Abstract
Fabry disease is an X-linked recessive inborn metabolic disorder characterized by systemic and vascular accumulation of globotriaosylceramide (Gb3) caused by a deficiency of the lysosomal enzyme α-galactosidase A (α-gal A). The condition is associated with an increased morbidity and mortality due to renal failure, cardiac disease, and early onset of stroke. Hemizygous males are primarily affected clinically with variable expression in heterozygous females. Gene-therapy trials have been initiated recently in α-gal A knockout mouse models of Fabry disease by using a variety of viral vectors. In the present investigation we administered single i.v. injections of 1 × 1010 genomes of recombinant adeno-associated virus (rAAV) encoding the human α-gal A gene driven by a modified chicken β-actin (CAG) promoter to α-gal A knockout (Fabry) mice. Transgenic mice were analyzed for expression of α-gal A activity and Gb3 levels in liver, kidney, heart, spleen, small intestine, lung, and brain. Administration of the rAAV-CAG-hAGA vector resulted in stable expression of α-gal A in organs of the Fabry mice for >6 months. α-Gal A activity in the organs became equal to or higher than that of wild-type mice. Accumulated Gb3 in the liver, heart, and spleen was reduced to that of wild-type mice with lesser but significant reductions in kidney, lung, and small intestine. Injection of the rAAV-CAG-hAGA construct into skeletal muscle did not result in expression of α-gal A in it or in other tissues. This study provides a basis for a simple and efficient gene-therapy approach for patients with Fabry disease and is indicative of its potential for the treatment of other lysosomal storage disorders.
Fabry disease is an inborn error of glycosphingolipid metabolism that is due to a deficiency of the lysosomal hydrolase α-galactosidase A (α-gal A, EC 3.2.1.22) (1). It is an X-linked lysosomal storage disorder with multisystem involvement resulting primarily from the accumulation of glycosphingolipids such as globotriaosylceramide (Gb3) with terminal α-galactosyl moieties in various organs (2). Progressive glycosphingolipid accumulation leads to clinical manifestations of the disease such as premature strokes, myocardial infarction, or terminal renal failure, often resulting in death in the fourth or fifth decade of life. The disease is primarily manifested in hemizygous males and usually to a lesser degree in heterozygous females.
In the past, treatment of patients with Fabry disease was largely palliative. Enzyme-replacement therapy has been shown recently to provide significant benefit to patients with this disorder (3–5). This treatment, however, may have limitations for long-term systemic correction. Therefore, gene therapy using a variety of delivery systems has been explored in the α-gal A knockout murine model of Fabry disease (6–12). Fabry disease seems to be an appropriate disorder for gene therapy because target cells are readily accessible, and comparatively low levels of enzyme production are needed to reduce accumulated Gb3 (2). More importantly, metabolic cooperativity is also manifested in Fabry disease mice because gene-expressing cells secrete α-gal A, which corrects bystander cells (13).
We previously demonstrated that the deficiency of α-gal A in the Fabry mouse model can be corrected for long periods of time by α-gal A gene transfer into the liver via the hepatic portal vein by using a recombinant adeno-associated virus (rAAV) vector (14). In the current study we explored the possibility of eliminating the need for injection of an α-gal A vector into the hepatic portal vein. We found that a single i.v. injection of an adenovirus-associated α-gal A vector containing the chicken β-actin (CAG) promoter can bring about above-normal production of α-gal A and reduction of accumulated Gb3 levels in multiple organs. These observations may lead to the development of a gene-therapy procedure for long-term correction for patients with Fabry disease.
Materials and Methods
Construction of Viral Vector.
The vector plasmid rAAV-CAG-hAGA was derived from the plasmid pAM/CAG-pL-WPRE-BGH poly(A) and constructed by cloning full-length human α-gal A cDNA into the unique BamHI–HindIII site of the vector backbone. Large-scale production of rAAV-CAG-hAGA vector was carried out in an adenovirus-free system by triple transfection of 293T cells with the vector plasmid and two additional plasmids carrying adenoviral helper functions and AAV rep and capsid functions. Transfection was performed by standard calcium-phosphate precipitation. After 48 h, cells were collected and lysed by freezing and thawing. rAAV-CGA-hAGA vector was purified by CsCl gradient centrifugation as described (15). The concentration of the viral vector was determined by quantitative PCR by using an ABI 7700 (Perkin–Elmer/Applied Biosystems).
Animals and Procedures.
Mice in the C57BL/6 × 129/svJ hybrid background were maintained and treated in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care in an accredited facility at the National Institutes of Health. The genotype of the mice was identified by PCR-based assays on tail biopsies (14). One injection of 1.0 × 1010 genomes in 200 μl of normal saline was administered to 11- to 12-week-old mice via the tail vein or into the quadriceps muscle. Blood samples were obtained every 2 weeks from the tail vein. After injection of rAAV-CAG-hAGA, four mice in each experimental group were killed at 4, 8, 12, and 24 weeks postinjection. Age-matched noninjected Fabry mice and wild-type mice were killed at similar time points. Liver, kidney, heart, spleen, lungs, small intestine, and brain were obtained and snap-frozen on dry ice. Organs were stored at −70°C before processing.
α-Gal A Assay and Quantitation of Gb3.
Tissue samples were homogenized with citrate-phosphate buffer (28 mM citric acid/44 mM disodium phosphate/5 mg/ml sodium taurocholate, pH 4.4). α-Gal A activity was measured fluorimetrically in triplicate at 37°C with 5 mM 4-methylumbelliferyl-α-d-galactopyranoside containing 0.1 M N-acetylgalactosamine, a specific inhibitor of N-acetylgalactosaminidase (16). After extraction and saponification as described (17), the glycolipid fraction obtained was dissolved in a minimum volume of chloroform/methanol (2:1) and analyzed by HPLC on a 150 × 4.6-mm Luna 3 silica column (Phenomenex, Torrance, CA). Glycolipids were eluted by using a solvent consisting of chloroform/methanol/water/ammonia (66:30:3.5:0.5) and determined quantitatively by using a Sedex 55 evaporative light-scattering detector (4). Standard Gb3 was obtained from Matreya (Pleasant Gap, PA).
Immunofluorescence analysis of Gb3 was performed as described (18). After the mice were killed, tissues were removed, and 10-μm-thick coronal sections were cut at 100-μm intervals. The samples were fixed with 2% paraformaldehyde, blocked, and treated with FITC-conjugated anti-Gb3 antibody (Seikagaku America, Rockville, MD). The stained tissues were examined with a Leica confocal laser microscope.
Western Blot Analysis.
The protein content of homogenized samples was determined by using a BCA kit (Pierce). Proteins (20 μg per lane) were separated by SDS/PAGE using 8% polyacrylamide gels, transferred to nitrocellulose membrane, and probed with rabbit anti-mouse antibody (a gift from Transkaryotic Therapies, Cambridge, MA) and visualized by using horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (Cappel) as the secondary antibody. Human α-gal A (Genzyme) was used as control at 0.004 ng per lane.
Immunoassay for α-Gal A Antibodies.
Circulating antibodies against α-gal A were assayed by ELISA as described (14, 19). Serum samples were collected before injection of rAAV-CAG-hAGA vector and every 2 weeks after injection.
Results
rAAV Construct.
The recombinant vector containing the modified CAG promoter (20, 21) was designed for liver-specific expression of α-gal A. The cDNA for α-gal A is driven by a CAG promoter flanked by the regulatory element WPRE and the polyadenylation site provided by the BGH poly(A). Before administration of the vector to the α-gal A-deficient mice, we examined the effectiveness of this virus in fibroblasts derived from patients with Fabry disease and in 293T human embryonic kidney cells. Fibroblasts and 293T cells were infected at multiplicities of infection of 50, 100, and 200 at 50–60% confluence. Both types of cells consistently showed ≈2.5-, 4-, and 7-fold higher α-gal A expression than the uninfected cells at the respective multiplicities of infection (data not shown). We determined the specificity of the expressed α-gal A message and the enzyme in the Fabry-patient fibroblasts and 293T cells with RT-PCR and Western blot analysis. These in vitro results confirmed that the rAAV-CAG-hAGA vector was capable of delivering a functional transgene to the cells. Further, the augmented content of Gb3 in fibroblasts from patients with Fabry disease was reduced by 70% at a multiplicity of infection of 50 and cleared completely at multiplicities of infection of 100 and 200 (data not shown).
Expression of the Human α-Gal A in Fabry Mice.
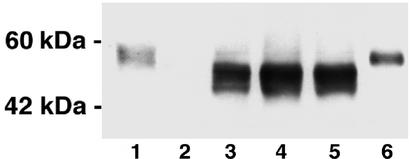
The expression of the human α-gal A protein was examined in α-gal A knockout mice by Western blot analysis. A robust signal was seen in liver (Fig. 1). Injected animals showed the specific human α-gal A band in all tissues except brain. There were minor differences in expression levels in the treated groups. No signal was seen in the Fabry mice that did not receive the transgene. The endogenous mouse α-gal A migrates slightly slower on SDS gels than human recombinant α-gal A, which is ≈50 kDa, and it therefore appears to be a slightly larger protein. All the organs tested except brain showed much higher α-gal A activity than that in noninjected Fabry mice (Table 1). α-Gal A activity in the liver, heart, and spleen in the injected Fabry mice was higher than that of the wild-type mice throughout the observation period. Liver and spleen showed the highest enzyme levels. Liver enzyme activity was consistently 5-fold higher than wild-type levels over the entire 24 weeks. In the kidney of injected mice, α-gal A was sustained at 60% of the activity observed in wild-type mice at 24 weeks after a single injection of vector. α-Gal A activity in the small intestines in the injected animals rose significantly over the noninjected Fabry mice but did not reach the level of the wild-type mice. Although α-gal A activity in the lungs of the injected Fabry mice was consistently less than in the wild-type mice, 20% of the normal level was attained. No α-gal A activity was observed in the brain of the noninjected or injected Fabry mice (data not shown). i.m. injection (single or multiple) of rAAV-CAG-hAGA did not produce any detectable α-gal A in the muscle or any visceral organs.
Figure 1.
Western blot analysis of α-gal A expression in the liver of wild-type and α-gal A knockout mice. Lane 1, liver lysates from wild-type mice; lane 2, noninjected α-gal A knockout mice; lanes 3–5, liver lysates from three separate α-gal A knockout mice i.v.-injected with rAAV-CAG-hAGA vector; lane 6, human α-gal A.
Table 1.
α-Gal A activity in the organs of wild-type, noninjected α-gal A knockout mice, and α-gal A knockout mice injected with rAAV-CAG-hAGA
| Weeks | Group of mice | Enzyme activity, nmol per h per mg of protein
|
|||||
|---|---|---|---|---|---|---|---|
| Liver | Kidney | Heart | Spleen | Lung | SI | ||
| 4 | Wild type | 24.5 ± 3.1 | 6.5 ± 0.3 | 1.0 ± 0.7 | 53.6 ± 28 | 13.6 ± 2.3 | 33.8 ± 1.1 |
| Noninjected Fabry | 1.1 ± 0.1 | 0.7 ± 0.0 | 0.2 ± 0.0 | 3.5 ± 1.1 | 0.7 ± 0.2 | 1.1 ± 0.1 | |
| Injected Fabry | 109.0 ± 40.0* | 2.0 ± 0.4* | 3.5 ± 0.8* | 34.1 ± 6.6† | 1.3 ± 0.2* | 3.5 ± 0.7* | |
| 8 | Wild type | 24.1 ± 4.6 | 6.2 ± 0.4 | 1.3 ± 0.1 | 47.2 ± 11 | 12.4 ± 1.1 | 21.1 ± 5.0 |
| Noninjected Fabry | 1.5 ± 0.4 | 0.9 ± 0.2 | 0.2 ± 0.0 | 4.3 ± 0.6 | 0.6 ± 0.1 | 1.1 ± 0.1 | |
| Injected Fabry | 183.0 ± 88.0* | 3.9 ± 1.7* | 3.3 ± 1.1† | 80.3 ± 42* | 1.9 ± 0.5* | 6.7 ± 2.7* | |
| 12 | Wild type | 22.2 ± 4.9 | 6.5 ± 0.1 | 1.4 ± 0.1 | 37.5 ± 5.6 | 13.5 ± 2.9 | 18.2 ± 0.8 |
| Noninjected Fabry | 1.1 ± 0.1 | 0.8 ± 0.1 | 0.2 ± 0.6 | 3.7 ± 0.7 | 0.8 ± 0.4 | 1.0 ± 0.2 | |
| Injected Fabry | 301.0 ± 285.0 | 3.8 ± 1.8 | 4.2 ± 1.9* | 82.7 ± 47.0 | 2.7 ± 1.1* | 7.5 ± 1.0 | |
| 24 | Wild type | 20.5 ± 6.3 | 8.8 ± 0.7 | 1.6 ± 0.2 | 38.1 ± 6.7 | 20.6 ± 8.6 | 27.1 ± 4.9 |
| Noninjected Fabry | 2.2 ± 1.1 | 0.9 ± 0.2 | 0.1 ± 0.0 | 3.6 ± 0.1 | 0.8 ± 0.4 | 0.7 ± 0.1 | |
| Injected Fabry | 113.0 ± 16.0† | 6.2 ± 1.2† | 5.2 ± 0.8† | 68.7 ± 13.0† | 2.0 ± 0.3† | 4.5 ± 2.1* | |
Time in weeks after injection of the vector is shown in the left column. Mean α-gal A activity ± SEM is shown for four mice at each time point. SI, small intestine.
P < 0.05 vs. noninjected Fabry mice.
P < 0.01.
Effect of Transgene Expression on Organ Levels of Gb3.
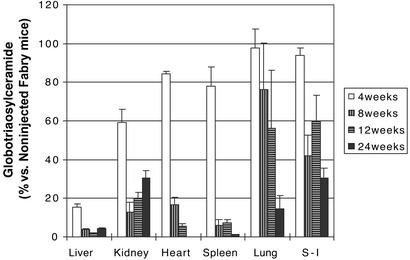
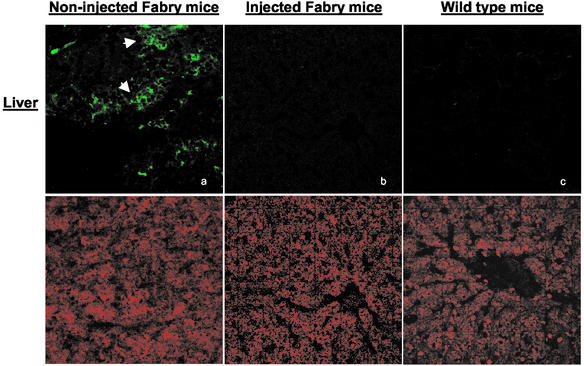
Impressive reduction of accumulated Gb3 occurred in all tissues after injection of rAAV-CAG-hAGA into the α-gal A knockout mice (Table 2). Dramatic reduction of Gb3 in the liver occurred by 4 weeks after injection of the vector. Heart and spleen of the injected mice show maximum reduction at 24 and 8 weeks postinjection, respectively (Fig. 2). Lipid reduction in the kidney was maximal at 8 and 12 weeks and thereafter appeared to reaccumulate to some extent. Gb3 levels in the lungs and small intestine also decreased after the injection of the vector but not to the extent of that in the other organs. The reduction of Gb3 in the liver, heart, and spleen was maintained up to 24 weeks postinjection. These observations were confirmed by using monoclonal anti-Gb3 antibody. Immunochemical staining confirmed minimal Gb3 in hepatocytes of the treated mice at 8 weeks posttreatment (Fig. 3). Typically, such staining in tissue sections from untreated Fabry mice show lysosomal inclusions. Gb3 clearance was also apparent in other tissues that were examined immunohistochemically, but the reduction was especially prominent in the liver.
Table 2.
Gb3 clearance in organs of wild-type mice, noninjected α-gal A knockout mice, and α-gal A knockout mice injected with rAAV-CAG-hAGA
| Weeks | Group of mice | Gb3 levels, nmol/mg protein
|
|||||
|---|---|---|---|---|---|---|---|
| Liver | Kidney | Heart | Spleen | Lung | SI | ||
| 4 | Wild type | 0.04 ± 0.0 | 1.03 ± 0.5 | 0.13 ± 0.1 | 0.0 ± 0.0 | 0.68 ± 0.0 | 0.21 ± 0.1 |
| Noninjected Fabry | 3.06 ± 0.7 | 8.41 ± 1.5 | 0.85 ± 0.1 | 3.85 ± 0.5 | 4.36 ± 0.9 | 8.91 ± 2.7 | |
| Injected Fabry | 1.95 ± 0.5* | 4.92 ± 0.4* | 0.72 ± 0.1* | 2.96 ± 0.2* | 4.2 ± 0.2* | 9.29 ± 0.7* | |
| 8 | Wild type | 0.15 ± 0.1 | 0.78 ± 0.3 | 0.09 ± 0.0 | 0.45 ± 0.0 | 0.33 ± 0.1 | 0.22 ± 0.1 |
| Noninjected Fabry | 4.18 ± 1.2 | 12.7 ± 5.8 | 1.65 ± 0.2 | 9.43 ± 1.3 | 1.75 ± 0.6 | 20.1 ± 7.1 | |
| Injected Fabry | 0.21 ± 0.1* | 1.52 ± 0.6* | 0.22 ± 0.0† | 0.55 ± 0.2† | 1.23 ± 0.3* | 9.01 ± 0.9* | |
| 12 | Wild type | 0.04 ± 0.0 | 0.84 ± 0.6 | 0.0 ± 0.0 | 0.47 ± 0.1 | 0.0 ± 0.0 | 0.14 ± 0.0 |
| Noninjected Fabry | 3.06 ± 0.1 | 6.52 ± 1.7 | 0.84 ± 0.3 | 15.9 ± 6.6 | 3.37 ± 0.8 | 14.9 ± 1.4 | |
| Injected Fabry | 0.04 ± 0.0† | 1.31 ± 0.5† | 0.43 ± 0.0* | 1.08 ± 0.2* | 1.75 ± 0.7 | 8.99 ± 2.9* | |
| 24 | Wild type | 0.08 ± 0.0 | 2.31 ± 1.1 | 0.02 ± 0.0 | 0.31 ± 0.0 | 0.18 ± 0.0 | 0.39 ± 0.1 |
| Noninjected Fabry | 4.18 ± 1.2 | 12.4 ± 1.2 | 2.24 ± 0.4 | 49.7 ± 10 | 4.85 ± 1.8 | 23.8 ± 4.9 | |
| Injected Fabry | 0.34 ± 0.1* | 5.02 ± 2.5† | 0.0 ± 0.0† | 0.72 ± 0.1† | 0.6 ± 0.2* | 7.97 ± 2.6† | |
Weeks after injection of the vector are shown in the left column. Mean Gb3 ± SEM is shown for four mice at each time point. SI, small intestine.
P < 0.05 vs. noninjected Fabry mice.
P < 0.01.
Figure 2.
Correction of Gb3 accumulation in α-gal A knockout mice. Levels of Gb3 are expressed as a percentage of the quantity in tissues from noninjected age-matched Fabry mice. Error bars indicate standard deviation. Four mice were analyzed at each time point. S-I, small intestine.
Figure 3.
Immunostaining for Gb3 in mouse liver after administration of rAAV-CAG-hAGA. Tissue specimens from noninjected Fabry mice, injected Fabry mice, and wild-type mice were analyzed at 4 weeks postinjection. Samples were examined by confocal microscopy (×40). (Upper) Gb3 staining with FITC-conjugated anti-Gb3 antibody. (Lower) Nuclear staining in the same sections with propidium iodide. There is intense Gb3 fluorescence in noninjected Fabry mice compared with that of injected Fabry or wild-type mice.
Body Weight.
Compared with wild-type mice of the same strain, Fabry mice exhibit a loss of body weight ranging from 8% to 10% by 10–20 weeks of age, and in older Fabry mice the weight decreases to 60–75% of age-matched wild-type mice (data not shown). Injected Fabry mice gained weight that appeared to be related to the increase in α-gal A activity and reduction of the body burden of Gb3. Fabry patients receiving enzyme-replacement therapy also show reversal of weight loss that is typical of patients with Fabry disease (4).
Antibody Production to α-Gal-A.
Almost all mice that received the rAAV-CAG-hAGA vector showed a low titer of anti-α-gal A antibody. One of the mice at 24 weeks postinjection developed an anti-α-gal A antibody titer of 1:10,240 with ≈50% inhibition of α-gal A activity. Low anti-α-gal A antibody titers are not uncommon in patients of Fabry diseases receiving enzyme-replacement therapy (4, 5). Appearance of antibody did not seem to affect the salutary responses of patients to the enzyme.
Discussion
We previously demonstrated that genetic modification of cells in the liver by administration of an α-gal A vector via the hepatic portal vein can result in systemic delivery of α-gal A (14). However, the clinical feasibility of the portal-vein delivery seems limited, particularly if it is necessary to perform the procedure repeatedly. In the present study, we used the robust properties of a CAG promoter that is in wide use for its strong and sustained transcriptional activity in rodent hepatocytes in vivo (22, 23). We now have demonstrated that significant production of α-gal A occurs after a single i.v. injection of an appropriately modified vector. Nakai et al. (24) reported that i.v. injection of AAV-hFIX vector bearing the cytomegalovirus-β-actin promoter resulted in a high copy number of mRNA in the liver. The resultant expression of hFIX in blood was higher than that produced by the EF1α promoter or the cytomegalovirus core promoter, and expression was maintained for >1 year in mice.
Expression of the α-gal A gene in the liver seems to be an attractive strategy for Fabry disease. A significant proportion of overexpressed lysosomal hydrolases are secreted into the blood. They are subsequently recaptured from the circulation by cellular uptake via the mannose-6-phosphate receptors on plasma membranes (25). Intravenous injection of α-gal A into Fabry mice corrects tissue enzyme levels and reduces Gb3 storage (26, 27). For a sustained long-term supply of enzyme, gene therapy may prove to be superior to intermittent injections of the enzyme.
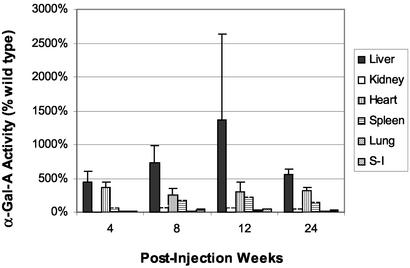
We were somewhat surprised that single or multiple i.m. injections of rAAV-CAG-hAGA failed to produce any detectable α-gal A in mouse quadriceps muscle or other visceral organs. A recent report has appeared in which an adeno-associated vector containing the α-gal A gene was injected into muscles of α-gal A knockout mice (28). The results obtained in the two studies are quite disparate. By i.v. administration of recombinant vector, we obtained α-gal A expression in various organs of the recipient mice that ranged from >2-fold over that in wild-type mice to as much as a 10-fold augmentation depending on the tissue (Fig. 4). In contrast, i.m. injection by Takahashi et al. (28) produced only a 6–10% increase of α-gal A activity over that in wild-type mice. Moreover, these authors report an increase in α-gal A activity in the brain of the recipient mice. We did not observe an increase of α-gal A activity in the brains of the recipient mice after i.v. injection of vector. Although the precise difference between the two constructs is not clear, the chicken β-actin promoter was present in their vector as well as in our investigation. Our rAAV-α-gal A contains the WPRE element that is known to play a critical role in enhancing the level and stability of an expressed recombinant protein (29). It will be important to define the differences between the two constructs that resulted in such variable expression of α-gal A. Muscle has been used for production of enzymes with AAV and adenovirus vectors; however, the secretion from muscle might be variable and depend on the nature of the enzyme. Previous studies showed that α-glucosidase (30) and β-glucuronidase (31) are secreted after i.m. injection of recombinant adenoviral and rAAV vectors. It is not clear whether the serotype of our vector (AAV-II) or nature of α-gal A itself precludes its expression in muscle tissue.
Figure 4.
α-Gal A enzyme activity in injected Fabry mice. Levels of increased α-gal A enzyme are expressed as a percentage of the quantity in tissues from age-matched wild-type mice. Error bars indicate standard deviation. Four mice were analyzed at each time point. S-I, small intestine.
In summary, we have shown that a single i.v. administration of a rAAV-CAG-hAGA to Fabry mice resulted in sustained expression of α-gal A and reduction in Gb3 storage in relevant organs. No immune response to compromise the effectiveness of treatment was observed.
These findings may lead to the development of effective gene therapy for patients with Fabry disease as well as for other lysosomal storage disorders.
Acknowledgments
We thank Zu-Xi Yu for help with confocal microscopy and Jay Chiorini for discussion on AAV production.
Abbreviations
- α-gal A
α-galactosidase A
- Gb3
globotriaosylceramide
- AAV
adeno-associated virus
- rAAV
recombinant AAV
- CAG
chicken α-actin
- hAGA
human α-gal A cDNA
References
- 1.Brady R O, Gal A E, Bradley R M, Martensson E, Warshaw A L, Laster L. N Engl J Med. 1967;276:1163–1167. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- 2.Desnick R J, Ioannou Y A, Eng C M. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Valle D, Sly W S, editors. New York: McGraw–Hill; 2001. pp. 3733–3744. [Google Scholar]
- 3.Schiffman R, Murray G J, Treco D, Daniel P, Sellow-moura M, Myers M, Quirk J M, Zirzow G C, Borowski M, Loveday K, et al. Proc Natl Acad Sci USA. 2000;97:365–370. doi: 10.1073/pnas.97.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiffmann R, Kopp J B, Austin H A, III, Sabnis S, Moore D F, Weibel T, Blow E, Brady R O. J Am Med Assoc. 2001;285:2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- 5.Eng C M, Guffon N, Wilcox W R, Germain D P, Lee P, Waldek S, Caplan L, Linthorst G E, Densick R J. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 6.Ohshima T, Murray G J, Swaim W D, Longenecker G, Quirk J M, Cardareli C O, Sugimoto Y, Pastan I, Gottesman M M, Brady R O, Kulkarni A B. Proc Natl Acad Sci USA. 1997;94:2540–2544. doi: 10.1073/pnas.94.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takenaka T, Hendrickson C S, Tworek D M, Tudor M, Schiffmann R, Brady R O, Medin J A. Exp Hematol (Charlottesville, Va) 1999;27:1149–1159. doi: 10.1016/s0301-472x(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 8.Ohsugi K, Kobayashi K, Itoh K, Sakuraba H, Sakuragawa N. J Hum Genet. 2000;45:1–5. doi: 10.1007/s100380050001. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler R J, Li C, Cherry M, Zhu Y, Hempel D, Rooijen N V, Ioannou Y A, Desnick R J, Goldberg M A, et al. Hum Gene Ther. 2002;13:935–945. doi: 10.1089/10430340252939041. [DOI] [PubMed] [Google Scholar]
- 10.Takenaka T, Murray G J, Qin G, Quirk J M, Oshima T, Qasba P, Clark K, Kulkarini A G, Brady R O, Medin J A. Proc Natl Acad Sci USA. 2000;97:7151–7520. doi: 10.1073/pnas.120177997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashley G A, Desnick R J, Gordon R E, Gordon J J W. J Invest Med. 2002;50:185–192. doi: 10.2310/6650.2002.33432. [DOI] [PubMed] [Google Scholar]
- 12.Li D, Ziegler R J, Cherry M, Lukason M, Desnick R J, Yew N S, Cheng S H. Mol Ther. 2002;5:745–754. doi: 10.1006/mthe.2002.0605. [DOI] [PubMed] [Google Scholar]
- 13.Medin J A, Tudor M, Simovitch R, Quirk J M, Jacobson S, Murray G J, Brady R O. Proc Natl Acad Sci USA. 1996;93:7917–7922. doi: 10.1073/pnas.93.15.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung S, Han P I, Limaye A, Xu R, Gelderman M P, Zerfas P, Tirumalai K, Murray G J, During M J, Brady R O, Qasba P. Proc Natl Acad Sci USA. 2001;98:2676–2681. doi: 10.1073/pnas.051634498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samulski R J, Chang L-S, Shenk T. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusiak J W, Quirk J M, Brady R O. J Biol Chem. 1987;253:184–190. [PubMed] [Google Scholar]
- 17.Ullman M D, McCluer R H. J Lipid Res. 1977;8:371–378. [PubMed] [Google Scholar]
- 18.Itoh K, Kotani M, Tai T, Suzuki H, Utsunomiya T, Inoue H, Yamada H, Sakuraba H, Suzuki Y. Clin Genet. 1993;44:302–306. doi: 10.1111/j.1399-0004.1993.tb03905.x. [DOI] [PubMed] [Google Scholar]
- 19.Liang H, Narum D L, Fuhrmann S R, Luu T, Sim B K. Infect Immun. 2000;68:3564–3568. doi: 10.1128/iai.68.6.3564-3568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazaki J, Takaki S, Araki K, Tashiro F, Tominaga A, Takatsu K, Yamamura K. Gene. 1989;79:269–277. doi: 10.1016/0378-1119(89)90209-6. [DOI] [PubMed] [Google Scholar]
- 21.Niwa H, Yamamura K, Miyazaki J. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 22.Kiwaki K, Kanegae Y, Saito I, Komaki S, Nakamura K, Miyazaki J I, Endo F, Matsuda I. Hum Gene Ther. 1996;17:821–830. doi: 10.1089/hum.1996.7.7-821. [DOI] [PubMed] [Google Scholar]
- 23.Kosuga M, Enosawa S, Li X, Suzuki S, Matsuo N, Yamada M, Roy-Chowdhury J, Koiwai O, Okuyama T. Cell Transplant. 2000;9:675–680. doi: 10.1177/096368970000900513. [DOI] [PubMed] [Google Scholar]
- 24.Nakai H, Herzog R W, Hagstrom J N, Walter J, Kung S H, Yang E Y, Tai S J, Iwaki Y, Kurtzman G J, Fisher K J, et al. Blood. 1998;15:4600–4607. [PubMed] [Google Scholar]
- 25.Kornfeld S, Mellman I. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- 26.Brady R O, Murray G J, Moore D F, Schiffmann R. J Inherited Metab Dis. 2001;24:18–24. doi: 10.1023/a:1012451320105. [DOI] [PubMed] [Google Scholar]
- 27.Ioannou Y A, Zeidner K M, Gordon R M, Desnick R J. Am J Hum Genet. 2001;68:14–25. doi: 10.1086/316953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi H, Hirai Y, Migita M, Seino Y, Fukuda Y, Sakuraba H, Kase R, Kobayashi T, Hashimoto Y, Shimada T. Proc Natl Acad Sci USA. 2002;99:13777–13782. doi: 10.1073/pnas.222221899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loeb J E, Cordier W S, Harris M E, Weitzman M D, Hope T J. Hum Gene Ther. 1999;10:2295–2305. doi: 10.1089/10430349950016942. [DOI] [PubMed] [Google Scholar]
- 30.Pauly D F, Johns D C, Matelis L A, Lawrence J H, Byrne B J, Kessler P D. Gene Ther. 1998;5:473–480. doi: 10.1038/sj.gt.3300609. [DOI] [PubMed] [Google Scholar]
- 31.Daly T M, Okuyama T, Vogler C, Haskins M E, Muzyczka N, Sands M S. Hum Gene Ther. 1999;10:85–94. doi: 10.1089/10430349950019219. [DOI] [PubMed] [Google Scholar]