Abstract
Kinesin is a double-headed motor protein that moves along microtubules in 8-nanometer steps. Two broad classes of model have been invoked to explain kinesin movement: hand-over-hand and inchworm. In hand-over-hand models, the heads exchange leading and trailing roles with every step, whereas no such exchange is postulated for inchworm models, where one head always leads. By measuring the stepwise motion of individual enzymes, we find that some kinesin molecules exhibit a marked alternation in the dwell times between sequential steps, causing these motors to “limp” along the microtubule. Limping implies that kinesin molecules strictly alternate between two different conformations as they step, indicative of an asymmetric, hand-over-hand mechanism.
Results from a variety of single-molecule experiments have furnished insights into the mechanochemical properties of kinesin motor proteins. Individual kinesin dimers move processively, making discrete 8-nm steps at stochastic intervals, and may take a hundred or more steps before releasing from the microtubule surface. Processive motion persists even in the presence of sustained external loads up to several pN (1–3), suggesting that some portion of the kinesin dimer remains bound to the microtubule at all times. Kinesin molecules move on the microtubule surface lattice along paths parallel to the protofilaments (4, 5), interacting with one binding site per tubulin dimer (6). Finally, kinesin moves in such a way as to hydrolyze exactly one adenosine triphosphate (ATP) molecule per 8-nm step (1, 7, 8). Two broad classes of stepping pattern are consistent with the foregoing observations: hand-over-hand models, in which the two heads step alternately, exchanging leading and trailing roles with each step, and inchworm models, in which a given head remains in the lead (9, 10).
The active portion of the kinesin motor is formed from a dimer of identical heavy chains, which fold into twin heads attached to a single common stalk (11). The two globular heads, which carry enzymatic activity and bind ATP and microtubules, are joined to the stalk through short (~13 amino acids) neck linker regions, consisting of single polypeptide chains (12). The heavy chains then intertwine to form a coiled-coil dimerization domain consisting of classic heptad repeats (11). On the basis of this structure, free rotation (i.e., swiveling) of an individual head could occur, in principle, about the neck linker, but head motions would be summed mechanically at the point where the heavy chains merge into a common stalk, precluding further independent rotation beyond that point.
A recent study by Gelles and co-workers (10) supplied compelling evidence that significant rotation of the kinesin stalk does not occur during stepping. A large rotation, up to 180°, should be imparted to the stalk in a class of hand-over-hand models termed “symmetric.” This class is symmetric in the sense that “the three-dimensional structure of the kinesin-microtubule complex is identical at the beginning and end of each ATP hydrolytic cycle, except that…the two subunits (and therefore the two heads) swap places” (10). An example of a symmetric hand-over-hand model with this property is one in which the dimer rotates about an axis perpendicular to the microtubule by half a revolution per step (13). On the basis of their negative findings, Hua et al. (10) ruled out the so-called symmetric hand-over-hand model, and instead proposed an inchworm mechanism wherein only one of the kinesin heads is active in hydrolyzing ATP, to account for the observation of one ATP hydrolysis per 8-nm step (1, 7, 8). However, the possibility of an asymmetric hand-over-hand motion was not formally excluded, provided that compensatory movements of the head and neck linker domains during stepping conspire to suppress the overall rotation of the common stalk. One such asymmetric mechanism has been proposed, for example, in which the kinesin-microtubule complex alternates between two conformations and the stepping motions of the two heads are distinctly different (14).
To discriminate among candidate models, we measured the stepwise motion of individual native and recombinant kinesin homodimers at high spatiotemporal resolution, using an optical force-clamp apparatus (2, 3, 15, 16). We found that the intrinsic stepping rates can alternate between two different values at each sequential step, causing kinesin molecules to “limp.” The strict kinetic alternation in stepping implies an alternation between two underlying molecular configurations, thereby excluding fully symmetric models, such as the inchworm and symmetric hand-over-hand mechanisms. Therefore, the discovery that kinesin limps, taken together with its other nanomechanical properties, implies that it advances by some form of asymmetric hand-over-hand mechanism. Recent work has established that an actin-based motor, myosin V, also moves hand-over-hand (although it is not yet known whether the motion is symmetric or asymmetric), raising the possibility that the mechanism may be common to dimeric, processive mechanoenzymes (17).
In our assays (18), we attached single kinesin molecules to microscopic beads (0.5 μm diameter) that served as markers for position and as handles to apply external forces (1–3, 15, 16, 19, 20). Using an optical trap, we captured individual freely diffusing beads carrying kinesin motors and placed them near microtubules immobilized on a cover glass, whereupon they bound and moved. Subsequent processive motion was tracked with nanometer-level precision. We used a feedback-controlled force clamp to apply a constant rearward load during motion, at a level below the kinesin stall force (~6 pN) (20 –22), an arrangement that greatly reduces Brownian fluctuations and improves spatiotemporal resolution (2, 3). The force clamp also allows comparatively long displacements to be scored (up to ~40 steps), facilitating statistical analysis. We studied the motions of His- and biotin-tagged recombinant kinesin derivatives of various lengths from fruit fly and human expressed in Escherichia coli, as well as native kinesin purified from squid.
A truncated derivative of Drosophila melanogaster kinesin (DmK401) containing two identical heads and a sufficient length of neck coiled-coil for dimerization (23) exhibited an obvious limp, alternating between slow and fast dwell times (where dwell times are defined as the time intervals between successive 8-nm advances). Characteristically, limping manifested itself as a tendency for steps in position recordings to occur in discrete pairs (Fig. 1A), even though individual dwell times are governed by a stochastic process and therefore show variability. Put somewhat differently, when the dwell times in any given record were numbered sequentially, the longer-lived dwell times tended to cluster systematically in either the even-or the odd-numbered subset. This clustering was not an experimental artifact, because limping was almost never observed with native kinesin protein purified from Loligo pealei (LpK) and studied under the same conditions (Fig. 1B).
Fig. 1.
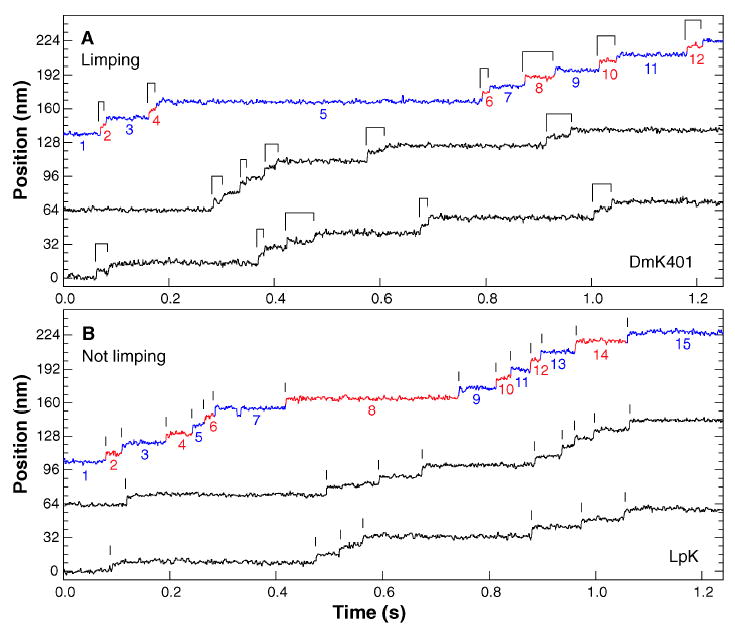
Representative high-resolution stepping records of position against time, showing the single-molecule behavior of kinesin motors under constant 4 pN rearward loads. (A) Limping motion of the recombinant kinesin construct, DmK401. The dwell intervals between successive 8-nm steps alternate between slow and fast phases, causing steps to appear in pairs, as indicated by the ligatures. (B) Nonlimping motion of native squid kinesin, LpK. No alternation of steps is apparent; vertical lines mark the stepping transitions. Slow and fast phase assignments, as described in the text, are indicated in color on the uppermost trace of each panel (blue and red, respectively), and the corresponding dwell intervals are numbered. All traces were median filtered with a 2.5-ms window.
Statistical analysis showed that the average dwell times for the slow and fast phases of DmK401 stepping were significantly different. Because the starting phase in any given record is not known a priori, the dwell intervals were assigned to either a “slow” or a “fast” phase by comparing the mean duration of the even-numbered subset of intervals to the mean duration of the odd-numbered subset for each record. All intervals in the subset with the greater mean were assigned to the slow phase; conversely, those from the subset with the lesser mean were assigned to the fast phase. Having made such an assignment for every record, we could then combine data from an ensemble of single molecules to generate global distributions for the fast and slow phases (Fig. 2A). The durations of both the slow and fast dwell times for DmK401 were distributed nearly exponentially (24) but with characteristic lifetimes that differed nearly sixfold, τslow = 136 ± 6 ms and τfast = 24 ± 1 ms. This disparity was also reflected in the limp factor, L, defined as the ratio of the mean duration of the slow phase to that of the fast phase in a given record (25). The distribution of L showed significant limping in most individual molecules but varied widely from bead to bead (and even from run to run): 63% of records had L > 4, and the average limp factor was = 6.45 ± 0.31 (Fig. 2B). Some individual DmK401 motors taking multiple runs generated consistently higher limp factors than did others, but the global distribution was broad and did not contain clearly separable populations of limping versus nonlimping molecules.
Fig. 2.

Stepping statistics for DmK401, LpK, and a computer simulation. (A) Experimental dwell time distributions for the slow (blue) and fast (red) phases of DmK401 motion, with statistical errors as indicated. Solid lines show single-exponential fits to the data, with time constants τslow = 136 ± 6 ms and τfast = 24 ± 1 ms. The first bin and bins with < 7 counts were not included in fits (these points are displayed without error bars); 2,948 intervals from 278 individual records are represented. (B) Experimental distribution of the limp factor, L, for individual records of DmK401 movement (black). (Inset) Histograms of the mean durations of the slow (blue) and fast (red) phases for individual records. (C) Same as in (A), but for LpK. The time constants are τslow = 90 ± 4 ms and τfast = 54 ± 2 ms. 2,523 dwells from 208 records are represented. (D) Same as in (B), but for LpK. Outliers with L > 6 (arrows) are caused by rare, limping LpK molecules. (E) Same as in (A), but for a computer simulation of an ideal Poisson stepper with a single characteristic stepping time (τ = 71 ms) that produced the time constants τslow = 87 ± 3 ms and τfast = 56 ± 2 ms. 2,230 dwells from 220 records are represented. (F) Same as in (B), but for the simulation.
In contrast to the clear-cut limping displayed by DmK401, limping was nearly absent from stepping records of native squid kinesin (Fig. 1B). The identical method of phase assignment was applied to generate global distributions for the slow and fast phase of stepping in LpK (Fig. 2C). The dwell times were again distributed nearly exponentially but with characteristic lifetimes, τslow = 90 ± 4 ms and τfast = 54 ± 2 ms, which differ far less than those of DmK401. Furthermore, the limp factors for LpK were distributed quite narrowly (compare Fig. 2, B and D), with just 8% of runs producing L > 4. The average limp factor was = 2.23 ± 0.14, which is only slightly higher than the value anticipated for a nonlimping stepper, estimated by computer simulations to be ~ 1.8.
We note that the systematic assignment of even or odd dwell intervals to either a slow or a fast phase introduces sampling bias for records of finite length, even when all the stepping times are drawn from the same statistical distribution. This bias occurs because the subset with the maximum sum is always selected for one phase and the minimum for the other. The sampling bias becomes negligible only in the limit of long runs containing a great many individual steps, or when the difference between the two phases is large compared with the stochastic variation in dwell times (as with DmK401). For shorter runs containing fewer steps on average, however, the bias can be estimated. Computer-simulated records for an ideal random stepper were analyzed, i.e., for a nonlimping Poisson process with dwell intervals drawn from a single exponential distribution (with lifetime τ = 71 ms) (26). For such a simulation, we obtained , = 1.79 ± 0.14, with τslow = 87 ± 3 ms and τfast = 56 ± 2 ms (Fig. 2, E and F), values quite similar to LpK.
We then tested a series of recombinant kinesin derivatives from Drosophila of increasing stalk length (Fig. 3A). As longer and longer sections of the coiled-coil stalk region were included in the expressed construct, the propensity to limp decreased monotonically (Fig. 3B). The largest construct tested, DmK871 (which includes 90% of the heavy chain), had a limp factor of 2.16 ± 0.17, statistically indistinguishable from native squid kinesin. Interestingly, the systematic increase in limp factor with shorter stalk length was attributable to an increase in the characteristic lifetime of the slow phase of stepping, whereas the timing of the fast phase appeared to remain invariant (Fig. 3C). This result suggests that the kinetics for one head alone are slowed in limping constructs, whereas the partner head retains normal enzymatic activity.
Fig. 3.

The effect of construct length on kinesin limping. (A) Cartoons showing the named structural features of kinesin dimers used in this work, with stalk lengths displayed approximately to scale and numbers of amino acids shown. (B) The mean limp factors, , for DmKHC constructs (black circles), full-length native LpK (open circle), and for a nonlimping Poisson stepper under conditions similar to our experiments (dashed lines, showing 63% confidence intervals), as a function of the construct length (number of amino acids). Points show the means and estimated errors [evaluated by bootstrapping (35)] for each distribution of not less than 141 limp factors. (C) The average durations of the slow (blue circles) and fast stepping phases (red triangles) as functions of the construct length for DmKHC derivatives (solid symbols) and native LpK (open symbols).
Limping was not restricted to recombinant Drosophila kinesin molecules. A bacterially expressed derivative of human kinesin with a C-terminal biotinylation tag (HsK413) (27) also limped. HsK413 produced a limp factor of 2.98 ± 0.25, significantly greater than that for a Poisson stepper or native squid kinesin but not as pronounced as the two comparably sized fruit fly constructs, DmK401 and DmK448 (Fig. 3B). On rare occasions (9 of 208 records), even selected squid kinesin molecules appeared to limp, producing outliers in Fig. 2D (arrows). Some of these outlier molecules limped consistently (28).
The fact that both native and bacterially expressed kinesin homodimers from several species can limp suggests that this behavior may reflect the common mechanism by which all processive kinesin molecules move. The alternation between short and long dwell times during limping directly reflects a corresponding alternation in the intrinsic rate for exiting the dwell state. This strict kinetic switching implies that the structural configuration of the kinesin-microtubule complex at the end of even-numbered dwell intervals differs from that of odd-numbered dwell intervals. Thus, the mechanism whereby kinesin advances must be asymmetric, in the sense that its underlying molecular conformations switch at every step. Symmetric mechanisms—by virtue of their symmetry—cannot account for switching. For example, inch-worm-type models will not limp without the ad hoc incorporation of additional (asymmetric) features, nor will symmetric hand-over-hand models of the type discussed by Gelles and co-workers (10).
The process by which kinesin motors advance is poorly understood in mechanistic detail, so it is not currently possible to assign limping to established structural features of its motion. However, some candidate mechanisms are immediately suggested by the molecular geometry of the kinesin dimer and its presumed relation to the microtubule during a hand-over-hand walk. Limping might be induced, in principle, in kinesin molecules with an axial misregistration between the α helices of their coiled-coil dimerization domains (29–31). Misregistration by a full heptad repeat would shift the globular heads relative to one another, effectively increasing the maximum neck linker length for one head by up to ~1 nm, i.e., from 3 to 4 nm. Such a shift could shorten the tether of the other head, generating an asymmetry. The head with the shorter tether would require additional time to find its forward microtubule binding site in a diffusional search (Fig. 4A), thereby slowing its kinetics relative to the partner head in situations where this step becomes rate-determining. The increased propensity of short constructs to limp, along with their large variation in limp factor, may be explained by their greater likelihood of axial misregistration, arising from the reduced thermodynamic stability of short coiled-coils.
Fig. 4.

Candidate models for limping in kinesin. (A) The misregistration model. Left (in register): The two-heads-bound state for native, non-limping kinesin, shown as it moves to the right along the microtubule lattice (tubulin α–β subunits, spaced pairwise by 8 nm, are indicated in gray and yellow). The coils of both heavy chains (blue and red ribbons) are in correct register. Middle and right (misregistered): A shift in registration of the coiled-coil by a single heptad changes the relative lengths of the head-neck linker regions by up to 1 nm. On alternate steps, this shift places one head (dark gray) farther from its binding site on the microtubule, reducing the stepping rate when this head attempts to take the lead (middle). When the partner head (light gray) steps, the shift is accommodated by slack in the longer tether (right). (B) The winding model. When one head (dark gray) steps forward, the neck coiled-coil (red and blue) is overwound relative to the relaxed state. When the partner head (light gray) leads, the coiled-coil is underwound. Asymmetry in torsional compliance slows the stepping associated with overwinding.
In an alternative picture, limping may be caused by an over- and underwinding of the coiled-coil region generated during hand-over-hand motion (Fig. 4B). Coiled-coils are thought to have an asymmetric torsional compliance because of their chirality (32); hence, the energetic barrier (along with the overall energetic cost) for winding the kinesin stalk from its relaxed, equilibrium configuration should differ with the handedness of rotation. In the event that such torsional barriers affect the rates of head advance, they could give rise to kinetic alternation. The relative difference in barrier height should be reduced with longer tethers, which are torsionally more compliant, perhaps accounting for the decrease in limping with increasing construct length (33).
None of the candidate mechanisms would seem to offer an immediate explanation for the experimental observation that only the time constant for the slow phase of limping is increased in shorter constructs, although such an effect may be incorporated with additional assumptions (34). Nevertheless, the mechanisms do suggest experimental approaches that should drive further single-molecule work to explore these possibilities. Regardless of the mechanism giving rise to it, however, the finding that kinesin molecules limp indicates that an asymmetric, hand-over-hand model supplies the correct description of kinesin stepping. Limping behavior may also complicate the interpretation of earlier biochemical kinetic experiments performed on populations of soluble kinesin dimers: Such molecules are generally derived from short, bacterially expressed constructs, in which the kinetic differences between the two heads are most pronounced.
Supplementary Material
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/1092985/DC1
Materials and Methods
Figs. S1 and S2
Table S1
References and Notes
References
- 1.Schnitzer MJ, Block SM. Nature. 1997;388:386. doi: 10.1038/41111. [DOI] [PubMed] [Google Scholar]
- 2.Visscher K, Schnitzer MJ, Block SM. Nature. 1999;400:184. doi: 10.1038/22146. [DOI] [PubMed] [Google Scholar]
- 3.Block SM, Asbury CL, Shaevitz JW, Lang MJ. Proc Natl Acad Sci USA. 2003;100:2351. doi: 10.1073/pnas.0436709100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelles J, Schnapp BJ, Sheetz MP. Nature. 1988;331:450. doi: 10.1038/331450a0. [DOI] [PubMed] [Google Scholar]
- 5.Ray S, Meyhofer E, Milligan RA, Howard J. J Cell Biol. 1993;121:1083. doi: 10.1083/jcb.121.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song YH, Mandelkow E. Proc Natl Acad Sci USA. 1993;90:1671. doi: 10.1073/pnas.90.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hua W, Young EC, Fleming ML, Gelles J. Nature. 1997;388:390. doi: 10.1038/41118. [DOI] [PubMed] [Google Scholar]
- 8.Coy DL, Wagenbach M, Howard J. J Biol Chem. 1999;274:3667. doi: 10.1074/jbc.274.6.3667. [DOI] [PubMed] [Google Scholar]
- 9.Svoboda K, Mitra PP, Block SM. Biophys J. 1995;68:69S. [PMC free article] [PubMed] [Google Scholar]
- 10.Hua W, Chung J, Gelles J. Science. 2002;295:844. doi: 10.1126/science.1063089. [DOI] [PubMed] [Google Scholar]
- 11.Vale RD, Fletterick RJ. Annu Rev Cell Dev Biol. 1997;13:745. doi: 10.1146/annurev.cellbio.13.1.745. [DOI] [PubMed] [Google Scholar]
- 12.Rice S, et al. Nature. 1999;402:778. doi: 10.1038/45483. [DOI] [PubMed] [Google Scholar]
- 13.Howard J. Annu Rev Physiol. 1996;58:703. doi: 10.1146/annurev.ph.58.030196.003415. [DOI] [PubMed] [Google Scholar]
- 14.Hoenger A, et al. J Mol Biol. 2000;297:1087. doi: 10.1006/jmbi.2000.3627. [DOI] [PubMed] [Google Scholar]
- 15.Lang MJ, Asbury CL, Shaevitz JW, Block SM. Biophys J. 2002;83:491. doi: 10.1016/S0006-3495(02)75185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visscher K, Block SM. Methods Enzymol. 1998;298:460. doi: 10.1016/s0076-6879(98)98040-5. [DOI] [PubMed] [Google Scholar]
- 17.Yildiz A, et al. Science. 2003;300:2061. doi: 10.1126/science.1084398. [DOI] [PubMed] [Google Scholar]
- 18.Materials and methods are available as supporting material on Science Online.
- 19.K. Svoboda, C. F. Schmidt, B. J. Schnapp, S. M. Block, Nature 365, 721 (1993). [DOI] [PubMed]
- 20.Svoboda K, Block SM. Cell. 1994;77:773. doi: 10.1016/0092-8674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 21.Meyhofer E, Howard J. Proc Natl Acad Sci USA. 1995;92:574. doi: 10.1073/pnas.92.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima H, Muto E, Higuchi H, Yanagida T. Biophys J. 1997;73:2012. doi: 10.1016/S0006-3495(97)78231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berliner E, Young EC, Anderson K, Mahtani HK, Gelles J. Nature. 1995;373:718. doi: 10.1038/373718a0. [DOI] [PubMed] [Google Scholar]
- 24.In the limit of very high loads approaching stall, or very low ATP concentrations, dwell intervals for kinesin are distributed exponentially because the stepping rate is dominated by a single rate-determining transition in the biochemical cycle (1–3). Under the conditions of the current work (4 pN rearward load and 2 mM ATP), the dwell interval distributions are expected to deviate slightly from pure exponentials (2, 3).
- 25.The limp factor, L, is a statistic derived from each individual record. The ratio of fast to slow phase lifetimes is an ensemble statistic derived from the combined data for many such records. The average limp factor therefore provides an alternative measure of the overall stepping asymmetry. Its expectation value is not identical to the global lifetime ratio because of the effect of finite run lengths.
- 26.The simulations contained runs with numbers of steps chosen to match closely those in our experiments (18).
- 27.Rosenfeld SS, Fordyce PM, Jefferson GM, King PH, Block SM. J Biol Chem. 2003;278:18550. doi: 10.1074/jbc.M300849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.For example, one such molecule generated 21 runs in all, 13 of which included more than eight dwell intervals, and were therefore scored (18). This molecule accounted for three of the nine outliers with L > 6, and the average limp factor for the molecule was 4.7.
- 29.Misura KM, Scheller RH, Weis WI. J Biol Chem. 2001;276:13273. doi: 10.1074/jbc.M009636200. [DOI] [PubMed] [Google Scholar]
- 30.Tripet B, Vale RD, Hodges RS. J Biol Chem. 1997;272:8946. doi: 10.1074/jbc.272.14.8946. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, et al. Nature. 2003;424:341. doi: 10.1038/nature01801. [DOI] [PubMed] [Google Scholar]
- 32.Bryant Z, et al. Nature. 2003;424:338. doi: 10.1038/nature01810. [DOI] [PubMed] [Google Scholar]
- 33.One quantity that varies systematically with the length of the construct in our experiments is the angle, θ, between the microtubule axis and the kinesin stalk, which experiences a force on its distal end acting through the center of the attached bead (fig. S2). This force has both rearward and upward components, and Howard and colleagues (13) have shown that the speed of kinesin movement can be affected by changes in the upward force. For the constructs used here, we estimate that θ ranges from 45° to 63° (table S1), which corresponds to a 26% maximal variation in upward loading, seemingly too small to account for a threefold change in limp factor. Moreover, small changes in loading would not be expected a priori to affect the two heads differentially.
- 34.For example, an asymmetric effect on the characteristic step times for limping constructs could be explained in the misregistration model by assuming that for correctly dimerized molecules, the rates of stepping are not normally limited by the rates at which heads can locate new binding sites. In the case of misregistration, however, the head with the shorter tether takes additional time to reach its new site, thereby slowing its rate of advance to such an extent that it becomes rate-determining for the slow phase. Correspondingly, the head with the longer tether takes less time to advance, but because this is not the limiting factor, there is no significant variation in the fast phase. A similar explanation could be invoked to explain asymmetry in the winding model. A high energetic penalty for overwinding could cause the slow step to become rate-determining, whereas the low penalty for underwinding could have little effect on the fast phase, during which other unidentified transitions would govern the stepping rate.
- 35.B. F. J. Manly, Randomization, Bootstrap, and Monte Carlo Methods in Biology (Chapman & Hall/CRC, Boca Raton, FL, 2001), pp. 34–67.
- 36.This study was initiated by C.L.A. but subsequently carried out with equal contributions from A.N.F and C.L.A. in the laboratory of S.M.B. We thank J. Gelles, W. Hancock, and S. Rosenfeld for generously providing expression plasmids and kinesin protein, and C. Spiess for assistance with protein expression. B. Weis, P. Harbury, D. Donoho, M. Filler, K. Slon, and members of our laboratory provided helpful advice and discussions. C.L.A. is supported by the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation (Fellowship DRG-1649). A.N.F. is supported by a Predoctoral Fellowship from the NSF. This work was supported by a grant from the National Institute of General Medical Sciences to S.M.B.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


