynn et al. (40) suggested that CAF modifies antithrombin III (ATIII) in the FCS present in their CD8+ cell culture fluids showing anti-HIV activity. The ATIII purified from these fluids has anti-HIV activity and is reduced in size. Geiben-Lynn et al. (40) suggested a CD8+ cell product with proteolytic or other processing activity could be involved. However, we observe CAF/CNAR activity under serum-free culture conditions (unpublished observations), indicating ATIII is not required.
In summary, our results suggest that the anti-HIV activity of CD8+ cells most likely involves, at least in part, a protease that affects a product of CD8+ cells. It is also conceivable that CNAR is mediated by two separate CD8+ cell anti-HIV mechanisms: one involving a protease and its substrate, and another involving an unidentified component not sensitive to protease inhibitors. The ultimate result of the antiviral activity is a block in virus transcription. These possibilities need to be considered as further work is directed at defining this important natural anti-HIV factor.
Acknowledgments
We thank Roland Orque, Noah Dowell, and Matthew Richey for technical assistance; Leyla Diaz, Kyle Bonneau, and Alan Landay for critical review of the paper; and Ann Murai for help in the preparation of the manuscript. This research was supported by National Institutes of Health Grants RO1-AI-30350 and GM 56531.
Abbreviations
- CNAR
CD8+ cell noncytotoxic antiviral response
- CAF
CD8+ cell antiviral factor
- RT
reverse transcriptase
- PHA
phytohemagglutinin
References
- 1.Levy J A. HIV and the Pathogenesis of AIDS. Washington, DC: Am. Soc. Microbiol.; 1998. [Google Scholar]
- 2.Levy J A. AIDS. 1993;7:1401–1410. doi: 10.1097/00002030-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Pantaleo G, Menzo S, Vaccarazza M, Graziosi C, Cohen O J, Demarest J F, Montefiori D, Orenstein J M, Fox C, Schrager L K, et al. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 4.Levy J A, Mackewicz C E, Barker E. Immunol Today. 1996;17:217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 5.Walker C M, Moody D J, Stites D P, Levy J A. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 6.Barker E, Bossart K N, Levy J A. Proc Natl Acad Sci USA. 1998;95:1725–1729. doi: 10.1073/pnas.95.4.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackewicz C E, Yang L C, Lifson J D, Levy J A. Lancet. 1994;344:1671–1673. doi: 10.1016/s0140-6736(94)90459-6. [DOI] [PubMed] [Google Scholar]
- 8.Mackewicz C E, Ortega H W, Levy J A. J Clin Invest. 1991;87:1462–1466. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landay A L, Mackewicz C, Levy J A. Clin Immunol Immunopathol. 1993;69:106–116. doi: 10.1006/clin.1993.1157. [DOI] [PubMed] [Google Scholar]
- 10.Gomez A M, Smaill F M, Rosenthal K L. Clin Exp Immunol. 1994;97:68–75. doi: 10.1111/j.1365-2249.1994.tb06581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castelli J C, Deeks S G, Shiboski S, Levy J A. Blood. 2002;99:4225–4227. doi: 10.1182/blood-2001-11-0078. [DOI] [PubMed] [Google Scholar]
- 12.Chen C H, Weinhold K J, Bartlett J A, Bolognesi D P, Greenberg M L. AIDS Res Hum Retroviruses. 1993;9:1079–1086. doi: 10.1089/aid.1993.9.1079. [DOI] [PubMed] [Google Scholar]
- 13.Copeland K F T, McKay P J, Rosenthal K L. AIDS Res Hum Retroviruses. 1995;11:1321–1326. doi: 10.1089/aid.1995.11.1321. [DOI] [PubMed] [Google Scholar]
- 14.Walker C M, Levy J A. Immunology. 1989;66:628–630. [PMC free article] [PubMed] [Google Scholar]
- 15.Mackewicz C E, Ortega H, Levy J A. Cell Immunol. 1994;153:329–343. doi: 10.1006/cimm.1994.1032. [DOI] [PubMed] [Google Scholar]
- 16.Mackewicz C E, Ridha S, Levy J A. AIDS. 2000;14:328–330. doi: 10.1097/00002030-200002180-00019. [DOI] [PubMed] [Google Scholar]
- 17.Mackewicz C E, Lieberman J, Froelich C, Levy J A. AIDS Res Hum Retroviruses. 2000;16:367–372. doi: 10.1089/088922200309241. [DOI] [PubMed] [Google Scholar]
- 18.Brinchmann J E, Gaudernack G, Vartdal F. J Acquired Immune Defic Syndr. 1991;4:480–488. [PubMed] [Google Scholar]
- 19.Clerici M, Balotta C, Trabattoni D, Papagno L, Ruzzante S, Rusconi S, Fusi M L, Colombo M C, Galli M. AIDS. 1996;10:1432–1433. doi: 10.1097/00002030-199610000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Paliard X, Lee A Y, Walker C M. AIDS. 1996;10:1317–1321. doi: 10.1097/00002030-199610000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Leith J G, Copeland K F T, McKay P J, Richards C D, Rosenthal K L. AIDS. 1997;11:575–580. doi: 10.1097/00002030-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Rubbert A, Weissman D, Combadiere C, Pettrone K A, Daucher J A, Murphy P M, Fauci A S. AIDS Res Hum Retroviruses. 1997;13:63–69. doi: 10.1089/aid.1997.13.63. [DOI] [PubMed] [Google Scholar]
- 23.Blazevic V, Heino M, Ranki A, Jussila T, Krohn K J E. AIDS. 1996;10:1435–1436. doi: 10.1097/00002030-199610000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Levy J A, Tobler L H, McHugh T M, Casavant C H, Stites D P. Clin Immunol Immunopathol. 1985;35:328–336. doi: 10.1016/0090-1229(85)90093-5. [DOI] [PubMed] [Google Scholar]
- 25.Greco G, Fujimura S H, Mourich D V, Levy J A. J Virol. 1999;73:1528–1534. doi: 10.1128/jvi.73.2.1528-1534.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackewicz C E, Garovoy M R, Levy J A. J Virol. 1998;72:10165–10170. doi: 10.1128/jvi.72.12.10165-10170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackewicz C E, Barker E, Levy J A. Science. 1996;274:1393–1395. doi: 10.1126/science.274.5291.1393. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman A D, Banapour B, Levy J A. Virology. 1985;147:326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- 29.Mackewicz C E, Blackbourn D J, Levy J A. Proc Natl Acad Sci USA. 1995;92:2308–2312. doi: 10.1073/pnas.92.6.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tateno M, Levy J A. Virology. 1988;167:299–301. doi: 10.1016/0042-6822(88)90084-0. [DOI] [PubMed] [Google Scholar]
- 31.Mackewicz C E, Orque R, Jung J, Levy J A. Clin Immunol Immunopathol. 1997;82:274–281. doi: 10.1006/clin.1996.4292. [DOI] [PubMed] [Google Scholar]
- 32.Brinchmann J E, Gaudernack G, Vartdal F. J Immunol. 1990;144:2961–2966. [PubMed] [Google Scholar]
- 33.Vu T, Hung D H, Wheaton V I, Coughlin S R. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 34.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 35.Baier M, Werner A, Bannert N, Metzner K, Kurth R. Nature. 1995;378:563. doi: 10.1038/378563a0. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Yu W, He T, Yu J, Caffrey R E, Dalmasso E A, Fu S, Pham T, Mei J, Ho J J, et al. Science. 2002;298:995–1000. doi: 10.1126/science.1076185. [DOI] [PubMed] [Google Scholar]
- 37.Mackewicz C E, Barker E, Greco G, Reyes-Teran G, Levy J A. J Clin Invest. 1997;100:921–930. doi: 10.1172/JCI119608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackewicz C E, Levy J A, Cruikshank W W, Kornfeld H, Center D M. Nature. 1996;383:488–489. doi: 10.1038/383488a0. [DOI] [PubMed] [Google Scholar]
- 39.Zanussi S, D'Andrea M, Simonelli C, Tirelli U, De Paoli P. AIDS. 1996;10:1431–1432. doi: 10.1097/00002030-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Geiben-Lynn R, Brown N, Walker B D, Luster A D. J Biol Chem. 2002;277:42352–42357. doi: 10.1074/jbc.M207079200. [DOI] [PubMed] [Google Scholar]
- 41. Mackewicz, C. E., Wang, B., Metkar, S., Richey, M., Froelich, C. & Levy, J. A. (2003) Blood, in press. [DOI] [PubMed]