Abstract
Heterogeneity of heart failure (HF) phenotypes indicates contributions from underlying common polymorphisms. We considered polymorphisms in the β1-adrenergic receptor (β1AR), a β-blocker target, as candidate pharmacogenomic loci. Transfected cells, genotyped human nonfailing and failing ventricles, and a clinical trial were used to ascertain phenotype and mechanism. In nonfailing and failing isolated ventricles, β1-Arg-389 had respective 2.8 ± 0.3- and 4.3 ± 2.1-fold greater agonist-promoted contractility vs. β1-Gly-389, defining enhanced physiologic coupling under relevant conditions of endogenous expression and HF. The β-blocker bucindolol was an inverse agonist in failing Arg, but not Gly, ventricles, without partial agonist activity at either receptor; carvedilol was a genotype-independent neutral antagonist. In transfected cells, bucindolol antagonized agonist-stimulated cAMP, with a greater absolute decrease observed for Arg-389 (435 ± 80 vs. 115 ± 23 fmol per well). Potential pathophysiologic correlates were assessed in a placebo-controlled trial of bucindolol in 1,040 HF patients. No outcome was associated with genotype in the placebo group, indicating little impact on the natural course of HF. However, the Arg-389 homozygotes treated with bucindolol had an age-, sex-, and race-adjusted 38% reduction in mortality (P = 0.03) and 34% reduction in mortality or hospitalization (P = 0.004) vs. placebo. In contrast, Gly-389 carriers had no clinical response to bucindolol compared with placebo. Those with Arg-389 and high baseline norepinephrine levels trended toward improved survival, but no advantage with this allele and exaggerated sympatholysis was identified. We conclude that β1AR-389 variation alters signaling in multiple models and affects the β-blocker therapeutic response in HF and, thus, might be used to individualize treatment of the syndrome.
Keywords: adenylyl cyclase, G protein-coupled receptor, genetics, myocardium, signaling
Familial clustering of physiologic and clinical phenotypes, reduced penetrance in familial cardiomyopathies, and marked interindividual variability in susceptibility, progression, and treatment response have suggested a potential disease-modifying role for common genetic variants in heart failure (HF). Nonsynonymous single-nucleotide polymorphisms, prevalent throughout the human genome, including some within important cardiovascular signaling pathways, have been proposed as a basis for such phenotypic heterogeneity (1). Few examples, however, have emerged where common polymorphisms have an impact of sufficient magnitude to affect therapeutic targeting.
The β1-adrenergic receptor (β1AR) is a member of the 7-transmembrane-spanning superfamily of receptors. β1ARs couple to the stimulatory heterotrimeric G protein Gs, acting to increase adenylyl cyclase activity and intracellular cAMP, and also act through non-cAMP-dependent pathways, culminating in various cell-specific intracellular events. The β1AR is the principal βAR subtype expressed on the cardiac myocyte, and, when occupied by agonist, mediates increases in contractility as well as cardiomyopathic effects (2, 3). Because the cardiomyopathic effects of chronic β1AR stimulation predominate in human HF, administration of β-blocking agents improves clinical outcomes, pathologic remodeling, and myocardial molecular abnormalities in the syndrome (3–5). Even though most β-blocker trials in HF have shown group mean beneficial effects, there is substantial interindividual variability in outcomes that is not explained by baseline clinical characteristics (4, 6, 7). We have considered that the basis of such variability may be genetic in nature, with the candidate gene being the major target for β-blockers, the β1AR.
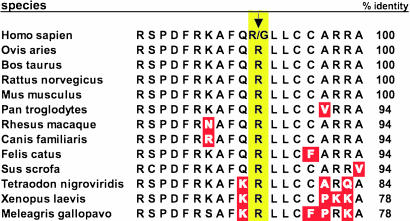
To approach this hypothesis, we had (8) resequenced the β1AR gene from several human cohorts and found a common nonsynonymous single-nucleotide variation at nucleotide 1,165 of the ORF, which results in either Arg or Gly being encoded at amino acid position 389. In the general population, the allele frequency of Arg-389 is ≈70% (homozygote frequency ≈50%) (8). This residue is localized in an intracellular loop composed of residues extending from the seventh transmembrane domain of the receptor to the membrane-anchored palmitoylated cysteine(s). Based on the crystal structure of bovine rhodopsin, this small intracellular β1AR loop is predicted to be an α-helix (9). As shown in Fig. 1, this region is highly conserved among β1ARs of various species. Some divergence is noted in lower species (e.g., Xenopus laevis), but, nevertheless, Arg in the analogous human position 389 is invariant in β1ARs of all species sequenced to date. Polymorphic variation at such a highly conserved residue has been considered one criterion for predicting altered function. We have thus assessed catecholamine-mediated signaling of the human Arg and Gly receptors in transfected fibroblasts expressing each receptor and found that the Arg receptor displays increased coupling to Gs and stimulation of adenylyl cyclase as compared with the Gly variant (8). In addition, we have overexpressed each human variant in the mouse heart via transgenesis and found enhanced baseline and β-agonist-stimulated contractility in the Arg, compared with Gly, hearts (10). Furthermore, only the Arg mice showed a time-dependent decrease in βAR signaling and ultimately exhibited HF. In a small, short-term, retrospective, non-placebo-controlled study in HF, we had shown that the increase in left ventricular ejection fraction (LVEF) after administration of the β-blocker carvedilol was greater for Arg vs. Gly patients (10). However, the change in LVEF (which, in our prior study, was derived from two measurements) is not predictive of cardiac remodeling or improvement in patient survival in long-term β-blocker trials (7). An additional complexity derived from the transgenic studies and cardiac adenylyl cyclase activities from end-stage hearts (10) suggest that the phenotype of the Arg (vs. Gly) receptor may be time-dependent. In the current report, we investigate the critical issue of the relevance of this variation for β-blocker therapy in HF, using multiple complementary approaches: human isolated cardiac tissue preparations, heterologously expressed receptors in model cells, and a large randomized placebo-controlled clinical trial of the β-blocker bucindolol in HF.
Fig. 1.
The β1AR-389 polymorphism is within a highly conserved intracellular region. Amino acid sequences from diverse species are shown aligned with human residues 379–397, with differences indicated in red. The human polymorphism is located at position 389 (yellow) in patients treated with bucindolol.
Results
β1AR Genotype Impacts Human Ventricular ex Vivo Contractile Responses.
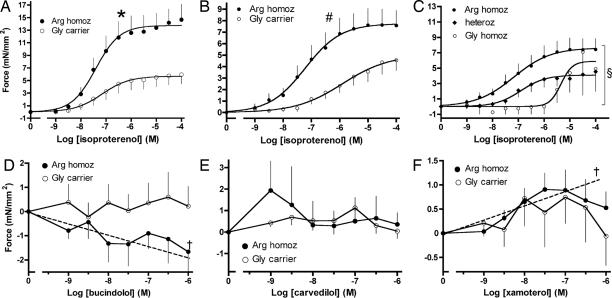
In these studies, isolated right ventricular trabeculae from human hearts were used to ascertain the effects of genotype on contraction by using the relevant tissue, under endogenous expression, in the absence and presence of HF. Such studies have not been previously reported, and a phenotypic difference would substantially strengthen the basis for the hypothesis that these two β1AR variants have relevance to human therapeutic responses. The preexplant LVEF in the nonfailing group was 0.61 ± 0.13 for Arg (n = 11) and 0.53 ± 0.15 for Gly (n = 11; P = 0.21) and, in the failing group, 0.21 ± 0.09 for Arg (n = 17) and 0.16 ± 0.07 for Gly (n = 14; P = 0.17). The other demographic characteristics of the hearts, including age, etiology, and sex, were comparable between the Arg and Gly groups (see Table 2, which is published as supporting information on the PNAS web site). Shown in Fig. 2 A and B are systolic tension responses to isoproterenol (ISO) in right ventricular trabeculae removed from nonfailing and failing human hearts, stratified by the β1AR-389 genotype. In nonfailing hearts, the maximal ISO responses differed between genotypes (Arg homozygotes 14.6 ± 2.4 vs. 6.1 ± 1.4 mN/mm2 for Gly carriers; P < 0.01), and the dose–response curves between submaximal ISO concentrations of 10 nM and 1 μM were different by analysis of covariance (ANCOVA) (mean difference 2.8 ± 0.3-fold, P = 0.028; P < 0.001 for curve-slope difference). Importantly, a similar difference was observed in trabeculae from failing hearts (Fig. 2B), where responses between the two genotypes differed between the linear portion of the log dose–response curves between submaximal ISO concentrations of 10 nM to 1 μM (mean of 4.3 ± 2.1-fold, P = 0.013; P < 0.001 for curve slope). There were two failing hearts that were Gly homozygotes, and, as shown in Fig. 2C, these two hearts had responses that were less sensitive to ISO stimulation than the 12 heterozygotes (P < 0.001 for slope, and P = 0.011 for force-response differences among the three groups). To determine whether these findings could be explained by a genotype-dependent differential expression of β1- or β2ARs, radioligand binding was performed with membranes from these preparations. Neither β1- nor β2AR densities were found to be different between β1-389 genotypes in either the nonfailing or failing groups. In nonfailing preparations, the densities were: Arg homozygotes, β1 = 92 ± 9.2 and β2 = 27 ± 3.7 fmol/mg vs., respectively, 97 ± 7.0 and 29 ± 6.8 fmol/mg in Gly carriers, all P > 0.50. As expected, in failing preparations, βAR receptor densities were decreased vs. nonfailing, but, nevertheless, there was no difference between the genotypes: β1 = 52 ± 6.6, β2 = 16 ± 2.6 fmol/mg vs., respectively, 39 ± 7.0 and 12 ± 1.8 fmol/mg in Gly carriers, all P > 0.12.
Fig. 2.
β1AR genotype and drug-response correlations in nonfailing and failing human ventricles. (A and B) Right ventricular trabeculae were used from 22 nonfailing (A) and 31 failing (B) human hearts as described in Methods. ∗, P < 0.001 for curve slope, P = 0.028 for force response differences vs. Gly. #, P < 0.001 for curve slope, P = 0.013 for force response differences vs. Gly. (C) ISO responses plotted by all β1 389 genotypes; §, P < 0.001 for slope, P = 0.011 for force-response differences among all three curves. (D–F) Failing trabeculae (n = 23) were precontracted with 10 μM forskolin, and the effects of bucindolol, carvedilol, or the partial agonist xamoterol were determined; †, slopes (dashed lines) of curve differ from zero at P < 0.01. See Tables 2 and 3 for patient characteristics.
To ascertain the potential for bucindolol to act as a partial agonist (sometimes referred to as an antagonist with intrinsic sympathomimetic activity), which may be detrimental in HF (11), similar studies were carried out with a group (n = 23) of failing hearts with bucindolol, carvedilol, and xamoterol. There were no differences between Arg homozygotes and Gly carriers with respect to demographics. (Table 3, which is published as supporting information on the PNAS web site). This group of right ventricular trabeculae again confirmed the coupling phenotype with the full agonist ISO similar to that shown in Fig. 2B (data not shown). As has been reported, β1AR partial agonism is best observed while trabeculae are submaximally precontracted with forskolin (12). As can be seen in Fig. 2D, bucindolol had no partial agonist effects, and, indeed, for Arg hearts, there was a small (maximum of 1.7 ± 1.0 mN/mm2) negative inotropic response that was statistically significant, as measured by curve slope (P = 0.01 compared with slope of 0). For the Gly hearts, the response to bucindolol was flat (slope P > 0.6). The maximal force generation between Arg and Gly hearts was not statistically different, with P = 0.17. In contrast, the β-blocker carvedilol (Fig. 2E) acted as a neutral antagonist in both Arg homozygotes and Gly carriers (each slope P > 0.60). For the β1AR partial agonist xamoterol (13), Arg hearts but not Gly showed small positive inotropic responses (Fig. 2F) as assessed by the slopes of the response curves (P < 0.001 for Arg homozygotes, P = 0.46 for Gly carriers; maximal response not different, P = 0.79). Collectively, the above data establish that the hyperresponsive phenotype of Arg-389 is observed in nonfailing and failing human hearts, with the potential to affect agonist or antagonist responses in HF. In addition, bucindolol exhibits no evidence of intrinsic sympathomimetic activity, even under conditions that can unmask weak partial agonist activity and, in fact, is an inverse agonist only in Arg hearts. And, finally, these data do not support the concept of a phenotypic switch (10), because end-stage Arg failing hearts show an increased response to agonist compared with Gly-389.
Functional Antagonism of Norepinephrine (NE)-Stimulated cAMP in Transfected Cells.
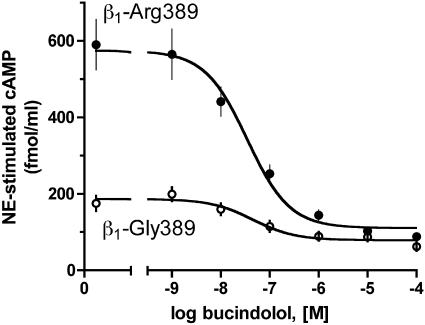
For these studies, transfected fibroblasts expressing equivalent levels (fmol/mg, n = 4) of the Arg (123 ± 19) and Gly (137 ± 16) human β1ARs were used. Basal levels of cAMP were 72 ± 8.5 and 59 ± 9.1 fmol per well (P < 0.01), with the Arg cells having higher basal levels, consistent with increased Gs coupling and spontaneous non-agonist-promoted activation. To examine functional antagonism, cells were exposed to 10 μM agonist NE, in the absence or presence of varying concentrations of bucindolol, and cAMP levels determined. Arg displayed a greater cAMP stimulation to agonist in the absence of bucindolol compared with Gly-389, which represents the primary phenotypes of the two receptors, as noted in ref. 8. Despite the substantially greater degree of NE-mediated stimulation of the Arg receptor, bucindolol effectively antagonized the response (Fig. 3). The difference in the absolute decrease in cAMP production afforded by bucindolol was greater for cells expressing Arg: bucindolol caused a maximal decrease of 435 ± 80 fmol/ml cAMP in Arg-389 cells compared with 115 ± 23 fmol/ml cAMP in Gly cells (P = 0.008; n = 4). The potency of bucindolol was not different for the response (EC50 = 46 ± 4.5 and 35 ± 11 nM, respectively, P = 0.94; n = 4). In addition, in [125I]cyanopindolol competition binding studies, the affinity for bucindolol was not different between Arg (pKi = 9.6 ± 0.04) and Gly receptors (pKi = 9.6 ± 0.11, n = 3). Finally, bucindolol alone in concentrations up to 10 μM resulted in no significant increases or decreases in cAMP accumulation for either receptor (Arg = 3 ± 3.2%, Gly = 9 ± 4.7% of the NE response, P > 0.05 vs. basal). These data thus show a greater cAMP-lowering effect of bucindolol with Arg cells because of the greater initial stimulation by agonist and the capacity of bucindolol to antagonize this robust stimulation in an efficacious manner.
Fig. 3.
Bucindolol effectively antagonizes the hyperfunctional β1-Arg-389 receptor. Transfected fibroblasts expressing equivalent levels of the receptors were stimulated with 10 μM NE, with simultaneous exposure to the indicated concentrations of bucindolol. ∗, P = 0.008 vs. Gly, n = 4.
β1-Arg-389 is the Favorable β-Blocker Response Allele in Human HF.
The results from the human ventricular strips and transfected cells prompted genotyping for this β1AR allele in patients from the β-Blocker Evaluation of Survival Trial (BEST), a trial of the β-blocker bucindolol in the treatment of Class III/IV HF, which included a placebo arm (6). One thousand forty patients consented to the DNA substudy. The characteristics of the patients in the substudy, grouped by β1AR genotype and treatment, are provided in Table 4, which is published as supporting information on the PNAS web site. These groups were highly comparable; of particular relevance is that we found no differences in age, sex, race, HF etiology, New York Heart Association (NYHA) class, or baseline heart rate, blood pressure, or LVEF among groups stratified by placebo, bucindolol treatment, or genotype. As expected, the number of homozygous Gly-389 individuals was relatively small (52 placebo, 42 bucindolol). Thus, two genotypes were used for comparison: Arg homozygotes and Gly carriers (see Methods). The four cohorts, grouped by treatment and genotype, each consisted of >200 subjects (Table 4).
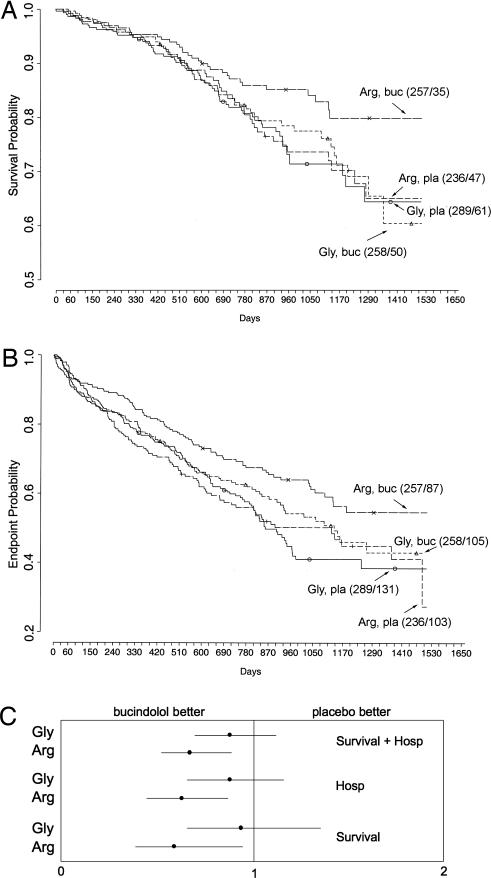
Survival of placebo- and bucindolol-treated patients stratified by β1AR-389 genotype is shown in the Kaplan–Meier curves displayed in Fig. 4A. Individual comparisons, adjusted for age, sex, and race, revealed that homozygous Arg bucindolol-treated patients had increased survival compared with Arg placebo-treated patients [hazard ration (HR) = 0.62, 95% confidence interval (C.I.) = 0.40–0.96, P = 0.03], an improvement of 38% in bucidolol over placebo. This same comparison in Gly carriers revealed no difference in survival curves (HR = 0.90, 95% C.I. = 0.62–1.30, P = 0.57), indicative of no treatment response to bucindolol. There was also an apparent influence of β1AR genotype on the HF exacerbations during bucindolol treatment, as measured by hospitalization because of HF. A decrease in hospitalizations with bucindolol treatment in homozygous Arg patients was observed compared with placebo patients with the same genotype (HR = 0.64, 95% C.I. = 0.46–0.88, P = 0.006). Gly-389 carriers showed no benefit of the drug compared with placebo in terms of hospitalizations (HR = 0.86, 95% C.I. = 0.64–1.15, P = 0.30). For the combined outcome of time to first HF hospitalization or death (Fig. 4B), a bucindolol-associated favorable treatment effect was evident for Arg patients compared with placebo (HR = 0.66, 95% C.I. = 0.50–0.88, P = 0.004), but it was not apparent in bucindolol-treated Gly-389 carriers vs. placebo (HR = 0.87, 95% C.I. = 0.67–1.11, P = 0.250). Given that there was some overlap in the 95% C.I.s (Fig. 4C) for the HRs for the three outcomes above between Arg and Gly, the test for interaction between β1-389 genotype and treatment was not statistically significant (P = 0.18, 0.17, and 0.15, respectively), but the trends all favored bucindolol with the Arg genotype. Several other outcomes (see Table 4) were considered in secondary analyses. Of particular note, there were no trends by genotype in the change in heart rate or LVEF during bucindolol treatment. There were no apparent trends in the adjudicated cause of death and genotype, although the number of events in the various categories limited power. Finally, adjustments for the uncommon position-49 polymorphism (Gly-49) had no significant effect on any of the above results (data not shown).
Fig. 4.
Kaplan–Meier analysis of endpoints in the placebo–bucindolol study stratified by treatment and β1AR genotype. (A) For survival, Arg homozygotes had an HR = 0.62, 95% C.I. = 0.40–0.96, P = 0.03. In contrast, for β1-Gly-389 carriers, the HR = 0.90, 95% C.I. = 0.62–1.30, P = 0.57. (B) For the combined endpoint of death or HF hospitalization, Arg homozygotes had an HR = 0.66, 95% C.I. = 0.50–0.88, P = 0.004. In contrast, for β1-Gly-389 carriers, the HR = 0.87, 95% C.I. = 0.67–1.11, P = 0.25. In parentheses next to each curve are the number of total subjects enrolled/the number of events at end of study. buc, bucindolol; pla, placebo. (C) Graphical representation of HRs and C.I.s by bucindolol and placebo is shown. Hosp, hospitalization.
An important issue for the current study is the known difference in the frequency of the Arg allele between blacks and nonblacks (14) and the fact that, in the entire BEST cohort (6), bucindolol’s mortality effect in blacks was less favorable than in nonblacks. In the current substudy, the allele frequency for Arg in the bucindolol group was found to be 0.73 in nonblacks vs. 0.62 in blacks. We thus considered whether the current findings are based simply on race rather than genotype. However, based on the number of blacks in the study (≈20%), the allele frequency difference would have needed to be >10-fold in order for Gly to be a meaningful surrogate for identification of blacks-only and to affect overall outcome. Furthermore, as noted above, the HRs were adjusted for race, and, nevertheless, an advantage was observed for Arg but not Gly patients. We also carried-out this same analysis excluding the black subjects, thus removing any potential for confounding by race. For mortality, Arg patients treated with bucindolol had an HR = 0.56, 95% C.I. = 0.34–0.90, P = 0.017, vs. placebo. The bucindolol treatment HR for Gly = 0.81, 95% C.I. = 0.53–1.24, P = 0.34, vs. placebo (interaction P = 0.11). Thus, even in the nonblacks, the Arg genotype is associated with a favorable trend but not the Gly genotype, and the minor differences in allele frequency of Arg in blacks did not confound the results of the entire substudy population.
Exaggerated Sympatholysis, Genotype, and the Response to Bucindolol.
Increased adrenergic activity supports compromised myocardial function but contributes to the progression of HF (15). This relationship between adrenergic activity and outcomes was observed in BEST, where a marked decrease of adrenergic activation (change in NE levels from baseline, termed exaggerated sympatholysis) was associated with increased mortality in bucindolol-treated patients (16). Unlike other β-blockers, bucindolol has potent sympatholytic properties, and, in BEST, 18% of patients treated with bucindolol exhibited exaggerated NE decreases at 3 months, which was associated with a 1.7-fold increased risk of subsequent mortality (16). Although the increased mortality risk of exaggerated sympatholysis is not completely understood, it probably involves unfavorable loss of adrenergically mediated contractility support to the failing heart. Patients who are Arg homozygous could, therefore, potentially “tolerate” the loss of NE signaling better than Gly carriers, because the former has enhanced agonist-promoted coupling to contractility (Fig. 2 A and B). Another potential mechanism by which Arg homozygotes could have a therapeutic advantage would be the greater absolute degree of antagonism afforded by bucindolol with the Arg receptor under conditions of elevated NE (Fig. 3). To determine which of these two mechanisms may have influenced the favorable therapeutic effect of bucindolol in Arg vs. Gly subjects, we compared mortality effects by baseline NE and by NE change at 3 months. In this analysis, the number of events was small once patients were stratified by treatment, genotype, and NE status, and, thus, we considered trends in the HRs as relevant indicators of a genotype effect on survival. These trends (Table 1), reveal that the HRs of Arg homozygotes to Gly carriers for mortality actually decreases with increasing baseline NE, suggesting a progressive advantage to bucindolol-treated Arg homozygotes with increasing baseline adrenergic drive. For the change in NE analysis, we compared mortality in Arg with Gly patients in three groups with an increased risk for mortality. These groups were previously identified by a likelihood analysis (16) and consisted of (i) those with increased mortality and a >244 pg/ml reduction in NE at 3 months of treatment), (ii) a reference group with little or no change in NE (−244 to +145 pg/ml) not found to be at risk for increased mortality, and (iii) a group at increased risk for mortality with >145 pg/ml increase in NE from bucindolol treatment. As can be seen in Table 1, there is no advantage to Arg homozygotes in the subgroup at increased risk for mortality from exaggerated sympatholysis (Group 1); the HR of 1.07 indicates a negligible advantage of Gly carriers in this group. On the other hand, as for baseline NE, there is a trend of decreasing HRs with increasing NE rise at 3 months. Indeed, in Group 3, the relative advantage of Arg over Gly amounted to 64% reduction in mortality. Taken together, these NE data suggest that the bucindolol therapeutic advantage for Arg variant is related to the degree of adrenergic activity that can be antagonized and not to protection against sympatholysis.
Table 1.
Relationship among NE levels, genotype, and survival in patients treated with bucindolol
| NE group | Mortality HR, Arg:Gly | 95% C.I. | Cox P value | No. of events |
|---|---|---|---|---|
| BSL NE (n) | ||||
| 64–356 (146) | 0.90 | 0.37, 2.22 | 0.82 | 19 |
| 358–545 (144) | 0.74 | 0.37, 1.51 | 0.41 | 31 |
| 546–2571 (149) | 0.68 | 0.32, 1.47 | 0.33 | 29 |
| Change in NE* (n) | ||||
| Group 1 (70) | 1.07 | 0.41, 2.78 | 0.89 | 17 |
| Group 2 (248) | 0.82 | 0.43, 1.59 | 0.56 | 36 |
| Group 3 (54) | 0.36 | 0.11, 1.15 | 0.08 | 14 |
The cutpoints for NE change in the three groups were determined from a previously published likelihood analysis (22) from BEST. These were (i) those with a >244 pg/ml reduction in NE with treatment that was associated with a 1.69-fold increased mortality, (ii) a reference group with little or no change in NE (−244 to +145 pg/ml) not found to be at risk for increased mortality, and (iii) those with a >145 pg/ml increase in NE with treatment associated with a 1.65-fold increased mortality. BSL, baseline.
*Change in NE from baseline after 3 months of bucindolol treatment.
Discussion
The potential for the β1AR genotype at position 389 to affect the clinical response to bucindolol was explored in the largest prospective, randomized, long-term, placebo-controlled β-blocker trial with accompanying archived DNA to date. These clinical results, as well as those from the human ex vivo functional studies and heterologously expressing cells represent findings not readily predicted by previous studies. In transgenic mice, the Arg genotype was associated with increased mortality from progressive HF that was not observed in this study, as the Kaplan–Meier curves of Arg and Gly patients on placebo were essentially superimposable (Fig. 4 A and B). This difference between the mouse and human studies is likely because of overexpression of the receptors acting to amplify the phenotypes. And, furthermore, we note that, even though Gly patients have lower β1AR signaling, it is not “protective,” which might be expected to improve survival compared with Arg in the placebo group. This finding implies that, as the pathological cycle of failure and increased NE develops, both Arg and Gly patients are above the threshold necessary for worsening function over time. But the absolute change in signaling imposed by β-blocker treatment portends greater improvement in Arg patients. We have previously reported that membranes from failing human Gly hearts have greater signaling to adenylyl cyclase than Arg, which, along with the transgenic studies, suggested a time-dependent switch in phenotypes between Arg and Gly receptors (10). Such a change in phenotype could have been manifested as a difference in responsiveness to β-blockade based on genotype as well as the timing of administration of the drug. However, in this work, we show by a more relevant measure (contractility) that Arg hearts remain hyperfunctional even at end-stage HF. The difference between the two studies is likely because of the fact that, in myocardial membrane adenylyl cyclase assays, a significant portion of the response to the non-subtype-specific agonist isoproterenol is due to stimulation of the β2AR (17), whereas, in ventricular contraction studies, the response is mediated primarily by β1AR (2). Thus, the current data, which use human ventricular tissue endogenously expressing these receptors and a physiologic readout, do not show a phenotypic switch, even at end stage. And furthermore, we note a genotype-dependent therapeutic response for Arg, regardless of age, severity, or length of disease at the time of initiating bucindolol therapy, which would not be expected if such a change in signaling occurred.
It has also been suggested that the efficacy of bucindolol in BEST might have been adversely affected because the agent could have intrinsic sympathomimetic activity (partial agonism) (18). Although studies with hearts from rat (19) and dog (20) indicate such actions, human studies have been equivocal. In normal human ventricular samples in culture where cAMP accumulation was used as the endpoint, partial agonist activity has been reported (18). These results have not been consistent, however, with human, failing ventricles. In one study (21), bucindolol had no positive inotropic effect in six ventricular strips studied. In another study of eight failing ventricular strips, three had positive inotropic responses, and five had negative inotropic responses (22). Another study in isolated preparations of failing human hearts found no evidence of intrinsic sympathomimetic activity (23). In human trials of HF, there is no evidence for bucindolol having partial agonist activity based on heart rate, contractility, or other cardiovascular measures (6, 24, 25). In this work, segregating the hearts by genotype, we note no significant partial agonist effect at either genotype and, indeed, a small inverse agonist effect with Arg hearts. In contrast, the partial agonist xamoterol acted as might be expected, showing small increases in contractility with Arg and less or no response with Gly. When carvedilol was used in contractility studies, we found no genotype-dependent differences in signaling, and the overall response was consistent with this β-blocker acting as a neutral antagonist at both receptors. Whether this finding portends a different genotype–outcome response with carvedilol as compared with bucindolol will need to be addressed with clinical trials.
Collectively, the results from these ex vivo, cell, and human studies strongly indicate that position-389 variants of the β1AR are predictors of the response to the β-blocker bucindolol in chronic HF. Given that bucindolol, metoprolol, carvedilol, and bisoprolol each have differences in βAR subtype specificity, sympatholysis, and other properties (3), the current results with bucindolol may not necessarily be generalized to other β-blockers. Of note, one published study in HF has found no association between β1AR genotype and the combined response of hospitalizations and death to treatment with metoprolol (26). In that study, however, ≈45% of the patients had mild HF (NYHA Class II), and the mean follow-up period was only 12 months. The lack of agreement between that study and ours may be due to this difference in severity, follow-up or event rates, or to differences in the properties of the two β-blockers (3). Interestingly, the Gly allele has recently been reported to be associated with an increased frequency of altering non-β-blocker medications during a metoprolol titration compared with Arg (27) as well as a poor LVEF response (28). On the other hand, another study found no association with LVEF or heart rate changes and genotype during treatment with either metoprolol or bisoprolol (29). Nor has β1AR genotype been found to be associated with the survival response to β-blockers after acute coronary syndrome (30).
In summary, we have shown that the Arg/Gly 389 polymorphism of the β1AR has a significant impact on agonist-mediated contractility in nonfailing and failing human hearts and the response to antagonists and partial agonists in the failing heart, directs the absolute degree of inhibition of signal transduction by bucindolol, and is associated with trends in the clinical response of bucindolol in patients with HF. These results, obtained by using multiple approaches, indicate that the response to this form of therapy can be influenced by β1AR genotype and sets the stage for the development of genomics-based treatments of chronic HF with antiadrenergic agents.
Methods
Studies were approved by the Institutional Review Boards or Animal Use and Care Committees of the University of Cincinnati, the University of Colorado, or the BEST DNA Oversight Committee.
Ex Vivo Human Ventricular Studies.
Nonfailing hearts were obtained from local potential organ donors whose hearts were not transplanted because of physical or ABO blood type incompatibility. Failing hearts were from patients with end-stage HF due to ischemic or nonischemic dilated cardiomyopathies who underwent cardiac transplantation. The contractile response of isolated, field-stimulated human trabeculae was assessed as described (2, 31, 32). Trabeculae of uniform size (1–2 × 6–8 mm) were mounted in 80-ml muscle bath chambers in Tyrode’s solution at pH 7.45 bubbled with 95% O2 and 5% CO2 at 37°C. Field stimulation by a 5-ms pulse at 10% above threshold was then applied, and, after equilibration, dose–response curves to ISO, bucindolol, or xamoterol were performed by using the indicated concentrations and application of increasing doses every 5 min. For studies of potential partial agonist effects of bucindolol, carvedilol, and xamoterol, 13 Arg-homozygous and 10 Gly-carrier ventricular preparations were precontracted with 10 μM forskolin, a condition which is optimal for detecting weak partial agonism (31, 32). Systolic tension at each dose was calculated as the stimulated tension in mN/mm2 minus baseline tension, and the entire dose–response curve was subjected to nonlinear curve fitting. A statistically significant negative or positive slope of the responses on grouped data was used to identify negative or positive inotropic effects, respectively. Differences in isoproterenol dose–response curves with respect to genotype were determined by analysis of covariance and a test for interaction between curve slopes, a method previously shown to be highly sensitive for weak responses (2, 31, 32).
Radioligand Binding, cAMP Assays.
Chinese hamster fibroblasts were stably transfected by using constructs representing the human β1AR with either Arg or Gly at encoded residue 389. Receptor expression and affinity for bucindolol were determined by radioligand binding studies with [125I]cyanopindolol ([125I]CYP) as described (8). Whole-cell cAMP accumulation studies were carried out by the [3H]adenine method, with exposure to the indicated agents for 15 min at 37°C. A crude membrane fraction was prepared from the free walls of right ventricles of all human hearts used in this study, and β1- and β2AR densities were measured by [125I]CYP binding as described (33).
Patient Population and Genotyping.
Phenotypic data and archived DNA were used from patients who participated in the BEST (6), who consented for DNA substudies. The study was a multicenter, randomized, long-term, placebo-controlled trial of the β-blocker bucindolol in patients with Class III/IV HF. The study design has been described in detail elsewhere (34). DNA was extracted from whole blood or myocardium by using standard techniques. Genotyping at the β1AR gene was performed by using methods exactly as described in detail in ref. 35.
Statistical Methods.
The systolic tension (force in mN/mm2) dose–responses, radioligand binding, and cAMP results were analyzed and compared as described above or in previous reports (8, 10, 32). These data are reported as mean ± SE. Because of the known low frequency of Gly homozygotes (14), the primary genotypes in the clinical trial were considered, a priori, as either Arg homozygous or Gly carriers (heterozygous or Gly homozygous. The primary endpoints were all-cause mortality, hospitalizations that were adjudicated by an endpoints committee (6) as being due to HF, and the combined endpoint of time to death or HF hospitalization. Cumulative survival curves were constructed by Kaplan–Meier methods (36). The Cox proportional-hazards regression model was used to examine the effects of treatment stratified by the indicated genotype. Results were adjusted for age, sex, and race. Because of the limited number of comparisons and a hypothesis that was based on the results we found in the cell-based and human ventricle studies (see Results), we considered P values <0.05 as significant, without adjustments for multiple comparisons. For the clinical characteristics of the patients, continuous variables are reported as mean ± SD, and comparisons were by t test or Wilcoxon rank-sum tests. Categorical variables are reported as proportions, and comparisons were by χ2 or Fisher’s exact tests.
Supplementary Material
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants HL052318, HL48013, HL071609, and HL077101. DNA samples were provided by the BEST DNA Bank, cosponsored by the NHLBI and the Department of Veterans Affairs Cooperative Studies Program.
Abbreviations
- β1AR
β1-adrenergic receptor
- C.I.
confidence interval
- HF
heart failure
- HR
hazard ratio
- ISO
isoproterenol
- LVEF
left ventricular ejection fraction
- NE
norepinephrine.
Footnotes
Conflict of interest statement: S.B.L. and J.D.P. are consultants and M.R.B. is an officer and equity holder in ARCA Discovery (Denver, CO).
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Liggett S. B. Nat. Med. 2001;7:281–283. doi: 10.1038/85411. [DOI] [PubMed] [Google Scholar]
- 2.Bristow M. R., Ginsberg R., Umans V., Fowler M., Minobe W., Rasmusen R., Zera P., Menlove R., Shah P., Jamieson S., et al. Circ. Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 3.Bristow M. Circulation. 2003;107:1100–1102. doi: 10.1161/01.cir.0000054530.87613.36. [DOI] [PubMed] [Google Scholar]
- 4.MERIT-HF Study Group. Lancet. 1999;353:2001–2006. [Google Scholar]
- 5.Packer M., Coats A. J. S., Fowler M. B., Katus H. A., Krum H., Mohacsi P., Rouleau J. L., Tendera M., Castaigne A., Roecker E. B., et al. N. Engl. J. Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 6.BEST Trial Investigators. N. Engl. J. Med. 2001;344:1659–1667. [Google Scholar]
- 7.van Campen L. C., Visser F. C., Visser C. A. J. Cardiovasc. Pharmacol. 1998;32:S31–S35. doi: 10.1097/00005344-199800003-00006. [DOI] [PubMed] [Google Scholar]
- 8.Mason D. A., Moore J. D., Green S. A., Liggett S. B. J. Biol. Chem. 1999;274:12670–12674. doi: 10.1074/jbc.274.18.12670. [DOI] [PubMed] [Google Scholar]
- 9.Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., et al. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 10.Perez J. M., Rathz D. A., Petrashevskaya N. N., Hahn H. S., Wagoner L. E., Schwartz A., Dorn G. W., II, Liggett S. B. Nat. Med. 2003;9:1300–1305. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- 11.The Xamatoterol in Severe Heart Failure Study Group. Lancet. 1990;336:1–6. [PubMed] [Google Scholar]
- 12.Jasper J. R., Michel M. C., Insel P. A. Mol. Pharmacol. 1990;37:44–49. [PubMed] [Google Scholar]
- 13.Nuttall A., Snow H. M. Br. J. Pharmacol. 1982;77:381–388. doi: 10.1111/j.1476-5381.1982.tb09309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore J. D., Mason D. A., Green S. A., Hsu J., Liggett S. B. Hum. Mutat. 1999;14:271. doi: 10.1002/(SICI)1098-1004(1999)14:3<271::AID-HUMU14>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 15.Port J. D., Bristow M. R. J. Mol. Cell. Cardiol. 2001;33:887–905. doi: 10.1006/jmcc.2001.1358. [DOI] [PubMed] [Google Scholar]
- 16.Bristow M. R., Krause-Steinrauf H., Nuzzo R., Liang C. S., Lindenfeld J., Lowes B. D., Hattler B., Abraham W. T., Olson L., Krueger S., et al. Circulation. 2004;110:1437–1442. doi: 10.1161/01.CIR.0000141297.50027.A4. [DOI] [PubMed] [Google Scholar]
- 17.Bristow M. R., Hershberger R. E., Port J. D., Minobe W., Rasmussen R. Mol. Pharmacol. 1989;35:295–303. [PubMed] [Google Scholar]
- 18.Andreka P., Aiyar N., Olson L. C., Wei J. Q., Turner M. S., Webster K. A., Ohlstein E. H., Bishopric N. H. Circulation. 2002;105:2429–2434. doi: 10.1161/01.cir.0000016050.79810.18. [DOI] [PubMed] [Google Scholar]
- 19.Deitchman D., Perhach J. L., Jr., Snyder R. W. Eur. J. Pharmacol. 1980;61:263–277. doi: 10.1016/0014-2999(80)90128-4. [DOI] [PubMed] [Google Scholar]
- 20.Willette R. N., Aiyar N., Yue T. L., Mitchell M. P., Disa J., Storer B. L., Naselsky D. P., Stadel J. M., Ohlstein E. H., Ruffolo R. R., Jr. J. Pharmacol. Exp. Ther. 1999;289:48–53. [PubMed] [Google Scholar]
- 21.Maack C., Bohm M., Vlaskin L., Dabew E., Lorenz K., Schafers H. J., Lohse M. J., Engelhardt S. Circulation. 2003;108:348–353. doi: 10.1161/01.CIR.0000080325.94345.8B. [DOI] [PubMed] [Google Scholar]
- 22.Maack C., Cremers B., Flesch M., Hoper A., Sudkamp M., Bohm M. Br. J. Pharmacol. 2000;130:1131–1139. doi: 10.1038/sj.bjp.0703400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hershberger R. E., Wynn J. R., Sundberg L., Bristow M. R. J. Cardiovasc. Pharmacol. 1990;15:959–967. doi: 10.1097/00005344-199006000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert E. M., Anderson J. L., Deitchman D., Yanowitz F. G., O’Connell J. B., Renlund D. G., Bartholomew M., Mealey P. C., Larrabee P., Bristow M. R. Am. J. Med. 1990;88:223–229. doi: 10.1016/0002-9343(90)90146-5. [DOI] [PubMed] [Google Scholar]
- 25.Bristow M. R., Roden R. L., Lowes B. D., Gilbert E. M., Eichhorn E. J. Clin. Cardiol. 1998;21:I-3–I-13. doi: 10.1002/clc.4960211303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White H. L., De Boer R. A., Maqbool A., Greenwood D., van Veldhuisen D. J., Cuthbert R., Ball S. G., Hall A. S., Balmforth A. J. Eur. J. Heart Failure. 2003;5:463–468. doi: 10.1016/s1388-9842(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 27.Terra S. G., Pauly D. F., Lee C. R., Patterson J. H., Adams K. F., Schofield R. S., Belgado B. S., Hamilton K. K., Aranda J. M., Hill J. A., et al. Clin. Pharmacol. Ther. 2005;77:127–137. doi: 10.1016/j.clpt.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Terra S. G., Hamilton K. K., Pauly D. F., Lee C. R., Patterson J. H., Adams K. F., Schofield R. S., Belgado B. S., Hill J. A., Aranda J. M., et al. Pharmacogenet. Genomics. 2005;15:227–234. doi: 10.1097/01213011-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 29.de Groote P., Helbecque N., Lamblin N., Hermant X., McFadden E., Foucher-Hossein C., Amouyel P., Dallongeville J., Bauters C. Pharmacogenet. Genomics. 2005;15:137–142. doi: 10.1097/01213011-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Lanfear D. E., Jones P. G., Marsh S., Cresci S., McLeod H. L., Spertus J. A. J. Am. Med. Assoc. 2005;294:1526–1533. doi: 10.1001/jama.294.12.1526. [DOI] [PubMed] [Google Scholar]
- 31.White M., Wiechmann R. J., Roden R. L., Hagan M. B., Wollmering M. M., Port J. D., Hammond E., Abraham W. T., Wolfel E. E., Lindenfeld J., et al. Circulation. 1995;92:2183–2189. doi: 10.1161/01.cir.92.8.2183. [DOI] [PubMed] [Google Scholar]
- 32.Bristow M. R., Ginsburg R., Minobe W., Cubicciotti R. S., Sageman W. S., Lurie K., Billingham M. E., Harrison D. C., Stinson E. B. N. Engl. J. Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 33.Lowes B. D., Minobe W., Abraham W. T., Rizeq M. N., Bohlmeyer T. J., Quaife R. A., Roden R. L., Dutcher D. L., Robertson A. D., Voelkel N. F., et al. J. Clin. Invest. 1997;100:2315–2324. doi: 10.1172/JCI119770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The BEST Steering Committee. Am. J. Cardiol. 1995;75:1220–1223. doi: 10.1016/s0002-9149(99)80766-8. [DOI] [PubMed] [Google Scholar]
- 35.Small K. M., Rathz D. A., Liggett S. B. Methods Enzymol. 2002;343:459–475. doi: 10.1016/s0076-6879(02)43152-7. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan E. L., Meier P. J. Am. Stat. Assoc. 1958;53:457–481. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.