Abstract
Vitamin C (ascorbic acid) is essential to prevent disease associated with connective tissue (e.g., scurvy), improves cardiovascular and immune cell functions, and is used to regenerate α-tocopherol (vitamin E). In contrast to most animals, humans lack the ability to synthesize ascorbic acid as a result of a mutation in the last enzyme required for ascorbate biosynthesis. Vitamin C, therefore, must be obtained from dietary sources and, because it cannot be stored in the body, it must be obtained regularly. Once used, ascorbic acid can be regenerated from its oxidized form in a reaction catalyzed by dehydroascorbate reductase (DHAR). To examine whether overexpression of DHAR in plants would increase the level of ascorbic acid through improved ascorbate recycling, a DHAR cDNA from wheat was isolated and expressed in tobacco and maize, where DHAR expression was increased up to 32- and 100-fold, respectively. The increase in DHAR expression increased foliar and kernel ascorbic acid levels 2- to 4-fold and significantly increased the ascorbate redox state in both tobacco and maize. In addition, the level of glutathione, the reductant used by DHAR, also increased, as did its redox state. These results demonstrate that the vitamin C content of plants can be elevated by increasing expression of the enzyme responsible for recycling ascorbate.
Keywords: ascorbic acid‖dehydroascorbate reductase‖redox state‖ glutathione‖nutrition
Vitamin C (ascorbic acid) is required for cardiovascular function, immune cell development, connective tissue, and iron utilization. Although plants and most animals can synthesize ascorbic acid (AsA), humans lack l-gulono-1,4-lactone oxidoreductase, which is required for the final step in AsA synthesis. Because AsA cannot be stored in the body, the vitamin must be acquired regularly from dietary sources, primarily from plants rich in AsA. As many plant foods are not rich in AsA, the ability to increase the level of this vitamin in more plant foods would increase their nutritive value.
The AsA biosynthetic pathway differs between plants and animals. In animals, d-glucose is converted to AsA through d-glucuronic acid, l-gulonic acid, and l-gulono-1,4-lactone, whereas in plants it is converted to AsA through l-galactose and l-galactono-1,4-lactone, the immediate precursor of AsA (1–3). AsA serves as an antioxidant or reductant in several pathways, including the xanthophyll cycle, where it is a cofactor of violaxanthin de-epoxidase, in the regeneration of vitamin E in animals, and in the detoxification of reactive oxygen species (4–8). Oxidation of AsA produces the short-lived radical monodehydroascorbate (MDHA), which is converted to AsA by MDHA reductase (MDHAR) or disproportionates nonenzymatically to AsA and dehydroascorbate (DHA; Fig. 1A). DHA undergoes irreversible hydrolysis to 2,3-diketogulonic acid (9) or is recycled to AsA by dehydroascorbate reductase (DHAR), which uses glutathione (GSH) as the reductant (Fig. 1A; refs. 3 and 10). Thus, DHAR allows the plant to recycle DHA, thereby recapturing AsA before it is lost. Because AsA is the major reductant in plants, DHAR serves to regulate their redox state.
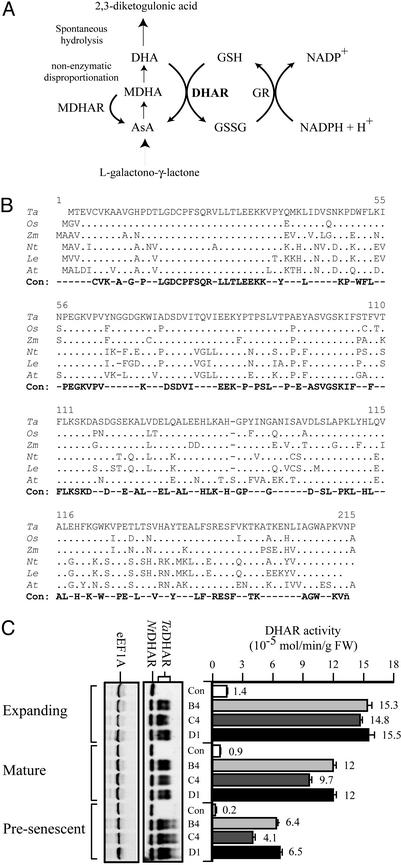
Figure 1.
Isolation of DHAR and overexpression in tobacco. (A) AsA is synthesized from l-galactono-1,4-lactone. AsA is oxidized to MDHA, which is converted to AsA by MDHAR or disproportionates nonenzymatically to AsA and DHA. DHA spontaneously hydrolyzes to 2,3-diketogulonic acid unless salvaged by DHAR, which uses GSH as the reductant. Oxidized glutathione (GSSG) is reduced by glutathione reductase (GR). (B) Alignment of the amino acid sequence of wheat (Triticum aestivum) DHAR with that of rice (Oryza sativa), maize (Zea mays), tobacco (N. tabacum), tomato (Lycopersicon esculentum), and Arabidopsis. Identity with wheat DHAR is indicated by dots, whereas positions that differ are shown; dashes represent gaps introduced to maintain alignment. The absolute consensus (Con) is also shown. GenBank accession numbers are as follows: wheat, AY074784; rice, AY074786; tobacco, AY074787; Arabidopsis, AY074785; maize, AW258053 and BE552888; and tomato, AW093721. (C) Western analysis of wheat DHAR expression in T1 tobacco (i.e., B4, C4, and D1) and a vector-only control plant (i.e., Con) is shown (Left). The presence of the His-tag wheat (TaDHAR) and endogenous tobacco (NtDHAR) proteins is indicated. Western analysis of eukaryotic elongation factor 1A (eEF1A) was used as a control. DHAR activity measured in each leaf type is represented by the histograms. The average and SDs are reported for three independent measurements. FW, fresh weight.
Recently, the metabolic engineering of plants to elevate the level of β-carotene (provitamin A) and α-tocopherol (vitamin E) has been reported (11, 12). Plants expressing l-gulono-1,4-lactone oxidoreductase from rat were reported to contain elevated AsA, although the pathway by which this occurred is unknown, and whether the ascorbate redox state was altered was not reported (13). Although AsA biosynthetic mutants have been described (14–17), there has been no report of increasing AsA levels and redox state through the manipulation of the plant-specific pathway. We show in this study that overexpression of DHAR in tobacco leaves and in maize leaves and kernels results in increased AsA content and a higher ratio of the reduced to oxidized form of AsA. Increased AsA content was accompanied by an increase in GSH, thus resulting in a substantial change in the intracellular redox state. These results demonstrate that the level of vitamin C in plants can be elevated through increased ascorbate recycling.
Materials and Methods
Isolation of DHAR cDNAs, Plant Transformation, and Western Analysis.
Full-length wheat and tobacco DHAR cDNAs were isolated from seedling cDNA expression libraries by using anti-wheat DHAR antiserum and the wheat cDNA (as a probe for the tobacco library), respectively. Full-length ESTs for rice, tomato, and Arabidopsis were identified in GenBank. The sequence for a full-length maize DHAR was constructed from two partial ESTs (accession nos. AW258053 and BE552888) that are identical in the region of their overlap. Recombinant wheat DHAR, with or without an N-terminal His-tag, was expressed from pET19b or pET11 (Novagen), respectively. Transgenic tobacco (Nicotiana tabacum, cv. Xanthi) expressing the His-tagged wheat DHAR from the cauliflower mosaic virus (CaMV) 35S promoter (in the binary vector, pBI101) was generated by using Agrobacterium tumefaciens, as described (18).
Transgenic maize containing the wheat DHAR (without a His-tag) under the control of the maize ubiquitin (Ub) promoter in pACH18 (19) or Shrunken 2 (Sh2) promoter (amplified as a 1.5-kbp fragment from B73 and substituted for the Ub promoter in pACH18) was generated by particle bombardment of embryogenic A188 X B73 (HiII) callus, as described (20). Cotransformation with the bar gene provided bialaphos selection for the transformed callus (21). Regenerants containing the Sh2-DHAR or Ub-DHAR constructs were identified by using PCR; DHAR expression was confirmed by activity assay and Western analysis. T0 plants were crossed with HiII, transgene-containing progeny identified, and the T1 plants were selfed.
Anti-DHAR antiserum raised against DHAR purified from wheat seedlings was used at 1:5,000 to 1:10,000 dilution in Western analysis, as described (22).
Enzyme Assays.
DHAR activity was assayed essentially as described (23). Tobacco or maize were ground in extraction buffer (50 mM Tris⋅HCl, pH 7.4/100 mM NaCl/2 mM EDTA/1 mM MgCl2), and soluble protein was obtained after a 5-min centrifugation at 13,000 rpm (Eppendorf centrifuge 5417C). DHAR was assayed from an equal amount of protein as described (24) in 50 mM K2HPO4/KH2PO4, pH 6.5/0.5 mM DHA/1 mM GSH, and its activity was followed by an increase in absorbance at 265 nm. Glutathione reductase (GR), MDHAR, ascorbate peroxidase (APX), l-galactono-1,4-lactone dehydrogenase (GLDH), superoxide dismutase (SOD), and catalase (CAT) activities were determined as described (25–29).
AsA, DHA, GSH, and Oxidized Glutathione (GSSG) Measurements.
AsA was measured as described (30). Leaves were ground in 2.5 M HClO4 and centrifuged at 13,000 rpm (Eppendorf centrifuge 5417C) for 10 min. Two volumes of 1.25 M Na2CO3 were added to the supernatant, and after centrifugation, 100 μl of the mixture was added to 895 μl of 100 mM K2HPO4/KH2PO4, pH 5.6. AsA was determined by the change in absorbance at 265 nm following the addition of 0.25 unit of ascorbate oxidase. The total amount of reduced and oxidized ascorbic acid (i.e., AsA and DHA) was determined by reducing DHA to AsA (in a reaction containing 100 mM K2HPO4/KH2PO4 at pH 6.5, 2 mM GSH, and 0.1 μg of recombinant wheat DHAR protein incubated at 25°C for 20 min) before measuring AsA. The amount of DHA was determined as the difference between these two assays. GSH and GSSG were determined from leaves, as described (31).
Results
Isolation of cDNAs Encoding DHAR.
With the exception of rice and a chloroplast-targeted DHAR from spinach (32, 33), DHAR cDNAs from plant species have not been reported. Consequently, a full-length wheat cDNA was isolated from a library by using anti-DHAR antiserum and was then used to screen a tobacco cDNA library. Full-length tomato, Arabidopsis, and rice ESTs encoding DHAR were identified, and a full-length maize EST was reconstructed from two partial ESTs. The rice EST identified in this study was identical to that reported (30). DHAR is conserved in molecular mass (23,358 Da in wheat) and composition among plant species (Fig. 1B). Recombinant wheat DHAR (with an N-terminal His-tag) exhibited substantial DHAR activity (9.1 μmol/min/μg) and was ≈40% as active as recombinant protein without a His-tag (23 μmol/min/μg).
Overexpression of DHAR in Tobacco.
To elevate DHAR expression in tobacco, the His-tagged wheat DHAR cDNA was introduced under the control of the cauliflower mosaic virus 35S promoter and DHAR-overexpressing regenerants identified. T1 progeny overexpressed wheat DHAR without altering expression of the endogenous DHAR, which thus served as an internal control for equal protein loading (Fig. 1C). Equal loading was verified by Western analysis of eEF1A (Fig. 1C). Wheat DHAR appeared as two bands where the lower one corresponded to the recombinant His-tagged form (data not shown), suggesting that the upper band may be modified in a way that retards its migration. T1 progeny exhibited up to an 11-fold increase in DHAR activity in expanding leaves (P < 0.001), up to a 13-fold increase in mature leaves (P < 0.01), and up to a 32-fold increase in presenescent leaves (P < 0.01; Fig. 1C). DHAR overexpression did not affect the growth rate or flowering time of the tobacco.
Increased DHAR Activity Increases AsA and Glutathione Content in Tobacco.
To determine the metabolic consequences of DHAR overexpression, the amount of AsA present in leaves was measured. The level of AsA increased up to 2.4-fold in expanding leaves (P = 0.01), 3.9-fold in mature leaves (P < 0.05), and 2.2-fold in presenescent leaves (P < 0.01) in DHAR-overexpressing plants (Fig. 2A). The redox status of ascorbate (i.e., AsA:DHA) increased from a ratio of 1.5 in control leaves to more than 4 in expanding leaves of DHAR-overexpressing plants (P < 0.001), and similar increases were observed in adult and presenescent leaves (Fig. 2A).
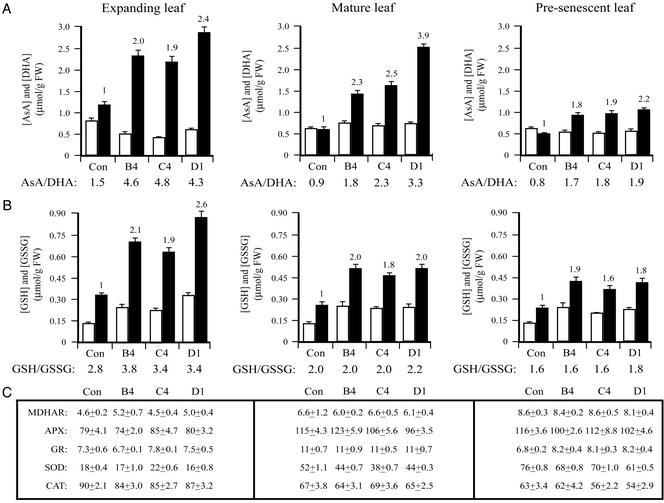
Figure 2.
Overexpression of DHAR increases ascorbic acid and glutathione in tobacco. (A) AsA and DHA. (B) GSH and GSSG. (C) MDHAR (10−8 mol of NAPDH oxidized per min per mg of protein), APX (10−8 mol of AsA oxidized per min per mg of protein), GR (10−8 mol of NAPDH oxidized per min per mg of protein), SOD (units to inhibit nitroblue tetrazolium (NBT) photoreduction by 50%), and CAT (10−6 mol of H2O2 reduced per min per mg of protein) activities were measured in expanding leaves, mature leaves exhibiting maximal photosynthetic activity, presenescent leaves of control (Con), and three T1 DHAR-overexpressing transgenics (i.e., B4, C4, and D1). The level of AsA or GSH (black histograms), DHA or GSSG (white histograms), and the enzyme activities were assayed in three independent replicates of leaves pooled from three independent plants, and the average and SD were reported. The fold increase in AsA or GSH relative to the control is indicated above the histograms. The ratio of AsA to DHA or GSH to GSSG in each line is indicated below each pair of histograms.
An increase in AsA could result from increased regeneration or biosynthesis. To investigate the latter possibility, the level of GLDH, which catalyzes the terminal step in ascorbate biosynthesis, was measured in expanding leaves where the largest increase in AsA was observed. GLDH activity in three DHAR-overexpressing lines, i.e., B4, C4, and D1 (8.6 ± 0.8, 9.1 ± 0.8, 8.6 ± 0.5 units, respectively) was not significantly different from that of control plants (9.3 ± 0.4 units), suggesting that the observed increase in AsA in DHAR-overexpressing plants did not result from increased biosynthesis.
Because GSH is used as the reductant by DHAR, the levels of reduced (GSH) and oxidized (GSSG) glutathione were determined. Interestingly, GSH increased up to 2.6-fold in expanding leaves (P < 0.05), up to 2.0-fold in mature leaves (P < 0.01), and up to 1.9-fold in presenescent leaves (P < 0.05; Fig. 2B), suggesting that increasing AsA may require and signal a corresponding increase in glutathione. The glutathione redox status (i.e., GSH:GSSG) increased significantly in expanding leaves (P < 0.05), but was little changed in older leaves. MDHAR activity was not significantly different between DHAR-overexpressing and control plants (Fig. 2C).
One possible explanation for the increase in GSH in DHAR-overexpressing plants could be that an increase in AsA induces an oxidative stress response that may include increases in the level of GSH and enzyme activities such as GR, APX, CAT, and SOD. Such a response was provoked in tobacco engineered to overexpress chloroplast-targeted γ-glutamylcysteine synthetase (γ-ECS), which resulted in increases in glutathione (32). However, DHAR-overexpressing plants did not exhibit any visible oxidative stress symptoms as were observed in γ-ECS-overexpressing plants (34). Moreover, no significant change in the activity of GR, APX, CAT, and SOD was detected, with the exception of a 10–25% decrease in SOD activity in mature and presenescent leaves (P < 0.05; Fig. 2C). These data suggest that oxidative stress is not increased and may, in fact, be decreased when DHAR is overexpressed.
Increased DHAR Activity Increases AsA and Glutathione Content in Maize.
To determine whether increasing DHAR expression can alter the redox status of an unrelated species, transgenic maize containing the wheat DHAR cDNA (without a His-tag) under the control of the maize ubiquitin (Ub) or Shrunken 2 (Sh2) promoter was generated. Leaves of T2 progeny confirmed by PCR to harbor the DHAR transgene exhibited a 25- to 50-fold increase in DHAR activity relative to the corresponding syngeneic lines (Fig. 3A). The level of AsA increased up to 1.8-fold (P < 0.01) in leaves from DHAR-overexpressing maize (Fig. 3B), similar to that observed in tobacco (Fig. 2A). The increase in AsA resulted in a 30–40% increase in the ascorbate redox state (P < 0.01; Fig. 3B) and was accompanied by a 1.9-fold increase in GSH (P < 0.001) and a 30–40% increase in the GSH redox state (P < 0.01; Fig. 3C), similar to that observed in tobacco (Fig. 2B).
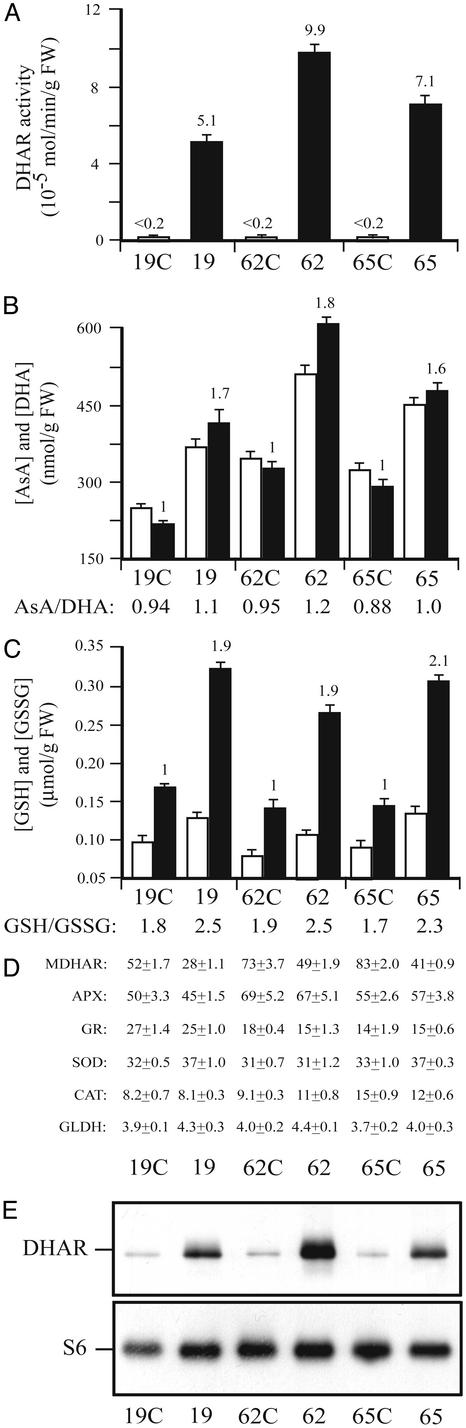
Figure 3.
Overexpression of DHAR increases ascorbic acid and glutathione content in maize leaves. (A) DHAR activity. (B) AsA and DHA. (C) GSH and GSSG. (D) MDHAR, APX, GR, SOD, CAT, and GLDH (10−8 mol of cytochrome c reduced per min per mg of protein) activities. (E) DHAR and ribosomal protein S6 were measured in expanding leaves of 4-week-old maize. AsA or GSH (black histograms), DHA or GSSG (white histograms), and enzyme activities were assayed in three independent assays, and the average and SD were reported. For each line, leaves from syngeneic (i.e., 19C, 62C, and 65C) and DHAR-expressing (i.e., 19, 62, and 65) individuals were assayed.
Maize leaves contain 10-fold more MDHAR activity than does tobacco (compare Fig. 3D to 2C), suggesting that MDHAR may be responsible for recycling AsA in maize. MDHAR activity was consistently reduced by ≈2-fold in DHAR-overexpressing leaves (P < 0.05). No significant change was observed in APX, GR, SOD, CAT, or GLDH activities (Fig. 3D), suggesting that the activity of oxidative stress-associated enzymes was unaltered in DHAR-overexpressing leaves.
T3 kernels expressing the wheat DHAR transgene exhibited a 20- to 106-fold increase in DHAR activity (Fig. 4A) and increased expression (Fig. 4E) relative to syngeneic kernels. As observed in leaves, the level of AsA in DHAR-overexpressing kernels was up to 1.9-fold higher than that in syngeneic kernels (P < 0.05) in which the amount of AsA varies by 11% between lines (data not shown; Fig. 4B). The increase in AsA (Fig. 4B) correlated with DHAR expression (Fig. 4A). Up to a 40% increase (P < 0.05) in the ascorbate redox state was observed. Similar increases in GSH, GSSG, and the glutathione redox state (P < 0.05) were observed (Fig. 4C) and correlated with DHAR expression (Fig. 4A). No significant change in MDHAR, APX, GR, SOD, or CAT activities was observed (Fig. 4D), and the grain developed and germinated normally.
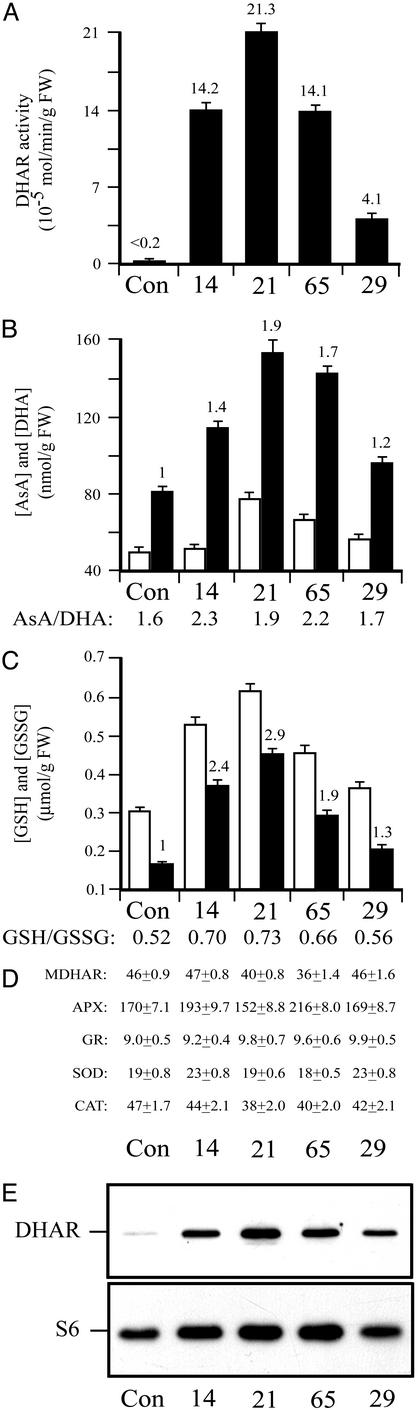
Figure 4.
Overexpression of DHAR increases ascorbic acid and glutathione content in maize kernels. (A) DHAR activity. (B) AsA and DHA. (C) GSH and GSSG. (D) MDHAR, APX, GR, SOD, and CAT. (E) DHAR and ribosomal protein S6 were measured in developing maize kernels. AsA or GSH (black histograms) and DHA or GSSG (white histograms) were assayed in three independent assays, and the average and SD were reported. For each line, kernels from a syngeneic line (Con) and four DHAR-expressing lines (i.e., 14, 21, 65, and 29) were assayed.
Discussion
We have demonstrated that the vitamin C content of plants can be increased by overexpressing DHAR, the enzyme responsible for regenerating ascorbic acid. The increase in ascorbate was surprising, given that this compound is the major antioxidant contributing to the redox status of the cell and that its synthesis is subject to feedback inhibition by the pool size of ascorbate (35). However, its concentration is light-dependent (36), demonstrating that the level of AsA can vary depending on internal and external conditions.
After the introduction of the wheat DHAR cDNA into tobacco or maize, DHAR activity increased 11- to 100-fold and was accompanied by increases in AsA and redox state. In expanding tobacco leaves, this more reducing environment was a consequence of an increase in AsA and a decrease in DHA. Similar increases were observed in maize leaves and kernels, demonstrating that changes in AsA can be made in photosynthetic and nonphotosynthetic organs. Although an increase in flux through the AsA biosynthetic pathway might account for the increase in AsA, no increase in GLDH, which catalyzes the last step of ascorbate biosynthesis, was observed.
The cellular concentration of AsA is determined by the rate of its synthesis and decay. DHA is rapidly hydrolyzed into 2,3-diketogulonic acid if not salvaged by DHAR. The increase in ascorbate in plants overexpressing DHAR can be understood through the recycling function of the enzyme, which would increase the likelihood that DHA is converted to AsA before being lost through its decay. Consequently, the increase in AsA in DHAR-overexpressing plants may result from enhanced rescue of DHA from its decay pathway and its efficient recycling back to AsA.
That increased DHAR activity leads to increased AsA recycling suggests that the amount of endogenous DHAR may be limiting. Similar limited expression was noted for γ-tocopherol methyltransferase (γ-TMT), which catalyzes the last step in α-tocopherol (vitamin E) biosynthesis, and its overexpression in Arabidopsis resulted in a shift from γ-tocopherol (i.e., the substrate) to α-tocopherol (i.e., the product; ref. 11). As with γ-TMT, limiting DHAR expression might enable plants to control their intracellular redox state, particularly in response to developmental cues or changes in their external environment.
In addition to an increase in AsA, an unexpected increase in the level and redox state of glutathione was also observed in DHAR-overexpressing plants. The increase in the redox status of ascorbate in DHAR-overexpressing plants might have been perceived as a stress to which the plant responds by increasing antioxidant concentration. Tobacco engineered to overexpress γ-ECS contained elevated glutathione levels but, paradoxically, exhibited chlorosis and necrosis (34). However, these growth defects were not correlated with the increase in glutathione but rather with a decrease in glutathione redox state. Although DHAR-overexpressing plants exhibit elevated levels of ascorbate and glutathione, they differ from γ-ECS-overexpressing plants, in that the redox state of both also increases. Moreover, DHAR-overexpressing plants do not exhibit any chlorosis, necrosis, or premature senescence, and exhibit no increase in GR, APX, CAT, or SOD activities, supporting the conclusion that an oxidative stress response had not been elicited. Although mutants affected in AsA biosynthesis exhibit slower shoot growth (36), increasing the level of AsA did not impair the growth of DHAR-overexpressing plants, suggesting that increasing ascorbate and its redox state is not detrimental to growth. The increase in glutathione that accompanies the increase in ascorbate suggests a coordinate balance between these two antioxidants that is not unexpected, given that glutathione is required by DHAR. Because glutathione is present at one to two orders of magnitude lower concentration than AsA (37), an increase in AsA may require a corresponding increase in glutathione, suggesting that changes in AsA may signal for changes in the glutathione pool size.
Although the current recommended dietary allowance for vitamin C is 75–90 mg, an increase to 200 mg has been suggested (38). Such an increase would require increased dietary emphasis of foodstuffs rich in ascorbate. Increased AsA achieved through the overexpression of DHAR represents a simple strategy by which ascorbate content can be enhanced in both the photosynthetic and nonphotosynthetic organs.
Acknowledgments
We thank Julia Bailey-Serres and Karen Browning for anti-S6 and anti-eEF1A antisera, respectively, and Sanghyun Lim for assistance with genotyping the transgenic maize lines. This work was supported by U.S. Department of Agriculture Grant NRICGP 2002-00743 and the University of California Agricultural Experiment Station.
Abbreviations
- AsA
ascorbic acid
- MDHA
monodehydroascorbate
- MDHAR
MDHA reductase
- DHA
dehydroascorbate
- DHAR
DHA reductase
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- GR
glutathione reductase
- APX
ascorbate peroxidase
- GLDH
l-galactono-1,4-lactone dehydrogenase
- SOD
superoxide dismutase
- CAT
catalase
- FW
fresh weight
Footnotes
References
- 1.Wheeler G L, Jones M A, Smirnoff N. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- 2.Smirnoff N, Wheeler G L. Crit Rev Biochem Mol Biol. 2000;35:291–314. doi: 10.1080/10409230008984166. [DOI] [PubMed] [Google Scholar]
- 3.Smirnoff N, Conklin P L, Loewus F A. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:437–467. doi: 10.1146/annurev.arplant.52.1.437. [DOI] [PubMed] [Google Scholar]
- 4.Asada K. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 5.Smirnoff N. Curr Opin Plant Biol. 2000;3:229–235. [PubMed] [Google Scholar]
- 6.Bratt C E, Arvidsson P O, Carlsson M, Akerlund H E. Photosynth Res. 1995;45:169–175. doi: 10.1007/BF00032588. [DOI] [PubMed] [Google Scholar]
- 7.Neubauer C, Yamamoto H Y. Photosynth Res. 1994;39:137–147. doi: 10.1007/BF00029381. [DOI] [PubMed] [Google Scholar]
- 8.Eskling M, Arvidsson P O, Akerlund H E. Physiol Plant. 1997;100:806–816. [Google Scholar]
- 9.Washko P W, Welch R W, Dhariwal K R, Wang Y, Levine M. Anal Biochem. 1992;204:1–14. doi: 10.1016/0003-2697(92)90131-p. [DOI] [PubMed] [Google Scholar]
- 10.Noctor G, Foyer C H. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 11.Shintani D, Dellapenna D. Science. 1998;282:2098–2100. doi: 10.1126/science.282.5396.2098. [DOI] [PubMed] [Google Scholar]
- 12.Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I. Science. 2000;287:303–305. doi: 10.1126/science.287.5451.303. [DOI] [PubMed] [Google Scholar]
- 13.Jain A K, Nessler C L. Mol Breeding. 2000;6:73–78. [Google Scholar]
- 14.Conklin P L, Williams E H, Last R L. Proc Natl Acad Sci USA. 1996;93:9970–9974. doi: 10.1073/pnas.93.18.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conklin P L, Pallanca J E, Last R L, Smirnoff N. Plant Physiol. 1997;115:1277–1285. doi: 10.1104/pp.115.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conklin P L, Norris S R, Wheeler G L, Williams E H, Smirnoff N, Last R L. Proc Natl Acad Sci USA. 1999;96:4198–4203. doi: 10.1073/pnas.96.7.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conklin P L, Saracco S A, Norris S R, Last R L. Genetics. 2000;154:847–856. doi: 10.1093/genetics/154.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraley R T, Rogers S G, Horsch R B, Sanders P R, Flick J S, Adams S P, Bittner M L, Brand L A, Fink C L, Fry J S, et al. Proc Natl Acad Sci USA. 1983;80:4803–4807. doi: 10.1073/pnas.80.15.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen A H, Quail P H. Transgenic Res. 1996;5:213–218. doi: 10.1007/BF01969712. [DOI] [PubMed] [Google Scholar]
- 20.Gordon-Kamm W J, Spencer T M, Mangano M L, Adams T R, Daines R J, Start W G, Obrien J V, Chambers S A, Adams W R, Willetts N G, et al. Plant Cell. 1990;2:603–618. doi: 10.1105/tpc.2.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Block M, Botterman J, Vandewiele M, Dockx J, Thoen C, Gossele V, Movva N R, Thompson C, Van Montagu M, Leemans J. EMBO J. 1987;6:2513–2518. doi: 10.1002/j.1460-2075.1987.tb02537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young T E, Ling J, Geisler-Lee J, Tanguay R L, Caldwell C, Gallie D R. Plant Physiol. 2001;127:777–791. [PMC free article] [PubMed] [Google Scholar]
- 23.Hossain M A, Asada K. Plant Cell Physiol. 1984;25:85–92. [Google Scholar]
- 24.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 25.Foyer C, Halliwell B. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- 26.de Pinto M C, Tommasi F, De Gara L. Plant Physiol Biochem. 2000;38:541–550. [Google Scholar]
- 27.Gainnopolitis C N, Pies S K. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aebi H. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 29.Jimenez A, Hernandez J A, Del Rio L A, Sevilla F. Plant Physiol. 1997;114:275–284. doi: 10.1104/pp.114.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foyer C H, Rowell J, Walker D. Planta. 1983;157:239–244. doi: 10.1007/BF00405188. [DOI] [PubMed] [Google Scholar]
- 31.Griffith O W. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 32.Urano J, Nakagawa T, Maki Y, Masumura T, Tanaka K, Murata N, Ushimaru T. FEBS Lett. 2000;466:107–111. doi: 10.1016/s0014-5793(99)01768-8. [DOI] [PubMed] [Google Scholar]
- 33.Shimaoka T, Yokota A, Miyake C. Plant Cell Physiol. 2000;41:1110–1118. doi: 10.1093/pcp/pcd035. [DOI] [PubMed] [Google Scholar]
- 34.Creissen G, Firmin J, Fryer M, Kular B, Leyland N, Reynolds H, Pastori G, Wellburn F, Baker N, Wellburn A, Mullineaux P. Plant Cell. 1999;11:1277–1292. doi: 10.1105/tpc.11.7.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pallanca J E, Smirnoff N. J Exp Bot. 2000;51:669–674. [PubMed] [Google Scholar]
- 36.Veljovic-Jovanovic S D, Pignocchi C, Noctor G, Foyer C H. Plant Physiol. 2001;127:426–435. [PMC free article] [PubMed] [Google Scholar]
- 37.Smirnoff N. Philos Trans R Soc London B. 2000;355:1455–1464. doi: 10.1098/rstb.2000.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine M, Conry-Cantilena C, Wang Y, Welch R W, Washko P W, Dhariwal K R, Park J B, Lazarev A, Graumlich J F, King J, Cantilena L R. Proc Natl Acad Sci USA. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]