Abstract
Neuroglobin (Ngb) is an O2-binding protein localized to cerebral neurons of vertebrates, including humans. Its physiological role is unknown but, like hemoglobin, myoglobin, and cytoglobin/histoglobin, it may transport O2, detoxify reactive oxygen species, or serve as a hypoxia sensor. We reported recently that hypoxia stimulates transcriptional activation of Ngb in cultured cortical neurons and that antisense inhibition of Ngb expression increases hypoxic neuronal injury, whereas overexpression of Ngb confers resistance to hypoxia. These findings are consistent with a role for Ngb in promoting neuronal survival after hypoxic insults in vitro. Here we report that in rats, intracerebroventricular administration of an Ngb antisense, but not sense, oligodeoxynucleotide increases infarct volume and worsens functional neurological outcome, whereas intracerebral administration of a Ngb-expressing adeno-associated virus vector reduces infarct size and improves functional outcome, after focal cerebral ischemia induced by occlusion of the middle cerebral artery. We conclude that Ngb acts as an endogenous neuroprotective factor in focal cerebral ischemia and may therefore represent a target for the development of new treatments for stroke.
The ability to sense and respond to hypoxia is a universal attribute of eukaryotic cells, but the role of this ability in protecting cells from ischemic insults and its potential for therapeutic application are unclear. One prominent feature of cellular adaptation to hypoxia or ischemia consists of the increased expression of hypoxia-inducible proteins (1), including proteins with the capacity to protect the brain from ischemic insults (2, 3). Examples include hypoxia-inducible factor-1 (4), erythropoietin (5), vascular endothelial growth factor (6), heme oxygenase-1 (7), and adrenomedullin (8). These proteins are likely to exert their neuroprotective effects through diverse mechanisms but their hypoxia-responsiveness depends ultimately on O2-binding proteins that can sense hypoxia and trigger appropriate cellular adaptations (9, 10).
The globins are a family of heme proteins that can bind, transport, scavenge, detoxify, and sense gases like O2 and NO (11). Four vertebrate globins have been identified. Hemoglobin, which differs from other vertebrate globins in occurring as a tetramer, is localized to erythrocytes and transports O2 between the lungs and other tissues. Myoglobin (Mgb) is monomeric and is localized to the cytoplasm of skeletal and cardiac myocytes. Cytoglobin or histoglobin (12, 13) is expressed widely in both neural and nonneural vertebrate tissues, but its functions are unknown. The recent discovery of neuroglobin (Ngb; ref. 14), which is expressed primarily in cerebral neurons (15), is induced by neuronal hypoxia and cerebral ischemia (16) and protects neurons from hypoxia in vitro (16), suggests that this protein may have a role in sensing or responding to neuronal hypoxia, which could have implications for the pathophysiology and treatment of stroke.
To test the hypothesis that Ngb has protective effects in cerebral ischemia, Ngb protein expression in rat brain was reduced by intraventricular administration of an Ngb antisense oligodeoxynucleotide (ODN) or increased by intracerebral administration of an Ngb-expressing adeno-associated virus (AAV) vector. Focal cerebral ischemia was then induced by occlusion of the middle cerebral artery (MCA), and histological and functional neurological outcomes were assessed. Our results point to a neuroprotective role for Ngb against cerebral ischemia in vivo.
Materials and Methods
Animals.
Adult male Sprague–Dawley rats (290–320 g) were used, and animal experiments were approved by the Buck Institute's Institutional Animal Care and Use Committee and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
ODNs.
A phosphorothioate antisense ODN labeled with fluorescein at the 5′ end and directed against the initial coding region of Ngb (5′-TCCGGGCGCTCCAT-3′, nucleotides 90–77) was designed based on the mouse Ngb GenBank sequence NM 022414. This and a sense sequence (5′-ATGGAGCGCCCGGA-3′, nucleotides 77–90) were synthesized commercially (Qiagen, Valencia, CA) and purified by HPLC. Rats were anesthetized with 4% isoflurane in 70% N2O/30% O2 and placed in a Kopf stereotaxic frame. The skull was exposed and a 1-mm burr hole was drilled over the left hemisphere, 1.5 mm lateral to the sagittal suture and 0.8 mm caudal to the bregma (17). ODNs (10 nmol/ml) or artificial cerebrospinal fluid (aCSF) were infused into the lateral ventricle at 1 μl/h for 72 h with an Alzet (Palo Alto, CA) minipump (18).
Western Blotting.
Cerebral cortical protein (100 μg) was electrophoresed on 12% SDS/PAGE gels and transferred to polyvinyldifluoridine membranes. Membranes were incubated overnight at 4°C with affinity-purified rabbit polyclonal anti-Ngb (1:2,000) raised against a synthetic peptide corresponding to amino acids 35–50 (NH2-CLSSPEFLDHIRKVML-COOH) of mouse Ngb (16). A horseradish peroxidase-conjugated anti-rabbit secondary Ab (Santa Cruz Biotechnology) and chemiluminescence substrate (NEN) were used to visualize immunolabeled bands.
Immunohistochemistry.
Brains were perfused with 0.9% saline and then 4% paraformaldehyde in PBS (pH 7.4) and embedded in paraffin. Immunocytochemistry was performed on 6-μm sections as described (19) by using rabbit polyclonal anti-mouse Ngb (1:200), mouse monoclonal anti-NeuN (Chemicon; 1:200), and mouse monoclonal anti-glial fibrillary acidic protein (Sigma; 1:200) as primary Abs and FITC-conjugated goat anti-rabbit IgG and rhodamine-conjugated goat anti-mouse IgG (Jackson ImmunoResearch; 1:200) as secondary Abs. Controls included omitting primary or secondary Ab. Fluorescence signals were detected with a Nikon E800 epifluorescence microscope at excitation/emission wavelengths of 535/565 (rhodamine) and 470/505 (FITC) nm. Results were recorded with a Magnifire digital color camera (Optronics International, Chelmsford, MA).
Focal Cerebral Ischemia.
Ischemia was induced by using the suture occlusion technique (19). Rats were anesthetized as described above and a 19-mm, 3-0 monofilament nylon suture was inserted via the left external and internal carotid arteries to occlude the MCA at its origin for 90 min, followed by reperfusion. Sham-operated control rats underwent identical surgery except that the suture was not inserted. Rectal temperature was maintained at 37.0 ± 0.5°C. Rats were killed 24 h later.
Measurement of Infarct Volume.
Coronal brain sections were stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC; 2-mm sections; ref. 20) or hematoxylin (30-μm sections) and used to determine infarct areas with the nih image program. Effects of edema and of differential tissue shrinkage were controlled for by subtracting the area of the uninfarcted (TTC- or hematoxylin-stained) tissue in the ischemic hemisphere from the area of the contralateral hemisphere (21). Infarct volume was determined by summing infarct areas multiplied by slice thickness.
Behavioral Testing.
Twenty-four hours after MCA occlusion, the neurological status of each rat was evaluated according to a neurological grading score based on motor, sensory, and reflex testing, in which higher scores indicated more severe impairment, as described (22).
AAV Vector Production and in Vitro Testing.
Full-length mouse Ngb cDNA was cloned into an AAV vector (pTR-UF 12d) containing a cytomegalovirus/chicken β-actin hybrid promoter, and AAV-Ngb vector was produced as described (23, 24). Human embryonic kidney 293 cells (25) and rat cortical neurons (16) were cultured as described and treated for 72–96 h with 1011 particles per well of AAV-Ngb or control (AAV-gfp) vector; cultured neurons were exposed to hypoxia, and neuronal viability was measured by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) as described (16).
AAV Vector Delivery in Vivo.
Rats were anesthetized as described previously and placed in stereotaxic frames with head holders. Two burr holes were drilled and AAV-Ngb or AAV-gfp (4 × 1013 particles per ml), or aCSF, was injected into the left cerebral cortex (0.48 mm anterior to the bregma, 5.5 mm lateral to the midline, 6 mm beneath the dura) and left striatum (0.48 mm anterior to the bregma, 3.5 mm lateral to the midline, 6 mm beneath the dura). At each site, a volume of 3 μl was injected over 2 min, the needle was partially withdrawn (to 3.5 mm beneath the dura for cortical injection and to 4.5 mm beneath the dura for striatal injection), the injection was repeated, and the needle was left in place for an additional 5 min. The needle was removed and bone wounds were closed with bone wax. Focal cerebral ischemia was produced 3 weeks later as described previously.
Results
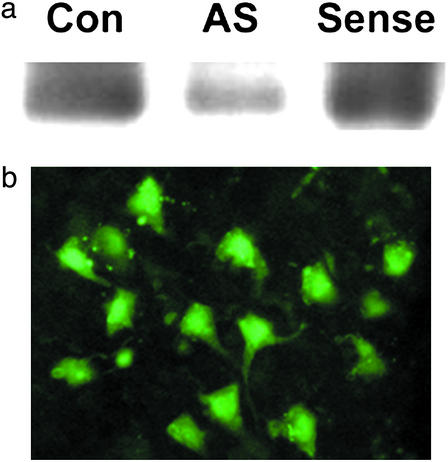
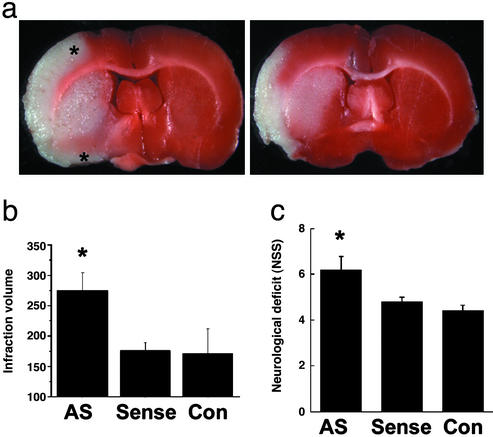
To investigate whether Ngb has a neuroprotective effect in cerebral ischemia in vivo, we used a phosphorothioate antisense ODN directed against the initial coding region of the target Ngb mRNA and a control sense sequence, both labeled with fluorescein at the 5′ end (16). Western blots showed that Ngb protein expression was reduced in cerebral cortex of rats that received a 72-h infusion of antisense ODN into the left lateral ventricle, compared with those given sense ODN or aCSF (128 mM NaCl/2.5 mM KCl/0.95 mM CaCl2/1.99 mM MgCl2) (Fig. 1a). Fluorescence microscopy confirmed successful delivery of the fluorescent ODNs to cerebral cortical neurons (Fig. 1b). Next, antisense or sense ODN or aCSF (control) was infused into the lateral ventricle for 72 h and, 24 h later, focal cerebral ischemia was induced by inserting a monofilament suture into the left MCA for 90 min, followed by reperfusion for 24 h (26). Antisense knockdown of Ngb expression was associated with a 56–60% increase in infarct volume (P < 0.02, n = 5 per group) compared with rats receiving an Ngb sense ODN or aCSF (Fig. 2 a and b). Antisense treatment also worsened postischemic neurological deficits (Fig. 2c), as determined by behavioral testing with a modified Neurological Severity Score, which has been shown to be useful for monitoring functional neurological outcome after focal cerebral ischemia (22). There were no differences among the three treatment groups in venous blood concentrations of Na+, K+, Ca2+, HCO3−, or hemoglobin; arterial blood pH, PO2, or PCO2; or mean arterial blood pressure.
Figure 1.
Ngb antisense ODN, given by the intracerebroventricular route, reduces Ngb expression in rat brain. (a) Western blot of Ngb expression in cerebral cortex from control, antisense-treated, and sense ODN-treated rats. (b) Immunohistochemical localization of fluorescein-labeled ODN (green) within cerebral cortical neurons.
Figure 2.
Ngb antisense ODN exacerbates focal cerebral ischemia in the rat. (a) Antisense-treated (Left) and sense ODN-treated (Right) brains after MCA occlusion and TTC staining, showing an increase in infarct area (white region at left; note greater superior and inferior extent of cortical infarction indicated by asterisks) in antisense-treated rats. (b) Infarct volume (mm3) increased by antisense ODN. (c) Neurological deficit worsened by antisense ODN. *, P < 0.05 compared with aCSF- (Con) and sense ODN-treated rats (n = 5–6; ANOVA and post hoc t test).
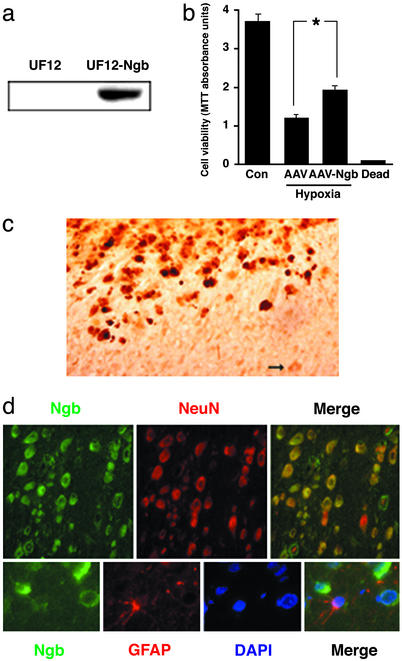
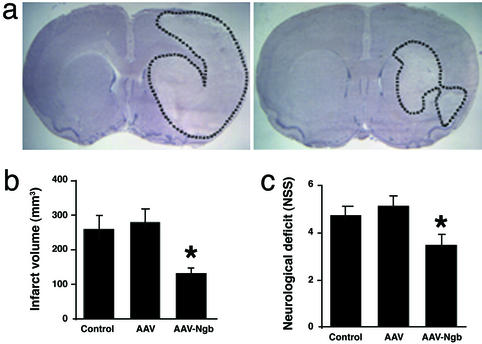
If Ngb is neuroprotective, then its overexpression should confer relative resistance to cerebral ischemia. To test this hypothesis, an AAV-Ngb vector was designed using pTR-UF12d with a cytomegalovirus enhancer and β-actin promoter, and with gfp as a reporter gene. In pilot studies, AAV-Ngb increased Ngb protein expression in 293 cells (Fig. 3a) and partially prevented hypoxia-induced cell death in primary cultures of cortical neurons in vitro (Fig. 3b). Intracerebral injection of AAV-Ngb but not control AAV-gfp vector into cerebral cortex and striatum was associated with increased expression of Ngb in cerebral cortical neurons (Fig. 3 c and d) and with a 49–52% decrease in the size of cerebral infarcts compared with rats receiving control AAV-gfp vector or aCSF (Fig. 4 a and b). The decrease in infarct size was attributable primarily to a decrease in the extent of cortical, rather than striatal, infarction, consistent with the cortical location of most salvageable (penumbral) tissue in this MCA occlusion model. There was also an improvement in neurological function in animals receiving the AAV-Ngb vector (Fig. 4c). As in the antisense experiments described previously, there were no differences among the three treatment groups in venous blood concentrations of Na+, K+, Ca2+, HCO3−, or hemoglobin; arterial blood pH, PO2, or PCO2; or mean arterial blood pressure.
Figure 3.
AAV-Ngb vector increases Ngb expression in vitro and in rat brain in vivo. (a) Western blot of Ngb expression in AAV-gfp control vector- (Left) and AAV-Ngb-transfected (Right) 293 cells. (b) AAV-Ngb partially protects cultured cortical neurons from hypoxic death, measured with MTT. (Dead, freeze-thawed cells). *, P < 0.05 (n = 4; ANOVA and post hoc t test). (c) Intracerebral injection of AAV-Ngb increases Ngb expression (top left) over basal levels (bottom right), as shown by immunohistochemistry with an anti-Ngb Ab (brown). Arrow, example of an uninfected cell with basal expression of Ngb. (d) Intracerebral administration of AAV-Ngb is associated with expression of Ngb in cortical neurons (Upper) but not astroglia (Lower). NeuN, neuronal nuclear antigen; GFAP, glial fibrillary acidic protein; DAPI, 4′,6-diamidino-2-phenylindole.
Figure 4.
Overexpression of Ngb reduces ischemic cerebral injury in the rat. (a) Intracerebral AAV-Ngb reduces infarct size, shown as outlined areas in hematoxylin-stained sections (Left, AAV-gfp control vector-treated; Right, AAV-Ngb-treated). (b) Infarct volume (mm3) reduced by AAV-Ngb. (c) Neurological deficit improved in AAV-Ngb-treated animals. *, P < 0.05 compared with aCSF control and AAV-gfp control vector-treated rats (n = 3–8; ANOVA and post hoc t test).
Discussion
The major finding of this study is that changes in Ngb expression result in corresponding changes in the severity of histological and functional deficits after focal cerebral ischemia, in a manner consistent with an endogenous neuroprotective action of Ngb. Although we described a neuroprotective effect of Ngb against neuronal hypoxia in vitro (16), this is our first demonstration of a function for Ngb in vivo.
How Ngb protects neurons from hypoxia or ischemia is unclear. The related protein Mgb seems to have several biological functions in myocytes. Mgb binds to and facilitates the intracellular diffusion of O2 from capillary blood to myocyte mitochondria (27), although the magnitude of its contribution to intracellular O2 transport is uncertain (28). Mgb-knockout mice have normal skeletal and cardiac muscle function and normal exercise capacity under normoxic and hypoxic conditions (29), which could argue against a role in O2 delivery, or could be due to the induction of multiple compensatory mechanisms, including increased coronary blood flow, coronary flow reserve, capillary density, and hematocrit (30). Another role for Mgb may be to scavenge intracellular NO, because Mgb binds NO and prevents downstream effects such as inhibition of cytochrome c oxidase (31). In support of this hypothesis, hearts from Mgb-knockout mice show increased sensitivity to the vasodilator and cardiodepressor effects of NO (32).
Considerably less is known about the mechanisms involved in hypoxic induction of Ngb or Ngb-mediated neuroprotection. In addition to hypoxia, neuronal Ngb expression can be increased by cobalt and deferoxamine (16), which suggests that hypoxia-inducible factor-1 might be responsible (33) but there is no direct evidence to support this possibility. In HN33 cells, hypoxic induction of Ngb is blocked by the mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK) inhibitor PD98059, implicating MEK in this process, whereas induction of Ngb expression by hemin involves protein kinase G and soluble guanylate cyclase (34).
Concerning the mechanism of neuroprotection, overexpression of Ngb in HN33 cells neither increases O2 consumption (which might be expected if Ngb enhanced O2 delivery to mitochondria) nor confers resistance to the cytotoxic effect of the NO donor, sodium nitroprusside (16). It is still possible, however, that intracellular O2 transport by Ngb has a role in neuroprotection or that Ngb can detoxify more physiological levels of NO or other reactive oxygen species. Alternatively, Ngb might serve as a hypoxia sensor to trigger more directly neuroprotective downstream events (35–37).
The protective effect of Ngb in cerebral ischemia raises the possibility that Ngb could be a suitable target for new therapies directed against stroke, a leading cause of neurological disability and death for which no widely effective treatment exists (38). As an example, drugs like hemin, which increases Ngb expression in vitro (34) and is used clinically in the treatment of acute intermittent porphyria (39), might be developed for this purpose.
Acknowledgments
This work was supported by National Institutes of Health Grant NS35965.
Abbreviations
- AAV
adeno-associated virus
- aCSF
artificial cerebrospinal fluid
- MCA
middle cerebral artery
- Mgb
myoglobin
- Ngb
neuroglobin
- ODN
oligodeoxynucleotide
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wenger R H. FASEB J. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- 2.Bergeron M, Yu A Y, Solway K E, Semenza G L, Sharp F R. Eur J Neurosci. 1999;11:4159–4170. doi: 10.1046/j.1460-9568.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- 3.Bernaudin M, Tang Y, Reilly M, Petit E, Sharp F R. J Biol Chem. 2002;277:39728–39738. doi: 10.1074/jbc.M204619200. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron M, Gidday J M, Yu A Y, Semenza G L, Ferriero D M, Sharp F R. Ann Neurol. 2000;48:285–296. [PubMed] [Google Scholar]
- 5.Sakanaka M, Wen T C, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z G, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panahian N, Yoshiura M, Maines M D. J Neurochem. 1999;72:1187–1203. doi: 10.1111/j.1471-4159.1999.721187.x. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe K, Takayasu M, Noda A, Hara M, Takagi T, Suzuki Y, Yoshia J. Acta Neurochir. 2001;143:1157–1161. doi: 10.1007/s007010100007. [DOI] [PubMed] [Google Scholar]
- 9.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara J M, Lane W S, Kaelin W G., Jr Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 10.Jaakkola P, Mole D R, Tian Y M, Wilson M I, Gielbert J, Gaskell S J, Kriegsheim A, Hebestreit H F, Mukherji M, Schofield C J, et al. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 11.Bunn H F, Poyton R O. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 12.Burmester T, Ebner B, Weich B, Hankeln T. Mol Biol Evol. 2002;19:416–421. doi: 10.1093/oxfordjournals.molbev.a004096. [DOI] [PubMed] [Google Scholar]
- 13.Trent J T, 3rd, Hargrove M S. J Biol Chem. 2002;277:19538–19545. doi: 10.1074/jbc.M201934200. [DOI] [PubMed] [Google Scholar]
- 14.Burmester T, Weich B, Reinhardt S, Hankeln T. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 15.Reuss S, Saaler-Reinhardt S, Weich B, Wystub S, Reuss M H, Burmester T, Hankeln T. Neuroscience. 2002;115:645–656. doi: 10.1016/s0306-4522(02)00536-5. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Jin K, Mao X O, Zhu Y, Greenberg D A. Proc Natl Acad Sci USA. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parmentier-Batteur S, Bohme G A, Lerouet D, Zhou-Ding L, Beray V, Margaill I, Plotkine M. J Cereb Blood Flow Metab. 2001;21:15–21. doi: 10.1097/00004647-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu S, Nagayama T, Jin K L, Zhu L, Loeffert J E, Watkins S C, Graham S H, Simon R P. J Cereb Blood Flow Metab. 2001;21:233–243. doi: 10.1097/00004647-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Jin K, Clark K R, Peel A L, Mao X O, Chang Q, Simon R P, Greenberg D A. Gene Ther. 2003;10:115–122. doi: 10.1038/sj.gt.3301868. [DOI] [PubMed] [Google Scholar]
- 20.Bederson J B, Pitts L H, Germano I M, Nishimura M C, Davis R L, Bartkowski H M. Stroke (Dallas) 1986;17:1304–1307. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 21.Swanson R A, Morton M T, Tsao-Wu G, Savalos R A, Davidson C, Sharp F R. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Chen J, Wang L, Lu M, Chopp M. Neurology. 2001;56:1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- 23.Zolotukhin S, Byrne B J, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski R J, Muzyczka N. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 24.Peel A L, Klein R L. J Neurosci Methods. 2000;98:95–104. doi: 10.1016/s0165-0270(00)00183-7. [DOI] [PubMed] [Google Scholar]
- 25.Klein R L, Meyer E M, Peel A L, Zolotukhin S, Meyers C, Muzyczka N, King M A. Exp Neurol. 1998;150:183–194. doi: 10.1006/exnr.1997.6736. [DOI] [PubMed] [Google Scholar]
- 26.Longa E Z, Weinstein P R, Carlson S, Cummins R. Stroke (Dallas) 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 27.Merx M W, Flogel U, Stumpe T, Godecke A, Decking U K, Schrader J. FASEB J. 2001;15:1077–1079. doi: 10.1096/fj.00-0497fje. [DOI] [PubMed] [Google Scholar]
- 28.Papadopoulos S, Endeward V, Revesz-Walker B, Jurgens K D, Gros G. Proc Natl Acad Sci USA. 2001;98:5904–5909. doi: 10.1073/pnas.101109798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garry D J, Ordway G A, Lorenz J N, Radford N B, Chin E R, Grange R W, Bassel-Duby R, Williams R S. Nature. 1998;395:905–908. doi: 10.1038/27681. [DOI] [PubMed] [Google Scholar]
- 30.Gödecke A, Flögel U, Zanger K, Ding Z, Hirchenhain J, Decking U K, Schrader J. Proc Natl Acad Sci USA. 1999;96:10495–10500. doi: 10.1073/pnas.96.18.10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunori M. Trends Biochem Sci. 2001;26:21–23. doi: 10.1016/s0968-0004(00)01698-4. [DOI] [PubMed] [Google Scholar]
- 32.Flögel U, Merx M W, Gödecke A, Decking U K, Schrader J. Proc Natl Acad Sci USA. 2001;98:735–740. doi: 10.1073/pnas.011460298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang G L, Semenza G L. Proc Natl Acad Sci USA. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Y, Sun Y, Jin K, Greenberg D A. Blood. 2002;100:2494–2498. doi: 10.1182/blood-2002-01-0280. [DOI] [PubMed] [Google Scholar]
- 35.Trent J T, 3rd, Watts R A, Hargrove M S. J Biol Chem. 2001;276:30106–30110. doi: 10.1074/jbc.C100300200. [DOI] [PubMed] [Google Scholar]
- 36.Dewilde S, Kiger L, Burmester T, Hankeln T, Baudin-Creuza V, Aerts T, Marden M C, Caubergs R, Moens L. J Biol Chem. 2001;276:38949–38955. doi: 10.1074/jbc.M106438200. [DOI] [PubMed] [Google Scholar]
- 37.Couture M, Burmester T, Hankeln T, Rousseau D L. J Biol Chem. 2001;276:36377–36382. doi: 10.1074/jbc.M103907200. [DOI] [PubMed] [Google Scholar]
- 38.Dirnagl U, Iadecola C, Moskowitz M A. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 39.Grandchamp B. Semin Liver Dis. 1998;18:17–24. doi: 10.1055/s-2007-1007136. [DOI] [PubMed] [Google Scholar]