Abstract
Gangliosides are sialic acid-containing glycosphingolipids that are present on all mammalian plasma membranes where they participate in recognition and signaling activities. We have established mutant mice that lack GM3 synthase (CMP-NeuAc:lactosylceramide α2,3-sialyltransferase; EC 2.4.99.-). These mutant mice were unable to synthesize GM3 ganglioside, a simple and widely distributed glycosphingolipid. The mutant mice were viable and appeared without major abnormalities but showed a heightened sensitivity to insulin. A basis for the increased insulin sensitivity in the mutant mice was found to be enhanced insulin receptor phosphorylation in skeletal muscle. Importantly, the mutant mice were protected from high-fat diet-induced insulin resistance. Our results show that GM3 ganglioside is a negative regulator of insulin signaling, making it a potential therapeutic target in type 2 diabetes.
Gangliosides are sialic acid-containing glycosphingolipids that are ubiquitously distributed on vertebrate plasma membranes. They are synthesized in the Golgi apparatus by the sequential transfer of carbohydrate residues onto a ceramide lipid anchor. A combinatorial biosynthetic pathway results in a large diversity of oligosaccharide structures on gangliosides (1). GM3 ganglioside is a key structure; it contains the simplest ganglioside oligosaccharide in this pathway (Fig. 1A), a trisaccharide composed of glucose, galactose, and sialic acid. It is the most widely distributed ganglioside among tissues, and it serves as a precursor for most of the more complex ganglioside species.
Figure 1.
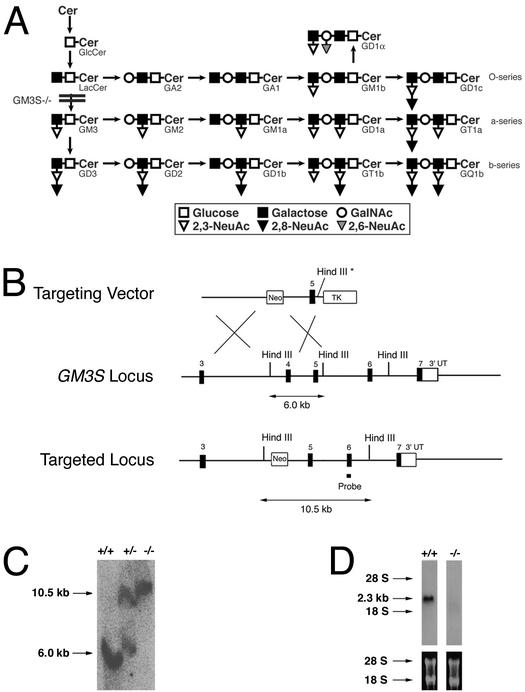
Genetic disruption of GM3 ganglioside synthesis. (A) Pathway of ganglioside synthesis showing the block in GM3S−/− mice. Cer, ceramide. (B) Strategy to disrupt GM3S locus. (Top) Targeting vector. Asterisk indicates that the HindIII site was disrupted. (Middle) Locus organization. (Bottom) Targeted locus. The approved gene name for GM3S is Siat9. (C) Southern blot of tail DNA showing HindIII cleaved DNA fragments corresponding to the wild-type (6.0 kb) and targeted (10.5 kb) GM3S alleles. (D) Northern blot of brain RNA from GM3S+/+ and GM3S−/− mice by using GM3S cDNA as a probe. Ethidium bromide-stained gel of RNA before blotting showing 28S and 18S RNA.
Gangliosides are involved in cell-signaling functions as ligands and as modulators of receptor activity (2–5). In the case of the insulin receptor, sialylparagloboside and the structurally related GM3 ganglioside have been found to inhibit the intrinsic tyrosine kinase activity of soluble receptors (6, 7). Furthermore, GM3 ganglioside depressed insulin-mediated signaling in cultured cells (6, 8). These results and the finding of enhanced expression of GM3 synthase in models of insulin resistance have led to the suggestion that GM3 ganglioside overexpression may play a role in the pathogenesis of type 2 diabetes through a negative modulation on insulin receptor signaling (6). To determine the physiological role of GM3 ganglioside and whether insulin receptor signaling is influenced by GM3 ganglioside in vivo, we established mice with a disrupted gene (GM3S; also known as Siat9) encoding GM3 synthase. We have found that mice without the capacity to synthesize GM3 ganglioside had enhanced phosphorylation of the skeletal muscle insulin receptor after ligand binding. Correspondingly, they showed heightened responses in glucose and insulin tolerance tests. Furthermore, these mutant mice were protected from high-fat diet-induced insulin resistance. The results indicate that GM3 ganglioside has an important role in regulating insulin sensitivity.
Materials and Methods
Generation of GM3S−/− Mice.
GM3S genomic DNA fragments were cloned from a 129/svev lambda library. A 6-kb ApaI–XbaI fragment corresponding to the third intron and a 3-kb KpnI–XbaI fragment containing exon 5 were used to construct the targeting vector. Conditions for targeting and creation of chimeric mice have been described (9). Mutant mice were maintained on a C57BL/6-129/svev mixed background by heterozygous matings to generate littermate controls. GM3S genotypes were determined by Southern blot and PCR analyses of genomic DNA isolated from ES cells and tail biopsies. For genotyping by Southern blot, DNA was digested with HindIII and hybridized with a probe derived from exon 6. For genotyping by PCR, the primers were 5′-AGCTCAGAGCTATGCTCAGGA-3′ (primer 1), 5′-TACCACATCGAACTGGTTGAG-3′ (primer 2), 5′-CAATAGATCTGACCCCTATGC-3′ (primer 3), and 5′-TCGCCTTCTTGACGAGTTCTTCTGAG-3′ (primer 4). Primers 1 and 2 detected the wild-type GM3S allele and amplified an ≈400-bp fragment. Primers 3 and 4 detected the mutant allele and amplified an ≈300-bp fragment. Forty-five cycles of 94°C (1 min), 60°C (1 min), and 72°C (1 min) were used for amplification.
Ganglioside Analysis.
Gangliosides were isolated as described (9). For sialidase treatment, the reaction mixture contained the following components in a total volume of 0.25 ml: potassium acetate buffer, pH 4.5, 25 μmol; BSA, 75 μg; calcium chloride, 0.25 μmol; 20 μg of substrate and 0.02 unit of Vibrio cholera sialidase. The solution was incubated for 1 h at 37°C, stopped by heating for 5 min at 100°C, and desalinated on RP-18 columns. Each lane of the TLC plate was loaded with the equivalent of 10 mg wet-weight of brain. The TLC solvent system was CHCl3/MeOH/0.22% CaCl2; 60/35/8 (vol/vol/vol). The ganglioside bands were detected with a phosphoric acid/copper sulfate reagent [15.6 g of CuSO4-(H2O)5 and 9.4 ml of H3PO4 (85%, wt/vol) in 100 ml of water]. The TLC plate was sprayed with the reagent and developed at 160°C for 15 min. Nanoelectrospray tandem mass spectrometry was accomplished as described (10).
Phosphorylation of the Insulin Receptor.
Experiments were carried out in mice fasted for 14 h. Insulin (5 units per animal; Sigma) was administered through the inferior vena cava under anesthesia. Two minutes after injection, hind limb muscle and adipose tissue were removed and incubated for 30 min on ice with lysis buffer (PBS, pH 7.4/1% Nonidet P-40/0.5% sodium adeoxycholate/0.1% SDS/1 mM PMSF/10 mM sodium orthovanadate/3 μg/ml aprotinin). Lysates were immunoprecipitated with an anti-insulin receptor-β subunit antibody (Santa Cruz Biotechnology). Immunoprecipitated samples were subjected to SDS/PAGE and then transferred to nitrocellulose membranes. The blots were probed sequentially, first with an antiphosphotyrosine antibody (PY99, Santa Cruz Biotechnology) and then with an anti-insulin receptor-β subunit antibody. Blots were developed by a chemiluminescence detection system (ECL, Amersham Pharmacia).
Glucose and Insulin Tolerance Tests, Hormone Measurements, and High-Fat Diet.
Glucose and insulin tolerance tests were performed in fasted mice (13–15 h) with i.p. injections of glucose (2 g of glucose per kg of body weight) or insulin (0.75 units of insulin per kg of body weight), respectively. For the glucose tolerance test, blood glucose values were measured immediately before and 15, 30, 60, and 120 min after glucose injection. For the insulin tolerance test, blood glucose levels were measured immediately before and 15, 30, and 60 min after insulin injection. To induce glucose intolerance, 6- to 8-week-old GM3S+/+ and GM3S−/− mice were placed on a 45% high-fat diet (D12451, Research Diets, New Brunswick, NJ) for 10 weeks (11).
Euglycemic–Hyperinsulinemic Clamps.
The clamp procedures have been described in detail elsewhere (12–14).
Statistical Analysis.
The statistical significance of differential findings between experimental groups and controls was determined by Student's t test and was considered significant if two-tailed P values were <0.05.
Results
Disruption of GM3 Synthase Gene.
To determine the physiological role of GM3 ganglioside and whether insulin receptor signaling is influenced by gangliosides in vivo, we established mice with a disrupted gene (GM3S) encoding GM3 synthase, an α2,3-sialyltransferase that transfers a sialic acid residue to lactosylceramide to yield GM3 ganglioside (Fig. 1A). To inactivate the GM3S locus, we designed a targeting vector for deletion of exon 4 from the gene (Fig. 1B). Correctly targeted ES cells were obtained and were used to generate chimeric male mice, which passed the disrupted allele to their offspring (Fig. 1C). Heterozygous matings produced viable mice homozygous for the mutation at the expected Mendelian frequency, indicating the absence of embryonic lethality. Northern blot analysis demonstrated a deficiency of the 2.3-kb GM3S transcript in brain (Fig. 1D), showing that the genetic modification produced a null allele and suggesting that the deletion of exon 4 caused destabilization and degradation of GM3S mRNA. A GM3S cDNA lacking the exon 4 sequence was expressed in COS-1 cells and was found to be incapable of producing GM3 synthase activity (not shown).
GM3S−/− mutant mice lived longer than 1 year. There were not significant differences in weight gain between mutant and littermate control mice. Both males and females were fertile. Histologic analysis of tissues from mutant mice revealed no apparent abnormalities in brain, liver, adipose tissue, and muscle.
Analysis of brain gangliosides in the GM3S−/− mice demonstrated a pattern consistent with an absence of GM3 synthase (Fig. 2). The major brain gangliosides present in GM3S+/+ mice (GM1a, GD1a, GD1b, and GT1b) were absent in the GM3S−/− mice. The synthesis of each of these ganglioside species requires the activity of GM3 synthase (Fig. 1A). The GM3S−/− mice expressed major ganglioside species that comigrated with GM1b and GD1α gangliosides of the 0-series (Figs. 1A and 2), which do not require the activity of GM3 synthase.
Figure 2.
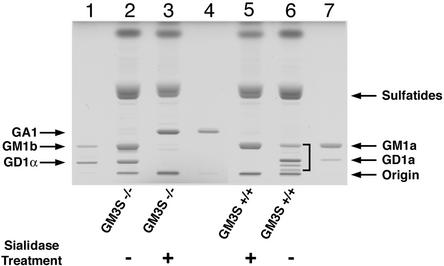
Brain gangliosides of GM3S−/− mice. Gangliosides were isolated from brains of GM3S+/+ and GM3S−/− mice. The samples were either untreated (−, lanes 2 and 6) or treated with sialidase (+, lanes 3 and 5). The positions of glycolipid standards are indicated (lane 1, GM1b, GD1α; lane 4, GA1; lane 7, GM1a, GD1a). The bracket (lane 6) indicates the normal major gangliosides in wild-type mice that include GM1a, GD1a, GD1b, and GT1b.
Analysis of acidic glycolipid fractions by electrospray–ionization tandem mass spectrometry provided spectra that confirmed the presence of GM1b and GD1α as well as GD1c in the brains of GM3S−/− mice. Using a triple-quattrupole instrument model Quattro II (micromass) equipped with a nanoelectrospray source, product ion spectra were obtained in negative mode with argon (3 × 10−3 mbar) as collision gas. Product ion spectra of the GM3S−/− ganglioside GM1 (m/z 1,544.8) showed fragments m/z 611 and 655 typical for GM1b but not for GM1a (collision energy = 42 eV). Product ion spectra of the GM3S−/− ganglioside GD1 (m/z 917.7 with z = 2e-) showed fragments m/z 370, 473, 475 {[NeuNAc-HexNAc − (2 H2O − H+)]−}, and 481 typical for GD1α but not for GD1a or GD1b (collision energy of 30 eV). In addition, the product ions m/z 380 and m/z 581 {[(NeuNAc)2 − H3O+]−} showed the presence of either GD1c or GD1b. The latter was excluded by TLC and the sialidase treatment experiment (Fig. 2). Brain gangliosides from GM3S+/+ and GM3S−/− mice were treated with V. cholera sialidase. On complex brain gangliosides, the sialidase will not act on the inner sialic acid transferred by GM3 synthase. As expected, digestion of brain gangliosides from GM3S+/+ mice with sialidase yielded the monosialoganglioside GM1a, which contains the inner sialic acid added by GM3 synthase (Figs. 1A and 2). Gangliosides from GM3S−/− mice, when treated with sialidase, were converted to GA1, the asialo form of GM1a. These results confirmed that the major gangliosides present in the brain of the GM3S−/− mice lacked the inner α2,3-linked sialic acid contributed by GM3 synthase.
Insulin Receptor Phosphorylation and Insulin Sensitivity.
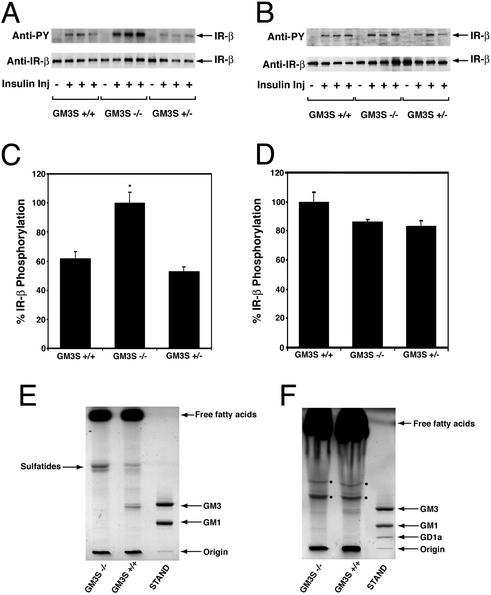
We next investigated the phosphorylation-mediated activation of the insulin receptor that occurs after interaction with insulin (15). At 2 min after injection of mice with insulin, the skeletal muscle insulin receptor β subunit in GM3S−/− mice was phosphorylated to a significantly greater extent than in GM3S+/+ or GM3+/− mice (Fig. 3 A and C; P < 0.05 by Student's t test). Interestingly, in the same mice no significant differences in the degree of phosphorylation of insulin receptor β subunit in adipose tissue was detected (Fig. 3 B and D). Thin-layer chromatographic analysis of acidic glycolipids revealed that GM3 ganglioside was detectable in both skeletal muscle and adipose tissue of GM3S+/+ mice (Fig. 3 E and F). In GM3S−/− mice, the ganglioside band corresponding to GM3 was absent.
Figure 3.
Enhanced insulin receptor phosphorylation in GM3S−/− mice. The insulin receptor β subunit was analyzed for phosphorylation status in skeletal muscle (A) and adipose tissue extracts (B) by Western blotting. GM3S+/+, GM3S+/−, and GM3S−/− mice, four in each group, were either not injected (−) or injected (+) with insulin. (A and B Upper) Immunoblots developed with antibody to phosphotyrosine (anti-PY). (A and B Lower) Same blot developed with antibody to insulin receptor β subunit (anti-IR-β). (C and D) Quantitation of phosphorylation of insulin receptor β subunit from Western blots of skeletal muscle (C) and adipose tissue (D). Level of phosphorylation (anti-PY) was normalized to amount of total receptor (anti-IR-β). *, P < 0.05 by Student's t test; GM3S−/− vs. GM3S+/+ or GM3S+/−. (E and F) TLC analysis of skeletal muscle (E) and adipose tissue (F) anionic lipids from GM3S+/+ and GM3S−/− mice. The anionic lipids were visualized by a phosphoric acid/copper sulfate reagent. Migration positions of free fatty acids, sulfatides, and ganglioside standards (STAND) are shown. The two bands denoted in the adipose tissue profile (F) by the filled circles are unidentified.
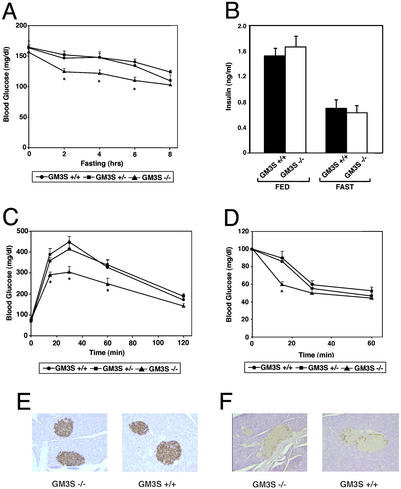
We investigated parameters of glucose homeostasis. Fed blood glucose levels of GM3S−/− mice were not significantly different from littermate GM3S+/+ or GM3S+/− mice (Fig. 4A). However, during a fast, the GM3S−/− group became hypoglycemic more rapidly than the other genotypes (Fig. 4A). Insulin levels, both fasting and fed, were not significantly different between GM3S−/− and GM3S+/+ mice (Fig. 4B). Differences between the GM3S−/− mice and the GM3S+/− and GM3S+/+ littermates were detected when a glucose tolerance test was administered (Fig. 4C). The blood glucose levels in the GM3S−/− mice were reduced more rapidly than in GM3S+/− and GM3S+/+ littermates after injection of a glucose solution. The differences were significant within the first 60 min of the test (P < 0.05 by Student's t test). By 120 min after injection, blood glucose levels in the GM3S−/− mice were no longer different from those of the other genotypes.
Figure 4.
Determinations of glucose homeostasis in GM3S−/− mice. (A) Blood glucose levels of male GM3S+/+, GM3S+/−, and GM3S−/− mice, 6–8 weeks old, during fasting (n = 6, +/+; n = 8, +/−; n = 9, −/−). *, P < 0.05 by Student's t test; GM3S−/− vs. GM3S+/+ or GM3S+/−. (B) Insulin levels of male GM3S+/+ and GM3S−/− mice, 6–8 weeks old, when fed and after fasting for 14 h (n = 12 each genotype). (C) Glucose tolerance tests of male GM3S+/+, GM3S+/−, and GM3S−/− mice, 6–8 weeks old (n = 14 each genotype). *, P < 0.05 by Student's t test; GM3S−/− vs. GM3S+/+ or GM3S+/−. (D) Insulin tolerance tests of male mice GM3S+/+, GM3S+/−, and GM3S−/− mice, 6–8 weeks old (n = 6,+/+; n = 8, +/−; n = 8, −/−). *, P < 0.05; GM3S−/− vs. GM3S+/+ or GM3S+/−. (E and F) Sections of pancreas from GM3S+/+ and GM3S−/− mice showing islets immunostained for insulin (E) and glucagon (F).
The GM3S−/− mice displayed an increased sensitivity to insulin when compared with controls. In an insulin tolerance test, the GM3S−/− mice became significantly more hypoglycemic 15 min after insulin injection than the GM3S−/− or GM3S+/+ mice (Fig. 4D; P < 0.05 by Student's t test). At the later time points, there was not a significant difference between the genotypes. Pancreatic islet morphology appeared normal in the mutant mice; no difference between islets of GM3S+/+ and GM3S−/− mice was detected after immunostaining for insulin and glucagon (Fig. 4 E and F).
Protection from High-Fat Diet-Induced Insulin Resistance.
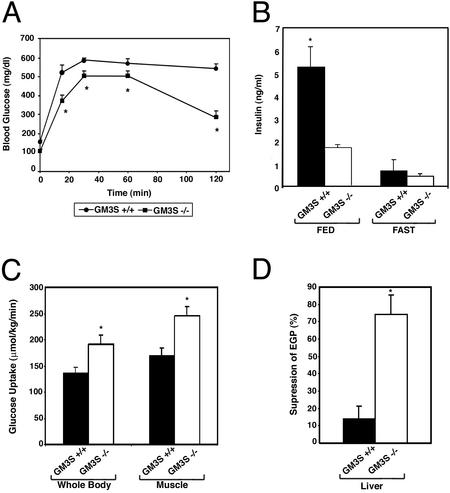
We placed GM3S−/− and GM3S+/+ mice on a 45% high-fat diet for 10 weeks in an attempt to induce insulin resistance (11). Body weight was not significantly different between genotypes after the high-fat diet regimen (not shown). GM3S+/+ mice after high-fat diet treatment were glucose intolerant as evidenced by an impaired ability to lower their blood glucose (Fig. 5A). The GM3S−/− mice placed on a 45% fat diet for the same period were significantly better in their lowering blood glucose to levels approaching baseline. Blood glucose levels at all time points after the glucose injection was administered were significantly different between the two groups (P < 0.05 by Student's t test). In addition, the fed insulin levels of the GM3S+/+ mice were significantly increased compared with the fed insulin levels of the GM3S−/− mice (Fig. 5B; P < 0.05 by Student's t test).
Figure 5.
GM3S−/− mice are protected from high-fat diet-induced insulin resistance. Groups of GM3S−/− and GM3S+/+ male mice were placed on a 45% high-fat diet for 10 weeks. The diet was started when the mice were 6–8 weeks old. (A) Glucose tolerance tests on GM3S+/+ and GM3S−/− mice after the high-fat diet (n = 8, +/+; n = 10, −/−). *, P < 0.05 by Student's t test; GM3S−/− vs. GM3S+/+. (B) Insulin levels of GM3S+/+ and GM3S−/− mice after the high-fat diet, when fed and after fasting for 14 h (n = 12 each genotype). *, P < 0.05 by Student's t test; GM3S−/− vs. GM3S+/+. (C) Whole body and skeletal muscle glucose uptake of GM3S+/+ and GM3S−/− mice after the high-fat diet as determined by euglycemic–hyperinsulinemic clamps (n = 4 each genotype). *, P < 0.05 by Student's t test; GM3S−/− vs. GM3S+/+. (E) The percent suppression of endogenous glucose production (EGP) in livers GM3S+/+ and GM3S−/− mice after the high-fat diet as determined by euglycemic–hyperinsulinemic clamps (n = 4 each genotype). *, P < 0.05 by Student's t test; GM3S−/− vs. GM3S+/+.
Euglycemic–hyperinsulinemic clamps were performed to determine relative tissue sensitivity to insulin in GM3S−/− and GM3S+/+ mice after the high-fat diet. Insulin-stimulated uptake of glucose, both whole body and by skeletal muscle, was found to be significantly higher in the GM3S−/− group than in the GM3S+/+ group (Fig. 5C; P < 0.05 by Student's t test). In addition, the ability of insulin to suppress endogenous glucose production in liver was significantly better in the mutant mice than in the wild-type mice (Fig. 5D; P < 0.05 by Student's t test). These results indicate that GM3S−/− mice were protected from the insulin resistance that GM3S+/+ mice develop from a high-fat diet.
Discussion
GM3 ganglioside has been described as a negative regulator of insulin receptor signaling. Mutant cells that express GM3 ganglioside display impaired insulin-dependent growth relative to parental cells expressing only lactosylceramide (8). Direct addition of GM3 ganglioside to 3T3-L1 adipocytes was shown to suppress insulin-stimulated phosphorylation of the insulin receptor (6). It had also been reported that GM3 ganglioside inhibited autophosphorylation of soluble receptors, suggesting a direct effect of ganglioside on receptor function (7). GM3 synthase knockout mice displayed enhanced ligand-induced insulin receptor phosphorylation and increased sensitivity in glucose and insulin tolerance tests, all indicative of a heightened insulin signaling response. These results suggest that GM3 ganglioside regulation of insulin receptor signaling is physiologically relevant.
The results illuminate a mode of insulin receptor regulation by gangliosides. Insulin receptor signaling emanates from glycosphingolipid-enriched membrane domains (rafts and caveolae) that contain gangliosides (2, 16–18). It is possible that protein:glycosphingolipid interactions within these membrane microdomains regulate insulin receptor signaling. Skeletal muscle, where receptor phosphorylation was enhanced, is the major site for glucose uptake (15). Interestingly, receptor phosphorylation was not detectably different in the adipose tissue of the knockout mice. This may reflect differences in glycolipid composition or in signaling pathways in the two tissues.
The absence of GM3 ganglioside conferred protection from high-fat diet-induced insulin resistance to the GM3S−/− mice. After a high-fat diet treatment, the mutant mice had lower insulin levels and were more responsive in a glucose tolerance test than wild-type mice. Heightened insulin sensitivity in the mutant mice, both in skeletal muscle and liver, was demonstrated by euglycemic–hyperinsulinemic clamps. Significant differences in insulin sensitivity between genotypes were not detected in clamp studies on mice fed a normal diet (data not shown). This may reflect an early kinetic difference in sensitivity, as seen in glucose and insulin tolerance tests, in the untreated mice that may not be detected in the clamp studies.
Our results, along with previous information, are consistent with the possibility that GM3 ganglioside expression may be relevant to insulin resistance in type 2 diabetes. Recently, it was shown that GM3 synthase mRNA and GM3 ganglioside were up-regulated by tumor necrosis factor-α (6), which is a cytokine elevated in obese individuals and has been suggested as a potential link between obesity and insulin resistance (19, 20). In addition, GM3 synthase mRNA was found to be significantly elevated in obese rodent models of insulin resistance compared with their lean counterparts (6). Our results showing that a lack of GM3 ganglioside protects mice from high-fat diet-induced insulin resistance raises the possibility that pharmacologic inhibition of GM3 ganglioside synthesis could serve as a therapeutic approach for insulin-resistant type 2 diabetes.
Acknowledgments
We thank Chuxia Deng for expertise in establishment of chimeric mice, Dr. M. Kiso (Department of Applied Bioorganic Chemistry, Gifu University, Gifu, Japan) for generosity in supplying us with synthetic ganglioside GM1b and GD1α standards, and Katie Prothero for help in preparing the figures. We are grateful to the Fonds der Chemischen Industrie for financial support.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kolter T, Proia R L, Sandhoff K. J Biol Chem. 2002;277:25859–25862. doi: 10.1074/jbc.R200001200. [DOI] [PubMed] [Google Scholar]
- 2.Hakomori S I. Proc Natl Acad Sci USA. 2002;99:225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hakomori S. Glycoconj J. 2000;17:627–647. doi: 10.1023/a:1011086929064. [DOI] [PubMed] [Google Scholar]
- 4.Allende M L, Proia R L. Curr Opin Struct Biol. 2002;12:587–592. doi: 10.1016/s0959-440x(02)00376-7. [DOI] [PubMed] [Google Scholar]
- 5.Miljan E A, Bremer E G. Sci STKE. 2002;2002:RE15. doi: 10.1126/stke.2002.160.re15. [DOI] [PubMed] [Google Scholar]
- 6.Tagami S, Inokuchi Ji J, Kabayama K, Yoshimura H, Kitamura F, Uemura S, Ogawa C, Ishii A, Saito M, Ohtsuka Y, et al. J Biol Chem. 2002;277:3085–3092. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]
- 7.Nojiri H, Stroud M, Hakomori S. J Biol Chem. 1991;266:4531–4537. [PubMed] [Google Scholar]
- 8.Tsuruoka T, Tsuji T, Nojiri H, Holmes E H, Hakomori S. J Biol Chem. 1993;268:2211–2216. [PubMed] [Google Scholar]
- 9.Liu Y, Wada R, Kawai H, Sango K, Deng C, Tai T, McDonald M P, Araujo K, Crawley J N, Bierfreund U, et al. J Clin Invest. 1999;103:497–505. doi: 10.1172/JCI5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandhoff R, Hepbildikler S T, Jennemann R, Geyer R, Gieselmann V, Proia R L, Wiegandt H, Grone H J. J Biol Chem. 2002;277:20386–20398. doi: 10.1074/jbc.M110641200. [DOI] [PubMed] [Google Scholar]
- 11.Steppan C M, Bailey S T, Bhat S, Brown E J, Banerjee R R, Wright C M, Patel H R, Ahima R S, Lazar M A. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 12.Haluzik M, Dietz K R, Kim J K, Marcus-Samuels B, Shulman G I, Gavrilova O, Reitman M L. Diabetes. 2002;51:2113–2118. doi: 10.2337/diabetes.51.7.2113. [DOI] [PubMed] [Google Scholar]
- 13.Youn J H, Kim J K, Buchanan T A. Diabetes. 1994;43:564–571. doi: 10.2337/diab.43.4.564. [DOI] [PubMed] [Google Scholar]
- 14.Kim J K, Michael M D, Previs S F, Peroni O D, Mauvais-Jarvis F, Neschen S, Kahn B B, Kahn C R, Shulman G I. J Clin Invest. 2000;105:1791–1797. doi: 10.1172/JCI8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saltiel A R, Kahn C R. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 16.Vainio S, Heino S, Mansson J E, Fredman P, Kuismanen E, Vaarala O, Ikonen E. EMBO Rep. 2002;3:95–100. doi: 10.1093/embo-reports/kvf010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simons K, Toomre D. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 18.Anderson R G. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 19.Moller D E. Trends Endocrinol Metab. 2000;11:212–217. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 20.Saltiel A R. Cell. 2001;104:517–529. doi: 10.1016/s0092-8674(01)00239-2. [DOI] [PubMed] [Google Scholar]