Abstract
CTL-associated antigen 4 (CTLA-4) engagement negatively regulates T cell activation and function and promotes immune tolerance. However, it has been difficult to explore the biology of selective engagement of CTLA-4 in vivo because CTLA-4 shares its ligands, B7-1 and B7-2, with CD28. To address this issue, we developed a Tg mouse expressing a single-chain, membrane-bound anti–CTLA-4 Ab (scFv) on B cells. B and T cells developed normally and exhibited normal phenotype in the steady state and after activation in these mice. However, B cells from scFv Tg+ mice (scαCTLA4+) prevented T cell proliferation and cytokine production in mixed lymphocyte reactions. Additionally, mice treated with scαCTLA4+ B cells had decreased T cell–dependent B cell Ab production and class switching in vivo after antigen challenge. Furthermore, expression of this CTLA-4 agonist protected NOD mice from spontaneous autoimmune diabetes. Finally, this disease prevention occurred in Treg-deficient NOD.B7-1/B7-2 double-knockout mice, suggesting that the effect of the CTLA-4 agonist directly attenuates autoreactive T cell activation, not Treg activation. Together, results from this study demonstrate that selective ligation of CTLA-4 attenuates in vivo T cell responses, prevents development of autoimmunity, and represents a novel immunotherapeutic approach for the induction and maintenance of peripheral tolerance.
Introduction
CTL-associated antigen 4 (CTLA-4, also known as CD152) has been well established as a negative regulator of T cell function (1–3). CTLA-4 ligation antagonizes early T cell activation, leading to decreased IL-2 production, inhibition of cell cycle progression, decreased induction of cyclins by anti-CD3, and modulation of TCR signaling (2, 4–6). Mice deficient for CTLA-4 develop lymphoproliferative disease and die within 4 weeks of birth (7, 8) adding further evidence for the critical role of CTLA-4 inhibition of T cell responses. More recently, CTLA-4 has been implicated in the function of Tregs, which suppress effector T cell activation and function (9, 10). In fact, it is clear that CTLA-4 plays a critical role in both the induction and maintenance of immune tolerance (11, 12). Thus, many efforts have focused on developing therapeutic approaches to selectively engage this pathway to treat autoimmunity and organ transplant rejection. Unfortunately, these attempts have been largely unsuccessful due to the complex biology of CTLA-4 (13). Early studies using Abs to target the CTLA-4 ligands B7-1 (also known as CD80) and B7-2 (also known as CD86) for immune regulation have been unreliable, as these ligands are shared with the positive costimulatory molecule CD28. Thus, rather than engaging CTLA-4 to turn off immunity, the ligands promote CD28/B7 interactions, resulting in enhanced T cell activation, expansion, and differentiation. Second, in vivo administration of anti–CTLA-4 Abs routinely acts as an antagonist of CTLA-4 function. For instance, treatment of autoimmune-prone mice with anti–CTLA-4 results in the exacerbation of diseases, including experimental autoimmune encephalomyelitis and autoimmune diabetes (14, 15). Thus, a new approach to directly engage the CTLA-4 molecule with an agonist signal is essential for treating autoimmunity.
CTLA-4 signaling requires cross-linking in conjunction with T cell receptor engagement. This requirement is a direct consequence of the fact that CTLA-4 functions by directly influencing TCR-mediated signal transduction by altering TCR-ζ chain phosphorylation and immune synapse formation (6, 16, 17). Thus, any effective negative signal following CTLA-4 engagement depends on the molecule being engaged, both physically and temporally, together with the TCR signal from the same APC (6). This most likely explains why in vivo treatment of mice with anti–CTLA-4 Abs acts as an antagonist, since even Fc receptor–mediated cross-linking would not likely occur with a cell bearing the relevant antigens. We hypothesized that effective delivery of a CTLA-4 agonist would depend on presentation of a specific CTLA-4 ligand (i.e., anti–CTLA-4 Ab) on a relevant APC-bearing cognate antigen. Thus, we developed a single-chain, membrane-bound anti–CTLA-4 Ab (scFv) that can be expressed directly on APCs. In previous studies, we demonstrated that this reagent was effective in vitro in blocking T cell activation and, as predicted, was effective when presented in cis, on the same APC, during TCR signaling (18). In this study, we have addressed the in vivo potential of such a reagent in an autoimmune setting. A construct was generated and expressed selectively on antigen-presenting B cells, which have been shown to be essential in secondary immunity and autoimmune diabetes in the NOD mouse strain (19). The model also takes advantage of the motility of B cells that traffic to sites of antigen priming and participate in autoimmune reactions at sites of inflammation. Using this Tg mouse model, we demonstrate that cell surface–bound anti–CTLA-4 does not alter normal B or T cell development or function but selectively inhibits antigen-specific T cell function in vivo and the development of autoimmune diabetes.
Results
Generation of membrane-bound anti–CTLA-4 Tg+ mice.
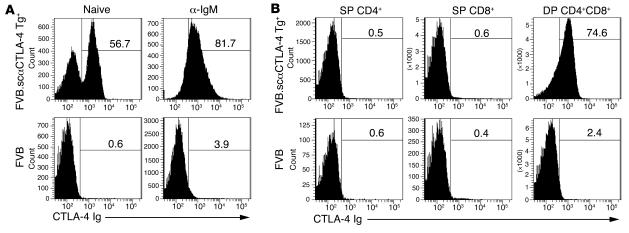
The anti–CTLA-4 (clone 4F10) single-chain, membrane-bound (4F10scFv) construct has been well characterized in vitro and shown to have profound inhibitory effects on T cell function (18, 20). These results prompted us to develop a Tg mouse model to test this novel construct in vivo. We chose to express the molecule on B cells since previous studies showed that the scFvmCTLA-4 was most effective when coexpressed on APCs, and B cells would be an effective cell population to selectively test antigen-specific responses. Thus, the IgM heavy-chain promoter and enhancer (PμEμ) was used to drive the expression of single-chain, anti–CTLA-4 (7M-4F10scFv) (21). In order to ensure that there would be no reverse signaling to the scFv-expressing cells, the construct was created using the transmembrane domain from B7-1, which lacked the C terminal intracytoplasmic tail (18, 20). The PμEμ 7M-4F10scFv B7-1 construct was microinjected into day 1 embryos isolated from FVB/n mice. B cell–specific 7M-4F10scFv B7-1 Tg+ mice (hereafter referred to as scαCTLA-4) developed normally according to Mendelian frequencies and without gross defects. Tg scαCTLA-4 expression was identified by flow cytometry using murine CTLA-4 Ig. scαCTLA-4 expression was found on B cells isolated from the spleen and peripheral lymph nodes. Approximately 55% of the B220+ B cells isolated from spleen of Tg mice were CTLA-4 Ig positive, with a range of 30%–60% (Figure 1A). This staining specificity was confirmed using human CTLA-4 Ig, which did not bind (data not shown). Stimulation of B cells with anti-IgM or LPS significantly increased the expression of scαCTLA-4 Tg+ to more than 80% of the B220+ B cells (Figure 1A). scαCTLA-4 expression was also detected on CD4+CD8+ double-positive (DP) thymocytes but not on single-positive (SP) CD4+ or CD8+ thymocytes (Figure 1B), peripheral CD4+ and CD8+ T cells, macrophages, or DCs (data not shown). It is also important to note that activated CD4+ and CD8+ T cells did not express scαCTLA-4 following anti-CD3 and anti-CD28 activation (data not shown).
Figure 1. Tg+ scαCTLA-4 expression.
Flow cytometric analysis was performed on splenocytes from scαCTLA-4 Tg+ mice and littermate controls using soluble murine fusion protein mCTLA-4 Ig and anti-B220. (A) Upper panels demonstrate scαCTLA-4 on naive Tg+ B cells and activated Tg+ B cells following anti-IgM stimulation (48 hours, 1 μg/ml) compared with FVB littermate negative controls (lower panels). All plots were gated on B220+ B cells. (B) Flow cytometric analysis was performed on thymocytes from scαCTLA-4 Tg mice and littermate controls as described above. Flow plots illustrate scαCTLA-4 expression in DP CD4+CD8+ thymocytes from scαCTLA-4 Tg+ mice, but not DP CD4+CD8+ thymocytes from FVB or SP CD4+ and SP CD8+ from scαCTLA-4 Tg+ or FVB Tg– controls. Results shown are representative of 3 independent experiments with ≥ 3 mice per group.
T and B cells develop normally in scαCTLA-4 Tg+ mice.
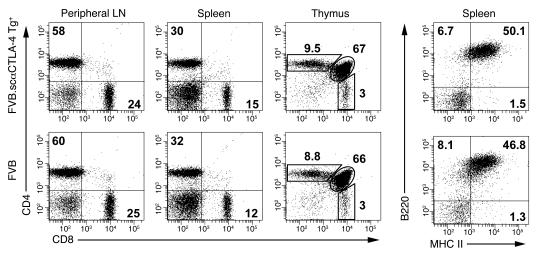
Despite scαCTLA-4 expression on DP thymocytes, T cell development appeared normal. This was evident in the thymus, with 70% CD4+CD8+ DP, 9% CD4+ SP, and 3% CD8+ SP thymocytes for both scαCTLA-4 Tg+ and littermate controls (Figure 2). Moreover, we did not find any changes in the peripheral T cells from lymphocytes nor significant changes in the total spleen cellularity, based on total numbers or composition. Approximately 55% B220+ B cells, 30% CD4+ T cells, and 15% CD8+ T cells were observed in both groups (Figure 2). Additionally, we did not find any differences in marginal zone, follicular B cells, or transitional B cells in Tg+ mice compared with controls (data not shown). CD4+ T cells constituted approximately 60% of the T cell composition of peripheral lymph nodes, with 25% being CD8+ T cells (Figure 2). These data indicated that although scαCTLA-4 expression was detected in the DP thymocytes, T and B cell development was normal.
Figure 2. Characterization of T cells and B cells in scαCTLA-4 Tg+ mice.
Flow cytometric analysis was performed to determine the percentage of CD4+ T cells, CD8+ T cells, and B220+ B cells in the spleen, peripheral lymph nodes, and thymus from FVB and FVB.scαCTLA-4 Tg+ mice. The number in each histogram quadrant represents the percentage of total cells. Results shown are representative of 3 independent experiments with ≥ 3 mice per group.
scαCTLA-4 Tg+ B and T cell function.
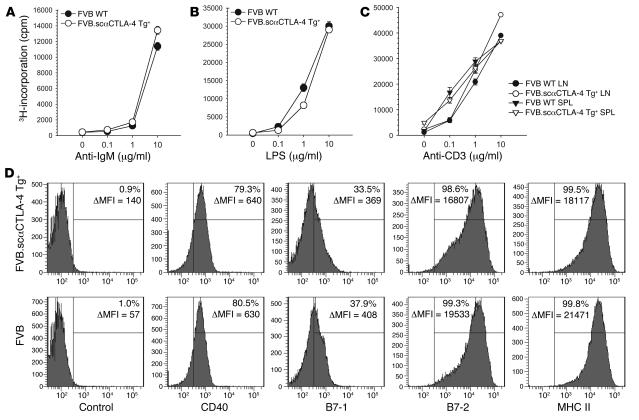
The functional response of splenocytes (Figure 3, A and B) or purified B cells (data not shown) from scαCTLA-4 Tg+ mice and littermate controls was compared using anti-IgM or LPS as mitogens. B cell proliferation, Ab production, IgM, MHC class II, CD40, B7-1, and B7-2 expression were measured. The expression of the Tg did not alter B cell responsiveness to BCR or innate stimuli (Figure 3, A and B). In addition, the surface expression of CD40, B7-1, B7-2, and MHC class II on naive and activated Tg+ and Tg– B cells was comparable at 24, 48, and 72 hours (Figure 3D and data not shown).
Figure 3. In vitro activation of B cells and T cells from scαCTLA-4 Tg+ mice.
(A) B and T cell proliferation was assessed from scαCTLA-4 Tg+ and controls using splenocytes following 0, 0.1, 1, or 10 μg/ml (A) anti-IgM, (B) LPS, or (C) anti-CD3 stimulation. (D) Flow cytometric analysis from scαCTLA-4 Tg+ and FVB controls after 48-hour culture with anti-IgM (1 μg/ml). Shaded histogram represents positive stain for B7-1, B7-2, CD40, and MHC II. Percentage positive above isotype control with mean fluorescence intensity (ΔMFI) is shown. Histograms are gated on B220+ cells. Data presented are representative of 3 independent experiments with ≥ 3 mice per group.
We next assessed the functional potential of the peripheral T cell compartment. Purified T cells from spleen and peripheral lymph nodes were stimulated with plate-bound anti-CD3. Peripheral T cells isolated from scαCTLA-4 Tg+ mice proliferated in a dose-dependent response to TCR engagement in a manner similar to control FVB T cells (Figure 3C). CD69 upregulation and CD62L downmodulation were also normal upon activation in Tg+ T and B cells (data not shown). Collectively, these data indicate that the Tg expression of scαCTLA-4 on B cells or DP thymic T cells did not significantly alter the development or function of peripheral B and T cells to polyclonal stimuli, including LPS, anti-IgM, or anti-CD3 and anti-CD28.
scαCTLA-4 Tg+ B cells inhibit T cell proliferation and cytokine production.
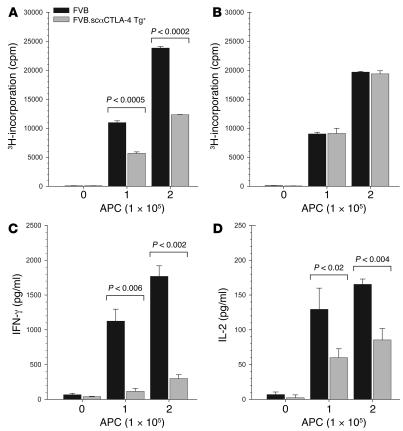
Next, we examined whether the expression of scαCTLA-4 on allogeneic B cells would alter T cell activation in vitro in a mixed lymphocyte reaction (MLR). Responder C57BL/6 T cells were stimulated with MHC-mismatched purified CD19+ B cells from FVB scαCTLA-4 Tg+ mice or littermate controls. T cell proliferation in response to scαCTLA-4 Tg+ allogeneic MHC was significantly lower (>50% reduction, P < 0.0002 at 1:5 responder/stimulator ratio or higher) compared with control B cells (Figure 4A). Total splenocytes from FVB and FVB.scαCTLA-4 were not as effective (30% reduction) at inhibiting T cell priming as purified B cells (50%), suggesting endogenous DC activated the alloreactive T cells (data not shown). These data also suggest that scαCTLA-4 function did not inhibit in trans although the 30% reduction in proliferation might suggest either a trans effect or, more likely, engagement of the activated T cells with antigen-bearing non-Tg B cells or DCs. In this regard, stimulation with purified scαCTLA-4 Tg+ B cells inhibited effector cytokine production in the allogeneic MLR. IFN-γ production was suppressed by 83% (P < 0.006) and IL-2 production by 68% (P < 0.02) (Figure 4, C and D). IL-4 and IL-10 were undetectable, indicating there was not a switch to a Th2-type response (data not shown). The reduced response was dependent on CTLA-4 expression on responding T cells, as C57BL/6 T cells from CTLA-4 KO mice responded equally to scαCTLA-4 Tg+ and Tg– B cell stimulations (Figure 4B). Thus, our results demonstrate that anti–CTLA-4 single-chain Abs expressed on APCs act as a CTLA-4 specific agonist to block responses of cognate T cells.
Figure 4. scαCTLA-4 Tg+ B cells inhibit allogeneic T cell proliferation and cytokine production during a MLR T cell response.
LPS-preactivated B cells from FVB WT control or FVB.scαCTLA-4 Tg+ mice were incubated with purified (A) C57BL/6 or (B) C57BL/6.CTLA-4 KO responder T cells in a MLR. Proliferation was assessed by tritiated thymidine incorporation. (C and D) Culture supernatant from WT C57BL/6 responder T cells demonstrates that scαCTLA-4 Tg+ B cells significantly reduced (C) IFN-γ and (D) IL-2 cytokine production after 48 hours of coculture. Responder T cells were cultured at 2 × 104 per well, and stimulator B cells were cultured at either 0, 1, or 2 × 105 per well as indicated. Results illustrated are representative of at least 2 independent experiments.
scαCTLA-4 inhibits T cell–dependent B cell Ab production in vivo.
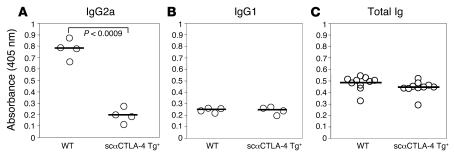
We next determined the effects of scαCTLA-4 on immunological responses to hapten-carrier complexes in vivo. WT FVB mice received dinitrophenyl-OVA–pulsed (DNP-OVA–pulsed) B cells from either scαCTLA-4 Tg+ mice or FVB controls (priming event). Seven days following initial priming, recipient mice received a boost of OVA antigen in CFA subcutaneously. DNP-specific Ab levels in sera were determined by using antigen-specific ELISA to determine levels of IgG1, IgG2a, and total Ig (days 0, 7, and 14). Mice immunized with antigen-pulsed B cells from the scαCTLA-4 Tg+ mice showed significantly decreased DNP-specific Abs (Figure 5A, P < 0.0009). There was a particular suppression of class switching to IgG2a, suggesting that coengagement of CTLA-4 during antigen exposure led to a profound suppression of Th1-dependent Ab production. We did not detect significant differences in levels of IgG1 anti-DNP Abs, indicating that scαCTLA-4 did not cause a switch to a Th2 Ab response (Figure 5B) consistent with the in vitro cytokine responses. Furthermore, we did not detect a significant change in total Ig levels, indicating that the effects of scαCTLA-4 were limited to antigen-specific responses (Figure 5C). These results demonstrate the important role CTLA-4 has in regulating T cell activity using a novel Tg approach that directly engages CTLA-4 on T cells in vivo influencing both T cell and T cell–dependent B cell responses.
Figure 5. scαCTLA-4 Tg+ B cells inhibit T cell–dependent B cell IgG2a Ab production in vivo.
scαCTLA-4 Tg and FVB littermate control B cells were activated in the presence of LPS (1 μg/ml) for 72 hours, loaded with DNP-OVA, and transferred to naive FVB recipients. Seven days following transfer of cells, recipient animals were boosted with 100 μg DNP-OVA in CFA injected subcutaneously. Fourteen days following B cell transfer, anti-DNP Ab production was measured using DNP-KLH–specific ELISA. Shown in A, anti-DNP serum IgG2a from 4 individual WT or scαCTLA-4 Tg+–treated mice. (B) Anti-DNP serum IgG1 from 4 individual WT or scαCTLA-4 Tg+–treated mice. (C) Total Ig from 10 individual WT or scαCTLA-4 Tg+–treated mice. The mean for each group is shown with a horizontal line. Results shown are representative of at least 2 independent experiments.
Effects of CTLA-4 in 2 models of autoimmune diabetes.
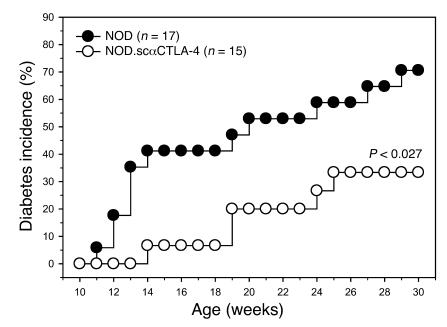
The scαCTLA-4 Tg+ mice were crossed onto the autoimmune diabetes-prone NOD mouse model to determine the effect of scαCTLA-4 B cell expression during the development of autoimmunity. Previous studies have shown that the development of diabetes in NOD mice is critically dependent on B cells but not Ab production, suggesting that B cells are prominent APCs in this setting (22, 23). NOD mice develop spontaneous diabetes between 12 and 30 weeks of age, with approximately 70% disease incidence in female mice in our colony. FVB.scαCTLA-4 Tg+ mice were backcrossed 10 generations to the NOD background. NOD.scαCTLA-4 Tg+ mice were characterized as described for the FVB.scαCTLA-4 Tg+ mice for B and T cell development and function. We found similar expression of the Tg on resting and activated B cells and on DP thymocytes and no expression on resting or activated CD4+ or CD8+ peripheral T cells (data not shown). scαCTLA-4 Tg expression was not detected in cells isolated from the pancreas (data not shown). CD19+ purified scαCTLA-4+ B cells were also tested in the MLR assay as previously described and were found to inhibit allogeneic T cell responder C57BL/6 purified T cells in vitro (data not shown) similarly to the FVB.scαCTLA-4 Tg+ cells (Figure 4). NOD.scαCTLA-4 and control NOD mice were monitored for the development of spontaneous diabetes. There was no statistical difference in insulin auto-Ab (IAA) responses in 5-, 10-, 20-, and 30-week-old NOD.scαCTLA-4 Tg+ mice as compared with NOD littermates (data not shown). However, there was a significant reduction of spontaneous diabetes in NOD.scαCTLA-4 Tg+ mice as compared with WT littermate NOD mice (Figure 6, P < 0.027). NOD diabetes incidence reached 70% (12 of 17) by 30 weeks of age while only 33% (5 of 15) of the NOD.scαCTLA-4 Tg+ mice were diabetic by the end of the study period (P < 0.027). There was also a significant delay in disease kinetics and time to 25% diabetes incidence. NOD control mice were diabetic by 12.2 weeks while the same incidence frequency in the NOD.scαCTLA-4 Tg+ mice did not occur until 19 weeks (P < 0.0077). Thus, expression of scαCTLA-4 Tg in NOD mice profoundly inhibited the development and progression of diabetes supporting a critical role for this pathway in autoimmunity.
Figure 6. NOD.scαCTLA-4 Tg+ mice are protected from spontaneous diabetes.
Female NOD.scαCTLA-4 Tg+ mice and NOD littermate controls were monitored for the development of spontaneous autoimmune diabetes. Diabetes incidence is shown here for NOD (n = 17) (filled circles) and NOD.scαCTLA-4 Tg+ (n = 15) (open circles). NOD diabetes incidence reached 70% (12 of 17) by 30 weeks of age while diabetes incidence in NOD.scαCTLA-4 Tg+ mice was significantly reduced to 33% (5 of 15) by 30 weeks of age (P < 0.027).
Mechanism of CTLA-4–mediated inhibition: scαCTLA-4 inhibition in NOD.B7 double-knockout mice in the absence of Tregs.
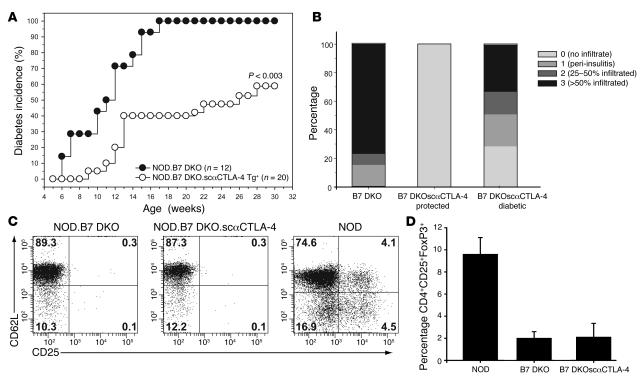
CTLA-4 has been proposed to be involved in Treg function (9). CTLA-4 blockade has been shown to inhibit Treg suppression (9) and abrogate Treg-mediated protection of autoimmune colitis and transplant rejection (24, 25). To determine if the scαCTLA-4 Tg blocks diabetes by directly inhibiting autoreactive T cells or affecting Treg activity, we took advantage of Treg-deficient NOD.B7-1.B7-2KO (NOD.B7 double-knockout [DKO]) mice. NOD.B7 DKO mice develop an accelerated form of autoimmune diabetes that begins as early as 2 weeks of age and progresses to 100 percent incidence (26). NOD.scαCTLA-4 Tg+ mice were bred with B7-deficient NOD.B7 DKO mice and monitored for diabetes development. As previously reported, diabetes was exacerbated in the NOD.B7 DKO mice with 100% incidence at 17 weeks. In contrast, ectopic expression of scαCTLA-4 suppressed the development of diabetes by 40% (Figure 7A, P < 0.003). Consistent with this finding, the Tg+ scαCTLA-4 mice that were protected from diabetes also had significantly less insulitis (Figure 7B). Although Tregs have been shown to be deficient in NOD.B7 DKO mice (26), it remained possible that the engagement of CTLA-4 by the agonist scαCTLA-4 restored Tregs in the NOD.B7 DKO.scαCTLA-4 Tg+ mice. We addressed this possibility by FACS analysis and determined that Tregs were not restored in NOD.B7 DKO.scαCTLA-4 Tg+ mice. Both B7 DKO groups had 0.3% CD4+CD25hiCD62L+ Tregs in comparison with 4% CD4+CD25hiCD62L+ Tregs in WT NOD mice (Figure 7C). Recent studies suggest that the putative transcription factor FoxP3 is the best marker of mouse Tregs. Therefore, we repeated the analysis of B7 DKO mice, utilizing intracellular staining of FoxP3 on CD4+CD25+ Tregs. As seen in Figure 7D, there is a significant decrease in the percentage of CD4+CD25+FoxP3+ Tregs in the NOD.B7 DKO mice as compared with the NOD mice (P < 0.0002). This defect in Treg numbers was not reconstituted in NOD.B7 DKO.scαCTLA4 Tg+ (P < 0.0004). Furthermore, there was no statistical difference in the number of FoxP3+ T cells between NOD.B7 DKO and NOD.B7 DKO.scαCTLA-4 Tg+ mice (P < 0.95). These results suggest that the inhibition of disease by scαCTLA-4 was not due to a restoration of Tregs; rather, the scαCTLA-4 Tg may directly inhibit autoreactive T cells.
Figure 7. scαCTLA-4 Tg+ NOD. B7 DKO mice are protected from accelerated diabetes.
Female NOD.B7 DKO and NOD.B7 DKO.scαCTLA-4 Tg+ mice were monitored for the development of spontaneous disease. (A) Diabetes incidence is shown here for NOD.B7 DKO (n = 12) (open circles) and NOD.B7 DKO.scαCTLA-4 Tg+ mice (n = 20) (filled circles). NOD.B7 DKO diabetes incidence reached 100% while diabetes incidence in NOD.B7 DKO.scαCTLA-4 Tg+ mice was significantly reduced to 60% by 30 weeks of age (P < 0.003). (B) NOD.B7 DKO.scαCTLA-4 Tg+ mice have decreased insulitis at 14 weeks of age. Pancreas from 14-week-old NOD.B7 DKO and scαCTLA-4 Tg NOD.B7 DKO mice were stained with H&E to determine clinical severity of insulitis. Pancreatic islets were scored for the presence of mononuclear infiltration: 0, no infiltrate; 1, peri-insulitis present; 2, 25–50% of the islet contained infiltrate; 3, more than 50% of the islet was infiltrated. Average percentages shown were determined from at least 100 islets from at least 3 mice per group. (C) Flow cytometric analysis was performed to determine the percentage of CD4+CD25+CD62L+ and (D) CD4+CD25+FoxP3+ Tregs in the lymphoid tissue of NOD.B7 DKO, NOD. B7 DKO.scαCTLA-4 Tg+, and NOD mice. The mean ± SD from at least 3 mice per group is shown. Results shown are representative of 3 independent experiments.
scαCTLA-4 Tg+ B cells inhibit the activation of autoreactive T cells in pancreatic lymph nodes.
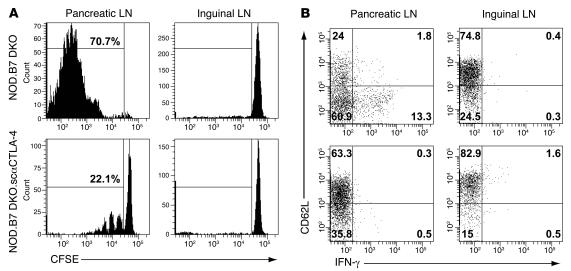
To further characterize T cell inhibition and test the hypothesis that CTLA-4 engagement directly inhibits autoreactive T cells, we compared islet antigen–specific T cell priming in the pancreatic LN of NOD.B7 DKO versus NOD.B7 DKO.scαCTLA-4 Tg+ recipients. Islet antigen–specific TCR Tg+ BDC2.5 Thy1.1 congenic CD4+CD25–CD62L+ LN cells were labeled with CFSE and adoptively transferred into prediabetic NOD.B7 DKO or NOD.B7 DKO.scαCTLA-4 Tg+ mice, and their proliferation was measured by CFSE dilution as a readout of in vivo priming (27). Antigen-specific Tregs were depleted to address the role of scαCTLA-4 on autoreactive effector T cells in the absence of regulation (9). As seen in Figure 8, 70.7% (65.3% ± 4.7 %) of the autoreactive BDC2.5 cells went into cycle in the pancreatic LN from NOD.B7 DKO recipient mice compared with only 22.1% (28.8% ± 10.7 %) in NOD.B7 DKO.scαCTLA-4 Tg+ recipients (Figure 8A). Moreover, BDC2.5 CD4+ T cells divided only 3 generations in NOD.B7 DKO.scαCTLA-4 hosts in contrast to 6 to 8 generations in the NOD.B7 DKO recipients (Figure 8A). There was no proliferation observed in antigen-deficient inguinal LN in either NOD.B7 DKO or NOD.B7 DKO.scαCTLA-4 Tg+ mice (Figure 8A). These results indicate that B cells bearing surface-bound anti–CTLA-4 suppress in vivo priming of autoreactive T cells in the absence of Tregs.
Figure 8. scαCTLA-4 Tg+ B cells inhibit the activation of autoreactive T cells in pancreatic lymph nodes.
BDC2.5.Thy1.1 CD4+ LN cells were labeled with CFSE and adoptively transferred to prediabetic 6-week-old NOD.B7 DKO or NOD.B7 DKO.scαCTLA-4 Tg+ mice. Pancreatic and inguinal LN cells were harvested from recipients (n = 3 for each group) 5 days later for analysis by flow cytometry for CFSE dye dilution as a readout of in vivo priming and proliferation. (A) Representative histogram CFSE plots of CD4+CD90.1+ T cells from NOD.B7 DKO and NOD.B7 DKO.scαCTLA-4 Tg+ recipients. (B) Direct assessment of in vivo differentiation and IFN-γ production measured by flow cytometry ex vivo. CD4+CD25– T cells from BDC2.5.Thy1.1.Yeti mice transferred to prediabetic NOD.B7 DKO or NOD.B7 DKO.scαCTLA-4 recipients (n = 3 for each group). Shown here is YFP (IFN-γ) and CD62L expression from CD4+CD90.1+ T cells from pancreatic and inguinal LNs. Results shown are representative of 2 independent experiments.
Next, we determined whether scαCTLA-4 similarly affects the ability of autoreactive BDC2.5 CD4+ T cells to differentiate and produce Th1 effector cytokines such as IFN-γ, which is known to be involved in disease pathogenesis (28). For this study, we utilized an IFN-γ reporter mouse in which the expression of yellow fluorescent protein (YFP) is under the control of the IFN-γ promoter (29). These targeted reporter mice were designated Yeti, for yellow-enhanced transcript for IFN-γ. Direct assessment of in vivo differentiation and IFN-γ production can thus be measured by flow cytometry ex vivo without the requirement of in vitro restimulation. We backcrossed these mice 12 generations to NOD and subsequently bred to NOD.BDC2.5.Thy1.1 TCR Tg+ mice to derive NOD.BDC2.5.Thy1.1.Yeti mice. The CD4+ T cells from BDC2.5.Thy1.1.Yeti mice were adoptively transferred to prediabetic NOD.B7 DKO or NOD.B7 DKO.scαCTLA-4+ recipients, and the number of YFP-expressing T cells in the pancreatic and inguinal LN were determined by FACS. It is important to note that freshly isolated CD4+ T cells from NOD.BDC2.5.Thy1.1.Yeti mice did not express YFP (data not shown). scαCTLA-4 Tg+ B cells blocked the IFN-γ production in the pancreatic LN 0.47% ± 0.06% compared with NOD.B7 DKO recipients with 10.3% ± 3% IFN-γ YFP–positive cells (Figure 8B). CD62L expression was also measured as a parameter of in vivo priming of CD4+ BDC2.5.Thy1.1+ gated cells from pancreatic and inguinal LN. IFN-γ–positive cells from the NOD.B7 DKO recipients downmodulated CD62L further, indicating in vivo activation in the absence of CTLA-4 signal. BDC2.5 cells from NOD.B7 DKO.scαCTLA-4 recipient mice did not express YFP, and only 35.8% (34.7% ± 1.4%) of the CD4+ cells from pancreatic LN downmodulated CD62L compared with 60.9 % (59.9% ± 2.3%) in controls (Figure 8B). The vast majority of CD4+ BDC2.5.Thy1.1 cells (~75%) from the inguinal LN in both groups did not express IFN-γ or downmodulate CD62L, indicating a lack of T cell priming as predicted (Figure 8B). Taken together, the results demonstrate that B cells expressing scαCTLA-4 can efficiently suppress proliferation and differentiation of autoreactive T cells in the draining LN in vivo (30).
Discussion
Although CTLA-4 engagement by B7 has been demonstrated to downregulate T cell responses in several systems, lack of appropriate reagents has prevented direct ligation in vivo to inhibit T cell function in autoimmune diseases. Rather, the majority of studies have utilized CTLA-4 antagonists or ectopic expression of B7 (which stimulate both CD28 and CTLA-4) to implicate the role of CTLA-4 in vivo (refs 14, 15 and reviewed in refs. 13, 31). In the studies presented here, we have generated a model to examine the effect of selective engagement of CTLA-4 during in vivo immune responses. This was accomplished by expressing a single-chain, membrane-bound scFv anti–CTLA-4 selectively on B cells. Our results demonstrate that scαCTLA-4 Tg+ expression did not affect T or B cell development but profoundly inhibited T cell responses to antigens presented by scαCTLA-4 Tg+ B cells in vitro and in vivo. Moreover, we show that selective engagement of CTLA-4 conferred significant protection against disease in 2 models of autoimmune diabetes, even in the absence of Tregs. The mechanism for this regulation was determined to be the prevention of autoreactive T cell expansion and development of effector function.
CTLA-4 has previously been demonstrated to regulate peripheral tolerance in several experimental systems (11, 12, 32). This regulation is at the level of T cell expansion and differentiation. In particular, we have previously shown that selective engagement of CTLA-4 using membrane-bound scαCTLA-4 inhibits antigen-specific T cell responses in vitro (18, 20). We have also shown that scαCTLA-4 can regulate CD8+ T cell–mediated tumor clearance in an in vivo tumor model (33). CD8+ T cells isolated from animals bearing scαCTLA-4 tumor cells were hyporesponsive ex vivo upon antigen rechallenge, indicating that CTLA-4 engagement resulted in tolerance (33). In this study, we have extended our previous findings to show that scαCTLA-4 expression on B cells inhibits CD4+ autoimmune responses in vivo. Two separate experimental models of autoimmune diabetes were used to address the efficacy of membrane-bound anti–CTLA-4 for disease prevention. We demonstrate that selective engagement of CTLA-4 conferred significant protection against disease in spontaneous NOD diabetes and even in the more aggressive autoimmune NOD.B7-1/B7-2 KO (B7 DKO) model. This CTLA-4 control may function at several levels, including T cell activation/proliferation, Th1/Th2 differentiation, and cell trafficking. Our data suggest that scαCTLA-4–mediated inhibition is at the level of CD4+ T cell activation/differentiation. We found a significant decrease in the percentage of autoreactive BDC2.5 CD4+ T cells proliferating in the NOD.B7 DKO.scαCTLA-4 Tg+ in pancreatic LN compared with controls, indicating that CTLA-4 engagement had a direct effect on antigen-specific T cell activation and expansion. CD8+NRP-V7+ tetramer–positive cells were examined in the NOD.scαCTLA-4 Tg+ mice, as these T cells have been shown to be increased during the progression of diabetes in NOD mice (34). There were no statistical differences in the number of CD8+NRP-V7+ antigen-specific cells in the NOD.scαCTLA-4 Tg+ mice as compared with NOD mice (data not shown). We further demonstrated scαCTLA-4 significantly limited the differentiation of diabetogenic T cells toward Th1 IFN-γ–producing cells. NOD.B7 DKO.scαCTLA-4 Tg+ mice had significant reduction of IFN-γ/YFP+ cells (0.5%) compared with controls (13.3%) in islet-draining pancreatic LN. These data indicate that CTLA-4 engagement significantly inhibits both the proliferation and Th1 differentiation in an antigen-specific response. The protection from disease in this setting appears to be a result of direct inhibition of effector T cell activation/differentiation rather than regulation, as the Treg deficiency observed in NOD.B7 DKO mice was not restored by the expression of the scαCTLA-4 Tg. It is interesting to note that reduced disease incidence correlated with decreased tissue infiltration (Figure 7B). This is not surprising unless you consider the fact that nearly all female NOD mice develop insulitis by 3 to 4 weeks of age (35). This lack of pancreatic infiltration indicates that CTLA-4 may function to prevent the trafficking and accumulation of inflammatory infiltrates, likely at the level of LN priming. It is important to note that the presence of the scαCTLA-4 Tg did not render these mice immunocompromised, as NOD.scαCTLA-4 Tg+ mice cleared cytomegalovirus in a manner similar to that observed in NOD mice. In these experiments, NOD.RAG KO mice were used to demonstrate that immunocompromised animals were unable to clear this viral infection (data not shown). Therefore, the scαCTLA-4–mediated disease inhibition and decreased insulitis in the absence of Tregs support the interpretation that CTLA-4 ligation functioned directly on the autoreactive T cells. These data are consistent with previous findings that CTLA-4 engagement results in a tolerant state, and only after blocking CTLA-4 do tissue infiltration and autoimmunity occur.
scαCTLA-4 Tg+ B cells prevent destructive insulitis and significantly affect NOD and NOD.B7 DKO mice from developing diabetes, suggesting B cells play a pivotal role in disease progression. B cells have been shown to be critical for the development of autoimmune diabetes in NOD mice, as B cell–deficient mice are protected (22, 23). Interestingly, B cell–deficient mice develop some insulitis, suggesting that diabetogenic responses can be initiated in the absence of B cells, likely via DCs. However, diabetes fails to progress in these mice, demonstrating a critical role of B cells in the amplification of autoimmune responses and disease progression (22). Wong et al. recently demonstrated that B cell antigen presentation, not Ig production, is essential for development of insulitis and diabetes (23). In fact, B cells may contribute to disease pathogenesis by further activating T cells in the pancreas or by enhancing epitope spreading where islet Ab-producing B cells selectively process apoptotic β cells to present new islet epitopes. Falcone et al. demonstrated a critical role for B cell antigen presentation and development of spontaneous responses to self antigens during the progression of autoimmune diabetes (19). Thus, it is possible that in our model, scαCTLA-4 Tg+ expression controlled diabetes by preventing B cell–dependent amplification of the anti-islet responses.
scαCTLA-4 significantly delayed disease onset and development of spontaneous diabetes in NOD mice. Although there was not complete protection, there is a significant consequence of engaging CTLA-4 during development of autoimmune diabetes. There are a number of possibilities as to why B cell expression of anti–CTLA-4 was not sufficient for complete disease protection. First, autoreactive T cells may interact with several APCs during diabetes initiation. Tg B cell expression of anti–CTLA-4 may raise the threshold for T cell activation. However, it is possible that some autoreactive T cells escape scαCTLA-4 regulation and encounter islet antigens presented by DCs or macrophage cells in the absence of CTLA-4. This possibility is supported by an in vitro MLR assay where total splenocytes were used to stimulate MHC mismatched T cells. In this assay, the presence of endogenous Tg nonbearing APCs partially restored T cell proliferation. These data further support the notion that the anti–CTLA-4 is working directly on T effectors and not indirectly through Tregs. A second possibility is that CTLA-4 may function to skew the T cell repertoire. However, based on previous studies by Allison and colleagues, it would be expected that, if anything, the repertoire would be broadened to include lower affinity clones that would escape CTLA-4 inhibition due to decreased action of the inhibitory receptor (36). A third possibility is that, over time, CTLA-4–resistant T cell clones emerge by overcoming the threshold of CTLA-4–mediated inhibition and become less dependent on costimulation. Autoimmune diabetes is a chronic progressive disease with autoreactive T cells accumulating within the target organ months prior to clinical manifestations (35). Given the disease kinetics, it is possible that autoreactive cells may develop mechanisms to overcome or bypass CTLA-4–mediated suppression. This idea is supported by the work of You et al., where equal frequencies of diabetogenic T cells were detected in 6-week-old prediabetic and overtly diabetic NOD mice (37). They proposed that the progression to overt disease correlates with a qualitative change in the pathogenic T cells that allows them to escape from regulation over time (37). Thus, in a chronic progressive autoimmune disease, such as type I diabetes, evolution of pathogenic T cells is likely to post significant challenge in designing novel immunosuppressive therapies.
Overall, we have demonstrated anti–CTLA-4 Abs anchored on the surface of APCs to be an effective means to suppress autoimmune responses. There are a number of ways that can be envisioned to adapt such an approach in clinical settings to prevent graft rejection and treat autoimmunity. The first is a gene therapy approach targeting APCs, including macrophages, DCs, and B cells, to express this construct with the intent that antigen-specific responses would be selectively engaged together with CLTA-4 inhibition. A second approach shown to be effective for targeting autoimmunity was the use of a bispecific Ab to simultaneously engage antigen and CTLA-4 (38). This approach was effective in a mouse model of thyroiditis using bispecific Abs against thyroid-stimulating hormone receptor and CTLA-4 (39). The major limitation for this strategy is the requirement of identifying and generating tissue-specific Abs against immunodominant antigens for each disease. The last approach to selectively target autoreactive and alloreactive T cells is to modify the membrane of APCs or grafted tissue with inhibitory molecules. Recent reports have demonstrated effective use of cell membrane–anchored FasL to prevent islet and cardiac graft rejection (40). This approach utilizes a protocol to biotinylate cells and “decorate” the membrane with a chimeric protein comprised of streptavidin and FasL (40). This is an attractive approach for the clinical setting for treatment of autoimmune disease and prevention of graft rejection. Patient cells, including B cells, macrophages, DCs and even islet grafts, could be biotinylated and modified with the addition of streptavidin scαCTLA-4. All of these therapeutic approaches are aimed toward developing a system in which antigen-specific inhibition using APCs is combined with CTLA-4–mediated inhibition.
Our results demonstrate profound inhibition of antigen-specific T and B cell responses by scαCTLA-4 Tg+ B cells both in vitro and in vivo. Moreover, we show that selective engagement of CTLA-4 conferred significant protection against an exacerbated form of autoimmune diabetes. These results suggest that simultaneous engagement of CTLA-4 during antigen presentation may be an effective regimen for treating autoimmune diseases and organ graft rejections. To this end, we have focused on the generation of a construct that results in deliberate ligation of CTLA-4 in vivo and represents what we believe to be a novel immunotherapeutic approach. This type of antigen-specific approach may be useful for treating autoimmunity and inducing peripheral tolerance and warrants further development and consideration.
Methods
Mice.
Female FVB, C57BL/6, and C3H mice were purchased from Jackson Laboratory. Female NOD mice were purchased from Taconic. NOD-BDC2.5 TCR Tg+ mice (27) were generously provided by C. Benoist and D. Mathis (Harvard Medical School, Boston, Massachusetts, USA), and C57BL/6.CTLA-4 KO mice were generously provided by A. Sharpe (8) (Harvard Medical School, Boston, Massachusetts, USA). IFN-γ reporter mice were generated on the C57BL/6 background and backcrossed 12 generations to NOD and subsequently bred to NOD.BDC2.5.Thy1.1 mice to generate NOD.BDC2.5.Thy1.1.Yeti mice as previously described (29). Mice were 2–8 weeks old at the initiation of the experiments. All mice were housed in a pathogen-free barrier facility at UCSF. Experiments complied with the Animal Welfare Act and NIH guidelines for the ethical care and use of animals in biomedical research and were approved by the UCSF Animal Care and Use Committee.
Abs.
Monoclonal Abs to murine CD4 (RM4-5), CD8a (Ly-2), CD19 (1D3), MHC II (KH114), IgM (R6-60.2), B220 (RA3-6B2), CD40 (3/23), B7-1 (16-10A1), B7-2 (GL-1), total Ig (R26-46), IgG1 (A85-1), IgG2a (R19-15), and isotype controls were purchased from BD Biosciences — Pharmingen. Monoclonal Abs to FoxP3 (FJK-16s) were purchased from eBioscience. Purified anti-mouse IgM was purchased from Jackson ImmunoResearch Laboratories Inc.
Generation of anti–CTLA-4 scFv Tg+ mice.
To obtain surface-immobilized anti–CTLA-4 Abs, a single-chain, membrane-bound anti–CTLA-4 Ab (7M-4F10scFvB7-1) was generated (18, 20). The cDNA encoding for 7M-4F10scFvB, a mutant clone with increased affinity for CTLA-4 (18, 20), was cloned into Bluescript plasmid PμEμ vector, a generous gift from J. Wilson (Princeton University, Princeton, New Jersey, USA), as previously described (21), to generate B cell expression of the ligand under the control of the IgM heavy chain enhancer and promoter. To introduce expression of anti–CTLA-4 in mice, the DNA fragment PμEμ.7M-4F10scFv-B7-1 was excised using Sal I sites and microinjected into day 1 embryos from FVB/n mice. Tg founders were identified by flow cytometry using mouse CTLA-4 (mCTLA-4) Ig in the presence of anti–B7-1 and anti–B7-2 Abs and backcrossed to NOD mice for 10 generations. Subsequently, the NOD.scαCTLA-4 Tg mice were crossed to NOD.B7 DKO mice.
Antigens.
DNP-OVA and DNP–keyhole limpet hemocyanin (DNP-KLH) were purchased from Genemed Synthesis Inc. The amino acid composition was verified by mass spectrometry, and purity (>98%) was assessed by HPLC.
Cell sorting.
scαCTLA-4 Tg+ B cells were stained with the fusion protein mCTLA-4 Ig (Genetics Institute Inc.) and with anti-B220, in the presence of anti–B7-1 and anti–B7-2 to block endogenous B7s. B220+ CTLA-4 Ig+ cells were sorted using a MoFlo cytometer high speed cell sorter (Dako). NOD.BDC2.5.Thy1.1 cells were stained with anti-CD4 FITC, anti-CD25 PE, and anti-CD62L APC, and the CD4+CD62L+CD25– T cells were sorted. All sorted populations had greater than or equal to 98% cell purity.
Activation of donor lymphocytes, cell culture, and transfer.
MoFlo-sorted WT FVB and scαCTLA-4 Tg B cells were activated by LPS (1 μg/ml, Sigma-Aldrich) in vitro for 72 hours, loaded with DNP-OVA and incubated for 4 hours at 37°C, and transferred to naive FVB recipients. Seven days following transfer of cells, recipient animals were immunized subcutaneously in the flank with DNP-OVA (100 μg/mouse) in CFA (Sigma-Aldrich) containing 4 mg/ml Mycobacterium tuberculosis (BD Diagnostics).
Murine cytomegalovirus infection.
NOD, NOD.scαCTLA-4 Tg+, and NOD.RAG KO mice were infected with 5 × 104 PFU CMV Smith strain. Fourteen days after infection, liver and spleen tissue was harvested, weighed, and homogenized, and viral plaque assays were performed using NIH 3T3 mouse embryonic fibroblast cells using serial dilutions.
Assessment of insulin auto-Abs.
Serum samples were collected from 5-, 10-, 20-, and 30-week-old NOD.scαCTLA-4 Tg+ and NOD control mice (n = 7–10/time point/group), and insulin auto-Abs were determined as previously described (41).
Assessment of diabetes and insulitis.
Blood glucose levels were measured from female NOD, NOD.scαCTLA-4 Tg+, NOD.B7 DKO, and NOD.B7 DKO.scαCTLA-4 Tg+ mice weekly with an Accu-Chek Active glucose meter (Roche Diagnostics). Mice were considered diabetic with measurements over 250 mg/dl. For histological analysis, pancreas was formalin fixed in 10% buffered formalin. Multiple 5-μm sections were stained with H&E and scored blindly for severity of insulitis as previously described (26).
CFSE adoptive transfer.
To measure in vivo proliferation, LN cells from BDC2.5.Thy1.1 mice were depleted of CD25+ cells using anti-CD25 (clone 7D4) and complement, followed by Ficoll density gradient centrifugation. Cells were labeled with 2.5 μM CFSE (Invitrogen), and 1 × 106 cells were intravenously transferred to recipient mice. Recipients were sacrificed 5 days later, and CFSE dilution of pancreatic and inguinal LN cells was measured by flow cytometry using a BD LSRII cytometer (BD Biosciences — Pharmingen).
ELISAs.
Assessment of cytokine production was tested for IL-2, IL-4, IL-10, and IFN-γ by commercial ELISA kits according to manufacturer-recommended protocol (Endogen, Pierce Biotechnology). Total Ig, IgG2a, IgG1, and IgM anti-DNP Ab production was measured at days 0, 7, and 14 following transfer of antigen-loaded WT and scαCTLA-4 Tg+ B cells. In brief, flat-bottom microtiter plates (Nunc) were coated with DNP-KLH (50 μg/ml) in PBS coating buffer and blocked with 2% BSA (Sigma-Aldrich) in PBS for 1 hour at room temperature; serum samples were subsequently added in triplicate. Biotinylated anti-Ig, anti-IgG2a, anti-IgG1, and anti-IgM (BD Biosciences — Pharmingen) were added and incubated for 2 hours. Plates were developed using streptavidin-peroxidase (Zymed) and OPD substrate (Sigma-Aldrich), and absorbance was read at 405 nm using a Vmax kinetic microplate reader (Molecular Devices).
In vitro T cell proliferation assays.
Cells were cultured in 96-well microtiter plates (Corning Inc.) at 5 × 105 viable cells/well in complete DMEM. Cells were pulsed with 1 μCi of 3H-TdR (ICN Radiochemicals) during the last 8 hours of a 96-hour culture, and 3H-TdR uptake was detected using a Packard TopCount Microplate Scintillation Counter (Packard Instruments Co.).
Statistics.
Statistical significance of cytokine levels, thymidine incorporation, and mean time to 25% disease incidence was analyzed using 2-tailed Student’s t test for comparisons of 2 means. Diabetes incidence statistical significance was analyzed using a nonparametric Wilcoxon signed-rank test. Values of P ≤ 0.05 were considered significant.
Acknowledgments
The authors would like to thank S. Jiang and C. McArthur for technical help with cell sorting; P. Wegfahrt for mouse handling; R. Takaki and L. Lanier for expert help with the CMV plaque assays; G. Eisenbarth and L. Cheng for performing the IAA assays; and C. Kadoch for technical assistance. We thank members of the Bluestone laboratory for critical review of this manuscript and helpful discussions. This work was supported by grants from the NIH (P01 AI035297, P30 DK063720, AI30663) and the Juvenile Diabetes Research Foundation (32004232).
Footnotes
Nonstandard abbreviations used: CTLA-4, CTL-associated antigen 4; DNP, dinitrophenyl; DP, double positive; KLH, keyhole limpet hemocyanin; mCTLA-4, mouse CTLA-4; MLR, mixed lymphocyte reaction; PμEμ, IgM heavy-chain promoter and enhancer; scαCTLA-4, single-chain anti–CTLA-4; scFv, single-chain antibody; SP, single positive; YFP, yellow fluorescent protein.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 116:2252–2261 (2006). doi:10.1172/JCI27856.
See the related Commentary beginning on page 2080.
References
- 1.Walunas T.L., et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 2.Walunas T.L., Bakker C.Y., Bluestone J.A. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krummel M.F., Allison J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krummel M.F., Allison J.P. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luhder F., Chambers C., Allison J.P., Benoist C., Mathis D. Pinpointing when T cell costimulatory receptor CTLA-4 must be engaged to dampen diabetogenic T cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12204–12209. doi: 10.1073/pnas.200348397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chikuma S., Imboden J.B., Bluestone J.A. Negative regulation of T cell receptor-lipid raft interaction by cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 2003;197:129–135. doi: 10.1084/jem.20021646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waterhouse P., et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 8.Tivol E.A., et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 9.Tang Q., et al. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur. J. Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 10.Tai X., Cowan M., Feigenbaum L., Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat. Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 11.Eagar T.N., Karandikar N.J., Bluestone J.A., Miller S.D. The role of CTLA-4 in induction and maintenance of peripheral T cell tolerance. Eur. J. Immunol. 2002;32:972–981. doi: 10.1002/1521-4141(200204)32:4<972::AID-IMMU972>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 12.Perez V.L., et al. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 13.Salomon B., Bluestone J.A. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 14.Vanderlugt C.L., et al. Treatment with intact anti-B7-1 mAb during disease remission enhances epitope spreading and exacerbates relapses in R-EAE. J. Neuroimmunol. 1997;79:113–118. doi: 10.1016/s0165-5728(97)00108-2. [DOI] [PubMed] [Google Scholar]
- 15.Luhder F., Hoglund P., Allison J.P., Benoist C., Mathis D. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J. Exp. Med. 1998;187:427–432. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egen J.G., Allison J.P. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- 17.Chikuma S., Bluestone J.A. CTLA–4: acting at the synapse. Mol. Interv. 2002;2:205–208. doi: 10.1124/mi.2.4.205. [DOI] [PubMed] [Google Scholar]
- 18.Griffin M.D., et al. Blockade of T cell activation using a surface-linked single-chain antibody to CTLA-4 (CD152). J. Immunol. 2000;164:4433–4442. doi: 10.4049/jimmunol.164.9.4433. [DOI] [PubMed] [Google Scholar]
- 19.Falcone M., Lee J., Patstone G., Yeung B., Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J. Immunol. 1998;161:1163–1168. [PubMed] [Google Scholar]
- 20.Griffin M.D., et al. Development and applications of surface-linked single chain antibodies against T-cell antigens. J. Immunol. Methods. 2001;248:77–90. doi: 10.1016/s0022-1759(00)00344-6. [DOI] [PubMed] [Google Scholar]
- 21.Wilson J.B., Weinberg W., Johnson R., Yuspa S., Levine A.J. Expression of the BNLF-1 oncogene of Epstein-Barr virus in the skin of transgenic mice induces hyperplasia and aberrant expression of keratin 6. Cell. 1990;61:1315–1327. doi: 10.1016/0092-8674(90)90695-b. [DOI] [PubMed] [Google Scholar]
- 22.Noorchashm H., et al. B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes. 1997;46:941–946. doi: 10.2337/diab.46.6.941. [DOI] [PubMed] [Google Scholar]
- 23.Wong F.S., et al. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes. 2004;53:2581–2587. doi: 10.2337/diabetes.53.10.2581. [DOI] [PubMed] [Google Scholar]
- 24.Read S., Malmstrom V., Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kingsley C.I., Karim M., Bushell A.R., Wood K.J. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J. Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 26.Salomon B., et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 27.Katz J.D., Wang B., Haskins K., Benoist C., Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 28.von Herrath M.G., Oldstone M.B. Interferon-gamma is essential for destruction of beta cells and development of insulin-dependent diabetes mellitus. J. Exp. Med. 1997;185:531–539. doi: 10.1084/jem.185.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stetson D.B., et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoglund P., et al. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J. Exp. Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenwald R.J., Freeman G.J., Sharpe A.H. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 32.Greenwald R.J., Boussiotis V.A., Lorsbach R.B., Abbas A.K., Sharpe A.H. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–155. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 33.Hwang K.W., et al. Cutting edge: targeted ligation of CTLA-4 in vivo by membrane-bound anti-CTLA-4 antibody prevents rejection of allogeneic cells. J. Immunol. 2002;169:633–637. doi: 10.4049/jimmunol.169.2.633. [DOI] [PubMed] [Google Scholar]
- 34.Moore A., Grimm J., Han B., Santamaria P. Tracking the recruitment of diabetogenic CD8+ T-cells to the pancreas in real time. Diabetes. 2004;53:1459–1466. doi: 10.2337/diabetes.53.6.1459. [DOI] [PubMed] [Google Scholar]
- 35.Anderson M.S., Bluestone J.A. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 36.Egen J.G., Kuhns M.S., Allison J.P. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 37.You S., et al. Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes. 2005;54:1415–1422. doi: 10.2337/diabetes.54.5.1415. [DOI] [PubMed] [Google Scholar]
- 38.Rao S., et al. Targeted delivery of anti-CTLA-4 antibody downregulates T cell function in vitro and in vivo. Clin. Immunol. 2001;101:136–145. doi: 10.1006/clim.2001.5119. [DOI] [PubMed] [Google Scholar]
- 39.Vasu C., Gorla S.R., Prabhakar B.S., Holterman M.J. Targeted engagement of CTLA-4 prevents autoimmune thyroiditis. Int. Immunol. 2003;15:641–654. doi: 10.1093/intimm/dxg061. [DOI] [PubMed] [Google Scholar]
- 40.Yolcu E.S., Askenasy N., Singh N.P., Cherradi S.E., Shirwan H. Cell membrane modification for rapid display of proteins as a novel means of immunomodulation: FasL-decorated cells prevent islet graft rejection. Immunity. 2002;17:795–808. doi: 10.1016/s1074-7613(02)00482-x. [DOI] [PubMed] [Google Scholar]
- 41.Yu L., et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1701–1706.. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]