Abstract
Twenty-four putative lipase/esterase genes of Mycobacterium tuberculosis H37Rv were expressed in Escherichia coli and assayed for long-chain triacylglycerol (TG) hydrolase activity. We show here that the product of Rv3097c (LIPY) hydrolyzed long-chain TG with high specific activity. LIPY was purified after solubilization from inclusion bodies; the enzyme displayed a K m of 7.57 mM and V max of 653.3 nmol/mg/min for triolein with optimal activity between pH 8.0 and pH 9.0. LIPY was inhibited by active serine-directed reagents and was inactivated at temperatures above 37 °C. Detergents above their critical micellar concentrations and divalent cations inhibited the activity of LIPY. The N-terminal half of LIPY showed sequence homology with the proline glutamic acid-polymorphic GC-rich repetitive sequences protein family of M. tuberculosis. The C-terminal half of LIPY possesses amino acid domains homologous with the hormone-sensitive lipase family and the conserved active-site motif GDSAG. LIPY shows low sequence identity with the annotated lipases of M. tuberculosis and with other bacterial lipases. We demonstrate that hypoxic cultures of M. tuberculosis, which had accumulated TG, hydrolyzed the stored TG when subjected to nutrient starvation. Under such conditions, lipY was induced more than all lipases, suggesting a central role for it in the utilization of stored TG. We also show that in the lipY-deficient mutant, TG utilization was drastically decreased under nutrient-deprived condition. Thus, LIPY may be responsible for the utilization of stored TG during dormancy and reactivation of the pathogen.
Tuberculosis caused by the organism Mycobacterium tuberculosis is one of the major public health threats in the world. The emergence of multidrug-resistant strains poses serious threats to the control of this disease due to the complex nature of second line drug treatment (1). Upon infection, the bacterium goes through an initial replicative phase inside the alveolar macrophages, after which it enters a nonreplicative, drug-resistant state of dormancy. This state of dormancy is probably induced by the environmental stress exerted upon the pathogen by the host’s immune response. The bacterium is able to survive in this dormant state for decades until the host’s immune system is weakened when it reactivates and causes the infectious disease (2). The current antimycobacterial drugs are able to kill only the actively replicating mycobacteria and do not clear the latent bacteria from the host (3). Thus, latency is a major problem in tuberculosis control. One-third of the world population is infected with the latent microorganism, and nearly 2 million deaths occur annually (4, 5). Individuals carrying a latent infection are estimated to harbor a 2–23% lifetime risk of reactivation (6).
There is strong evidence that fatty acids may be the energy source for the mycobacterium during persistence (7–10). However, the source of these fatty acids is not known. We proposed that M. tuberculosis stores energy in the form of triacylglycerol (TG)3 as it prepares to go into dormancy in a fashion similar to hibernating animals and microbial spores that use TG for long-term storage of energy. Previously, we identified 15 novel triacylglycerol synthase genes belonging to a nonclassical diacylglycerol acyltransferase family in M. tuberculosis, some of which are induced under in vitro conditions simulating dormancy and cause TG accumulation inside the bacterial cell (7). Utilization of the energy stored as TG requires its hydrolysis and the release of fatty acids for catabolism by β-oxidation. Although the M. tuberculosis genome contains 21 genes annotated as putative lipase/esterase genes (11) and 94 gene products would contain the α/β-hydrolase fold characteristic of lipases/esterases (12), there has been no report on the identification of an enzyme possessing long-chain TG hydrolase activity from M. tuberculosis. Recently, the products of Rv1399c (LIPH) and Rv3487c (LIPF) were cloned and characterized, but no long-chain TG hydrolysis was detected (13, 14).
Here we report on the screening of 21 annotated and three additional putative lipase/esterase gene products from M. tuberculosis for long-chain TG hydrolase activity and show that the newly identified Rv3097c gene product, which we named LIPY, is active as a true lipase. LIPY (PE-PGRS63), which is a member of the PE-PGRS protein family, is a lipase belonging to the hormone-sensitive lipase (HSL) family and hydrolyzes long-chain TG with high specific activity. We also show that lipY was up-regulated to a much higher level than the transcripts of other lipase/esterase-like genes when mycobacteria that accumulated TG during an in vitro dormancy-like state were subjected to TG utilizing conditions indicating that this lipase might play a role in the utilization of TG for survival through dormancy. We show here that the ability to hydrolyze stored TG was drastically diminished in a lipY-disrupted mutant (Δ-lipY). This is the first report on the characterization of a long-chain TG lipase from M. tuberculosis with experimental evidence for its vital physiological role and also the first report of a PE-PGRS protein showing enzymatic activity.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Culture Conditions
M. tuberculosis H37Rv (ATCC 25618) and Δ-lipY mutant were grown in Middlebrook 7H9 (supplemented with 0.05% Tween 80, 10% oleic acid/albumin/dextrose/catalase enrichment, and 0.2% glycerol) and in Dubos/Tween 80/albumin medium (prepared from Dubos broth base and Dubos medium albumin as per the manufacturer’s instructions). All media were purchased from Difco. Escherichia coli DH5α and BL21 Star (DE3) (Invitrogen) used as host strains for cloning and expression experiments were grown on Luria-Bertani broth or agar, and, when required, antibiotics were added to the culture media at the following concentrations: carbenicillin or kanamycin, 50 μg/ml; hygromycin B, 150 μg/ml for E. coli or 75 μg/ml for M. tuberculosis. Other chemicals and antibiotics were from Sigma, Fisher, and Calbiochem. DNA restriction and modifying enzymes were procured from New England Biolabs (Beverly, MA).
Induction of Lipase Genes and TG Utilization in M. tuberculosis Strains under Nutrient Starvation after Hypoxic Stress
M. tuberculosis H37Rv and Δ-lipY mutant cells were grown under a hypoxic condition essentially as previously described to induce the accumulation of TG inside the mycobacterial cell (7). M. tuberculosis cells were inoculated into Middlebrook 7H9, grown aerobically at 37 °C in roller bottles to an A 600 of 0.8, and were used to inoculate Dubos/Tween/albumin medium to an A 600 of 0.015, grown up to an A 600 of 0.06 and distributed in tubes or in single neck wolf bottles (Chemglass) with a 0.5 head space to culture volume ratio, sealed with rubber sleeve caps, and slowly stirred on a magnetic stirrer for hypoxic growth up to 12 days. Harvested cells were divided into three sets. The first set was preserved at −80 °C and was used as control (0 h). The harvested cells of the second set were washed and resuspended in phosphate-buffered saline (PBS) and incubated at 37 °C for 6 h. The cells of the third set were washed, resuspended in normal 7H9/OADC/Tween (7H9) medium instead of PBS, and served as a nutrient supplemented control. These differentially treated cells were used to measure the induction of lipase gene transcripts by semiquantitative RT-PCR and to measure the utilization of stored TG. RNA isolation and semiquantitative RT-PCR were performed as described before (7), using the primers shown in Table 1.
TABLE 1.
Primers used for RT-PCR analyses of transcripts of lipase genes induced in M. tuberculosis
| Gene | Primer pairs (5′–3′)a |
|---|---|
| lipC (Rv0220) | F: GGTAAGCACCTCAAGCCGCTCGGC |
| R: GCGCTGAACCACTACCCGCTCCAG | |
| lipD (Rv1923) | F: GGTGTTCAGCGGGCGAGCGAGTTC |
| R: GGCGCAACCACCGTCACTCCTCAC | |
| lipE (Rv3775) | F: CTAGACGCCGTCACGGCAACCGGC |
| R: ACTGGGCGCGACCAGCGGCACATC | |
| lipF (Rv3487c) | F: CGACGGCGCTGGGCGGGTGGTGCT |
| R: CGCGCCACGGCTTGCGGCGCGAGGT | |
| lipG (Rv0646c) | F: CTGATCATGGGCCTGGGCGCCCAG |
| R: GGACCCGTGAGCAGCGCCAGCAGC | |
| lipH (Rv1399c) | F: GACCTTCACCGCGGCCGACGGTGTC |
| R: TGCGTGCAACGCCCTCTTCAGCGCC | |
| lipI (Rv1400c) | F: GGGATCGAGGCCGTGCGCCAGCGGT |
| R: GCACAACCCGTAGCGCCACCAGCCC | |
| lipJ (Rv1900c) | F: CGCACGGTCGAGGACACCAGCACC |
| R: ACCCGGTAGGCGCCCAGGTTCGTC | |
| lipK (Rv2385) | F: GCCAGACGGGCCGTGGGGGATATG |
| R: CCCACCTGGATCAGCGTCGGTGGC | |
| lipL (Rv1497) | F: CCCGTGACCAGCTCCCGCGACAAG |
| R: GCGGAGCGCTGGCGGTGTATCTCG | |
| lipM (Rv2284) | F: TGGGTGACCGGTGAGGCGTCGAGG |
| R: AGTGCGGGCGGTCTCCTGGCTGGT | |
| lipN (Rv2970c) | F: GCATGTGGACACGGCGTGTGCAGG |
| R: AACCGGCACAGCGCGTCATGGGTG | |
| lipO (Rv1426c) | F: ACCCCTGGACCGGTGCTCGAAGCG |
| R: GGATCAGGGAGTCGTGGCGGCCGT | |
| lipP (Rv2463) | F: GGACCAGCTCCATGTGCTCGCGGC |
| R: GGGCTCGTGCGCGTCGGAGTTCAC | |
| lipQ (Rv2485c) | F: CCGGCGAACAGTCAGAGGCTGCCC |
| R: GGGCGTTGGGGAGCTCAGCGTAGG | |
| lipR (Rv3084) | F: CCACTGTTCGCTTCCCGCCGGCTG |
| R: TCGTCGCCCGCGGTCTGCATGACG | |
| lipS (Rv3176c) | F: CGCACCGGGCGAGCGCGCTCATCAG |
| R: CCTCGGCGAGCCGATCGGGCTGCTC | |
| lipT (Rv2045c) | F: ACGGCCACCGGCATCGTTGAAGGC |
| R: CGCTGGGCTTTCCGAGATCGCCCTGG | |
| lipU (Rv1076) | F: GTGTTGCCGGCGGACGGCACTCGA |
| R: GACGCAACGAGCGGATCGCCTCGG | |
| lipV (Rv3203) | F: CCCATCGCCGCACCCGATCTGCTGG |
| R: GCGCGGTCCCAGTCGACTGCGGATC | |
| lipW (Rv0217c) | F: ATCGCCGTCGTCACCCCACGACAG |
| R: CCGCATCGCCAAGATATGCCCGCC | |
| lipX (Rv1169c) | F: TTTGTCACCACACGGCCCGATTCG |
| R: GCGCGGTTGGCTAATTCGGTGAGC | |
| lipY (Rv3097c) | F: AGCCGCTGCCGAGGACGAGGTGTC |
| R: CGTCCCGGCAGTGCCTCCTTCCTG | |
| lipZ (Rv1834) | F: CCGAGTGTCCGGGAGTGGCGTGAC |
| R: AGCCTCGACCTGCGGGTAGTGGCC |
F, forward; R, reverse.
Cells from the three sets were autoclaved, and total lipids were extracted with chloroform/methanol (2:1; v/v) as previously described (7). The 12-day-old wild type M. tuberculosis hypoxic culture (10 ml) was also used for labeling with 1 μCi of [14C]oleic acid (specific activity, 55 Ci/mol; American Radiolabeled Chemicals) for 1 h, and then the cells were washed with PBS before incubating in PBS for the next 6 h. Lipids were analyzed by silica gel TLC using n-hexane/diethyl ether (9:1; v/v), and the radioactivity in the silica gel corresponding to the TG band was measured using a liquid scintillation counter (Packard). An autoradiogram of the TLC was prepared. The amount of TG was visualized by dichromate/sulfuric acid charring of the TLC plates as described before (15). The charred TLC plate was also scanned for quantification of TG accumulation by using the AlphaImager 2200 Gel Doc system (AlphaInnotech).
Generation of the lipY-disrupted Mutant of M. tuberculosis H37Rv
The lipY gene was disrupted by allelic exchange using specialized transducing phage as described (16). The disruption construct of lipY was made by sequential cloning of a 953-bp 5′-flank (consisting of the first 38 bp of lipY open reading frame (ORF) and a 915-bp sequence upstream of lipY ORF) and a 789-bp 3′-flank (consisting of last 4 bp of lipY ORF and 785 bp of sequence downstream of lipY) of lipY, on either side of res-hygres gene cassette in the cosmid pYUB854. The two flanks were generated by PCR amplification using M. tuberculosis H37Rv genomic DNA as template by introducing AflII and XbaI and HindIII and SpeI sites on the ends of the 5′-flanks and 3′-flanks, respectively, for directional cloning into pYUB854 (Table 2). The PacI-digested recombinant pYUB854 containing the lipY disruption construct was introduced in phasmid phAE159, and the recombinant transducing phage obtained after packaging was used to transduce M. tuberculosis. lipY disruption by allelic exchange was confirmed by PCR analyses using specific sets of primers and Southern hybridization (Table 2). Ten hygromycin-resistant clones were screened by PCR using a set of primers (Δ-F and Δ-R) designed from the deleted part of the lipY gene. The allelic exchange by double crossover was confirmed with two sets of primers, each representing a outwardly directed hyg primer (H1 and H2) and a primer (primers E and F) in the mycobacterial genome beyond the flanking gene sequences used for making the homologous arms of the disruption construct.
TABLE 2.
PCR primers used for lipY disruption in M. tuberculosis H37Rv
| Primer pair | Sequence |
|---|---|
| Primer pairs to amplify 5′ and 3′-flanks of lip Y | |
| 5′-flank | |
| A | 5′-GCTTAAGATGCCGTAGGACCCG-3′ |
| B | 5′-GGTCTAGAGACATCACCTCCGGC-3′ |
| 3′-flank | |
| C | 5′-GGAAGCTTACTCGGTATCGCCGC-3′ |
| D | 5′-GGACTAGTGGTGCAAAGTCCGGG-3′ |
| Primer pair from the deleted segment | |
| Δ-F | 5′-GTGCAGGCATTGACAGGCGCGGCC-3′ |
| Δ-R | 5′-CCAGGTCCCCACATCGAGCCACGG-3′ |
| Primer pairs to amplify genomic flanks in the mutants | |
| 5′-flank | |
| E | 5′-GTGACCGGGAGATCCGAGCAGAGG-3′ |
| H1 | 5′-TGAGGCGATGGTGGTGTCGATGCT-3′ |
| 3′-flank | |
| H2 | 5′-GGAACTGGCGCAGTTCCTCTGGGG-3′ |
| F | 5′-CCAAGGGCTGGGGTGCACAACTCC-3′ |
DNA Isolation and Southern Blotting
Mycobacterial genomic DNA was isolated by the guanidine thiocyanate method as described (17). DNA samples were digested with suitable restriction enzyme, separated by electrophoresis in 1% agarose gel, transferred to nylon membranes (Nytran Plus; Schleicher and Schuell), and hybridized with [α-32P]dCTP-labeled probes. Probe labeling and preparation were performed using Rediview [α-32P]dCTP and the Rediprime II random priming labeling kit (Amersham Biosciences) as per the manufacturer’s instructions.
Cloning and Expression of M. tuberculosis Lipase/Esterase Genes in E. coli
The 24 ORFs were amplified from the genomic DNA of M. tuberculosis H37Rv by PCR using Pfu Turbo Hotstart DNA polymerase (Stratagene) and cloned into pET200 d-TOPO expression vector (Invitrogen). The directionally cloned fragments were completely sequenced to confirm the sequence integrity of each expression construct. The constructs were used to transform competent cells of E. coli BL21 Star (DE3) (Invitrogen), and the proteins were expressed as N-terminal His6-tagged fusion proteins after induction with isopropyl 1-thio-β-d-galactopyranoside according to the manufacturer’s protocols. Total cell lysates from induced cultures were prepared in 0.1 m Tris·HCl, pH 8.0, containing 1 mm DTE and used for screening for TG hydrolase activity of all lip gene products. Untransformed host cell lysate was used as control.
Solubilization and Purification of LIPY
In our attempts to produce soluble LIPY protein, we performed isopropyl 1-thio-β-d-galactopyr-anoside induction in E. coli BL21 Star (DE3) cells at 16, 24, and 37 °C for 4 h and 12 h. Inductions were also carried out without using isopropyl 1-thio-β-d-galactopyranoside in Dual Media (Zymo Research). In all cases, virtually all of the expressed protein was insoluble. To solubilize the insoluble protein, His-tagged LIPY was expressed in 500-ml cultures by induction with 1 mm isopropyl 1-thio-β-d-galactopyranoside at 37 °C for 12 h in LB broth. Total cell lysates were centrifuged at 10,000 × g, and the pellet was solubilized in a buffer containing 1% (w/v) sodium lauroyl sarcosine, 2 mm SDS, 25 mm triethanolamine, 1.5 mm CaCl2, 50 mm sodium phosphate buffer, pH 7.0, 300 mm NaCl, and 50 μg/ml aprotinin with shaking at 330 rpm at 10 °C for 1 h. The solution was clarified by centrifugation at 16,000 × g for 20 min at 4 °C, and the supernatant was loaded onto a 5-ml bed volume cobalt affinity resin (TALON; BD Biosciences). Unbound proteins and detergents were removed by washing the resin with 10 bed volumes of bind/wash buffer (50 mm sodium phosphate buffer, pH 7.0, 300 mm NaCl) followed by 10 bed volumes of bind/wash buffer containing 10 mm imidazole. The bound protein was eluted in 5 bed volumes of bind/wash buffer containing 150 mm imidazole followed by 5 bed volumes of bind/wash buffer containing 250 mm imidazole, and 5-ml fractions were collected. Aliquots of fractions collected at each step of the solubilization and purification process were analyzed on 12% SDS-PAGE followed by Coomassie staining.
Lipase Assays
Lipase activity was measured by the release of [14C]oleic acid from [14C]triolein (55 mCi/mmol; American Radiolabeled Chemicals) using a modified method of Belfrage and Vaughan (18). The reaction mixture contained 50–100 μg of protein from total cell lysates, 0.2 μCi of [14C]triolein, 20 mm triolein, 2% gum arabic, 1 μg of bovine serum albumin, 100 mm NaCl, and 0.1 m Tris·HCl, pH 8.0, in a total volume of 100 μl. Triolein was emulsified in gum arabic and aliquoted into the reaction mixture at the indicated concentrations prior to the addition of enzyme. After incubation at 37 °C for 2 h, the reaction mixture was extracted with 1 ml of chloroform/methanol/hexane (1.25:1.41:1, v/v/v) following the addition of 200 μl of 0.1 m NaHCO3, pH 10.5. The radioactivity released into the upper aqueous phase was measured by liquid scintillation counting. Alternatively, the reaction mixture was acidified with 50 μl of 6 n HCl and extracted with 1 ml of chloroform/methanol (2:1, v/v). The lipids in the lower organic phase were resolved on silica TLC plates developed in hexane/diethyl ether/acetic acid (65:35:2, v/v/v), and the radioactivity corresponding to the internal oleic acid standard was determined by liquid scintillation counting.
To investigate substrate specificity, sonicated emulsions of 20 mm [14C]diolein or [14C]dioleoyl phosphatidylcholine or [14C]dioleoyl phosphatidylethanolamine or [14C]hexadecylpalmitate were used. To measure pH stability, the purified enzyme was preincubated in the appropriate buffer for 15 min at 24 °C prior to the assay at 37 °C for 2 h. Temperature stability was measured by preincubating purified LIPY at the indicated temperature for 15 min prior to assay at 37 °C. The effects of serine-directed reagents like diethyl-p-nitrophenyl phosphate (E-600), phenylmethanesulfonyl fluoride, detergents like SDS, polyethylene glycol tert-octylphenyl ether (Triton X-100), polyethylene glycol sorbitan monolaurate (Tween 20), and various salts were determined by preincubating the purified LIPY for 15 min at 24 °C with the indicated concentration of effector prior to the assay at 37 °C for 2 h.
RESULTS
Identification and Cloning of M. tuberculosis Lipase/Esterase Genes
M. tuberculosis probably uses fatty acids as a carbon source during its dormancy inside the host (8, 10). Previously, we identified 15 triacylglycerol synthase genes in the genome of the pathogen, several of which are induced under culture conditions that lead to a dormancy-like state resulting in TG accumulation within the bacterial cell (7). Utilization of stored TG would require a true lipase. However, no mycobacterial gene that encodes long chain TG hydrolase has been identified and characterized. We screened the M. tuberculosis genome for the presence of genes encoding such enzymes. The M. tuberculosis genome contains 21 ORFs annotated as putative lipase/esterase genes. We used the sequence of a putative TG lipase from M. tuberculosis strain W17 in the NCBI data base (U76006.1; accession number NCBI AAB18414) to screen the M. tuberculosis H37Rv genome for homologous genes. This approach identified three additional ORFs, Rv1169c, Rv3097c, and Rv1834, that we designate as lipX, lipY, and lipZ, respectively, to be consistent with the present nomenclature of all of the other lip genes in the M. tuberculosis genome. We included these three genes along with the 21 previously annotated lip genes in our screen for genes encoding true lipases.
Screening of M. tuberculosis Lipase/Esterase Gene Products for Long-chain TG Hydrolase Activity
The 24 selected M. tuberculosis ORFs encoding putative lipase/esterase proteins were expressed in E. coli, and total cell lysates were assayed for triolein hydrolase activity. Lipase activity above untransformed control was normalized for the expression level of each recombinant protein in the total cell lysate. Triolein hydrolase activity in our standard radiometric assay at pH 8.0 was by far the highest in the lysates of recombinant E. coli expressing LIPY followed by LIPC, LIPL, and LIPX that showed much lower activities followed by lysates expressing LIPK and LIPG that had even lower levels of activity. All other lip gene products showed little or no triolein hydrolase activity (Table 3).
TABLE 3. Long-chain TG hydrolase activity of expressed lip genes.
Lipase genes were expressed in E. coli BL21 cells, and lysates were assayed for TG hydrolase activity with 14C-labeled triolein as the substrate. A statistical analysis of the data was performed using the one-way two-tailed t test. The data passed the test with 99% confidence (p ≤ 0.0001) and are presented as mean with S.D. from at least three independent experiments.
| Gene product | TG hydrolase activity (mean ± S.D.) |
|---|---|
| nmol/mg/min | |
| LIPY | 41.0 ± 7.3 |
| LIPC | 2.7 ± 0.8 |
| LIPL | 1.1 ± 1.0 |
| LIPX | 0.7 ± 0.5 |
| LIPK | 0.6 ± 0.4 |
| LIPG | 0.4 ± 0.1 |
| LIPP | 0.14 ± 0.06 |
| LIPE | 0.12 ± 0.06 |
| LIPT | 0.07 ± 0.02 |
| LIPW | 0.05 ± 0.01 |
| LIPD | 0.05 ± 0.01 |
| LIPJ | 0.04 ± 0.02 |
| LIPZ | 0.04 ± 0.01 |
| LIPO | 0.04 ± 0.01 |
| LIPQ | 0.03 ± 0.02 |
| LIPR | 0.03 ± 0.02 |
| LIPS | 0.02 ± 0.01 |
| LIPM | 0 |
| LIPF | 0 |
| LIPH | 0 |
| LIPI | 0 |
| LIPN | 0 |
| LIPU | 0 |
| LIPV | 0 |
Utilization of Stored TG and Induction of Lipase Genes by Starvation in M. tuberculosis Cells Grown under Hypoxic Conditions
In order to test the hypothesis that lipase(s) release fatty acids from TG stores under nutrient-deprived conditions that might be encountered during dormancy, we cultured M. tuberculosis under hypoxic conditions for 12 days, a condition that was previously shown to cause TG accumulation (7), and subsequently incubated these cells in a starvation medium (PBS) to test whether the stored TG was utilized under such conditions. To label the stored TG, we incubated M. tuberculosis cells held under hypoxia for 12 days with [14C]oleic acid for 1 h, and then the labeled cells were incubated in PBS for 6 h. After incubation, about 50% of the labeled TG was utilized under nutrient-deprived conditions (Fig. 1A ). Accumulation of TG during hypoxia and utilization during starvation was also clearly seen by dichromate/sulfuric acid charring of the silica gel-TLCs of the total lipids (Fig. 1B ). The intensity of the TG band showed about 50% decrease after 6 h of incubation in PBS. Cells incubated in nutrient-rich medium, used as a control, did not utilize significant amounts of the stored TG (Fig. 1C ).
FIGURE 1. TG utilization by M. tuberculosis wild type and lipY mutant cells under nutrient starvation condition.
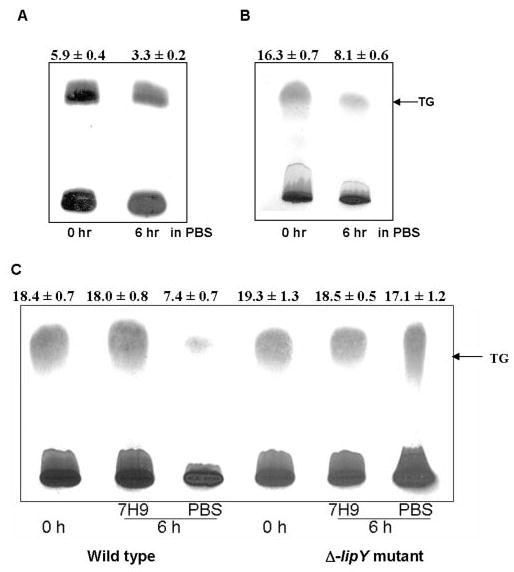
A, autoradiogram; B, dichromate/sulfuric acid charring of lipids showing utilization of TG by wild type M. tuberculosis in PBS medium. After 12 days of hypoxic growth, cells were incubated with 1 μCi of [14C]oleic acid for 1 h, and the cells were washed with PBS prior to a 6-h incubation in the same medium. C, dichromate/sulfuric acid charring of lipids showing utilization of TG accumulated during 12 days of hypoxic growth of M. tuberculosis (wild type) and lipY mutant cells at 0 h and by 6-h incubation in PBS and nutrient-rich (7H9) media. Lipids were separated on Silica gel TLC using n-hexane/diethyl ether (9:1, v/v) as developing solvent. A, an autoradiogram is shown from a typical experiment, and the incorporation values of 14C into TG is shown as a percentage of the total 14C administered. B and C, charred TLC chromatograms are shown from a typical experiment, and the intensity of the TG band was determined in arbitrary units by the AlphaImager 2200 Gel Doc system. Above each panel, the values are given as ± S.E. of three independent measurements.
During the TG utilization conditions, the lipase genes involved in the release of fatty acids from TG would be expected to be induced. To test for this possibility, the transcript levels of all of the 24 selected ORFs were measured by semiquantitative RT-PCR analyses. The transcript level of each lip gene before starvation (0 h), after starvation (6h-PBS), and after incubation in nutrient-rich medium (6h-7H9) are expressed as a fraction of the 23 S rRNA transcript level of the same sample (Fig. 2A ). Most of the lipase genes showed induction of transcript level during incubation for 6 h in PBS. The highest level of induction after 6 h of starvation was seen in the transcripts of lipY, lipE, lipC, lipZ, lipL, and lipT. Since the lip gene products manifested very different degrees of TG hydrolase activity, the possible relative contributions of the lipase genes to the hydrolysis of stored TG within the mycobacterial cell were assessed by multiplying the transcript level after 6 h of starvation with the triolein hydrolase activity of each expressed gene product. Such an analysis showed that LIPY had by far the greatest potential for hydro-lyzing in vivo stores of TG in the mycobacterium under such conditions (Fig. 2B ).
FIGURE 2. RT-PCR assessment of the induction of lip genes upon starvation (A) and estimation of the potential contribution to TG hydrolysis (B).
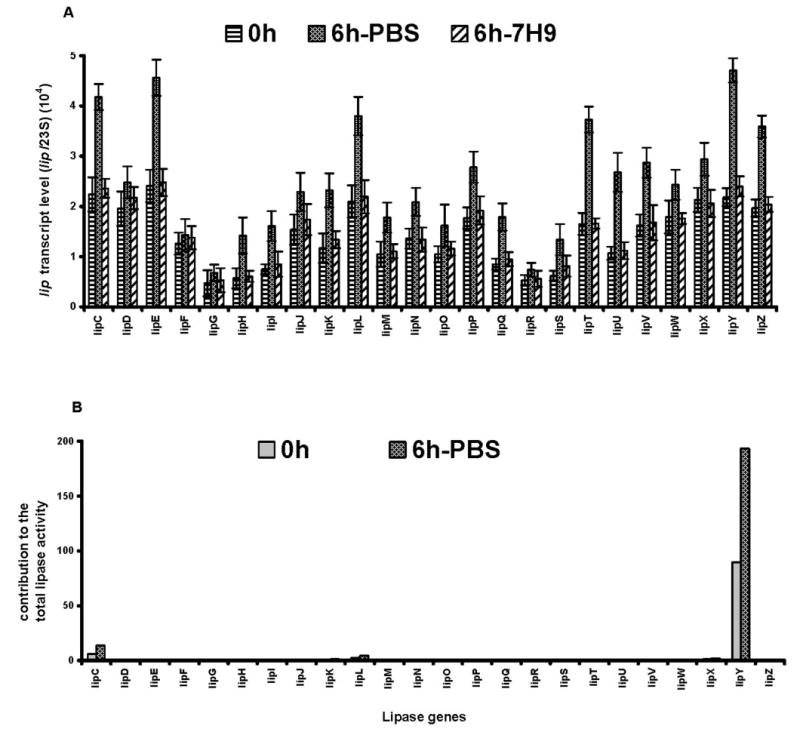
M. tuberculosis H37Rv was incubated in PBS or in 7H9 medium for 6 h after TG accumulation by hypoxic growth for 12 days. Transcript levels of lips are shown as a fraction of 23 S rRNA transcripts (mean ± S.D. from three independent experiments). The methods used for quantitation and experimental details are given under “Experimental Procedures.” B, the potential relative contribution of each lip gene product to the total lipase activity was estimated by multiplying transcript level with the lipase activity of each gene product expressed in E. coli.
Disruption of lipY in M. tuberculosis
LIPY showed the highest capacity to hydrolyze long chain TG among all of the probable lipase gene products of M. tuberculosis cloned and expressed in E. coli. Moreover, when the organism was subjected to a nutrient-deprived state after the cells had accumulated TG under hypoxia, lipY was found to be the most highly induced gene. These results suggested that LIPY is most likely to be the major lipase involved in the hydrolysis of the stored TG. To test for this possibility, we generated a lipY knock-out mutant of M. tuberculosis (Fig. 3). lipY was disrupted by allelic exchange using specialized transducing recombinant mycobacteriophage phAE159 (16). In the constructed lipY deletion allele, 1258 of 1314 bp of total lipY gene sequence was replaced with a hygromycin resistance gene cassette (res-hyg-res) flanked by res (resolvase recognition sequence) sequences. Several mutants were tentatively identified as lipY-disrupted mutant (Δ-lipY), since an 804-bp sequence in the deleted lipY segment could not be amplified by PCR (Fig. 3C ). Further PCR analysis of the flanking regions of the deleted part of the gene confirmed the deletion at the correct orientation by homologous recombination (Fig. 3C ). A 1330-bp 5′-flank (primer pair − E + H1) and a 1007-bp 3′-flank (primer pair − H2 + F) could be amplified from the selected disruptants, but no product could be amplified from the wild type genomic DNA (Fig. 3, B and C ). Southern blot of EcoRI-restricted genomic DNA of five putative ΔlipY mutants when hybridized with a 804-bp probe generated from the deleted sequence of the gene did not show any hybridization, whereas the wild type control showed the hybridized band (Fig. 3D ). lipY transcript was absent in Δ-lipY mutant, and the level of induction of the transcripts of the other lip genes was similar in the Δ-lipY and the wild type when both were incubated in PBS or in 7H9 medium (data not shown).
FIGURE 3. Generation of a lipY-deficient mutant of M. tuberculosis .
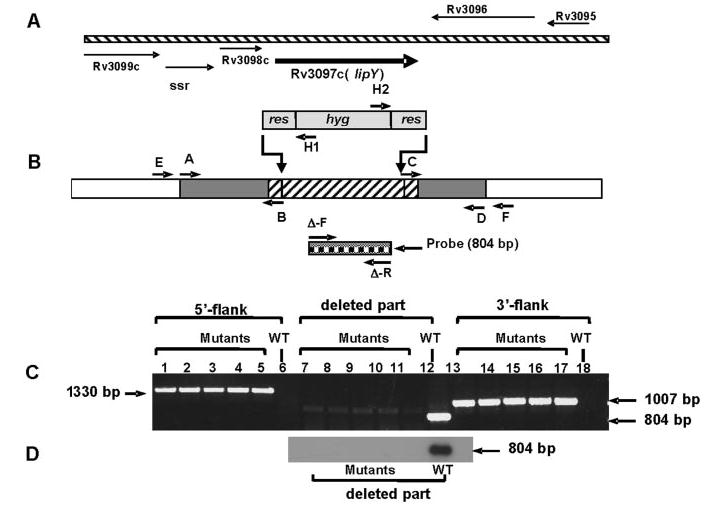
A, genomic organization of lipY (Rv3097c). B, schematic representation of the disruption construct for lipY. A hatched region with gray flanks depicts the genome sequence of lipY and its flanking regions used to make the disruption construct. Primer pairs A/B and C/D were used to generate the 5′- and 3′-flanks of the disruption construct. The part of the lipY gene (upward diagonal hatch) replaced with the res-hyg-res gene cassette is shown. Primer pairs E/H1, H2/F, and Δ-F/Δ-R (solid square check box) were used for PCR analysis of homologous recombination as described under “Results.” C, PCR analysis of 5′-flank (lanes 1–5, Δ-lipY mutants; lane 6, wild type), deleted part of the gene (lanes 7–11, Δ-lipY mutants; lane 12, wild type) and 3′-flank (lanes 13–17, Δ-lipY mutants; lane 18, wild type). D, Southern blot hybridization of five Δ-lipY mutant clones and wild type probed with the part of the deleted sequence of lipY. WT, wild type.
TG Utilization by lipY-deficient Mutant of M. tuberculosis
M. tuberculosis wild type and Δ-lipY mutant cells were subjected to TG utilizing condition as described above. When subjected to starvation by incubating in PBS, TG utilization in the lipY mutant was drastically decreased, compared with that in the wild type (Fig. 1C ). Also, no significant TG hydrolysis could be detected when the cells were incubated in a nutrient-rich medium (7H9) as a control.
Purification of LIPY
Since LIPY showed the highest potential for hydrolyzing the TG stored inside the M. tuberculosis cell, we purified LIPY and characterized its activity. LIPY was expressed as a His6-tagged fusion protein in E. coli at 16, 24, and 37 °C under various conditions of induction and was found to partition into the 16,000 × g pellet after cell lysis in all cases. Therefore, we solubilized LIPY from this pellet using a low concentration of detergents that did not inhibit the activity of LIPY as determined by preliminary assays with total cell lysates containing recombinant LIPY (data not shown). The clarified supernatant from the solubilized 16,000 × g pellet contained a large quantity of recombinant LIPY (Fig. 4, lane 2) and was used to purify LIPY by cobalt affinity chromatography. The His6-tagged LIPY protein was bound to the TALON resin and was eluted in the 150 mm imidazole elution step (Fig. 4). The recombinant LIPY migrated on SDS-PAGE with an apparent molecular weight that was slightly lower than the theoretically predicted value of 45 kDa. The purified enzyme eluted from a precalibrated Superose-6 gel filtration column just after the void volume, suggesting that the purified, recombinant LIPY exists as aggregates (data not shown).
FIGURE 4. SDS-PAGE analysis of the expression and purification of LIPY.
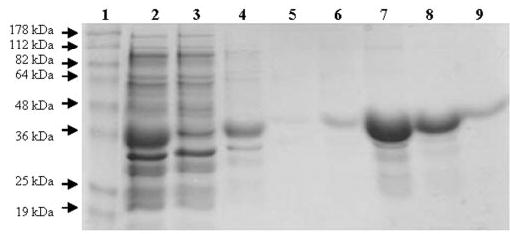
His-tagged LIPY was expressed in E. coli BL21 cells, solubilized from the 16,000 × g pellet from cell lysate and purified by cobalt affinity chromatography (TALON) as described under “Experimental Procedures.” Protein samples were loaded on a 12% SDS-polyacrylamide gel under reducing conditions. Lane 1, benchmark prestained molecular weight markers (Invitrogen); lane 2, 16,000 × g supernatant from solubilized inclusion bodies; lane 3, flow-through fraction from TALON resin; lanes 4 and 5, 10 mm imidazole wash; lanes 6 –9, 150 mm imidazole eluted fractions.
Biochemical Characterization of the TG Hydrolase Activity of LIPY
The purified recombinant LIPY protein showed very high activity in our standard radiometric triolein hydrolysis assay. Lipase activity increased linearly with time and protein concentration (Fig. 5, A and B ). LIPY displayed typical Michaelis-Menten kinetics (Fig. 5C ), and the apparent K m and V max values were calculated to be 7.57 mM and 653.3 nmol/mg/min, respectively, from the rectilinear double-reciprocal plot. LIPY hydrolyzed [14C]diolein at a lower rate (316.8 ± 9.0 nmol/mg/min). LIPY did not show any fatty acid release when incubated with phosphatidylcholine, phosphatidyl ethanolamine, or hexadecyl palmitate (data not shown). LIPY displayed optimal activity between pH 8.0 and pH 9.0 (Fig. 5D ). The effect of inhibitors like E-600, which is an organo-phosphorous compound that irreversibly inhibits various esterases and is known to target serine esterases/lipases, was tested on LIPY. E-600 inhibited LIPY by 99.5% at 0.5 μm (Fig. 6A ), and phenylmethanesulfonyl fluoride at 5 mm inhibited LIPY activity by 75% (Fig. 6B ). The temperature stability of LIPY was investigated by preincubation of the purified protein at the indicated temperature for 15 min. The lipase activity of LIPY dropped off sharply when the enzyme was held at 50 °C or higher (Fig. 6C ). LIPY was very stable in storage and retained nearly all of its original activity even after 60 days at 4 °C and after 4 cycles of freezing at −20 °C followed by thawing. The effect of various detergents on LIPY was investigated. Lipase activity was stimulated slightly by SDS at concentrations up to 2 mm, above which the activity was severely inhibited (Fig. 7A ). Triton X-100 at 0.1% stimulated the activity, but higher concentrations were inhibitory (Fig. 7B ). The inhibition by SDS was partially reversed by Triton X-100, and 20% of the original activity was recovered (data not shown). Tween 20 inhibited LIPY at all concentrations from 0.1 to 2.0% (Fig. 7C ). Many lipases require calcium for activity, but LIPY was inhibited by CaCl2. CoCl2, MnCl2, ZnCl2, and MgCl2 also inhibited the activity. However, NaCl, KCl, sodium acetate, and potassium acetate enhanced the activity (Table 4).
FIGURE 5. Characterization of triolein hydrolase activity of purified LIPY.
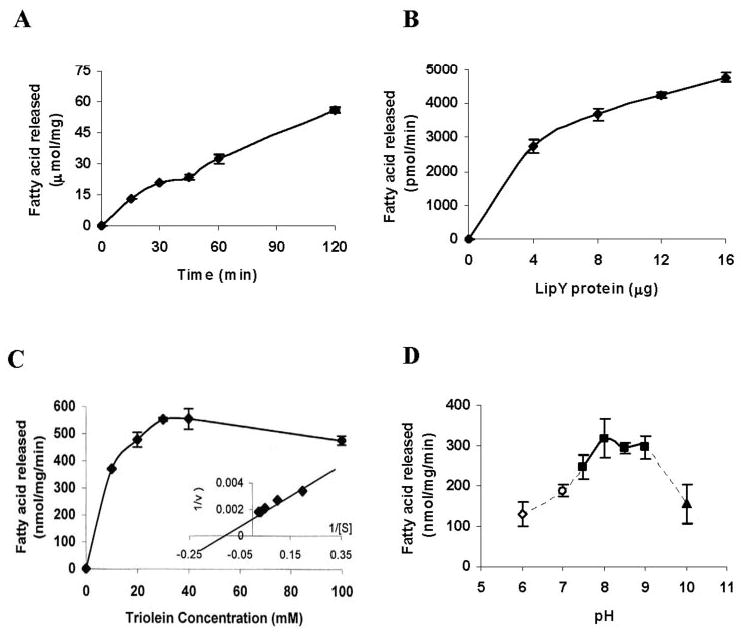
A, time course of [14C]triolein hydrolysis at pH 8.0 was determined using 8 μg of purified LIPY protein per assay. B, protein concentration dependence of TG hydrolysis (incubation time, 2 h). C, substrate concentration dependence of enzyme activity (incubation time, 2 h). D, effect of pH on lipase activity. LIPY was incubated for 15 min at room temperature in 0.1 m citrate-phosphate buffer, pH 6.0, or 0.1 m potassium phosphate buffer, pH 7.0, or 0.1 m Tris-HCl, pH 7.5/pH 8.0/pH 8.5/pH 9.0, or 0.1 m glycine-NaOH buffer, pH 10.0, prior to the assay. Values are mean ± S.D. for three replicates.
FIGURE 6.
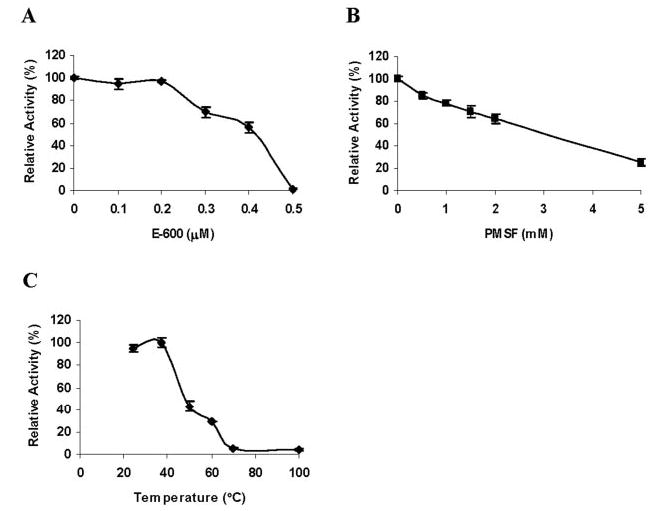
Effect of E-600 (A), phenylmethanesulfonyl fluoride (B), and temperature on TG hydrolysis by LIPY. LIPY was incubated with the indicated concentration of inhibitor for 15 min at room temperature prior to assay at 37 °C. C, LIPY was incubated at the indicated temperature for 15 min prior to the assay. Values are mean ± S.D. from three separate experiments.
FIGURE 7. Effect of detergents on LIPY activity.
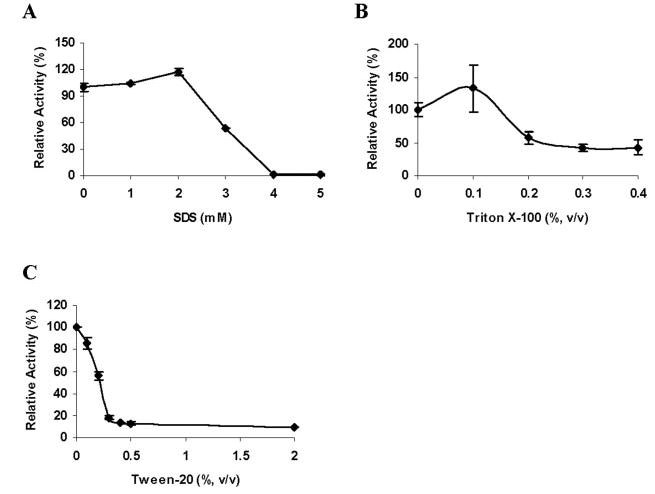
Purified LIPY was preincubated with the indicated concentrations of SDS (A), Triton X-100 (B), or Tween 20 (C) for 15 min at room temperature prior to assay. Activity relative to control is given as mean ± S.D. from three replicates.
TABLE 4. Effect of salts on LIPY activity.
Purified LIPY protein was incubated for 15 min at room temperature with the indicated concentrations of salts and then assayed radiometrically for triolein hydrolase activity as described under “Experimental Procedures.”
| Effector | Concentration | Activity (mean ± S.D.) |
|---|---|---|
| m m | nmol/mg/min | |
| None | 643.3 ± 65.0 | |
| NaCl | 200 | 681.3 ± 87.2 |
| KCl | 50 | 675.2 ± 103.5 |
| 100 | 686.8 ± 68.8 | |
| MgCl2 | 50 | 306.3 ± 111.1 |
| 100 | 219.9 ± 34.3 | |
| ZnCl2 | 10 | 358.7 ± 4.2 |
| 50 | 5.1 ± 2.5 | |
| MnCl2 | 50 | 354.3 ± 136.5 |
| 100 | 93.8 ± 7.8 | |
| CoCl2 | 10 | 424.8 ± 21.2 |
| 50 | 22.9 ± 9.9 | |
| CaCl2 | 50 | 405.1 |
| 100 | 433.8 | |
| 200 | 103.7 ± 29.8 | |
| CH3COO·K | 50 | 692.6 ± 57.0 |
| 100 | 749.0 ± 73.3 | |
| CH3COO·Na | 50 | 681.7 ± 80.3 |
| 100 | 729.3 ± 96.5 |
LIPY, a Member of the HSL Family
Thirteen of the 24 putative lipase/esterases can be classified as lipases belonging to the HSL family (12), and LIPY was the only protein of the 24 with a putative TG hydrolase activity as annotated in the data base (available on the World Wide Web at au.expasy.org/cgi-bin/niceprot.pl?P77909). The product of lipY would encode a protein with a predicted molecular mass of 45 kDa and a pI of 4.5. However, it showed only 9–21% global amino acid identity with the other lipase/esterase-like proteins in the mycobacterial genome (Table 5). Pairwise alignment of the amino acid sequence of LIPY with 35 representative lipases from all of the eight reported families of bacterial lipases (20) indicated that LIPY shared only 12–23% global amino acid identity with known bacterial lipases. However, LIPY possesses the conserved active site motif GDSAG characteristic of the HSL family. Since the crystal structures of the Bacillus subtilis brefeldinA esterase and the Archaeoglobus fulgidus carboxylesterase, which belong to the HSL family, have been elucidated (21, 22), we used the Cn3D version 4.1 software from the Entrez System at NCBI to produce a structure-based sequence alignment of LIPY with the members of the HSL family. The alignment of conserved domains produced by Cn3D was then used to align other selected members of the HSL family by the ClustalW multiple sequence alignment program. The multiple sequence alignment output from ClustalW was then adjusted manually to achieve maximum similarity between the amino acid sequences. As shown in Fig. 8, this alignment revealed a high degree of similarity between the C-terminal half of LIPY and other members of the HSL family. This region of high similarity includes the catalytic domain with the consensus pentapeptide GDSAG, containing the active serine residue and the strictly conserved HGGG motif of unknown function (23) located immediately upstream of the active site consensus motif. The aspartate and histidine residues of the active site are also conserved with the other members of the HSL family.
TABLE 5. Lipase/Esterase-like proteins in M. tuberculosis .
Amino acid identities of lipase genes were compared by pairwise alignment with LIPY, which had the highest TG hydrolase activity, using the ALIGN software program at Genestream (available on the World Wide Web at www2.igh.cnrs.fr/bin/align-guess.cgi).
| Gene product | Identity | Theoretical molecular mass | pI | Conserved active site residues |
|---|---|---|---|---|
| % | kDa | |||
| LIPYa (Rv3097c) | 100.0 | 45.0 | 4.5 | GDSAG |
| LIPP (Rv2463) | 21.4 | 42.8 | 6.0 | |
| LIPM (Rv2284) | 21.0 | 46.7 | 9.6 | GGSAG |
| LIPL (Rv1497) | 19.5 | 45.8 | 9.3 | |
| LIPD (Rv1923) | 19.0 | 47.2 | 6.7 | |
| LIPH (Rv1399c) | 18.9 | 33.9 | 4.3 | GWSLG |
| LIPE (Rv3775) | 18.8 | 45.3 | 8.6 | |
| LIPU (Rv1076) | 18.5 | 31.7 | 6.3 | GDSAG |
| LIPW (Rv0217c) | 18.4 | 32.2 | 8.3 | GASAG |
| LIPO (Rv1426c) | 18.3 | 46.1 | 10.5 | GGSAG |
| LIPI (Rv1400c) | 18.2 | 34.0 | 4.6 | GDSAG |
| LIPQ (Rv2485c) | 18.2 | 45.2 | 8.9 | GGSAG |
| LIPN (Rv2970c) | 18.1 | 40.1 | 6.4 | GDSAG |
| LIPG (Rv0646c) | 17.9 | 32.9 | 9.9 | GASMG |
| LIPK (Rv2385) | 17.9 | 32.9 | 8.1 | |
| LIPF (Rv3487c) | 17.8 | 29.4 | 7.7 | GDSAG |
| LIPT (Rv2045c) | 17.6 | 56.1 | 8.7 | GESAG |
| LIPZa (Rv1834) | 17.4 | 31.6 | 9.4 | |
| LIPR (Rv3084) | 17.1 | 32.6 | 9.9 | GDSAG |
| LIPC (Rv0220) | 16.7 | 44.3 | 10.4 | GCSAG |
| LIPJ (Rv1900c) | 16.4 | 49.7 | 5.4 | |
| LIPV (Rv3203) | 15.8 | 23.6 | 4.5 | GHSFG |
| LIPS (Rv3176c) | 15.2 | 35.2 | 6.4 | |
| LIPXa (Rv1169c) | 8.7 | 10.8 | 5.9 |
We identified these genes as putative lipases as described under “Experimental Procedures.”
FIGURE 8. Structure-based alignment of the conserved domains of LIPY with lipases from the HSL family.
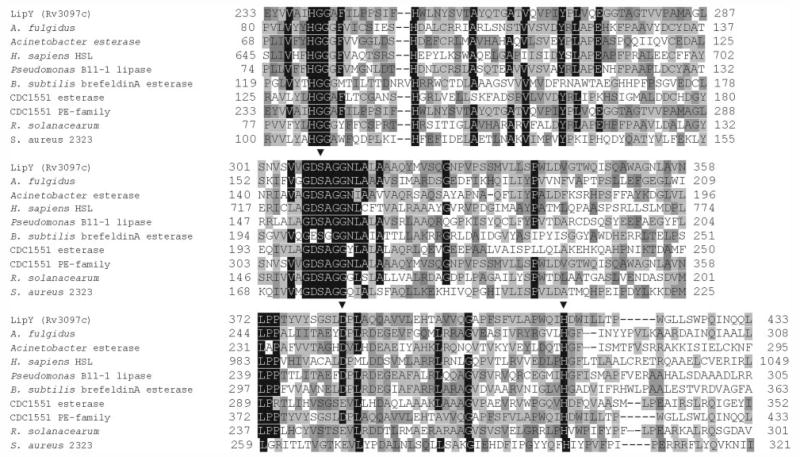
The multiple sequence alignment produced by Cn3D was used to align other members of the HSL family with LIPY using ClustalW, and the output was manually adjusted for optimal alignment. The sequences selected for alignment with LIPY are A. fulgidus carboxylesterase (GI 17943077), Acinetobacter esterase (GI 34559428), human HSL (GI 21328445), Pseudomonas B11–1 lipase (GI 2853612), B. subtilis brefeldin A esterase (GI 414722), M. tuberculosis CDC1551 esterase (GI 15840511), M. tuberculosis CDC1551 PE family protein (GI 15842668), R. solanacearum putative esterase/lipase (GI 17545158), and S. aureus hypothetical protein (GI 15928114). Black shading indicates residues conserved in seven or more aligned sequences, and shades of gray are used to indicate residues conserved in a few sequences. ▾, amino acids belonging to the catalytic triad. The ClustalW program was accessed from the European Bioinformatics Institute site on the World Wide Web at www.ebi.ac.uk/clustalw/.
DISCUSSION
Microbial populations grow exponentially while there is an abundant supply of nutrients, but starvation causes Gram-positive bacteria to generate dormant spores and enter a phase of low metabolic activity called dormancy in which the cells remain viable for long periods of time. M. tuberculosis exhibits such an ability to survive within the host in the dormant state for several decades and reactivate when conditions are favorable to cause the infectious form of the disease. Many fundamental questions concerning the dormant and reactivation phases of M. tuberculosis inside the host remain unanswered, and primary among them is one on the nature of the energy source for the bacillus during these stages.
The M. tuberculosis genome has more than 150 genes encoding enzymes involved in fatty acid degradation (11), strongly suggesting that M. tuberculosis uses host lipids during in vivo growth. In Mycobacterium species the glyoxylate cycle has been associated with the “persistence” locus (3), which is conserved between animal and plant pathogens that have the ability to switch diet inside the host (24). In vitro grown bacteria preferred carbohydrates as the energy source, but M. tuberculosis grown in vivo showed a preference for fatty acids (10). Macrophages infected with M. bovis BCG showed a marked decrease in the total TG (25).
Previously, we demonstrated that several nonclassical triacylglycerol synthase genes are induced in M. tuberculosis under hypoxic culture conditions, leading to the accumulation of TG. We proposed that TG is stored by the organism as it goes into dormancy for utilization during dormancy and reactivation (7). Despite the established Cornell mouse model of dormancy and reactivation (26) and the Wayne hypoxic model of nonreplicative persistence (27), there is a lack of an in vitro model of reactivation that induces the mycobacterium to utilize endogenously stored TG. Microarray analysis of the response of M. tuberculosis proline glutamic acid/proline proline glutamic acid genes to environmental change indicated that lipY (PE-PGRS63) was induced at least 2-fold inside interferon-γ-activated macrophage and repressed at least 2-fold in the stationary phase (28). A recent study on genome-wide changes in M. tuberculosis during nutrient starvation indicated that lipC, lipU, lipT, and Rv1169c (lipX) were up-regulated upon starvation (29). When M. tuberculosis was subjected to a 15-min acid shock, lipR and lipX were found to be up-regulated among other genes (30). In another report, the Mycobacterium avium homolog of lipL was reported to be up-regulated in cultured macrophages and in mice (31).
We used a hypoxic culture condition that caused the mycobacterium to accumulate TG followed by a nutrient starvation condition that we thought might compel the organism to utilize the endogenously stored TG. Release of energy stored in TG requires the activity of long-chain TG hydrolase(s). Although the purification of an intracellular, cell wall-associated long-chain TG lipase from Mycobacterium phlei was reported several decades ago (32), there has been no report in the literature on the identification of a long-chain TG hydrolase from M. tuberculosis. We report here on the identification and characterization of the lipY (Rv3097c) product, which is such an enzyme demonstrating a high specific activity for trioleoylglycerol hydrolysis and the identification of lipL, lipG, lipC, lipK, and Rv1169c products, which possess low levels of long-chain TG hydrolase activity. Purified LIPY hydrolyzed long-chain TG with high specific activity. Optimal activity was observed between pH 8.0 and pH 9.0, and the activity was inhibited by active serine-directed reagents. LIPY was very stable when stored at 4 or −20 °C for 60 days and subjected to four freeze-thaw cycles but was inactivated at temperatures above 50 °C. This remarkable stability in storage may be explained in part by the absence of cysteine residues. LIPY was completely inactivated by SDS above its critical micellar concentration, and the activity was not recovered completely by the addition of Triton X-100. In contrast, the cutinase from Fusarium solani pisi, which possessed a single disulfide bridge, was reported to recover its original activity by the addition of Triton X-100 after SDS treatment (33). LIPY did not require divalent cations for activity and did not hydrolyze phospholipids or wax esters. LIPY is a member of the PE-PGRS family, which is composed of nearly 100 members and is unique to mycobacteria (19). The N-terminal half of LIPY is homologous to proteins belonging to the PE-PGRS family, and the C-terminal half possesses domains conserved with the HSL family of lipases. To the best of our knowledge, LIPY is the first PE-PGRS family protein reported to have enzymatic activity. An analysis of the genomes of other mycobacterial species revealed that M. bovis BCG and M. tuberculosis CDC1551 each have a respective homolog of Rv3097c (lipY). However, the corresponding homolog in M. leprae is a pseudogene. We could not find homologs in the genomes of M. avium and M. smegmatis.
We have previously shown that hypoxic M. tuberculosis cultures accumulate TG (7). In the present study, we have shown that the TG, accumulated under hypoxic growth, was utilized when the cells were starved in PBS. Both radiolabeling and chemical analysis showed that under such nutrient starvation conditions, TG was efficiently utilized by M. tuberculosis cells. TG utilization by M. tuberculosis cells under such conditions suggests a possible role for the lipases in the mycobacterial genome. Most of the lip genes were found to be up-regulated during starvation after TG accumulation, but no significant induction of lip genes could be detected when the cells with accumulated TG were incubated in nutrient-rich 7H9 medium. Among the highly induced genes,lipYshowed the highest level of induction and a combination of the induction level and specific lipase activity indicates that lipY could be the primary lipase gene involved in the utilization of stored TG. In fact, lipY-deficient mutant had a significantly decreased ability to hydrolyze TG when grown under TG utilization conditions. Moreover, no other lip gene showed a compensatory up-regulation in the Δ-lipY cells under starvation in PBS. The very low TG hydrolase activity detected in the Δ-lipY mutant could be the collective contribution by all or some of the other lip genes and thus validates the central role of lipY in TG hydrolysis. To the best of our knowledge, this is the first demonstration of the utilization of stored TG by M. tuberculosis cells, and LIPY emerges as the main long chain TG hydrolase involved in TG utilization during the nutrient starvation. We postulate that the hydrolysis of TG, stored as the pathogen enters dormancy, by LIPY provides fatty acids as a source of carbon and energy for the bacterium, and thus LIPY may play a critical metabolic role during dormancy and reactivation of the pathogen.
Acknowledgments
We thank Chang-Muk Lee for statistical analysis of the lipase activity data.
Footnotes
This work was supported in part by National Institutes of Health Grants AI46582 and AI35272.
The abbreviations used are: TG, triacylglycerol; HSL, hormone-sensitive lipase; PBS, phosphate-buffered saline; RT, reverse transcription; PE-PGRS, proline glutamic acid-polymorphic GC-rich repetitive sequences; ORF, open reading frame.
References
- 1.The WHO/IUATLD Global Project on Anti-tuberculosis Drug Resistance Surveillance (2004) Anti-tuberculosis Drug Resistance in the World: Third Global Report, pp. 13–25, World Health Organization, Geneva, Switzerland
- 2.Dannenberg, Jr., A. M., and Rook G. A. W. (1994) in Tuberculosis: Pathogenesis, Protection and Control (Bloom, B. R., ed) pp. 459–483, American Society for Microbiology, Washington D. C.
- 3.Honer zu Bentrup K, Russell DG. Trends Microbiol. 2001;9:597–605. doi: 10.1016/s0966-842x(01)02238-7. [DOI] [PubMed] [Google Scholar]
- 4.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. J Am Med Assoc. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (2005) Global Tuberculosis Control: Surveillance, Planning, Financing, pg. 24, World Health Organization, Geneva, Switzerland
- 6.Zahrt TC. Microbes Infect. 2003;5:159–167. doi: 10.1016/s1286-4579(02)00083-7. [DOI] [PubMed] [Google Scholar]
- 7.Daniel J, Deb C, Dubey VS, Sirakova TD, Abomoelak B, Morbidoni HR, Kolattukudy PE. J Bacteriol. 2004;186:5017–5030. doi: 10.1128/JB.186.15.5017-5030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munoz-Elias EJ, McKinney JD. Nat Med. 2005;11:638–644. doi: 10.1038/nm1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell DG. Nat Cell Biol. 2003;5:776–778. doi: 10.1038/ncb0903-776. [DOI] [PubMed] [Google Scholar]
- 10.Segal W, Bloch H. J Bacteriol. 1956;72:132–141. doi: 10.1128/jb.72.2.132-141.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 12.Hotelier T, Renault L, Cousin X, Negre V, Marchot P, Chatonnet A. Nucleic Acids Res. 2004;32:D145–D147. doi: 10.1093/nar/gkh141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canaan S, Maurin D, Chahinian H, Pouilly B, Durousseau C, Frassinetti F, Scappuccini-Calvo L, Cambillau C, Bourne Y. Eur J Biochem. 2004;271:3953–3961. doi: 10.1111/j.1432-1033.2004.04335.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Wang JD, Li ZF, Xie J, Yang YP, Zhong Y, Wang HH. Protein Expression Purif. 2005;42:59–66. doi: 10.1016/j.pep.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Sirakova TD, Thirumala AK, Dubey VS, Sprecher H, Kolattukudy PE. J Biol Chem. 2001;276:16833–16839. doi: 10.1074/jbc.M011468200. [DOI] [PubMed] [Google Scholar]
- 16.Bardarov S, Bardarov S, Jr, Pavelka MS, Jr, Sambandamurthy V, Larsen M, Tufariello J, Chan J, Hatfull G, Jacobs WR., Jr Microbiology. 2002;148:3007–3017. doi: 10.1099/00221287-148-10-3007. [DOI] [PubMed] [Google Scholar]
- 17.Derbyshire, K. M., and Bardarov, S. (2000) in Molecular Genetics of Mycobacteria (Hatfull, G. F., and Jacobs, W. R., Jr., eds) pp. 93–107, American Society for Microbiology Press, Washington, D. C.
- 18.Belfrage P, Vaughan M. J Lipid Res. 1969;10:341–344. [PubMed] [Google Scholar]
- 19.Brennan MJ, Delogu G. Trends Microbiol. 2002;10:246–249. doi: 10.1016/s0966-842x(02)02335-1. [DOI] [PubMed] [Google Scholar]
- 20.Arpigny JL, Jaeger KE. Biochem J. 1999;343:177–183. [PMC free article] [PubMed] [Google Scholar]
- 21.Wei Y, Contreras JA, Sheffield P, Osterlund T, Derewenda U, Kneusel RE, Matern U, Holm C, Derewenda ZS. Nat Struct Biol. 1999;6:340–345. doi: 10.1038/7576. [DOI] [PubMed] [Google Scholar]
- 22.De Simone G, Menchise V, Manco G, Mandrich L, Sorrentino N, Lang D, Rossi M, Pedone C. J Mol Biol. 2001;314:507–518. doi: 10.1006/jmbi.2001.5152. [DOI] [PubMed] [Google Scholar]
- 23.Jaeger KE, Dijkstra BW, Reetz MT. Annu Rev Microbiol. 1999;53:315–351. doi: 10.1146/annurev.micro.53.1.315. [DOI] [PubMed] [Google Scholar]
- 24.Vereecke D, Cornelis K, Temmerman W, Holsters M, Goethals K. Trends Microbiol. 2002;10:485–488. doi: 10.1016/s0966-842x(02)02457-5. [DOI] [PubMed] [Google Scholar]
- 25.Jackson SK, Stark JM, Taylor S, Harwood JL. Br J Exp Pathol. 1989;70:435–441. [PMC free article] [PubMed] [Google Scholar]
- 26.McCune RM, Feldmann FM, Lambert HP, McDermott W. J Exp Med. 1966;123:445–468. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wayne LG, Sohaskey CD. Annu Rev Microbiol. 2001;55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 28.Voskuil MI, Schnappinger D, Rutherford R, Liu Y, Schoolnik GK. Tuberculosis (Edinb) 2004;84:256–262. doi: 10.1016/j.tube.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Mol Microbiol. 2002;43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 30.Fisher MA, Plikaytis BB, Shinnick TM. J Bacteriol. 2002;184:4025–4032. doi: 10.1128/JB.184.14.4025-4032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danelishvili L, Poort MJ, Bermudez LE. FEMS Microbiol Lett. 2004;239:41–49. doi: 10.1016/j.femsle.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Paznokas JL, Kaplan A. Biochim Biophys Acta. 1977;487:405–421. doi: 10.1016/0005-2760(77)90212-0. [DOI] [PubMed] [Google Scholar]
- 33.Kolattukudy, P. E. (1984) in Lipases (Borgstrom, B., and Brockman, H., eds) pp. 47–504, Elsevier Science Publishers, Amsterdam, The Netherlands


