Abstract
Background
It has been hypothesized that alterations of the serotonergic system contribute to neuropsychiatric symptoms in Alzheimer disease (AD). Cellular expressions of the two serotonergic receptors 5-HT2A and 5-HT6 have therefore been determined by immunohistochemistry in the prefrontal cortex of patients with AD (n=6) and normal age-matched controls (n = 7).
Results
In normal aging patients, 5-HT2A label was mainly observed in large pyramidal cells, but to a lesser extent also in small pyramidal cells and in stellate cells of cortical layers II-VI. In AD, a similar distribution was observed, but density of positive cells was significantly reduced by 33%. In aging control patients, the 5-HT6 receptor was expressed by pyramidal cells and occasional stellate cells, not only of layers II-V, but also of layer I, where a distinct label was observed in neurons and surrounding fibers. 5-HT6 receptor expression in AD patients had the same pattern, but was significantly decreased by 40%.
Conclusion
Our results indicate that a decline in neurons expressing 5-HT2A, but also 5-HT6 receptors may play a role in the etiopathology of neuropsychiatric symptoms in AD.
Background
Alzheimer disease (AD), the most common cause of dementia in the elderly, is clinically characterized by progressive cognitive impairment associated with severe neuropsychiatric disturbances. These behavioral and psychological symptoms of dementia (BPSD) include hallucinations, delusions, aggressive behavior, overactivity, anxieties and affective disturbances [1,2]. Whereas the decline in cognitive functions can be largely related to cholinergic dysfunction arising from disruption of basal forebrain cholinergic pathways (cholinergic hypothesis) [1,3,4], impaired balance between several neurotransmitters has been implicated in the pathogenesis of BPSP [2,5-7], with serotonin (5-HT) playing a pivotal role [2,8-10]. The actions of 5-HT are mediated through seven major receptors classes, 5-HT1–7, comprising a total of 14 distinct mammalian 5-HT receptor subtypes (for review, see [11]). The 5-HT2A receptor has attracted most interest because of its possible participation in behavioral alterations in AD. 5-HT2A is localized in the cortex and caudate and is involved in anxiety [10]. 5-HT2A receptors mediate the psychotomimetic effects of hallucinogens [11-13], and alterations in binding characteristics to this receptor have been observed in the prefrontal cortex of patients suffering from psychiatric diseases, e.g. schizophrenia [14,15], depression [16] and suicide [17]. Electrophysiological evidence suggests that 5-HT2A receptors are involved in the 5-HT-induced increase in excitatory postsynaptic potentials [12,18] and play an important role in the working memory process [13]. There is indication that 5-HT2A also participates in the etiopathology of AD. Serotonin increases the secretion of amyloid precursor protein (APP) through activation of 5-HT2A receptors [19]. 5-HT2A receptor binding is decreased in AD (for review, see [9,10]), and polymorphic variations have been described for the 5-HT2A gene that may be risk factors for hallucinations [20], aggression [21] and major depression [22] in AD. Much less is known about the 5-HT6 receptor, the most recent 5-HT receptor to be identified. Many antidepressants and antipsychotics are antagonists of the 5-HT6 receptor [15,23]. It has been shown to influence acetylcholine release in the frontal cortex [24] and may play a role in cognition deficits and in some form of anxiety [23]. A recent autoradiographic study examining [125I]-SB-258585 binding in autopsy specimens of AD patients indicates lowered 5-HT6 receptor density in the frontal and temporal cortices [5]. Association of a 5-HT6 receptor gene polymorphic variant and late-onset AD has been observed in the Chinese population [25], but not in Germans [26].
Surprisingly, although 5-HT2A and 5-HT6 receptor binding in AD has been studied extensively [2,5,9,10], there is no information on the expression of these receptors at the cellular level. We have therefore examined the expression of the 5-HT2A and 5-HT6 receptors in the brains of AD and of normal aging control patients by immunohistochemistry. Because the prefrontal cortex is of particular importance for the etiopathology of BPSD [1,8,12,14,16,27-31] and because neuropsychiatric symptoms in AD are associated with reduced metabolism [32,33] and perfusion [34] in Brodmann area 10, we have chosen this brain region for examination.
Methods
Tissue samples
The brains were obtained from a total of 13 autopsy cases with the consent of a close relative and with full approval by the ethical committees of Guangzhou hospital authorities and of the Chinese University of Hong Kong. Six of the specimens were from individuals with clinically and pathologically diagnosed senile dementia of the Alzheimer's type (mean age: 88 years, see table 1), seven specimens were from individuals, matched for age, gender and postmortem delay, who had no history of neurological diseases (normal control). Drug history was recorded for all patients, who had only received antibiotic treatment, but no psychopharmacological medication. Alzheimer Disease was clinically diagnosed according to the "National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association" (NINCDS-ADRA) criteria and the "Diagnostic and Statistical Manual of Mental Disorders", Fourth Edition, (DSM-IV-R) criteria.
Table 1.
Profiles of Alzheimer (AD) and matched normal aging patients
| Subject | Diagnosis | Sex | Age | Cause of death |
| 1 | Normal | F | 83 | Pneumonia |
| 2 | Normal | F | 86 | Gastrointestinal bleeding |
| 3 | Normal | M | 82 | Pneumonia |
| 4 | Normal | M | 93 | Sudden death |
| 5 | Normal | F | 72 | Sudden death |
| 6 | Normal | F | 85 | Pneumonia |
| 7 | Normal | F | 81 | Pneumonia |
| 8 | Alzheimer | M | 82 | Subarachnoid hemorrhage |
| 9 | Alzheimer | F | 91 | Septicemia |
| 10 | Alzheimer | F | 96 | Pneumonia |
| 11 | Alzheimer | F | 82 | Pneumonia |
| 12 | Alzheimer | F | 86 | Pneumonia |
| 13 | Alzheimer | F | 89 | Sudden death |
Whole brains were removed with an average postmortem delay of six hours, and tissue samples of the anterior prefrontal cortex (Brodmann's area 10) were dissected. Specimens were fixed overnight in 4% paraformaldehyde in phosphate-buffered saline (PBS; ph 7.4), dehydrated in graded ethanol, cleared with xylene and embedded in paraffin. 6-μm-thick serial sections were cut in the coronal plane, 90° perpendicular to the surface to avoid sectioning artifacts. Two sets of slides were obtained for each individual.
Immunohistochemistry
All steps were carried out at room temperature unless stated otherwise. Mounted sections were dewaxed, rehydrated and predigested with 0.1% trypsin (BDH Laboratory Supplies, Poole, U.K.) in 0.05M tris buffered saline containing 0.1% CaCl2 (pH 7.6; 37°C; 20 min) followed by two rinses (5 min) in 0.01M PBS for antigen retrieval. The endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in absolute methanol for 30 min, followed by another three rinses in PBS (5 min each). To suppress non-specific binding, sections were then incubated for 1h in 2 % normal blocking serum (VectastainR ABC Kit, Vector Laboratories, Burlingame, CA) in 0.3 % triton/PBS. Thereafter, sections were incubated overnight with the primary polyclonal antibodies: either goat anti-human serotonin 2A receptor (Santa Cruz sc-15073, Santa Cruz Biotechnology Inc., Sta. Cruz, CA) or goat anti-human serotonin 6 receptor (Santa Cruz sc-26668). The sections were then washed with three rinses of PBS containing 0.05 % Tween 20 (5 min each) and incubated with biotinylated secondary antibody in blocking solution (1:200) for 30 min (PK6105, anti-goat IgG, VectastainR ABC Kit, Vector Laboratories, Burlingame, CA). Sections were washed three times in PBS again (5 min) and incubated with ABC Reagent (VectastainR ABC Kit, Vector Laboratories, Burlingame, CA) for 30 min. The immunocytochemical staining signals were visualized by incubating the sections in 0.05% of the substrate 3'3'-diaminobenzidine tetrahydrochloride (DAB) in PBS containing 0.01% H2O2. All stainings included negative controls with omission of the primary antibody, which did not show any immunoreaction. The sections were washed with distilled water and then counterstained with 0.5% cresyl fast violet in 0.1M sodium acetate (pH 3.5, 5 min). They were differentiated with 95% ethanol, dehydrated, cleared and covered in Permount® (Fisher Scientific, Hampton, VA). Additional sections were stained with routine methods, including H&E, Nissl and Bielschowsky silver impregnation for tangle staging [35,36].
All cases underwent a standardized post-mortem neuropathological assessment of AD using established criteria including Braak staging [35]. Four brain regions were examined: prefrontal (area 10), occipital (area 17), entorhinal (area 28) and hippocampal cortices. Diagnosis was confirmed by the characteristic presence of amyloid plaques, neurofibrillary tangles (NFT) and neuronal degeneration. In addition, the presence of β-amyloid in the plaques was ascertained by immunohistochemistry using the rabbit polyclonal Anti-Amyloid Peptide β, Cleavage site 42 (Aβ42), antibody (A1976, Sigma, St. Louis, MU) [37]. All AD cases had neocortical NFT with involvement of both isocortical association areas and additional involvement of primary cortical (area 17) areas (Braak stage V-VI). In control patients, deposition of NFT was negligible in the entorhinal cortex and absent in the hippocampus as well as the neocortex (Braak stage = I). To exclude Dementia with Lewy bodies, all brain specimens were scrutinized for Lewy bodies, and only cases devoid of Lewy bodies were included in the study.
Statistics
For qualitative and quantitative evaluation, sections were observed under a photomicroscope (Axioplan 2 Photomicroscope, Zeiss, Germany). The number of 5-HT2A- and 5-HT6-immunoreactive cells was counted in 30 random selected 700 μm2 fields of normal aged and AD patients. 4–5 sections per individual were evaluated, sparing areas containing amyloid plaques. It has been shown in AD patients [38] that astrocytes in and around Aβ plaques are strongly positive for 5-HT2A receptor protein, whereas astrocytes in control patients do not display any 5-HT2A immunoreaction. Amyloid plaques were therefore excluded from the quantitative assessment, because the aim of our study was to count neurons expressing 5-HT receptors. Measurements were expressed as means ± SEM. For statistical comparison, the values were subjected to a one-way analysis of variance (ANOVA), demonstrating that the four data groups exhibited equal variance, but were not distributed normally. We have then applied the One Way non-parametric ANOVA (Kruskal-Wallis test) and the Mann Whitney Rank sum test, yielding the same results. A p value below 0.05 was considered significant.
Results
In the prefrontal cortex of normal aging patients, 5-HT2A receptor immunoreaction was observed in cortical layers II-VI. Large pyramidal neurons of layer V constituted the majority of immunoreactive cells, characterized by a strongly labeled cytoplasm and a moderately stained proximal part of the apical dendrite (Figs. 1a, c). In addition, smaller pyramidal cells and scattered stellate-shaped cells in the other layers were immunoreactive. Stellate cells had a strongly stained cytoplasm and labeled thin processes radiating to the side of the ovoid cell bodies and most likely represented interneurons. A few multipolar cells in layer VI were also 5-HT2A receptor positive. In the underlying white matter, occasional 5-HT2A receptor immunoreactive fiber bundles were observed. AD patients showed a similar distribution of 5-HT2A receptor label in the prefrontal cortex, i.e. staining in the cell bodies and apical dendrites of large pyramidal cells and in the soma and tender processes of a few scattered interneurons (Figs. 1b, d). However, numerical density of 5-HT2A receptor immunoreactive cells was significantly (p=0.001) decreased by 33% in the frontal cortex of AD patients (Fig. 2), as compared to normal aging patients. Reduction affected both pyramidal cells and cells of stellate morphology. In addition, labeled cells were also seen within and close to amyloid plaques. An association between 5-HT2A receptors and NFT was not observed.
Figure 1.
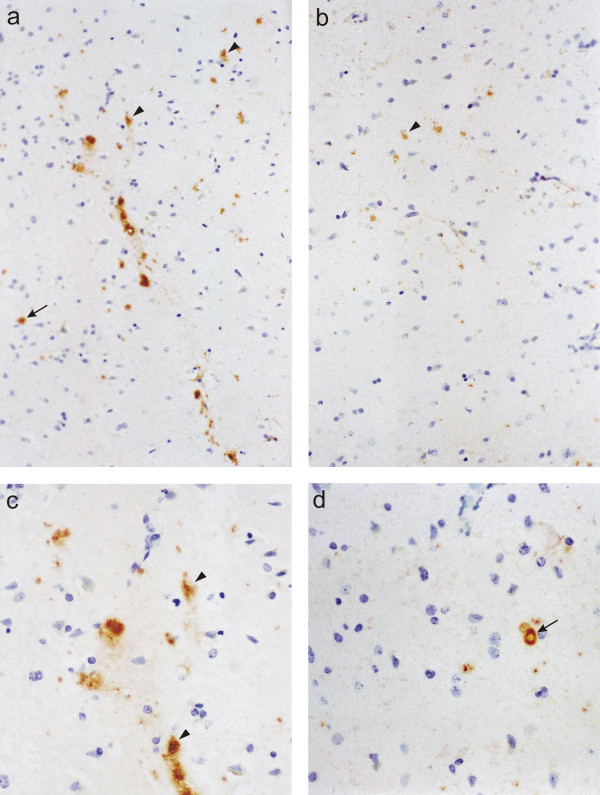
5-HT2A receptor. Immunoreaction for the 5-HT2A receptor in the prefrontal cortex of a normal aging (a, c) and an Alzheimer patient (b, d), cresyl violet counterstain. Both large pyramidal cells (arrowheads) and small interneurons (arrows) are stained. Note the reduction in labeled cells in the cortex of the Alzheimer patient (b, d). a, b: x200; c, d; x400.
Figure 2.
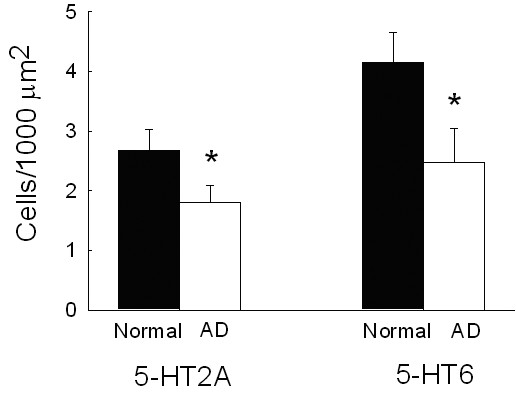
Cell densities. Numerical density of 5-HT2A and 5-HT6 receptor immunoreactive neurons in the prefrontal cortex of normal aging (n=7) and Alzheimer (AD) patients (n=6). Depicted are means ± SEM. Significant differences (p ≤ 0.05) are marked by an asterisk (*)
Distribution of 5-HT6 receptor immunoreaction in the prefrontal cortex of normal aging patients was slightly different from that of the 5-HT2A receptor. As for the 5-HT2A receptor, the most prominent label was observed in the cell bodies and the proximal apical dendrites of large pyramidal neurons, and occasionally, the soma and fine processes of scattered stellate cells were stained as well (Figs. 3a, c). In addition, 5-HT6 receptor immunoreaction was also detected in neurons of the molecular layer (layer I). These cells had a bipolar strongly labeled cell body and numerous immunoreactive processes radiating into the molecular layer (Fig. 3d). In contrast, hardly any 5-HT6 receptor immunoreactive cells were observed in layer VI. Occasional immunoreactive fiber bundles were seen in the underlying white matter. In summary, density of 5-HT6 receptor positive neurons was significantly higher than that of 5-HT2A receptor immunoreactive cells (p=0.05). In the prefrontal cortex of AD patients, 5-HT6 receptor label showed the same overall distribution (Figs. 3b, e), but numerical density of 5-HT6 receptor immunoreactive cells was again significantly (p=0.001) reduced, as compared to normal aging patients (Fig. 2). Both pyramidal and stellate cells were decreased by about 40%. There was no association between 5-HT6 receptors and NFT.
Figure 3.
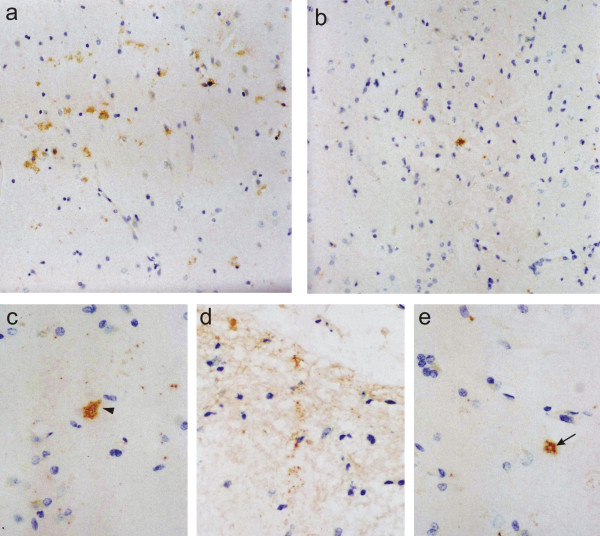
5-HT6 receptor. 5-HT6 receptor immunoreactive cells in the prefrontal cortex of normal aging (a, c, d) and Alzheimer patients (b, e), cresyl violet counterstain. Label is observed in large pyramidal cells (arrowhead) and small interneurons (arrow). Note positive cells and dense immunoreactive fibers in the molecular layer (d). In the cortex of the Alzheimer patient (b, e), significantly fewer cells are labeled than in that of the normal aging patient (a, c, d). a, b: x200; c, d, e; x400.
Discussion
The present study has been undertaken in order to determine changes in cellular distribution of two serotonin receptors, 5-HT2A and 5-HT6, in the prefrontal cortex of AD patients as compared to normal age-matched individuals. Our observation in aging (control) patients, showing 5-HT2A immunoreactivity in the cell bodies and the apical dendrites of pyramidal cells as well as in stellate-shaped cells, is consistent with previous studies in young humans [17], primates [18,39] and rodents [40,41]. However, we have detected relatively few labeled cells, and we are currently testing if the paucity in 5-HT2A receptors is attributable to the very advanced age of our patients or to the technique employed. A considerable age-related reduction in the number of cortical 5-HT2A binding sites in the frontal lobe has been described by numerous authors (see [9] for review). In contrast to 5-HT2A, relatively little is known on the distribution of the 5-HT6 receptor in the mammalian neocortex. 5-HT6 receptor binding sites have been detected by receptor autoradiography in the gray matter of the prefrontal cortex of healthy human adults [5,42], but there are no studies on the cellular level. We have been able to show that the 5-HT6 receptor is expressed both by pyramidal cells and by stellate-shaped cells located in cortical layers I-V. We have observed very little 5-HT6 immunoreaction in layer VI, but a distinct 5-HT6 label in layer I, with staining detected not only in a dense network of fibers, but also in neurons. This is somewhat at variance with a study on the rat neocortex, where 5-HT6 receptor mRNA has been detected by in-situ hybridization in layers II-VI, but not in layer I [43]. This discrepancy is, however, not surprising, given the marked inter-species differences described [44].
To our knowledge, this is the first immunohistochemical study of 5-HT2A receptor expression in AD. The reduction observed corroborates the results of previous 5-HT2A receptor binding studies showing decreased binding of 3 [H] ketanserin [45-47] and 3 [H] spiperone [48] in postmortem specimens of the frontal cortex of AD patients and in PET imaging studies [49]. Contrasting reports describing unaltered 3 [H] ketanserin binding [50], which are at variance with our results, may be attributable to recent psychotropic medication, which had not been administered to our patients. Reduced 5-HT2A receptor binding in AD has been explained by a loss of interneurons [51]. In contrast, our immunohistochemical data indicate that both 5-HT2A immunoreactive pyramidal and stellate-shaped cells, i.e. interneurons, are affected in AD. Moreover, we have demonstrated that the density of neurons expressing the 5-HT6 receptor is reduced to a similar extent, corroborating autoradiographic studies revealing decreased binding to 5-HT6 receptors in the prefrontal cortex of AD patients [5]. Our observation of reduced density of neurons expressing 5-HT2A and 5-HT6 receptors, which is not accompanied by similar alterations in GABAergic markers [6], points at a selective vulnerability of neurons receiving serotonergic input. This decrease is associated with several other abnormalities of the serotonergic system in AD. A marked depletion in 5-HT and its metabolite 5-Hydroxyindole Acetic Acid (5-HIAA) has been described in the frontal and temporal cortices of AD patients [2,28,52], which is most likely attributable to a reduction in serotonergic projection fibers. The underlying cause appears to be a significant loss of neurons in the dorsal and median raphe nuclei [53,54], which are the source of serotonergic nerve terminals and which, in addition, are a preferential site for neurofibrillary tangle formation [55]. It is very likely that the decrease in neurons expressing 5-HT2A and 5-HT6 receptors is related to this loss of serotonergic fibers. However, it may either reflect a process of primary cortical degeneration [4,56] leading to presynaptic disturbance of the serotonergic system, or be secondary to the decrease in serotonergic afferents.
Several lines of evidence indicate that serotonergic dysfunction in AD has important functional consequences. In a study using retrospective data, loss in 5-HT2A binding has been confined to AD patients with aggressive symptoms [57]. When cognitive function is assessed antemortem, 5-HT2A receptors are lost in the frontal and temporal cortex only in patients with severe, but not with mild to moderate dementia [47]. The decrease in the expression of 5-HT6 receptors in the prefrontal cortex of AD patients can be correlated to the extent of aggressive behavior [5]. The number of 5-HT uptake sites is significantly reduced in the frontal and temporal cortex of AD patients with persistent depression, anxiety and overactivity, compared with AD patients without these symptoms [52], and 5-HT levels in the prefrontal cortex (Brodmann 10) of AD correlate with overactivity [2].
Conclusion
Our results of normal aging patients, showing that 5-HT2A and 5-HT6 receptors are expressed by both pyramidal cells and stellate-shaped neurons, indicate that serotonergic fibers exert their influence upon these two neocortical cell types not only through the 5-HT2A, but also the 5-HT6 receptor. The significant 33–40% reduction in cells immunoreactive for 5-HT2A and 5-HT6 receptors observed in AD patients, affecting both large pyramidal cells and interneurons, points at a severely compromised serotonergic system in AD, involving not only serotonergic projection fibers, but also their corresponding receptors. This decline in receptors most likely contributes to the development of neuropsychiatric symptoms in AD.
Authors' contributions
Dietrich E. Lorke performed the evaluation of the slides, interpreted the results and wrote the manuscript.Gang Lu performed immunohistochemistry and documented the results.Eric Cho performed the quantitative and statistical analyses. David T. Yew conceived and planned the study, performed the neuropathological examination and evaluated the slides.
Abbreviations
AD: Alzheimer disease
BPSD: behavioral and psychological symptoms of dementia
5-HT: serotonin
5-HT2A: serotonergic receptor 2A
5-HT6: serotonergic receptor 6
NFT: neurofibrillary tangles
PBS: phosphate-buffered saline
SEM: standard error of the mean
Acknowledgments
Acknowledgements
The authors wish to thank Samuel Wong for skilful assistance in preparing the photographs and Stephanie Yeung for typing the manuscript.
Contributor Information
Dietrich E Lorke, Email: lorke@uaeu.ac.ae.
Gang Lu, Email: lugang@surgery.cuhk.edu.hk.
Eric Cho, Email: eric-cho@ana.cuhk.edu.hk.
David T Yew, Email: david-yew@cuhk.edu.hk.
References
- Cummings JL, Back C. The cholinergic hypothesis of neuropsychiatric symptoms in Alzheimer's disease. Am J Geriatr Psychiatry. 1998;6:S64–S78. doi: 10.1097/00019442-199821001-00009. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Gil-Bea FJ, Diez-Ariza M, Chen CP, Francis PT, Lasheras B, Ramirez MJ. Cholinergic-serotonergic imbalance contributes to cognitive and behavioral symptoms in Alzheimer's disease. Neuropsychologia. 2005;43:442–449. doi: 10.1016/j.neuropsychologia.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Giacobini E. Cholinergic function and Alzheimer's disease. Int J Geriatr Psychiatry. 2003;18:S1–S5. doi: 10.1002/gps.935. [DOI] [PubMed] [Google Scholar]
- Arendt T. Synaptic plasticity and cell cycle activation in neurons are alternative effector pathways: the 'Dr. Jekyll and Mr. Hyde concept' of Alzheimer's disease or the yin and yang of neuroplasticity. Prog Neurobiol. 2003;71:83–248. doi: 10.1016/j.pneurobio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Hirst WD, Chen CP, Lasheras B, Francis PT, Ramirez MJ. Differential involvement of 5-HT(1B/1D) and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer's disease. Neuropsychopharmacology. 2004;29:410–416. doi: 10.1038/sj.npp.1300330. [DOI] [PubMed] [Google Scholar]
- Yew DT, Li WP, Webb SE, Lai HW, Zhang L. Neurotransmitters, peptides, and neural cell adhesion molecules in the cortices of normal elderly humans and Alzheimer patients: a comparison. Exp Gerontol. 1999;34:117–133. doi: 10.1016/S0531-5565(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. The electrophysiology of prefrontal serotonin systems: therapeutic implications for mood and psychosis. Biol Psychiatry. 1998;44:1118–1127. doi: 10.1016/S0006-3223(98)00036-5. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Smith G, DeKosky ST, Pollock BG, Mathis CA, Moore RY, Kupfer DJ, Reynolds CF., III Serotonin in aging, late-life depression, and Alzheimer's disease: the emerging role of functional imaging. Neuropsychopharmacology. 1998;18:407–430. doi: 10.1016/S0893-133X(97)00194-2. [DOI] [PubMed] [Google Scholar]
- Lanctôt KL, Herrmann N, Mazzotta P. Role of serotonin in the behavioral and psychological symptoms of dementia. J Neuropsychiatry Clin Neurosci. 2001;13:5–21. doi: 10.1176/jnp.13.1.5. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/S0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev. 2000;31:302–12. doi: 10.1016/S0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B, Hussain T, Hayes W, Scarr E, Kitsoulis S, Hill C, Opeskin K, Copolov DL. Changes in serotonin2A and GABA(A) receptors in schizophrenia: studies on the human dorsolateral prefrontal cortex. J Neurochem. 1999;72:1593–1599. doi: 10.1046/j.1471-4159.1999.721593.x. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Amargòs-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT(1A) and 5-HT(2A) receptors in depression. Rev Psychiatr Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Pandey SC, Pesold C, Roberts RC, Conley RR, Tamminga CA. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry. 2002;159:419–429. doi: 10.1176/appi.ajp.159.3.419. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action ofhallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch RM, Deng M, Growdon JH, Wurtman RJ. Serotonin5-HT2a and 5-HT2c receptors stimulate amyloid precursor protein ectodomain secretion. J Biol Chem. 1996;271:4188–4194. doi: 10.1074/jbc.271.8.4188. [DOI] [PubMed] [Google Scholar]
- Holmes C, Arranz MJ, Powell JF, Collier DA, Lovestone S. 5-HT2A and 5-HT2C receptor polymorphisms and psychopathology in late onset Alzheimer's disease. Hum Mol Genet. 1998;7:1507–1509. doi: 10.1093/hmg/7.9.1507. [DOI] [PubMed] [Google Scholar]
- Assal F, Alarcon M, Solomon EC, Masterman D, Geschwind DH, .Cummings JL. Association of the serotonin transporter and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer disease. Arch Neurol. 2004;61:1249–1253. doi: 10.1001/archneur.61.8.1249. [DOI] [PubMed] [Google Scholar]
- Holmes C, Arranz M, Collier D, Powell J, Lovestone S. Depression in Alzheimer's disease: the effect of serotonin receptor gene variation. Am J Med Genet B Neuropsychiatr Genet. 2003;119:40–43. doi: 10.1002/ajmg.b.10068. [DOI] [PubMed] [Google Scholar]
- Branchek TA, Blackburn TP. 5-HT6 receptors as emerging targets for drug discovery. Annu Rev Pharmacol Toxicol. 2000;40:319–334. doi: 10.1146/annurev.pharmtox.40.1.319. [DOI] [PubMed] [Google Scholar]
- Riemer C, Borroni E, Levet-Trafit B, Martin JR, Poli S, Porter RH, Bos M. Influence of the 5-HT6 receptor on acetylcholine release in the cortex: pharmacological characterization of 4-(2-bromo-6-pyrrolidin-1- ylpyridine-4-sulfonyl)phenylamine, a potent and selective 5-HT6 receptor antagonist. J Med Chem. 2003;46:1273–1276. doi: 10.1021/jm021085c. [DOI] [PubMed] [Google Scholar]
- Kan R, Wang B, Zhang C, Yang Z, Ji Shun, Lu Z, Zheng C, Jin F, Wang L. Association of the HTR6 polymorphism C267T with late-onset Alzheimer's disease in Chinese. Neurosci Lett. 2004;372:27–29. doi: 10.1016/j.neulet.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Thome J, Retz W, Baader M, Pesold B, Hu M, Cowen M, Durany N, Adler G, Henn FA, Rösler M. Association analysis of HTR6 and HTR2A polymorphisms in sporadic Alzheimer's disease. J Neural Transm. 2001;108:1175–1180. doi: 10.1007/s007020170007. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–453. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- Palmer AM, Francis PT, Benton JS, Sims NR, Mann DM, Neary D, Snowden JS, Bowen DM. Presynaptic serotonergic dysfunction in patients with Alzheimer's disease. J Neurochem. 1987;48:8–15. doi: 10.1111/j.1471-4159.1987.tb13120.x. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- Stutzmann GE, Marek GJ, Aghajanian GK. Adenosine preferentially suppresses serotonin2A receptor-enhanced excitatory postsynaptic currents in layer V neurons of the rat medial prefrontal cortex. Neuroscience. 2001;105:55–69. doi: 10.1016/S0306-4522(01)00170-1. [DOI] [PubMed] [Google Scholar]
- Puig MV, Celada P, Artigas F. Serotonergic control ofprefrontal cortex. Rev Neurol. 2004;39:539–547. [PubMed] [Google Scholar]
- Sultzer DL, Brown CV, Mandelkern MA, Mahler ME, Mendez MF, Chen ST, Cummings JL. Delusional thoughts and regional frontal/temporal cortex metabolism in Alzheimer's disease. Am J Psychiatry. 2003;160:341–349. doi: 10.1176/appi.ajp.160.2.341. [DOI] [PubMed] [Google Scholar]
- Holthoff VA, Beuthien-Baumann B, Kalbe E, Lüdecke S, Lenz O, Zündorf G, Spirling S, Schierz K, Winiecki P, Sorbi S, Herholz K. Regional cerebral metabolism in early Alzheimer's disease with clinically significant apathy or depression. Biol Psychiatry. 2005;57:412–421. doi: 10.1016/j.biopsych.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Benoit M, Clairet S, Koulibaly PM, Darcourt J, Robert PH. Brain perfusion correlates of the apathy inventory dimensionsof Alzheimer's disease. Int J Geriatr Psychiatry. 2004;19:864–869. doi: 10.1002/gps.1163. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 2004;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Kril JJ, Halliday GM. Practical measures to simplify the Braak tangle staging method for routine pathological screening. Acta Neuropathol (Berl) 2000;99:199–208. doi: 10.1007/PL00007425. [DOI] [PubMed] [Google Scholar]
- Yew DT, Wong HW, Li WP, Lai HW, Yu WH. Nitric oxide synthase neurons in different areas of normal aged and Alzheimer's brains. Neuroscience. 1999;89:675–686. doi: 10.1016/S0306-4522(98)00383-2. [DOI] [PubMed] [Google Scholar]
- Wu C, Singh SK, Dias P, Kumar S, Mann DM. Activated astrocytesdisplay increased 5-HT2a receptor expression in pathological states. Exp Neurol. 1999;158:529–533. doi: 10.1006/exnr.1999.7105. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol. 2000;417:337–348. doi: 10.1002/(SICI)1096-9861(20000214)417:3<337::AID-CNE7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Cornea-Hébert , Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(SICI)1096-9861(19990628)409:2<187::AID-CNE2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- East SZ, Burnet PW, Leslie RA, Roberts JC, Harrison PJ. 5-HT6 receptor binding sites in schizophrenia and following antipsychotic drug administration: autoradiographic studies with 125I]SB-258585. Synapse. 2002;45:191–199. doi: 10.1002/syn.10097. [DOI] [PubMed] [Google Scholar]
- Ward RP, Hamblin MW, Lachowicz JE, Hoffman BJ, Sibley DR, Dorsa DM. Localization of serotonin subtype 6 receptor messenger RNA in the rat brain by in situ hybridization histochemistry. Neuroscience. 1995;64:1105–1111. doi: 10.1016/0306-4522(94)00439-C. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW, Medhurst AD. Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol Pharmacol. 2003;64:1295–1308. doi: 10.1124/mol.64.6.1295. [DOI] [PubMed] [Google Scholar]
- Cross AJ, Crow TJ, Ferrier IN, Johnson JA, Bloom SR, Corsellis JA. Serotonin receptor changes in dementia of the Alzheimer type. J Neurochem. 1984;43:1574–1581. doi: 10.1111/j.1471-4159.1984.tb06081.x. [DOI] [PubMed] [Google Scholar]
- Bowen DM, Najlerahim A, Procter AW, Francis PT, Murphy E. Circumscribed changes of the cerebral cortex in neuropsychiatric disorders of later life. Proc Natl Acad Sci U S A. 1989;86:9504–9508. doi: 10.1073/pnas.86.23.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MK, Tsang SW, Alder JT, Keene J, Hope T, Esiri MM, Francis PT, Chen CP. Loss of serotonin 5-HT2A receptors in the postmortem temporal cortex correlates with rate of cognitive decline in Alzheimer's disease. Psychopharmacology (Berl) 2005;179:673–677. doi: 10.1007/s00213-004-2077-2. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Altered cortical serotonergic binding. Arch Neurol. 1989;46:138–140. doi: 10.1001/archneur.1989.00520380038010. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Greer PJ, Cantwell MN, Houck PR, Mulsant BH, Ben-Eliezer D, Lopresti B, DeKosky ST, Reynolds CF., III PET imaging of serotonin type 2A receptors in late-life neuropsychiatric disorders. Am J Psychiatry. 1999;156:1871–1878. doi: 10.1176/ajp.156.12.1871. [DOI] [PubMed] [Google Scholar]
- Dewar D, Graham DI, McCulloch J. 5 HT2 receptors in dementia of Alzheimer type: a quantitative autoradiographic study of frontal cortex and hippocampus. J Neural Transm Park Dis Dement Sect. 1990;2:129–137. doi: 10.1007/BF02260900. [DOI] [PubMed] [Google Scholar]
- Procter AW, Lowe SL, Palmer AM, Francis PT, Esiri MM, Stratmann GC, Najlerahim A, Patel AJ, Hunt A, Bowen DM. Topographical distribution of neurochemical changes in Alzheimer's disease. J Neurol Sci. 1988;84:125–140. doi: 10.1016/0022-510X(88)90118-9. [DOI] [PubMed] [Google Scholar]
- Chen CP, Alder JT, Bowen DM, Esiri MM, McDonald B, Hope T, Jobst KA, Francis PT. Presynaptic serotonergic markers in community-acquired cases of Alzheimer's disease: correlations with depression and neuroleptic medication. J Neurochem. 1996;66:1592–1598. doi: 10.1046/j.1471-4159.1996.66041592.x. [DOI] [PubMed] [Google Scholar]
- Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ, Folstein MF, Price DL. Neuropathology of aminergic nuclei in Alzheimer's disease. Prog Clin Biol Res. 1989;317:353–365. [PubMed] [Google Scholar]
- Chen CP, Eastwood SL, Hope T, McDonald B, Francis PT, Esiri MM. Immunocytochemical study of the dorsal and median raphe nuclei in patients with Alzheimer's disease prospectively assessed for behavioural changes. Neuropathol Appl Neurobiol. 2000;26:347–355. doi: 10.1046/j.1365-2990.2000.00254.x. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Kemper T. Nucleus raphe dorsalis in dementia of the Alzheimer type: neurofibrillary changes and neuronal packing density. J Neuropathol Exp Neurol. 1984;43:359–368. doi: 10.1097/00005072-198407000-00001. [DOI] [PubMed] [Google Scholar]
- Li WP, Chan WY, Lai HW, Yew DT. Terminal dUTP nick end labeling (TUNEL) positive cells in the different regions of the brain in normal aging and Alzheimer patients. J Mol Neurosci. 1997;8:75–82. doi: 10.1007/BF02736774. [DOI] [PubMed] [Google Scholar]
- Procter AW, Francis PT, Stratmann GC, Bowen DM. Serotonergic pathology is not widespread in Alzheimer patients without prominent aggressive symptoms. Neurochem Res. 1992;17:917–922. doi: 10.1007/BF00993268. [DOI] [PubMed] [Google Scholar]


