Abstract
Purpose
To determine the distribution of slow-cycling cells, which are detected as label-retaining cells (LRCs), in mouse lens epithelium during post-natal development.
Methods
Pregnant Balb/c mice were injected intraperitoneally (twice daily), with tritiated thymidine (3H-TdR) beginning at 17 days gestation until birth. At birth, the in utero-labeled neonatal mice were injected subcutaneously with 3H-TdR (twice daily) for 3 days. Mice were sacrificed weekly for the first month and then at three week intervals up to 18.5 weeks (chase periods). Eyes were removed and processed for autoradiography. Small scrape wounds were made on the anterior surface of the lens of mice that had been “chased” for 18.5 weeks. Twenty-four hours later, wounded mice received a single injection of BrdU.
Results
Immediately after the in-utero/post-natal labeling period, 100% of the lens epithelial cells incorporated 3H-TdR, and all were heavily labeled. With time, the number of LRCs declined so that only 13% of the lens epithelial cells were labeled at 18.5 weeks. At this time the heaviest labeled cells were exclusively found in the central zone and represented 2-3% of the total LRCs. In contrast, lightly-labeled cells were found in both the central and germinative zones. Following wounding, the heavily-labeled LRCs incorporated BrdU indicating that these cells were healthy and could be recruited to proliferate.
Conclusion
The heavily-labeled LRCs, located exclusively in the central region, represent cells that divide very infrequently during homeostasis (putative stem cells). Upon perturbation, these cells can proliferative. The lightly-labeled LRCs, located in the central and germinative zones, cycle more frequently than the heavily-labeled LRCs. These LRCs may be phenotypically indistinguishable from stem cells and maintain the normal proliferative needs of the lens. A third population of actively cycling cells exists primarily in the germinative zone and represents the transit amplifying cells, which have a limited proliferative potential.
Keywords: stem cells, transient amplifying cells, wound repair, epithelial heterogeneity
Introduction
The lens consists of two main cell types, epithelial cells and fiber cells. The epithelial cells are a monolayer of cuboidal cells located at the anterior surface that can be grouped into an anterior or central and a pre-equatorial or peripheral region. Epithelial cells in the central region are considered to be mitotically quiescent, whereas the cells in the peripheral region are mitotically active 1. Due to its proliferative status, the peripheral region is also known as the germinative zone 2. The epithelial cells in the germinative zone eventually transition into post-mitotic cells, which differentiate, elongate, lose their organelles and become the crystalline-rich, enucleate fiber cells 3. It is apparent that under experimental conditions (i.e. inactivation of p53 and pRB), cells in the transitional zone have a certain degree of proliferative capacity 4.
The adult lens is unique in that cells are, for the most part, continuously produced with negligible cell loss 5; therefore this tissue expands throughout the lifetime of the organism 6. Homeostasis in such self- or continuously-renewing systems is maintained through a hierarchy of cells with varying proliferative capacities. Such a hierarchy consists of stem cells with unlimited proliferative capacity, young transit amplifying (TA) cells with a large proliferative capacity, and mature TA cells having a limited proliferative ability (for reviews see 7–9). Stem cells are a subpopulation of cells that have a large tissue repair capacity; they are morphologically and biochemically primitive and they are assumed to divide relatively infrequently in adult tissues 10. Upon division, in average one of the two stem cell progenies leave the stem cell niche (a specialized microenvironment) and become a transit amplifying (TA) cell that has only limited proliferative capability 11. One of the most reliable ways to identify epithelial stem cells takes advantage of the fact that these cells are normally infrequently or slow-cycling, and hence can be identified experimentally as the so-called “label-retaining cells” (LRCs) 12–18. This is done by continuously exposing cells to a DNA precursor such as tritiated thymidine (3H-TdR) or bromodeoxyuridine (BrdU); this results in the labeling of all the dividing cells, including the occasionally dividing stem cells. Following a chase period, which usually lasts 4 to 8 weeks, the rapidly cycling TA cells lose most, if not all of their label due to dilution, while cells that cycle slowly (the stem cells) retain the label; in this manner the stem cells can be detected as LRCs. Application of this technique to the eye has resulted in the concept that: (i) the limbal epithelium is the exclusive site of the corneal epithelial stem cells 14,19; (ii) the fornical epithelium is a site that is enriched in epithelial stem cells 18; and (iii) the proximal portion of the meibomian gland duct is the site of the meibomian gland stem cells 20. This technique has been modified and applied to the chick and teleost retinas and LRCs have been localized to the peripheral portions of these tissues 21,22. Surprisingly, there is no information about the distribution of LRCs in the lens epithelium.
It is frequently reported that the centrally located lens epithelial cells exhibit no mitotic activity 6,23–26. However, a small (0.02 – 0.09) percentage of proliferating cells have been shown to exist in the centrally located region of the lens epithelium of young (6-12 week old) mice and rats 27,28. During investigations on the cell kinetic properties of the mouse anterior segmental epithelia, we observed that following a single pulse administration of either 3H-TdR or BrdU, an occasional lens epithelial cell in the central zone incorporated these nucleosides. To confirm and extend these observations, we used continuous labeling strategies to characterize the proliferative nature of the lens epithelial cells. We observed that a hierarchal organization of proliferation exists in the adult lens epithelium with the slowest or rarely cycling cells located in the central region and the more actively proliferating cells detected in the peripheral or germinative zone. Furthermore, following perturbation both the slow-cycling and the actively-cycling lens epithelial cells could be induced to proliferate.
Materials and Methods
Animal care and use conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All animal protocols were approved by the Institutional Animal Care and Use Committee of Northwestern University School of Medicine.
Detection of Label-Retaining Cells
To detect label-retaining lens epithelial cells, we injected pregnant female Balb/c mice (Charles River, Wilmington, MA), intraperitoneally, with 5μCi/gm body weight of 3H-TdR (specific activity, 82.7Ci/mmol, Perkin Elmer, CA) twice daily (8:00AM and 5:00PM) from day 17 of gestation to birth. At birth, we injected the in utero-labeled neonatal mice subcutaneously with 5μCi/gm body weight of 3H-TdR twice daily for the first three days of life. These time intervals represent the “chase” periods. We sacrificed mice (n=4) 60 minutes after the last injection (time 0), weekly for the first month and then at three week intervals up to 4.5 months. We removed the entire eye including the surrounding eyelid tissues, and identified lens epithelial cells that retained the label as label-retaining cells (LRCs) by autoradiography. We determined the distribution of LRCs at each weekly interval by counting at least 1000 nuclei from five different sections from each mouse (n=4). LRCs were classified in the following manner: cells containing 3–8 grains over their nuclei were scored as lightly-labeled and cells with > than 8 grain/nucleus were designated as heavily-labeled. Only those sliver grains directly over the nucleus were counted; silver grains within the vicinity of the nucleus or the epithelial cell were not counted. To determine the distribution of lightly and heavily labeled LRCs within the lens epithelium, we divided anterior lens capsule into three areas, a central region consisting of ~120 epithelial cells and two equivalent peripheral or germinative regions containing ~ 50 cells on each side. We did not observe a change in the total number of lens epithelial cells during this study (neonatal – 18.5 weeks). However, the cells became markedly flattened during development (see Results below), most likely due to the increase in the size of the lens.
Detection of Rapidly-Cycling Cells
To identify the rapidly cycling transit amplifying (TA) cells, we injected adult (7 week old) mice (n=4) intraperitoneally with BrdU (50μg/g body weight). After a 24 hour period to ensure exit from the “S” phase, the same mice were injected with 10 μCi/g of 3H-TdR. Mice were sacrificed 1 hour later, andthe eyes and eyelids were processed for paraffin sectioning. We detected BrdU by immunohistochemistry and 3H-TdR by autoradiography as previously described 19. It has been estimated that following an intraperitoneal injection, over 80% of the BrdU not incorporated into tissues, is degraded within an hour in the liver 29. Thus, like 3H-TdR, BrdU is only available for a short period of time before it is rapidly removed from the eye 30.
Stimulation of Slow-cycling Cells
Prior to stimulation, we anesthetized groups of LRC-containing mice (n=4) that were chased for 18.5 weeks with gamma-hydroxybutyric acid (i.p. injection of 100 μl of 10%sol. in PBS). We inserted a 30-gauge needle into the peripheral portion of the anterior chamber and a small scrape or puncture was made in the anterior surface of the lens. Twenty-four hours post-stimulation, we injected these mice intraperitoneally with BrdU (50μg/g body weight). One hour later, we sacrificed the mice and processed the eyes for immunohistochemistry and autoradiography 19.
Detection of Doubled-Labeled Cells
To demonstrate multiple divisions by lens epithelial cells, lens epithelial scrape wounds were made as described above. Twenty-four hours post-wounding, we injected adult mice (n = 4) intraperitoneally with BrdU (50μg/g body weight). After a 24 hour chase, mice were injected intraperitoneally with 3H-TdR (10 uCi/g body weight). Mice were sacrificed 60 minutes following the injection of 3H-TdR and eyes were excised, fixed and processed for immunohistochemistry and autoradiography 19.
Results
Actively cycling transit amplifying (TA) cells are present in the central and germinative zones of the lens epithelium
To assess the proliferative status of the resting adult lens epithelium, we detected cells in the S phase of DNA synthesis by 2 consecutive pulse labels. The first label was BrdU, which was followed by 3H-TdR; the labels were 24 hours apart. We observed that most of the BrdU- and 3H-Tdr-labeled lens epithelial cells were in the germinative zones (Figs. 1A, C); however, an occasional BrdU-labeled cell, or a cell containing silver grains was also seen in the central zone of the lens epithelium (Figs. 1A, B). We did not observe any double (pulse)-labeled cells, indicating that cells that were tagged during the first labeling were not making DNA 24 hours later 19.
Figure 1.
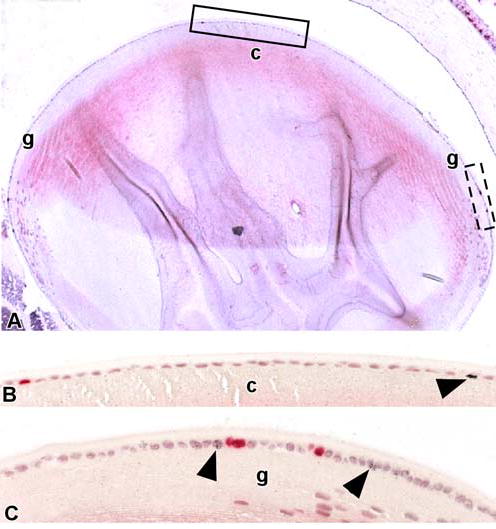
Actively cycling cells are present in the central and germinative zones of the mouse lens epithelium. (A) Paraffin section of the eye of an adult mouse that had received a single intraperitoneal injection of BrdU followed 24 hours later by an injection of 3H-TdR. BrdU-labeled or 3H-TdR-labeled nuclei were detected in the central (c) and germinative (g) zones of the lens epithelium. (B) A portion of the central (c) zone within the solid rectangle is shown at higher magnification; note the lens epithelial cells with BrdU-labeled (red nuclei) and 3H-TdR-labeled nuclei (arrowhead). (C) A portion of the germinative (g) zone within the dashed rectangle is shown at higher magnification. Cells with BrdU-labeled and 3H-TdR-labeled nuclei (arrowheads) are more prevalent in this region.
A Hierarchy of Proliferation Exists in the Lens Epithelium
In order to accurately detect LRCs, most if not all of the proliferating cells should incorporate the nucleoside at the beginning of the chase period. At birth, the neonatal mouse lens epithelium is rapidly proliferating with 20% of the cells in S phase 31. Since this proliferation rate progressively decreases to where less than 1% of adult lens epithelial cells are proliferating at any time 31,32, we employed a protocol that included both in-utero and neonatal labeling to maximize the labeling of the lens epithelial cells. In an initial series of experiments, we injected female mice twice daily with 3H-TdR from day 17 of gestation until birth and assessed the degree of 3H-TdR that was incorporated in the lens epithelium of the newborn mice. This labeling protocol resulted in the tagging of ~ 75% of the lens epithelial cells (data not shown). To increase the percentage of lens epithelial cells that incorporated 3H-TdR, we injected newborn mice that had been labeled in-utero, twice daily with 3H-TdR for the first three days of life. In neonatal mice labeled in such a manner, ~ 100% of the lens epithelial cells incorporated 3H-TdR and all were heavily labeled (Fig. 2). At this time (3 days post-natal), all the lens epithelial cells were cuboidal in shape (Fig. 2); however, by three weeks of age the epithelial cells in the central zone appeared flattened (Fig. 3B). Cells in the germinative zone closest to the central region were also flattened (Fig. 3A), whereas cells nearest the equator maintained a cuboidal shape (Fig. 1A, C, 6). There were no signs of weight loss, growth retardation, coat change and/or illness in the in utero/neonatally-labeled mice compared with unlabeled control mice during the course of the experiment (18.5 weeks), indicative that the labeling protocol did not adversely influence the development of these mice.
Figure 2.
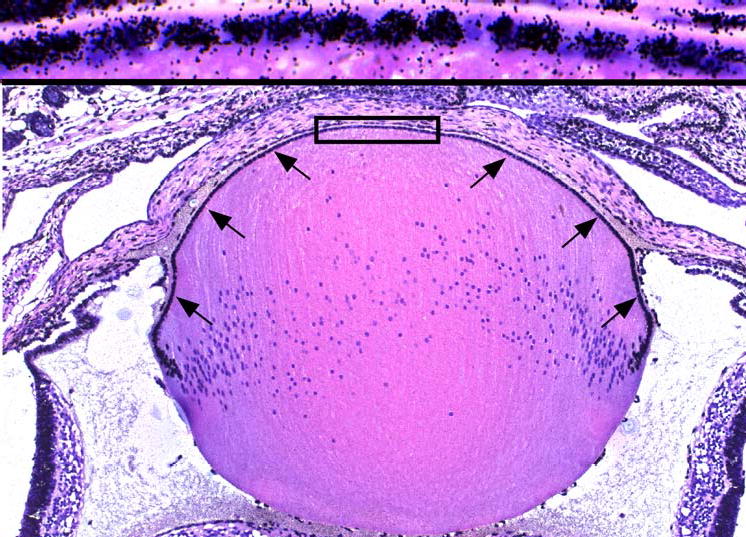
In utero/post-natal delivery of tritiated thymidine (3H-Tdr) labels all proliferating lens epithelial cells. A paraffin section of the eye of a 3-day-old mouse that had received 3H-Tdr twice daily in utero from day 17 gestation until birth, and then injected subcutaneously with 3H-Tdr twice daily for three days; note the uniform labeling of all lens epithelial cells (arrows). Area with in the rectangle shows the uniformly heavily labeled, cuboidal-shaped lens epithelial cells at a high magnification.
Figure 3.
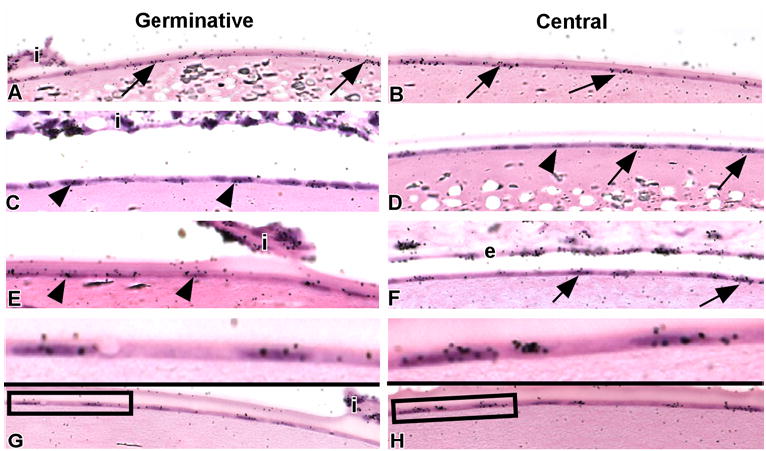
The temporal distribution of heavily-labeled and lightly-labeled cells within the central and germinative zones of the lens epithelium. (A) Heavily-labeled (> 8 silver grains/nucleus) cells were counted in the germinative and central regions and expressed as a percentage of cells within each region. A marked decrease is seen in the heavily-labeled cells in both regions over time. Note that by 12 weeks heavily-labeled cells are exclusively located in the central zone. (B) Lightly-labeled (3-8 silver grains/nucleus) cells were counted in the germinative and central regions and expressed as a percentage of cells within each region. A marked increase in the percentage of lightly-labeled cells was noted in both zones at the 2-week interval, reflecting the active proliferation and dilution of label within the heavily-labeled cells (A) during this period of lens development. The percentage of lightly-labeled cells in both zones continues to gradually decline with time. Interestingly, more lightly-labeled cells are present in the central versus the germinative zones at the later time periods.
Figure 6.
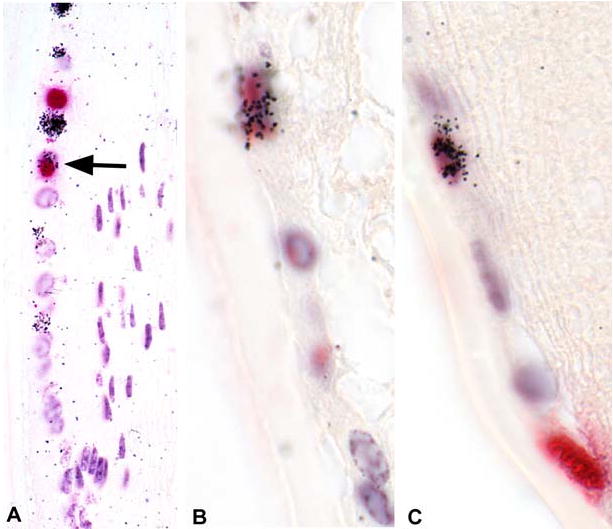
Wounding shortens the cell cycle time of actively cycling lens epithelial cells in the germinative zone. Twenty-four hours after lens scrape wounds were created, mice received a pulse of BrdU intraperitoneally. Twenty-four hours later, these mice received another pulse of 3H-TdR, were sacrificed 1 hour later, and eyes were processed for BrdU immunohistochemistry and autoradiography. Within the germinative zone (A-C), actively cycling (TA) cells are detected as BrdU stained (red) or cells with silver grains in the nuclei. Occasionally double-labeled cells (arrow in panel A; cells in panels B,C) are detected, indicating that a TA cell has undergone two divisions with 24-hours.
Following a 2-week chase, a significant reduction in overall labeling was noted within the lens epithelium (Table 1), typified by the reduction in heavily-labeled cells (Fig. 4A). This reduction is due, in part, to dilution of the label due to the rapidly proliferating nature of the epithelial cells in all regions of the developing lens 31. In addition, many of the labeled, lens epithelial cells transit out of the epithelium as they differentiate into fiber cells to meet the needs of the rapidly growing lens 2,3. Such dilution of the label, accounts for the increase noted in the number of lightly-labeled LRCs between the first and second week in both central and germinative zones (Fig. 4B). After a 6 week chase (Table 1, Figs. 3C, D, 4), ~27% of the lens epithelial cells still retained some level of 3H-TdR; however, only 5% of these LRCs were heavily labeled (Figs. 3D, 4A). The number of 3H-TdR-labeled lens epithelial cells continued to decline over time (Table 1); after an 18.5 week chase, only 13% of the lens epithelial cells retained the label (Figs. 3G,H, 4A,B). At this time the heaviest labeled cells were exclusively found in the central zone (Table 1, Fig. 3H, 4A) and represented 2–3% of the total LRCs. Interestingly, most of the lightly-labeled LRCs were observed in the central region (Table 1, Figs. 3D,F, Fig 4B), although some lightly-labeled LRCs were also detected in the germinative zone (Figs. 3G, 4B). The temporal distribution of the LRCs indicates that a hierarchy of proliferation exists in the lens epithelium with: (i) the slowest cycling (heavily-labeled LRCs) cells located exclusively in the central zone; (ii) their progeny, the lightly-labeled LRCs present in the central and germinative zones; and (iii) the actively cycling (non-LRCs) cells primarily located in the germinative zone.
Table 1.
Temporal Distribution of Heavily- and Lightly-labeled Cells (LRCs) Within the Lens Epithelium
|
Heavily - labeled Cells Mean ± SD |
Lightly - labeled Cells Mean ± SD |
|||
|---|---|---|---|---|
| Weeks* | Central | Germinative | Central | Germinative |
| 1 | 120±20 | 90±8 | 14±6 | 9±2 |
| 2 | 23±8 | 31±12 | 51±5 | 42±7 |
| 3 | 22±11 | 11±5 | 41±5 | 48±9 |
| 4 | 7±1 | 5±3 | 35±4 | 40±5 |
| 5 | 4±2 | 4±2 | 27±5 | 26±8 |
| 6 | 8±4 | 4±3 | 29±6 | 21±6 |
| 9 | 8±4 | 6±4 | 24±3 | 30±6 |
| 12 | 5±2 | 0±0 | 18±3 | 2±2 |
| 18.5 | 6±3 | 0±0 | 17±4 | 4±1 |
Time after the last injection of 3H-Tdr into neonatal mice that were labeled in utero. This is the “chase” period.
Figure 4.
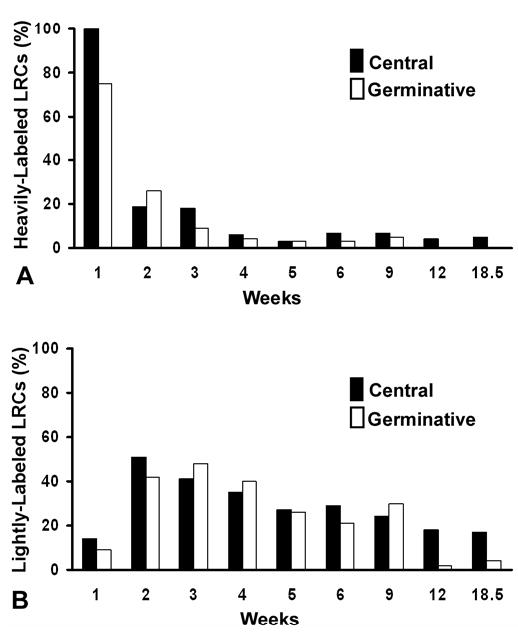
The lens epithelium is heterogeneous with respect to the distribution of LRCs. Mice labeled with an in utero/post-natal protocol were sacrificed after 3 (A,B), 6 (C,D), 12 (E,F), and 18.5 (G,H) weeks. Paraffin sections were processed for autoradiography. At the 3-week interval (A,B), heavily labeled LRCs (arrows) are noted in both germinative (A) and central zones (B) of the lens epithelium. At the 6-week interval (C,D), both heavy- (arrows) and lightly-labeled (arrowheads) LRCs were noted in the central zone (D), whereas lightly-labeled LRCs predominated in the germinative (C) zone. At the 12- (E,F) and 18.5- (G,H) week intervals, heavily-labeled LRCs (arrows) were exclusively found in the central zone (F,G); lightly-labeled LRCs (arrowheads) were routinely observed in the germinative zone (E,G) at these times. Areas within the rectangles show lightly-labeled (G) and heavily-labeled LRCs (H) at high magnification. i, portion of iris for orientation; e, portion of corneal endothelium adjacent to the lens.
Perturbation Stimulates LRCs in the Central Region to Proliferate
One of the characteristics of LRCs is that they can be induced to proliferate following perturbation 14,19. To ascertain whether the heavily-labeled LRCs in the central region of the lens were healthy and could proliferate, we wounded the lens epithelium of groups of mice (n=4) that were continuously labeled with 3H-TdR and then chased for 18. 5 weeks. Twenty-four hours post-wounding, we administered a pulse of BrdU to detect the actively proliferating cells and sacrificed the mice 1 hour later. After wounding, there was a 4- to 5-fold increase in the number of lens epithelial cells that incorporated BrdU (~2-3 cells in non-wounded lens versus ~ 8–10 cells in wounded lens; compare Figs. 5A,B with 5E,F). Many of the BrdU-labeled cells were located in the central zone (Figs. 5A-C), which is the region where the heavily-labeled LRCs were concentrated. We also observed an occasional 3H-TdR-labeled cell that incorporated BrdU, and hence was double-labeled (Fig. 5D). This indicated that centrally-located lens LRCs could be recruited to proliferate in response to a wound and thus were healthy.
Figure 5.
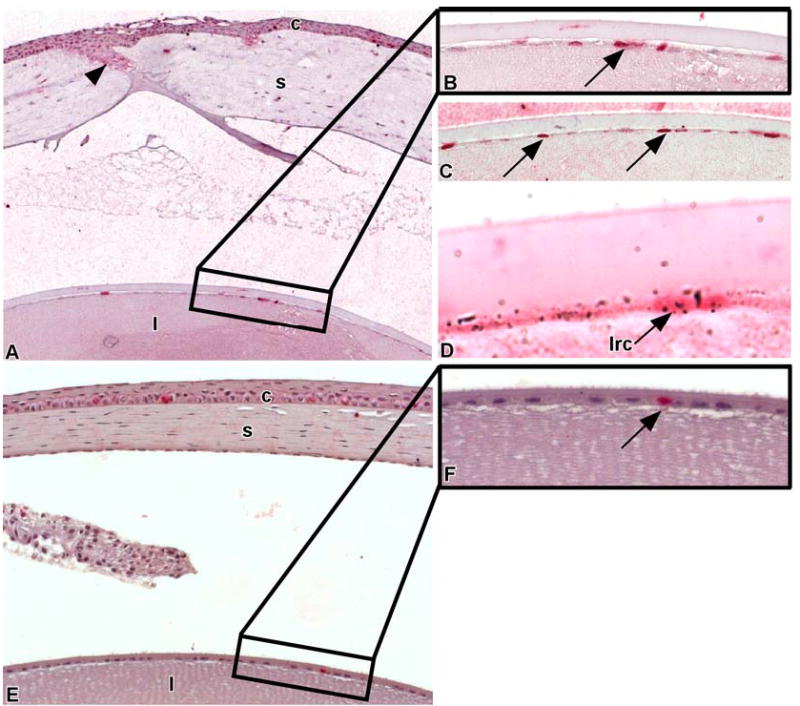
Wounding stimulates the central lens epithelial cells to proliferate. Scrape wounds were created on the lenses of mice that were labeled with an in utero/post-natal protocol and chased for 18.5 weeks. Twenty-four hours post-wounding, BrdU was injected intraperitoneally to tag proliferating cells. These mice were sacrificed 24 hours later (A) and paraffin sections were processed for BrdU immunohistochemistry and autoradiography. Arrowheads demarcate wound area. Note the increased number of BrdU labeled cells in corneal (c) and lens (l) epithelia. A portion of the central zone of the lens epithelium (area in the rectangle) containing BrdU labeled cells (arrow) is shown in higher magnification (B). (C) Paraffin section of a portion of the central zone of the lens epithelium from another mouse 24-hrs post-wounding showing numerous flattened lens epithelial cells stained with BrdU (arrows). (D) A central lens epithelial cell is double-labeled, containing both 3H-TdR and BrdU. This indicates that LRCs (lrc) are healthy and can divide following wounding. (E) A portion of a non-wounded, resting cornea (c) and lens (l) that were labeled with a single intraperitioneal injection of BrdU and sacrificed 24 hours later. A portion of the central zone of the lens epithelium (area in the rectangle) containing a single BrdU-labeled cell (arrow) is shown in higher magnification (F).
Wounding Shortens the Cell Cycle Time of Lens Epithelial Cells in the Germinative Zone
To ascertain whether the cells in the germinative zone cycled faster following perturbation, we used a double-labeling protocol where mice were wounded and 24 hours later, received a single pulse of BrdU. After an additional 24 hour chase, these mice received a single pulse of 3H-TdR and were sacrificed 1 hr later. Incorporation of either of these nucleosides occurs in cells that are actively cycling (e.g., TA cells). After wounding most of the cells incorporated either BrdU or 3H-TdR (Fig. 6A-C); however, some cells in the germinative region had nuclei that contained BrdU (red stain) as well as silver grains (3H-TdR) and hence were double-labeled (Fig. 6A-C). It has been estimated that the mitotically active population consists largely of cells that cycle once every 17–20 days 28. Thus for double-labeling to occur, the BrdU-labeled cells had to traverse the cell cycle within 24 hours in order to incorporate the 3H-TdR. The presence of such cells in the germinative zone indicates that these TA cells can indeed shorten their cell cycle time and also have the capacity for multiple cell divisions.
DISCUSSION
Kinetic Heterogeneity and Lens Epithelial Stem Cells
We and others have established that the detection of LRCs is one of the most reliable means of identifying stem cells 12–18. Due to the extremely low frequency of division for the lens epithelial cells, many cells retain their label and hence fall into the category of LRCs. Nonetheless, we were able to demonstrate that a hierarchy of cell proliferation exists within the lens epithelium with the slowest cycling cells, detected as the heavily-labeled LRCs, located exclusively in the central region. We propose that these cells are putative lens epithelial stem cells that divide very infrequently during homeostasis. However, upon perturbation, these cells can enter the proliferative pool and provide progeny that will supply the central and germinative zones with cells capable of further division capacity (the lightly-labeled LRCs). The relatively small (2-3%) number of heavily-labeled LRCs in the adult lens epithelium is consistent with the idea that stem cells comprise a small subpopulation of the tissue 33. In an earlier study, a small percentage (~1.2%) of adult mouse lens epithelial cells were observed to be labeled 20 days after a receiving a single pulse of 3H-TdR, and these cells were deemed to cycle slowly 28. The central zone contained the fewest number (~0.1%) of labeled cells, whereas the outer periphery (germinative zone) contained the largest number (~1.1%) of labeled cells. This distribution of labeled lens epithelial cells reflects the general labeling pattern seen after administration of a single pulse of nucleoside to an adult mouse; the number of centrally labeled cells is most likely and underestimation as: (i) only a single pulse of 3H-TdR was given and thus only those actively cycling cells were labeled; and (ii) the percentage of actively cycling lens epithelial cells in an 8-12 week old mouse is extremely low 28,31.
The lightly-labeled LRCs, located in the central and germinative zones divide more frequently than the heavily-labeled LRCs; however, they too possess proliferative capacity. These lightly labeled cells are: (i) the immediate progeny of the putative stem cells; (ii) may be phenotypically indistinguishable from stem cells 9; and (iii) are analogous to the “young” TA cells located at the peripheral corneal epithelium 19, and in the infundibulum of the hair follicle 10,34. Finally, a third population of more actively cycling cells exists primarily in the germinative zone and represents the transit amplifying (TA) cells. Following their last division, these TA cells differentiate into the lens fiber cells. Therefore we propose a model of “stem cell (heavily- labeled LRC) → stem cell/TA cell (lightly-labeled LRC) → TA cells → post-mitotic cell (lens fiber cell)” for the lens epithelium as contrasted with the scheme of “stem cell → TA cell → post-mitotic cell”, which has been proposed as the model for many self-renewing systems 10,11.
The recent finding that lens epithelial cells within the central and germinative regions have the same amount of telomerase activity 23, is consistent with the idea that cells within these zones have considerable proliferative capacity. Telomerase synthesizes and maintains telomeres, the DNA units located at the ends of chromosomes that protect the chromosome from premature loss of genes due to cell division 35,36. Telomerase activity is associated with stem cells (for reviews see 37,38), as well as actively dividing cells 39. It has recently been reported that when hTERT, the catalytic subunit of telomerase, was overexpressed in human limbal and corneal keratinocytes, the lifespan of the limbal keratinocytes was extended, whereas such overexpression had no effect on the corneal keratinocytes 40. This suggests that telomerase activity is associated with cells invested with high proliferative capacity (e.g., stem cells and “young” TA cells), but not with more mature TA cells 40. Since heavily-labeled LRCs (putative stem cells) are in the central zone and lightly-labeled LRCs (putative stem/TA cells) are in both the central and germinative zones of the lens, it is not surprising that the same amount of telomerase activity was found in these regions 23.
In addition to the kinetic heterogeneity that we observed in the lens epithelium, there have been reports of heterogeneity with respect to the presence of tyrosine-kinase and G-protein coupled receptor systems on lens epithelial cells 24. In preparations of whole human lens, the G-protein coupled agonist acetylcholine, only elicited large changes in cytosolic Ca+2 in the central epithelium but not in the equatorial region. In contrast, the tyrosine-kinase-linked growth factors EGF, TGFα and PDGF-AG produced large responses in the equatorial cells but did not elicit responses in the central cells 24. Similarly, total and active Src family kinases were higher at the equator than the central region of the lens epithelium 26. Inhibition of Src kinase activity resulted in a decrease in the proliferative status of the lens epithelium 26,41. This preferential distribution of tyrosine-kinases and receptors to the peripheral region has been postulated to be related to the active proliferation and differentiation that takes place in this zone. It does not however, explain the proliferative activity that is detected in lens epithelial cells of the resting central region or the perturbation-induced proliferative responses observed in the central lens epithelial cells 42.
Strategies of Epithelial Repair in the Lens
Our finding that the LRCs in the central zone of the lens epithelium enter the proliferative pool following wounding, confirms and extends earlier studies demonstrating that mechanical wounding initiates a burst of proliferation in the normally quiescent cells of the central zone of the lens epithelium 42. The recruitment of slow-cycling (LRCs) cells into the proliferative pool in response to external stimuli is one of the properties that have been ascribed to stem cells (for reviews see 8,11,33). For example, 24 hours following a central corneal epithelial scrape wound or the topical application of phorbol myristate to the anterior surface to mice, approximately 50% of the LRCs in the limbal epithelium were stimulated to divide as evidenced by their incorporation of a pulse of 3H-TdR 19. Likewise, following stimulation by the topical application of phorbol myristate, LRCs located in the proximal ductal epithelium of the meibomian gland showed a “red-speckled” pattern, which resulted from the dilution of BrdU due to cell division. In addition, some LRCs were observed to have incorporated a pulse of 3H-TdR, thereby further demonstrating the ability of these LRCs to divide 20. In the hair follicle, the normally slow-cycling cells in the bulge have been shown to divide in response to signals that initiate the hair growth cycle 34.
Our demonstration that some the actively proliferating cells in the germinative zone of the lens can be double-labeled following perturbation indicates that these cells shorten their cell cycle time after perturbation. Such double-labeling following wounding has been observed in the TA populations of the peripheral corneal epithelium 19, and represents a second means by which epithelia respond to the requirement of more cells. A further implication of the finding that cells in the germinative zone of the lens can be double-labeled is that these cells are capable of multiple rounds of DNA synthesis before differentiating into the lens fiber cells 2,3,28,32.
Is There a Stem Cell Niche in the Lens?
The preferential location of slow-cycling cells in the lens epithelium is consistent with the situation in other tissues where a non-random distribution of slow-cycling cells (LRCs) has been observed 14–19,34,43. Such a non-random distribution of LRCs has been attributed to the presence of a stem cell niche, or specialized microenvironment that maintains and/or protects stem cells (reviewed in 44,45). The lens epithelial cells rest on lens fiber cells and it is not evident whether differences exist in the biochemistry and/or molecular biology of the underlying fiber cells of the central versus germinative regions. However, the central epithelial cells do not appear to be functionally coupled to the underlying fiber cells, nor do they have typical gap junction structures on the apical membranes facing the fibers 46,47. In contrast, the germinative cells do have junctional complexes and appear to have some coupling to the fiber cells 47,48. It is not clear whether this type of coupling is related to the presence of the heavily- versus lightly-labeled LRCs. Evidence is also lacking whether regional differences or gradients exist in the composition of the aqueous humor that bathes the surface of the lens epithelial cells. Interestingly, a gradient in the nuclear and cytoplasmic expression of lens epithelium derived growth factor (LEDGF) has been noted in the rat lens. Epithelial cells in the central region displayed the highest nuclear staining expression followed by the cells in the germinative region; the cells of the equatorial bow region had the lowest expression of LEDGF 49. Message for LEDGF was also detected in the flattened cells of the anterior (central) region as well as in the cells of the germinative zone 49. LEDGF has been shown to be survival factor for mouse lens epithelial cells 50,51, thus it makes good biological sense that this factor is preferentially expressed in the region of the lens with the slowest cycling (putative stem cells) cells and perhaps this factor is involved in maintaining the “stemness” of these cells.
Acknowledgments
Supported by National Eye Institute Grant EY06769 (RML).
References
- 1.Beebe DC. The control of lens growth: relationship to secondary cataract. Acta Ophthalmologica - Supplementum 1992:53–7. [DOI] [PubMed]
- 2.Zelenka PS, Gao C-Y, Rampalli A, et al. Cell cycle regulation in the lens: proliferation, quiescence, apoptosis and differentiation. Prog in Ret and Eye Res. 1997;16:303–322. [Google Scholar]
- 3.Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–53. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen MM, Potter SJ, Griep AE. Deregulated cell cycle control in lens epithelial cells by expression of inhibitors of tumor suppressor function. Mechanisms of Development. 2002;112:101–13. doi: 10.1016/s0925-4773(01)00644-x. [DOI] [PubMed] [Google Scholar]
- 5.Ishizaki Y, Voyvodic JT, Burne JF, et al. Control of lens epithelial cell survival. Journal of Cell Biology. 1993;121:899–908. doi: 10.1083/jcb.121.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhat SP. The ocular lens epithelium. Bioscience Reports. 2001;21:537–63. doi: 10.1023/a:1017952128502. [DOI] [PubMed] [Google Scholar]
- 7.Alonso L, Fuchs E. Stem cells of the skin epithelium. Proc Nat Acad Sci. 2003;100 (Suppl 1):11830–5. doi: 10.1073/pnas.1734203100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavker RM, Tseng SCG, Sun T-T. Corneal epithelial stem cells at the limbus:looking at some old problems from a new angle. . Exp Eye Res. 2004;78:433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, ptfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 10.Lavker RM, Sun T-T. Epidermal stem cells: properties, markers, and location. Proc Nat Acad Sci USA. 2000;97:13473–13475. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potten CS, Booth C. Keratinocyte stem cells: a commentary. J Invest Dermatol. 2002;119:888–99. doi: 10.1046/j.1523-1747.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- 12.Bickenbach JR. Identification and behavior of label-retaining cells in oral mucosa and skin. J Dent Res. 1981;60:1611–1620. doi: 10.1177/002203458106000311011. [DOI] [PubMed] [Google Scholar]
- 13.Braun KM, Niemann C, Jensen UB, et al. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241–55. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 14.Cotsarelis G, Cheng SZ, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 15.Cotsarelis G, Sun T-T, Lavker RM. Label-retaining cells reside in the bulge of the pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 16.Morris RJ, Potten CS. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J Invest Dermatol. 1999;112:470–475. doi: 10.1046/j.1523-1747.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- 17.Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei ZG, Cotsarelis G, Sun T-T, et al. Label-retaining cells are preferentially located in fornical epithelium: implications on conjunctival epithelial homeostasis. Invest Ophthalmol Vis Sci. 1995;36:236–46. [PubMed] [Google Scholar]
- 19.Lehrer MS, Sun T-T, Lavker RM. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. . J Cell Sci. 1998;111:2867–2875. doi: 10.1242/jcs.111.19.2867. [DOI] [PubMed] [Google Scholar]
- 20.Lavker RM, Treet J, Sun T-T. Label-retaining cells (LRCs) are preferentially located in the ductal epithelium of the meibomian gland: implications on the mucocutaneous junctional (mcj) epithelium of the eyelid. Invest Ophthalmol Vis Sci 2003;44:E-3781.
- 21.Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220:197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- 22.Otteson DC, D'Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232:62–76. doi: 10.1006/dbio.2001.0163. [DOI] [PubMed] [Google Scholar]
- 23.Colitz CM, Davidson MG, Mc GM. Telomerase activity in lens epithelial cells of normal and cataractous lenses. Exp Eye Res. 1999;69:641–9. doi: 10.1006/exer.1999.0739. [DOI] [PubMed] [Google Scholar]
- 24.Collison DJ, Duncan G. Regional differences in functional receptor distribution and calcium mobilization in the intact human lens. Invest Ophthalmol & Vis Sci. 2001;42:2355–63. [PubMed] [Google Scholar]
- 25.Nguyen MM, Nguyen ML, Caruana G, et al. Requirement of PDZ-containing proteins for cell cycle regulation and differentiation in the mouse lens epithelium. Mol Cell Biol. 2003;23:8970–81. doi: 10.1128/MCB.23.24.8970-8981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamiya S, Delamere NA. Studies of tyrosine phosphorylation and Src family tyrosine kinases in the lens epithelium. Invest Ophthalmol Vis Sci. 2005;46:2076–81. doi: 10.1167/iovs.04-1199. [DOI] [PubMed] [Google Scholar]
- 27.McAvoy JW. Cell division, cell elongation and the co-ordination of crystallin gene expression during lens morphogenesis in the rat. Journal of Embryology & Experimental Morphology. 1978;45:271–81. [PubMed] [Google Scholar]
- 28.Rafferty NS, Rafferty KA., Jr Cell population kinetics of the mouse lens epithelium. Journal of Cellular Physiology. 1981;107:309–15. doi: 10.1002/jcp.1041070302. [DOI] [PubMed] [Google Scholar]
- 29.Kriss J, Reuesz The distribution and fate of bromodeoxyuridine and bromodeoxycitidine in the mouse and rat. Can Res. 1962;22:254–265. [PubMed] [Google Scholar]
- 30.Maenza RM, Harding CV. Disappearance of tritiated thymidine and tritiated water from the anterior chamber of the rabbit eye. Nature. 1962;196:786–787. [Google Scholar]
- 31.Tseng H, Matsuzaki K, Lavker RM. Basonuclin in murine corneal and lens epithelia correlates with cellular maturation and proliferative ability. Differentiation. 1999;65:221–227. doi: 10.1046/j.1432-0436.1999.6540221.x. [DOI] [PubMed] [Google Scholar]
- 32.Mukulicih AG, Young RW. Cell proliferation and displacement in the lens epithelium of young rats injected with tritiated thymidine. Invest Ophthalmol Vis Sci. 1963;2:344–354. [PubMed] [Google Scholar]
- 33.Miller SJ, Lavker RM, Sun T-T. Keratinocyte stem cells of cornea, skin, and hair follicle: common and distinguishing features. Dev Biol. 1993;4:217–240. [Google Scholar]
- 34.Taylor G, Lehrer MS, Jensen PJ, et al. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 35.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–73. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 36.Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521–9. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- 37.Harrington L. Does the reservoir for self-renewal stem from the ends? Oncogene. 2004;23:7283–9. doi: 10.1038/sj.onc.1207948. [DOI] [PubMed] [Google Scholar]
- 38.Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, and cancer. J Clin Invest. 2004;113:160–8. doi: 10.1172/JCI20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bickenbach JR, Vormwald-Dogan V, Bachor C, et al. Telomerase is not an epidermal stem cell marker and is downregulated by calcium. J Invest Dermatol. 1998;111:1045–52. doi: 10.1046/j.1523-1747.1998.00420.x. [DOI] [PubMed] [Google Scholar]
- 40.Pellegrini G, Dellambra E, Paterna P, et al. Telomerase activity is sufficient to bypass replicative senescence in human limbal and conjunctival but not corneal keratinocytes. Eur J Cell Biol. 2004;83:691–700. doi: 10.1078/0171-9335-00424. [DOI] [PubMed] [Google Scholar]
- 41.Walker JL, Zhang L, Menko AS. Transition between proliferation and differentiation for lens epithelial cells is regulated by Src family kinases. Developmental Dynamics. 2002;224:361–72. doi: 10.1002/dvdy.10115. [DOI] [PubMed] [Google Scholar]
- 42.Miller RC, Riley EF. Retention of labelled DNA precursor by murine cells after a single administration of tritium labelled thymidine. Cell Tissue Kinet. 1986;19:519–25. doi: 10.1111/j.1365-2184.1986.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 43.Morris RJ, Fischer SM, Slaga TJ. Evidence that the centrally and peripherally located cells in the murine epidermal proliferative unit are two distinct cell populations. J Invest Dermatol. 1985;84:277–281. doi: 10.1111/1523-1747.ep12265358. [DOI] [PubMed] [Google Scholar]
- 44.Fuchs E, Tumbar T, Guasch G. Socializing with their neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 45.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–30. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 46.Bassnett S, Kuszak JR, Reinisch L, et al. Intercellular communication between epithelial and fiber cells of the eye lens. J Cell Sci. 1994;107:799–811. doi: 10.1242/jcs.107.4.799. [DOI] [PubMed] [Google Scholar]
- 47.Prescott A, Duncan G, Van Marle J, et al. A correlated study of metabolic cell communication and gap junction distribution in the adult frog lens. Exp Eye Res. 1994;58:737–46. doi: 10.1006/exer.1994.1071. [DOI] [PubMed] [Google Scholar]
- 48.Rae JL, Bartling C, Rae J, et al. Dye transfer between cells of the lens. J Memb Biol. 1996;150:89–103. doi: 10.1007/s002329900033. [DOI] [PubMed] [Google Scholar]
- 49.Kubo E, Singh DP, Fatma N, et al. Cellular distribution of lens epithelium-derived growth factor (LEDGF) in the rat eye: loss of LEDGF from nuclei of differentiating cells. Histochem Cell Biol. 2003;119:289–99. doi: 10.1007/s00418-003-0518-3. [DOI] [PubMed] [Google Scholar]
- 50.Singh DP, Ohguro N, Chylack LT, Jr, et al. Lens epithelium-derived growth factor: increased resistance to thermal and oxidative stresses. Invest Ophthalmol Vis Sci. 1999;40:1444–51. [PubMed] [Google Scholar]
- 51.Singh DP, Ohguro N, Kikuchi T, et al. Lens epithelium-derived growth factor: effects on growth and survival of lens epithelial cells, keratinocytes, and fibroblasts. Biochem Biophys Res Comm. 2000;267:373–81. doi: 10.1006/bbrc.1999.1979. [DOI] [PubMed] [Google Scholar]


