Abstract
Soybean nodulin 26 is expressed and targeted to the symbiosome membrane of nitrogen-fixing nodules, where it forms an aquaporin channel with a modest water transport rate. In this study, we show that the phosphorylation of nodulin 26 on Ser-262, which is catalyzed by a symbiosome membrane–associated calcium-dependent protein kinase, stimulates its intrinsic water transport rate. Furthermore, using a phosphospecific antibody, we have elucidated the developmental appearance and regulation of nodulin 26 phosphorylation in vivo. Although nodulin 26 protein is detected first in differentiating infected cells (16 days), phosphorylated nodulin 26 does not become pronounced until infected cell maturation (25 days). Phosphorylation is sustained at steady state levels until entry into senescence. Nodulin 26 phosphorylation is enhanced further by osmotic stresses (water deprivation and salinity). Thus, the phosphorylation of nodulin 26 coincides with the establishment of mature nitrogen-fixing symbiosomes, is regulated by osmotic stresses that induce calcium-signaling pathways, and appears to be part of the adaptive responses of infected cells to osmotic challenge.
INTRODUCTION
Under conditions of limiting soil nitrogen, legumes can be infected by bacteria of the Rhizobiaceae family, resulting in the development of a symbiotic nitrogen-fixing organ, the nodule (reviewed by Gualtieri and Bisseling, 2000; Stougaard, 2000; Hirsch et al., 2001). During this process, the bacteria become enclosed within specialized infected cells within the core of the nodule. Most of the cytosolic volume of these cells is occupied by large numbers of organelles referred to as symbiosomes (Roth et al., 1988). The symbiosome consists of three major components: the nitrogen-fixing rhizobia bacteroids, the external symbiosome space, and a membrane of plant origin known as the symbiosome membrane.
The symbiosome membrane is highly specialized and participates in the exchange of metabolites (the efflux of fixed nitrogen and the uptake of a carbon source in the form of dicarboxylates) between the endosymbiont and the plant host as well as serving to protect the bacteroid from plant host defense responses (Udvardi and Day, 1997; Day et al., 2001). During the biogenesis of the symbiosome, a number of plant-encoded nodule-specific gene products (nodulins) are targeted to the symbiosome membrane, where they are presumed to aid in the establishment and maintenance of symbiosis (Fortin et al., 1985; Panter et al., 2000). Nodulin 26 is a symbiosome membrane–specific protein that is the major protein component of this membrane (Fortin et al., 1987; Weaver et al., 1991). Nodulin 26 is a member of the major intrinsic protein (MIP) superfamily of water and solute channels, and it confers high water permeability on the soybean symbiosome membrane (Rivers et al., 1997; Dean et al., 1999). In addition, nodulin 26 is the major phosphorylation target for a calcium-dependent protein kinase (CDPK), which also resides on the symbiosome membrane (Weaver et al., 1991). These multifunctional protein kinases are now recognized as the principal targets of calcium signals and catalyze the phosphorylation of multiple proteins, including membrane channels, pumps, and a number of metabolic enzymes (reviewed by Harmon et al., 2000). Phosphorylation of nodulin 26 occurs on one residue, Ser-262, which resides within the cytosolic C-terminal tail of the protein (Weaver and Roberts, 1992).
The findings that nodulin 26 is produced specifically during the formation of the symbiosome membrane, is a major component of this symbiotic interface, and is responsible for the high water permeability of this membrane suggest a specialized role in symbiosis, perhaps in osmoregulation (Rivers et al., 1997; Dean et al., 1999). Furthermore, the finding that nodulin 26 is phosphorylated by a CDPK suggests that these potential activities are subject to regulation by calcium signal transduction. However, the functional effects of phosphorylation as well as the nature of the developmental and environmental signals that regulate nodulin 26 phosphorylation have remained obscure. Here, we show that the phosphorylation of nodulin 26 is accompanied by an increase in its water permeability. Furthermore, using a phosphorylation-specific antibody, we have elucidated the appearance and regulation of nodulin 26 phosphorylation in vivo. Overall, phosphorylation accompanies the maturation of the nodule and is regulated in response to osmotic signals, supporting a role for nodulin 26 in the osmoregulation of the symbiosome membrane.
RESULTS
Comparison of the Water Permeability of Wild-Type Nodulin 26 and Phosphorylation Mutants in Oocytes
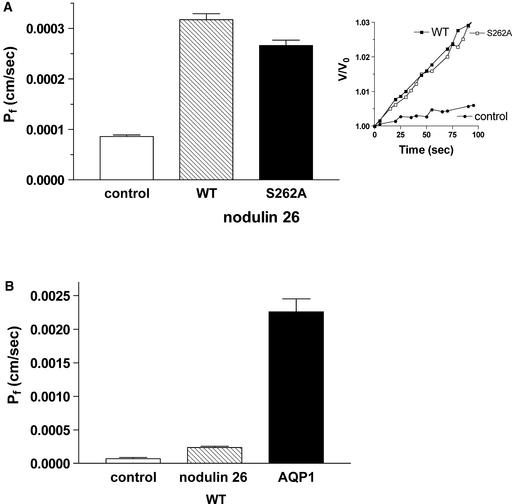
Upon expression in Xenopus laevis oocytes, wild-type soybean nodulin 26 and a mutant nodulin 26, S262A, which possesses an Ala substitution at the phosphorylation site (Ser-262), showed identical water permeability properties, increasing the osmotic water permeability (Pf) of the oocyte plasma membrane between threefold and fourfold compared with controls (Figure 1A). Oocytes expressing wild-type and S262A nodulin 26 also showed a decrease in activation energy as well as sensitivity to Hg2+ (data not shown), consistent with protein-facilitated water flux. Thus, the presence of a Ser or Ala at position 262 had no apparent effect on Pf. Although nodulin 26 expression in oocytes resulted in facilitated water transport, the overall change in water permeability was much less than that conferred by the expression of human aquaporin 1 (Figure 1B), consistent with the large disparity in the single-channel rates of these two MIP proteins (Rivers et al., 1997; Dean et al., 1999).
Figure 1.
Comparison of Osmotic Water Permeabilities of Oocytes Expressing Nodulin 26 and a Nodulin 26 Phosphorylation Null Mutant.
Xenopus oocytes were injected with 46 ng of the indicated complementary RNA, and oocyte swelling assays were performed by videomicroscopy as described in Methods. Error bars show standard errors of the mean (n = 6).
(A) Representative oocyte swelling profile (inset) and calculated osmotic water permeabilities of control oocytes or oocytes expressing wild-type nodulin 26 (WT) or the Ala-262 (S262A) mutant.
(B) Comparison of water permeabilities of control oocytes or oocytes expressing wild-type nodulin 26 (WT) or human aquaporin 1 (AQP1).
Analysis of Phosphorylation Effects in Oocytes
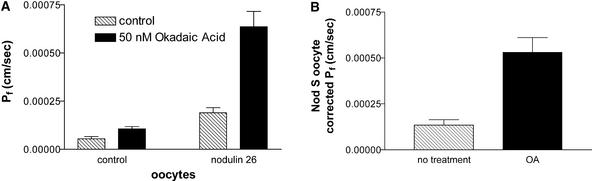
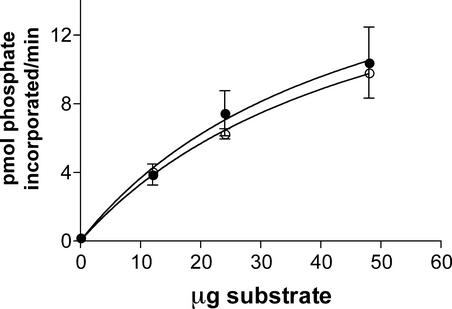
Oocytes treated with 50 nM of the general protein phosphatase type 1 and 2A inhibitor okadaic acid (OA) showed between a threefold and fourfold increase in Pf (0.00053 cm/s) compared with untreated controls (0.00013 cm/s) (Figure 2). Based on this result, it was proposed that nodulin 26 is a target for an oocyte protein kinase and that treatment with OA shifted the equilibrium of the protein to a phosphorylated state. Although nodulin 26 is phosphorylated in planta by CDPK (Weaver et al., 1991; Weaver and Roberts, 1992), it is less clear which protein kinase might phosphorylate the protein in oocytes, because animal tissues lack this enzyme (Harmon et al., 2000). Ser-262 within the hydrophilic C-terminal domain of nodulin 26 resembles sequences recognized by animal protein kinase Cα (PKCα) (Chakravarthy et al., 1991). Furthermore, another MIP protein, aquaporin 0, is phosphorylated by PKC on an identically placed Ser residue within its C terminus (Lampe and Johnson, 1989). Consistent with these observations, CK-15, a synthetic peptide substrate that contains the 14 C-terminal residues in nodulin 26, including the phosphorylation motif and Ser-262 (Weaver et al., 1991), was phosphorylated in vitro by PKCα in a manner indistinguishable from that of histone III, a commonly used substrate to assay this enzyme (Figure 3). In addition, the ectopic addition of PKCα to isolated symbiosome membranes also resulted in the phosphorylation of nodulin 26 (data not shown). These observations suggest that nodulin 26 is a substrate for PKC and that this enzyme might be a surrogate protein kinase that phosphorylates nodulin 26 in oocytes.
Figure 2.
Effect of OA on the Water Permeability of Oocytes Expressing Wild-Type Nodulin 26.
Xenopus oocytes expressing wild-type nodulin 26 complementary RNA or control oocytes were assayed by the swelling assay as described for Figure 1. The oocytes then were reequilibrated in isoosmotic Ringer's solution, treated with 50 nM OA as described in Methods, and reassayed. Error bars show standard errors of the mean (n = 10).
(A) Water permeability of nodulin 26 or water-injected (control) oocytes before (cross-hatched bars) or after (solid bars) OA treatment.
(B) Permeability of nodulin 26 oocytes after correction for basal oocyte water permeability.
Figure 3.
Phosphorylation of the C-Terminal Sequence of Nodulin 26 with PKCα.
A peptide (CK-15) comprising the hydrophilic C-terminal domain of nodulin 26 including the conserved CDPK phosphorylation site (Ser-262) was tested as a substrate for PKCα (closed circles). A histone IIIS preparation was tested as a positive control (open circles). Error bars show standard errors of the mean.
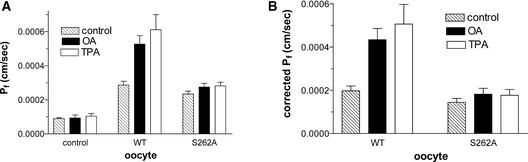
To investigate this possibility further, the effects of the PKC agonist phorbol-12-myristate-13-acetate (TPA) was tested on oocytes expressing wild-type nodulin 26 or the phosphorylation null mutant nodulin 26 S262A (Figure 4). Treatment of wild-type oocytes with either 10 nM TPA or 25 nM OA selectively enhanced the Pf of wild-type oocytes but did not affect water-injected controls or oocytes expressing nodulin 26 S262A (Figure 4). These data strongly suggest that OA and TPA result in the phosphorylation of nodulin 26 at Ser-262 and increase water permeability.
Figure 4.
Phorbol Ester and OA Effects on Nodulin 26 and Nodulin 26 Phosphorylation Null Mutant.
Oocytes expressing the indicated complementary RNAs (nodulin 26 wild type [WT] or nodulin 26 phosphorylation null mutant [S262A]) were prepared and assayed as described for Figure 1 with either no treatment (control; cross-hatched bars) or after treatment with 10 nM TPA (open bars) or 25 nM OA (closed bars). Error bars show standard errors of the mean (n = 14).
(A) Water permeabilities of various oocytes.
(B) Permeabilities corrected for the basal flux of the oocyte plasma membrane.
Effect of Nodulin 26 Phosphorylation on the Water Permeability of Soybean Symbiosomes
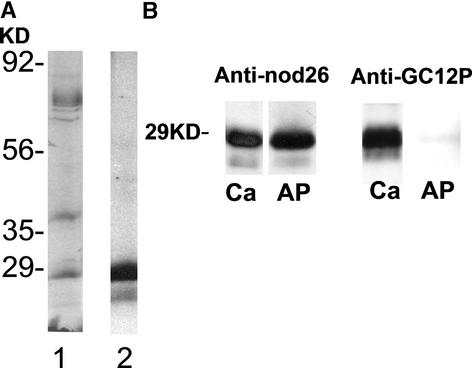
Although these findings provide strong evidence for the phosphorylation regulation of nodulin 26 water flux, we tested this hypothesis further by analyzing the effects of phosphorylation on the activity of native soybean nodulin 26 in isolated symbiosome membrane vesicles (Figure 5). To determine the phosphorylation status of nodulin 26, an antibody (anti-GC12P) that reacts specifically with phosphorylated nodulin 26 was generated against a peptide antigen containing the phosphorylated Ser-262 epitope. Nodulin 26 isolated from symbiosome membranes showed high reactivity with this antibody, which can be enhanced even further by incubation of symbiosome membranes with Ca2+ and ATP (which results in the phosphorylation of nodulin 26 by a symbiosome membrane–associated CDPK [Weaver et al., 1991]) (Figure 5A). Incubation of symbiosome membranes with calf intestinal phosphatase resulted in the loss of the protein gel blot signal with the phospho-specific anti-GC12P antibodies, whereas the overall immunoreactivity with anti-nodulin 26 antibodies was not affected (Figure 5B). Thus, treatments for the preparation of phosphorylated and dephosphorylated nodulin 26 were established.
Figure 5.
Preparation of Symbiosome Membrane Vesicles with Phosphorylated or Dephosphorylated Nodulin 26.
Soybean symbiosome membrane vesicles with phosphorylated or dephosphorylated nodulin 26 were prepared as described in Methods.
(A) Nodulin 26 phosphorylated in situ with CDPK by incubation of isolated symbiosome membranes (4 μg) with 1 mM CaCl2 and γ-32P-ATP was subjected to SDS-PAGE on 12.5% (w/v) polyacrylamide gels. Lane 1, Coomassie blue–stained gel; lane 2, autoradiogram of the gel shown in lane 1.
(B) Protein gel blot comparison of soybean symbiosome nodulin 26 phosphorylated in situ with CDPK (Ca) or dephosphorylated by phosphatase treatment (AP) with antibodies against nodulin 26 protein (anti-nod26) or with antibodies specific for the phosphorylated form of the protein (anti-GC12P).
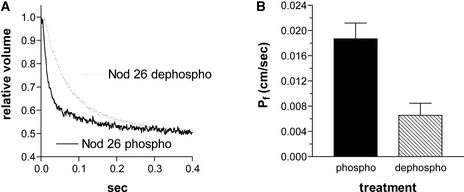
Analysis of the stopped-flow properties of the vesicles after these treatments showed that dephosphorylation of nodulin 26 was accompanied by a 70% reduction in symbiosome membrane water permeability (from a Pf of 0.019 cm/s [sem = 0.0025] to 0.007 cm/s [sem = 0.0019]) (Figure 6). Overall, these data support the findings with oocytes and suggest that the phosphorylation of nodulin 26 enhances its water permeability.
Figure 6.
Phosphorylation of Nodulin 26 Enhances the Water Permeability of Symbiosome Membrane Vesicles.
(A) Stopped-flow fluorimetric trace showing the time course of the change in internal volume of carboxyfluorescein-loaded symbiosome membrane vesicles containing phosphorylated (solid line) or dephosphorylated (dashed line) nodulin 26 upon doubling the medium osmolarity.
(B) Comparison of the calculated symbiosome membrane Pf from stopped-flow traces similar to those in (A). Error bars show standard errors of the mean (n = 3).
Nodulin 26 Phosphorylation Is Regulated Developmentally and by Osmotic Stress
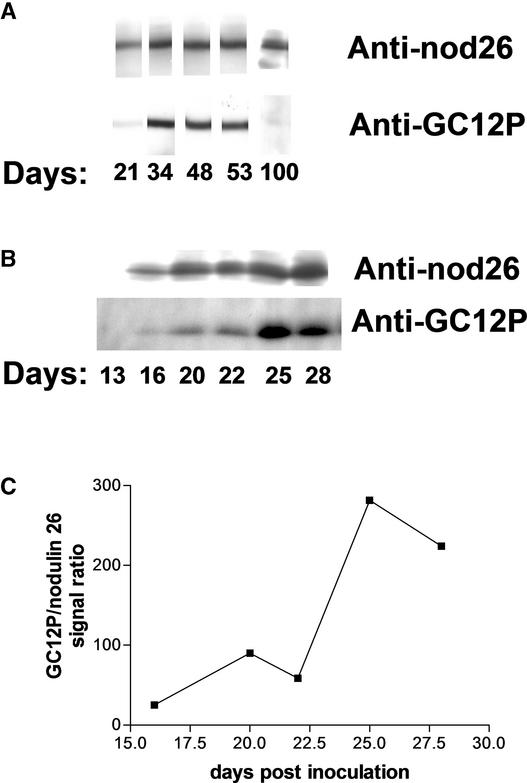
To assay the amount of nodulin 26 and its phosphorylation state in soybean nodule samples, membrane samples from developing soybean nodules were assayed by protein gel blot analysis with anti-nodulin 26 and anti-GC12P antibodies (Figure 7). Nodulin 26 protein became apparent at 16 days after planting, accumulated rapidly, and was sustained at a high level throughout the lifetime of the nodule (Figure 7). However, the phosphorylation of nodulin 26 lagged behind its appearance and did not reach a peak until 25 days after inoculation, at a time when nodules matured and were fully developed (Figure 8). Phosphorylation patterns remained high until very late developmental stages (after flowering), at which time they were virtually undetectable, even though high levels of nodulin 26 still were present (Figure 7).
Figure 7.
Developmental Expression Patterns of Nodulin 26 Protein and Phosphorylation State.
Twenty-microgram samples of soybean nodule membranes were collected and assayed by protein gel blot analysis for nodulin 26 protein content (anti-nod26) and phosphorylation state (anti-GC12P).
(A) Blots from a wide developmental profile (21 to 100 days after planting).
(B) Results from a separate experiment are shown, with a longer film exposure time focused on the crucial times for the onset of nodulin 26 protein production and the appearance of phosphorylation.
(C) Relative ratios of the phosphorylation (anti-GC12P) and protein (anti-nod26) signals from densitometry.
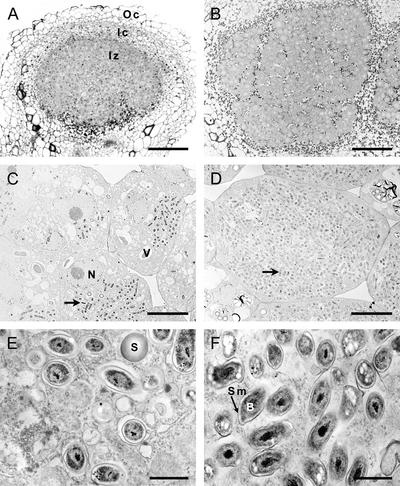
Figure 8.
Morphology of Developing Soybean Nodules.
Morphology of nodules from 13- or 25-day-old plants was investigated by bright-field microscopy and transmission electron microscopy.
(A) and (B) Bright-field images of toluidine blue-stained cross-sections of 13-day-old nodules (A) or 25-day-old nodules (B), with the outer cortex (Oc), inner cortex (Ic), and central infected zone (Iz) indicated.
(C) and (D) Low-magnification electron micrographs of infected cells of 13-day-old nodules (C) or 25-day-old nodules (D), with symbiosomes indicated by arrowheads. Residual vacuole (V) and nucleus (N) are indicated in the immature 13-day-old infected cells.
(E) and (F) High-magnification electron micrographs of representative symbiosomes from 13-day-old nodules (E) and 25-day-old nodules (F), with the symbiosome membrane (Sm) and bacteroids (B) indicated. Prominent poly-β-hydroxybutyrate granules, which become prevalent in 25-day-old nodule bacteroids, are visible as white staining bodies (F). S indicates a starch granule in immature 13-day-old infected cells.
Bars = 190 μm ([A] and [B]), 9 μm ([C] and [D]), and 1 μm ([E] and [F]).
Microscopic examination of developing nodules revealed that between 13 and 16 days after inoculation, a central infection zone was apparent with immature, differentiating infected cells, which showed the vesiculation of the large central vacuole and small numbers of symbiosomes that contained single bacteroids (Figure 8). By contrast, at 25 days, nodules showed larger differentiated infected cells with greater numbers of symbiosomes containing mature bacteroids, as indicated by the accumulation of poly-β-hydroxybutyrate granules (Figure 8). Overall, the developmental pattern suggests that nodulin 26 protein production occurs early in symbiosome biogenesis and that phosphorylation lags behind its synthesis, becoming apparent in mature, functional nitrogen-fixing symbiosomes.
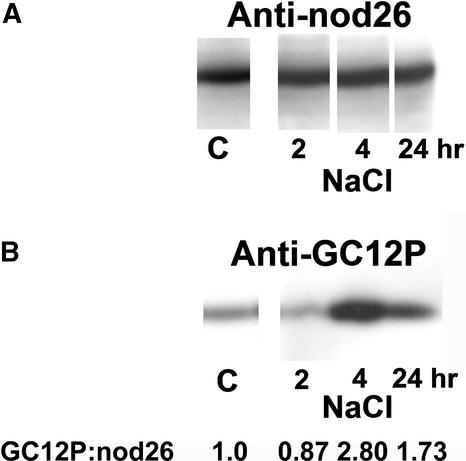
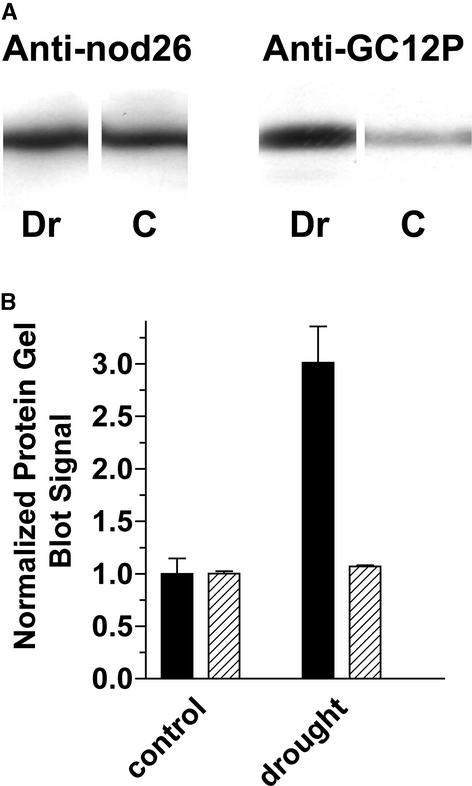
Because calcium signaling has been implicated in plant responses to osmotic signals (Knight et al., 1997), and considering the high sensitivity of nodules to osmotic stress signals (Pankhurst and Sprent, 1975; Weisz et al., 1985; Serraj et al., 1994), the effects of salinity stress on nodulin 26 phosphorylation in mature nodules were addressed. Exposure of 33-day-old nodulated soybeans to saline conditions (0.3 M NaCl) resulted in no detectable change in the overall levels of nodulin 26 protein (Figure 9A, anti-nodulin 26 signal), whereas the phosphorylation levels of the protein (Figure 9B, anti-GC12P signal) were enhanced as early as 4 h after treatment and remained high at 24 h after stimulus. Water deprivation of nodules showed similar results, with little change in nodulin 26 protein levels, whereas phosphorylation levels increased threefold (Figure 10). Interestingly, younger plants (<21 days) that showed low endogenous levels of phosphorylated nodulin 26 also lacked the ability to respond to salinity stress, suggesting that phosphorylation under normal or stress conditions was acquired only after nodule maturation (data not shown). Overall, these results show that the phosphorylation of nodulin 26 is dependent on the developmental state of the nodule and is enhanced by osmotic stress stimuli (drought or salinity).
Figure 9.
Phosphorylation of Nodulin 26 Is Stimulated by Salinity.
Membrane samples were prepared from nodules from 33-day-old control plants (C) and 33-day-old plants at 2, 4, and 24 h after watering with 0.3 M NaCl. Chemiluminescent autoradiograms of protein gel blots of 20-μg membrane samples for nodulin 26 protein content (anti-nod26) (A) and phosphorylation state (anti-GC12P) (B) are shown. The normalized ratio of anti-GC12P/anti-nod26 signals is indicated below (B).
Figure 10.
Phosphorylation of Nodulin 26 Is Stimulated by Water Deprivation Conditions.
Membrane samples (20 μg/lane) from nodules from 48-day-old well-watered control plants (C) or drought-stressed plants (Dr) were assayed by protein gel blot analysis for nodulin 26 protein content (anti-nod26) and phosphorylation state (anti-GC12P).
(A) Chemiluminescent autoradiogram of a protein gel blot.
(B) Comparison of the relative amounts of nodulin 26 protein (from densitometry of the anti-nod26 signal; cross-hatched bars) and the relative phosphorylation state (from the ratio of the anti-GC12P/anti-nod26 protein gel blot signals; solid bars) of nodulin 26. Data were normalized to the average signal obtained for control plants. Error bars show standard errors of the mean from three biological replicates.
DISCUSSION
Nodulin 26, among the first plant MIPs to be identified (Sandal and Marcker, 1988), is unique in that it is expressed solely in nitrogen-fixing legume nodules, where it is targeted specifically to the symbiosome membrane (Fortin et al., 1987; Weaver et al., 1991), the interface of bacteria-plant symbioses. Nodulin 26 is structurally and functionally distinct from other plant MIPs and is the archetype of a subfamily of plant MIPs known as the nodulin-like intrinsic membrane proteins (NIPs) (Johanson et al., 2001). NIPs are expressed in vegetative and reproductive tissues of nonlegumes (Weig et al., 1997; Johanson et al., 2001), suggesting a larger role in plant water relations than the symbiotic role of nodulin 26. Nodulin 26 (Rivers et al., 1997; Dean et al., 1999) and other NIP proteins (Weig et al., 1997; Guenther and Roberts, 2000; Weig and Jakob, 2000) share the general properties of multifunctional transport of water and uncharged solutes such as glycerol and have a low rate of water transport compared with most water-selective aquaporins (Rivers et al., 1997; Dean et al., 1999). Homology modeling based on MIP crystal structures (Fu et al., 2000; Sui et al., 2001) reveal a unique “hybrid” signature of four amino acids within the selectivity filter of the NIP pore that are proposed to account for these transport properties (Wallace et al., 2002).
Numerous MIP proteins are subject to phosphorylation, and the effects on activity are varied, ranging from the stimulation (e.g., the plant MIPs αTIP [Maurel et al., 1995] and PM28A [Johansson et al., 1998]) to the inhibition of water transport (e.g., mammalian AQP4 [Han et al., 1998; Zelenina et al., 2002]) and alterations in water permeability by affecting membrane trafficking and subcellular localization (e.g., AQP4 [Madrid et al., 2001] and AQP2 [Nielsen et al., 1995; Fushimi et al., 1997]). The present study shows that phosphorylation of nodulin 26 enhances its water permeability both upon expression in Xenopus oocytes and in symbiosome membrane vesicles. This finding supports the previous observations of Niemietz and Tyerman (2000) that the incubation of symbiosome membrane vesicles with ATP coincides with an increase in Pf. An examination of the C-terminal region of other NIPs shows that a subset possess a conserved CDPK phosphorylation sequence similar to that of nodulin 26 (Table 1). This observation, and the present findings with nodulin 26, suggest that phosphorylation by CDPK may be a common mechanism for the regulation of plant NIPs, an important feature considering their proposed low intrinsic water permeability.
Table 1.
Comparison of C-Terminal Domains of the Nodulin 26 Subfamily
| Proteina | Arabidopsis Gene No. | Phosphorylation Site (C Terminal)b |
|---|---|---|
| Nod 26 | I-T-K-S-A-S-F-L-K | |
| LIMP2 | I-T-K-N-V-S-F-L-K | |
| NIP1;1 | At4g18030 | I-T-K-S-G-S-F-L-K |
| NIP1;2 | At4g18910 | I-T-K-S-G-S-F-L-K |
| NIP2;1 | At2g34390 | F-S-K-T-G-S-S-H-K |
| NIP2;1-like | At2g29870 | F-S-K-T-G-S-S-H-K |
| NIP3;1 | At1g31880 | None found |
| NIP4;1 | At5g37810 | L-T-K-S-A-S-F-L-R |
| NIP4;2 | At5g37820 | L-T-K-S-A-S-F-L-R |
| NIP5;1 | At4g10380 | None found |
| NIP6;1 | At1g80760 | None found |
| NIP7;1 | At3g06100 | T-R-P-C-P-S-P-V-S-P-S |
Soybean nodulin 26 and Lotus japonicus LIMP2 sequences are from Weaver et al. (1991) and Guenther and Roberts (2000). NIP proteins from Arabidopsis are based on Johanson et al. (2001). NIP2;1-like is a gene related to NIP2;1 that encodes one-half of a MIP repeat.
Homologous regions within the hydrophilic C-terminal regions of each protein that contain phosphorylation sites. The Ser predicted to be phosphorylated is underlined. NIP1, NIP2, and NIP4 proteins possess predicted CDPK phosphorylation sequences similar to those shown to be phosphorylated in nodulin 26 and LIMP2. NIP7;1 contains a unique sequence that possesses regions similar to those recognized by mitogen-activated protein kinases (Clark-Lewis et al., 1991).
Using a specific antibody against the phosphoepitope of nodulin 26, we established the appearance of phosphorylated nodulin 26 during nodule organogenesis. Consistent with its characterization as a late nodulin gene based on mRNA expression data (Fortin et al., 1987), nodulin 26 protein appears in the immature infected cell, coinciding with the endocytosis of the bacteroids and enclosure into symbiosomes. During this period of nodule organogenesis, there is a concomitant breakdown of the central vacuole and a large increase in membrane biosynthesis to accommodate the expanding infected cell and symbiosome membrane production (Verma and Hong, 1996). The phosphorylation of nodulin 26 lags behind its synthesis and coincides with the maturation of the infected cell and bacteroid differentiation, consistent with the onset of nitrogen fixation. Under normal physiological conditions, nodulin 26 phosphorylation levels are maintained at a steady state level until very late stages (after flowering), when nodule senescence normally ensues as a result of the diversion of photosynthate for reproductive functions (Sutton, 1983). Overall, these data argue that nodulin 26 production accompanies the formation of the symbiosome membrane and that nodulin 26 is an integral component of the symbiosome throughout the lifetime of the nodule. However, phosphorylation is manifested in mature differentiated soybean nodules only before entry into senescence.
Phosphorylation during this middle phase of nodule development is maintained at a relatively constant level but is stimulated further by two environmental stresses: salinity and drought. These osmotic stress signals induce a calcium-signaling response in plants (Knight et al., 1997). Part of the response involves an increase in the expression of CDPKs (Urao et al., 1994), which are among the calcium-signaling targets involved in salt and drought stress adaptation (Sheen, 1996; Saijo et al., 2000). MIP proteins are subject to regulation by drought and salinity, but this regulation usually is exhibited by changes in gene expression, leading to alterations in the levels or localization of plant MIP proteins (Johansson et al., 2000; Maurel et al., 2002; Tyerman et al., 2002). By contrast, we found that nodulin 26 protein levels did not change in response to osmotic stress but that phosphorylation levels were altered. In this manner, nodulin 26 is similar to the PM28A plasma membrane intrinsic protein of spinach, which shows enhanced water permeability upon phosphorylation and is stimulated by changes in water potential (Johansson et al., 1998).
What is the physiological significance of nodulin 26 phosphorylation with regard to nodule physiology and membrane function? The inner volume of the infected cell is largely occupied by symbiosomes. The calcium-dependent phosphorylation of nodulin 26 is predicted to increase the water permeability of the symbiosome membrane. Under conditions of normal nodule physiology, this would attenuate the rate of water or solute flux in response to the changing osmotic conditions and metabolism of the symbiosome and the infected cell. This could serve an osmoregulatory function to buffer the volume of the infected cell cytosol to changes in external osmolarity by mediating the rapid reversible uptake and release of water. In addition, nodule development and maintenance are extremely sensitive to environmental and metabolic stresses. Osmotic stress results in morphological and biochemical changes that lead to the rapid cessation of nitrogen fixation (Pankhurst and Sprent, 1975; Weisz et al., 1985; Serraj et al., 1994), the induction of genes involved in stress adaptation (Delauney and Verma, 1990), and the accumulation of compatible solutes such as Pro and sugars, presumably to restrict water loss (Kohl et al., 1991; Irigoyen et al., 1992). Changes in membrane permeability coordinated with the accumulation and compartmentation of compatible solutes may be part of the osmotic adjustment, aiding in the maintenance of cell turgor and redirecting tissue water flow toward critical cells (Bohnert et al., 1995). Thus, the increase in the phosphorylation of nodulin 26 in response to salinity may represent part of a larger adaptation response of nodules to osmotic stress.
METHODS
Molecular Cloning Techniques/Xenopus Oocyte Expression
The soybean (Glycine max) nodulin 26 wild-type cDNA and phosphorylation null mutant with a substitution of an Ala for Ser-262 (S262A) were generated as described previously (Lee et al., 1995) and were cloned downstream of the T3 promoter of the pXBG-ev1 Xenopus laevis expression vector (Preston et al., 1992; Rivers et al., 1997; Dean et al., 1999; Guenther and Roberts, 2000). Capped nodulin 26 complementary RNA was generated by in vitro transcription with T3 RNA polymerase, and stage-V and -VI oocytes from Xenopus were injected with 46 ng of complementary RNA or an equivalent volume of sterile water (negative control) and were cultured at 18°C in frog Ringer's solution (96 mM NaCl, 2 mM KCl, 5 mM MgCl2, 5 mM Hepes-NaOH, pH 7.6, 0.6 mM CaCl2, and 200 mosmol/kg) supplemented with 100 μg/mL penicillin-streptomycin and 50 μg/mL tetracycline for 3 days before assay (Guenther and Roberts, 2000). The osmotic water permeability of Xenopus oocytes was determined at 18°C by video microscopic imaging of oocytes placed in 30% hypoosmotic (60 mosmol/kg) frog Ringer's solution (Guenther and Roberts, 2000). The rate of swelling, (dV/V0)/dt, was used to determine the osmotic water permeability (Pf):
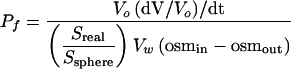 |
(1) |
where V0 is the surface area of the oocyte at time 0, osmin is the osmolarity in the oocyte, osmout is the osmolarity of the bath medium, Vw is the partial molar volume of water (18 cm3/mol), Sreal is the actual surface area of the oocyte, and Ssphere is the area calculated by assuming that the oocyte is a sphere. Based on morphological estimates, an Sreal/Ssphere value of 9 was used to correct for the increase in the surface area of the oocyte plasma membrane caused by the presence of folds and microvilli (Zampighi et al., 1995).
To test the effects of phorbol esters or okadaic acid, oocytes were incubated in 100% Ringer's solution containing either 10 nM phorbol-12-myristate-13-acetate (Calbiochem) or 25 to 50 nM okadaic acid (Calbiochem) for 30 min before assay.
Plant Growth and Treatments
Soybean (cv Bragg) was germinated and inoculated with Bradyrhizobium japonicum USDA110, grown in growth chambers (18-h light period, 21°C/6-h dark period, 19°C) in vermiculite, and watered during alternative weeks with water or Herridge's solution as described previously (Weaver et al., 1991; Guenther and Roberts, 2000). Drought stress was administered by withholding water from 28-day-old soybean plants. Control plants were grown under identical conditions except that the Herridge's solution/water regime was maintained. Salinity stress was administered by watering nodulated soybean plants with 0.3 M NaCl (Weig et al., 1997). Nodules were collected, quick-frozen on dry ice, and stored at −80°C until membrane extraction and analysis.
Symbiosome Membrane Isolation and Assay
Symbiosomes were isolated from 28-day-old nodules by a Percoll step gradient method (Weaver et al., 1991). Symbiosome membrane vesicles were prepared and loaded with 20 mM carboxyfluorescein, 25 mM 3-(N-morpholino)-propanesulfonic acid (Mops)–NaOH, pH 7.0, 3 mM MgSO4, and 200 mM mannitol (262 mosmol/kg) (Rivers et al., 1997). The Pf of symbiosome membrane vesicles was determined by abruptly doubling the osmolarity of the extravesicular solution by injection into an equal volume of 25 mM Mops-NaOH, pH 7.0, 3 mM MgSO4, and 700 mM sucrose and monitoring the time-dependent quenching of entrapped carboxyfluorescein by stopped-flow measurements with a Bio-Logic (Claix, France) model SFM-3 fluorimeter with a MPS-51 power supply fitted with a TC-100/10 cuvette, 15-mL syringes, and a HDS mixer; this apparatus was run at a total flow rate of 2 mL/s (80 μL/injection) with a measured dead time of 7 ms (Dean et al., 1999). The fluorimeter was controlled by Bio-Kine Software V3.20 (Claix, France), and fluorescence data (λex = 490 nm, emission filtered with a 515-nm cutoff filter) were collected (1000 points) at time intervals of 0.1 and 1 ms. At least 10 individual traces were averaged to reduce the signal-to-noise ratio and were fitted to a single exponential, and the amplitude and end-point values were used to relate relative fluorescence to relative volume based on a linear relationship between fluorescence and intravesicular volume (Harris et al., 1990) and the assumption that the internal volume of the vesicle reaches 50% of the initial volume at the end of the experiment. The Pf was calculated from the time course of relative fluorescence as described previously (Rivers et al., 1997; Dean et al., 1999) using the osmotic water permeability equation:
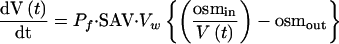 |
(2) |
where V(t) is the relative volume of the vesicles at time t, Pf is osmotic water permeability, SAV is the vesicle surface area-to-volume ratio, Vw is the molar volume of water (18 cm3/mol), and osmin and osmout are the initial osmolarities of the solution inside and outside the vesicle, respectively. SAV was calculated from vesicle diameters determined by 90° light-scattering intensity using a Brookhaven Instruments model BI-200 goniometer (Holtsville, NY).
For analysis of the effects of phosphorylation on Pf, isolated vesicles (13.1 μg protein/mL) were incubated at 30°C for 30 min in either 25 mM Mops-NaOH, pH 7.0, 3 mM MgSO4, 200 mM mannitol, and 3 mM ATP or 25 mM Mops-NaOH, pH 7.0, 3 mM MgSO4, 200 mM mannitol, and 30 units/mL calf intestinal phosphatase (Promega). The level of phosphorylation was assessed by protein gel blot analysis using a phosphorylation-specific antibody as discussed below. For analyses of the phosphorylation state of nodulin 26 during nodule development and in response to stress stimuli, a modification of the membrane purification protocol of Christiansen et al. (1995) was adopted. Nodules were ground on ice with a pestle in 250 mM sucrose, 50 mM Hepes-KOH, pH 7.5, 20 mM EDTA, 20 mM EGTA, 10 mM NaF, 5 mM sodium orthovanadate, 1% (w/v) polyvinylpyrrolidone-40, 20 mM isoascorbic acid, 0.2% (w/v) BSA, 5 mM DTT, 5 μg/mL leupeptin, and 0.5 mM phenylmethylsulfonyl fluoride. Extracts were centrifuged at 100g for 10 min. The supernatant then was passed through a 25-gauge needle three times and was centrifuged at 700g for 20 min. The 700g supernatant then was centrifuged at 100,000g for 1 h. The pellet was resuspended in 100 μL of 330 mM sucrose, 5 mM potassium phosphate, pH 7.8, 2 mM EDTA, 2 mM EGTA, 10 mM NaF, 5 mM sodium orthovanadate, 5 μg/mL leupeptin, and 0.5 mM phenylmethylsulfonyl fluoride. Samples were frozen in dry ice and stored at −80°C until protein gel blot analyses.
Immunochemical Techniques
Phosphorylation-specific antibodies against nodulin 26 were prepared in New Zealand White rabbits using the GC12P synthetic peptide (Sigma Genosys) containing the phosphorylation epitope CEITKNVS(PO4) FLKG. GC12P peptide was dissolved in 0.4 mL of 50 mM NaPO4, pH 7.2, and was coupled covalently via its sulfhydryl group to keyhole limpet hemocyanin that was preactivated with m-maleimidobenzoyl N-hydroxysuccinimide (MBS) (Pierce Endogen Chemicals, Rockford, IL). Antibodies were prepared against the conjugate (100 μg) by immunization of New Zealand White rabbits as described by Weaver et al. (1991). Antibodies against nonphosphorylated epitopes were removed by passing anti-GC12P serum (1 mL of serum per 0.5 mL of resin) over an agarose resin with an immobilized peptide with the same sequence lacking a phosphoserine (CREITKNVSFLKGI). Affinity purification of the remaining antibodies was performed on a second resin with immobilized GC12P as described by Harlow and Lane (1988). Column eluents were concentrated and exchanged into 10 mM NaPO4, 0.14 M NaCl, and 0.01 M KCl, pH 7.2 (PBS) using a Centricon 10 ultrafilter (Amicon, Beverly, MA). The final product was stored at −20°C. Resins containing peptides were generated by covalently coupling to ω-aminohexyl agarose (Sigma) by m-maleimidobenzoyl N-hydroxysuccinimide (Pierce Endogen Chemicals) using the general approach described by Weaver et al. (1991). Antibodies to full-length soybean nodulin 26 were prepared as described previously (Zhang and Roberts, 1995).
Protein gel blot detection was performed by a modification of previous methods (Weaver et al., 1991; Zhang and Roberts, 1995). After SDS-PAGE on 12.5% (w/v) polyacrylamide gels, protein or membrane samples were transferred electrophoretically to Immobilon-P polyvinylidene fluoride membranes (Millipore, Bedford, MA). Blotted membranes were blocked for 2 h in 10% (w/v) nonfat dry milk and 1% (v/v) goat serum in PBS. Primary antibody and secondary antibody (0.2 μg/mL horseradish peroxidase–coupled goat anti-rabbit IgG) incubations were performed for 1 h at 37°C in PBS, 0.5% (w/v) nonfat dry milk, and 1% (v/v) goat serum. After antibody incubations, the membranes was washed with PBS and 0.05% (v/v) Tween 20 and then with a final wash of PBS, 0.05% (v/v) Tween 20, 0.05% (w/v) SDS, and 0.1% (w/v) Triton X-100 at room temperature with three changes. Chemiluminescent detection was performed by incubation of membranes with 10 mL of 1.25 mM luminol, 0.2 mM p-coumaric acid, and 0.009% (v/v) hydrogen peroxide in 0.1 M Tris-HCl, pH 8.5, followed by autoradiography.
Analytical Methods
Protein kinase assays were performed as described by Weaver et al. (1991) using a synthetic peptide that contains the C-terminal 14 amino acids of soybean nodulin 26, including the unique site of phosphorylation, Ser-262 (Weaver et al., 1991; Weaver and Roberts, 1992). Reactions (50 μL) consisted of peptide substrate (7.2 μg), 1 mM γ-32P-ATP (500 dpm/pmol), 25 mM Mops-NaOH, pH 7.0, 10 mM magnesium acetate, 0.2 mM CaCl2, 0.2 mM ATP, 20 μg/mL l-α-1,2-dioleoylglycerol, 100 μg/mL phosphatidylserine, and 4.5 ng of recombinant human protein kinase C (Panvera, Madison, WI). Assays were performed at 30°C for 30 min, and the incorporation of phosphate was determined as described previously (Weaver et al., 1991). The osmolarity of all solutions was determined by freezing-point depression using the Osmette S Automatic Osmometer (Precision Systems, Natick, MA). Protein analyses were performed using the bicinchoninic acid assay (Pierce Endogen Chemicals).
For microscopy, nodules were dissected into 2- to 3-mm pieces with a razor blade and placed into Carson's formalin fixative (Fisher Scientific) at 4°C. The tissue samples then were washed in water and postfixed for 90 min in 50 mM potassium phosphate, pH 6.8, and 2% (w/v) osmium tetroxide. After several rinses in distilled water, the samples were dehydrated in a graded acetone series and infiltrated with Spurr's resin. A Reichert Om-U3 ultramicrotome (Wetzlar, Germany) was used to cut semithin (1 μm) and thin sections. Semithin sections were adsorbed to glass slides and stained with 1% (w/v) toluidine blue in 1% (w/v) borax for 1 min and then examined with a Nikon Eclipse 600 microscope (Tokyo, Japan) using bright-field optics. Thin sections were picked up on uncoated 400-mesh thin-bar copper grids, stained sequentially with saturated uranyl acetate in 50% (v/v) methanol and lead citrate, and examined with a Hitachi H-800 transmission electron microscope (Tokyo, Japan) at 100 kV.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes.
Acknowledgments
This work was supported in part by National Science Foundation Grant MCB-9904978 and U.S. Department of Agriculture National Research Initiative Competitive Grants Program Award 9703548 to D.M.R.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.009787.
References
- Bohnert, H.J., Nelson, D.E., and Jensen, R.G. (1995). Adaptations to environmental stresses. Plant Cell 7, 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy, B.R., Bussey, A., Whitfield, J.F., Sikorska, M., Williams, R.E., and Durkin, J.P. (1991). The direct measurement of protein kinase C (PKC) activity in isolated membranes using a selective peptide substrate. Anal. Biochem. 196, 144–150. [DOI] [PubMed] [Google Scholar]
- Christiansen, J.H., Rosendahl, L., and Widel, I.S. (1995). Preparation and characterization of seal inside-out peribacteroid membrane vesicles from Pisum sativum L. and Glycine max L. root nodules by aqueous polymer two-phase partitioning. Plant Physiol. 147, 175–181. [Google Scholar]
- Clark-Lewis, I., Sanghera, J.S., and Pelech, S.L. (1991). Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis-activated myelin basic protein kinase. J. Biol. Chem. 266, 15180–15184. [PubMed] [Google Scholar]
- Day, D.A., Poole, P.S., Tyerman, S.D., and Rosendahl, L. (2001). Ammonia and amino acid transport across symbiotic membranes in nitrogen-fixing legume nodules. Cell. Mol. Life Sci. 58, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, R.M., Rivers, R.L., Zeidel, M.L., and Roberts, D.M. (1999). Purification and functional reconstitution of soybean nodulin 26: An aquaporin with water and glycerol transport properties. Biochemistry 38, 347–353. [DOI] [PubMed] [Google Scholar]
- Delauney, A.J., and Verma, D.P. (1990). A soybean gene encoding delta 1-pyrroline-5-carboxylate reductase was isolated by functional complementation in Escherichia coli and is found to be osmoregulated. Mol. Gen. Genet. 221, 299–305. [DOI] [PubMed] [Google Scholar]
- Fortin, M.G., Morrison, N.A., and Verma, D.P. (1987). Nodulin-26, a peribacteroid membrane nodulin is expressed independently of the development of the peribacteroid compartment. Nucleic Acids Res. 15, 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin, M.G., Zelechowska, M., and Verma, D.P.S. (1985). Specific targeting of membrane nodulins to the bacteroid-enclosing compartment in soybean nodules. EMBO J. 4, 3041–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, D., Libson, A., Miercke, L.J., Weitzman, C., Nollert, P., Krucinski, J., and Stroud, R.M. (2000). Structure of a glycerol-conducting channel and the basis for its selectivity. Science 290, 481–486. [DOI] [PubMed] [Google Scholar]
- Fushimi, K., Sasaki, S., and Marumo, F. (1997). Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J. Biol. Chem. 272, 14800–14804. [DOI] [PubMed] [Google Scholar]
- Gualtieri, G., and Bisseling, T. (2000). The evolution of nodulation. Plant Mol. Biol. 42, 181–194. [PubMed] [Google Scholar]
- Guenther, J.F., and Roberts, D.M. (2000). Water-selective and multifunctional aquaporins from Lotus japonicus nodules. Planta 210, 741–748. [DOI] [PubMed] [Google Scholar]
- Han, Z., Wax, M.B., and Patil, R.V. (1998). Regulation of aquaporin-4 water channels by phorbol ester-dependent protein phosphorylation. J. Biol. Chem. 273, 6001–6004. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1988). Antibodies: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Harmon, A.C., Gribskov, M., and Harper, J.F. (2000). CDPKs: A kinase for every Ca2+ signal? Trends Plant Sci. 5, 154–159. [DOI] [PubMed] [Google Scholar]
- Harris, H.W., Jr., Handler, J.S., and Blumenthal, R. (1990). Apical membrane vesicles of ADH-stimulated toad bladder are highly water permeable. Am. J. Physiol. 258, F237–F243. [DOI] [PubMed] [Google Scholar]
- Hirsch, A.M., Lum, M.R., and Downie, J.A. (2001). What makes the rhizobia-legume symbiosis so special? Plant Physiol. 127, 1484–1492. [PMC free article] [PubMed] [Google Scholar]
- Irigoyen, J., Emerich, D.W., and Sanches-Diaz, M. (1992). Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 84, 55–60. [Google Scholar]
- Johanson, U., Karlsson, M., Johansson, I., Gustavsson, S., Sjovall, S., Fraysse, L., Weig, A.R., and Kjellbom, P. (2001). The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 126, 1358–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson, I., Karlsson, M., Johanson, U., Larsson, C., and Kjellbom, P. (2000). The role of aquaporins in cellular and whole plant water balance. Biochim. Biophys. Acta 1465, 324–342. [DOI] [PubMed] [Google Scholar]
- Johansson, I., Karlsson, M., Shukla, V.K., Chrispeels, M.J., Larsson, C., and Kjellbom, P. (1998). Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 10, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, H., Trewavas, A.J., and Knight, M.R. (1997). Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 12, 1067–1078. [DOI] [PubMed] [Google Scholar]
- Kohl, K.H., Kennelly, E.J., Zhu, Y., Schubert, K.R., and Shearer, G. (1991). Proline accumulation, nitrogenase (C2H2 reducing) activity, and activities of enzymes related to proline metabolism in water stressed nodules. J. Exp. Bot. 42, 831–837. [Google Scholar]
- Lampe, P.D., and Johnson, R.G. (1989). Phosphorylation of MP26, a lens junction protein, is enhanced by activators of protein kinase C. J. Membr. Biol. 107, 145–155. [DOI] [PubMed] [Google Scholar]
- Lee, J.W., Zhang, Y., Weaver, C.D., Shomer, N.H., Louis, C.F., and Roberts, D.M. (1995). Phosphorylation of nodulin 26 on serine 262 affects its voltage-sensitive channel activity in planar lipid bilayers. J. Biol. Chem. 270, 27051–27057. [DOI] [PubMed] [Google Scholar]
- Madrid, R., Le Maout, S., Barrault, M.B., Janvier, K., Benichou, S., and Merot, J. (2001). Polarized trafficking and surface expression of the AQP4 water channel are coordinated by serial and regulated interactions with different clathrin-adaptor complexes. EMBO J. 20, 7008–7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel, C., Javot, H., Lauvergeat, V., Gerbeau, P., Tournaire, C., Santoni, V., and Heyes, J. (2002). Molecular physiology of aquaporins in plants. Int. Rev. Cytol. 215, 105–148. [DOI] [PubMed] [Google Scholar]
- Maurel, C., Kado, R.T., Guern, J., and Chrispeels, M.J. (1995). Phosphorylation regulates the water channel activity of the seed-specific aquaporin α-TIP. EMBO J. 14, 3028–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, S., Chou, C.L., Marples, D., Christensen, E.I., Kishore, B.K., and Knepper, M.A. (1995). Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc. Natl. Acad. Sci. USA 92, 1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemietz, C.M., and Tyerman, S.D. (2000). Channel-mediated permeation of ammonia gas through the peribacteroid membrane of soybean nodules. FEBS Lett. 465, 110–114. [DOI] [PubMed] [Google Scholar]
- Pankhurst, C.E., and Sprent, J.I. (1975). Effects of water stress on the respiratory and nitrogen fixing activity of soybean root nodules. J. Exp. Bot. 26, 287–304. [Google Scholar]
- Panter, S., Thomson, R., de Bruxelles, G., Laver, D., Trevaskis, B., and Udvardi, M. (2000). Identification with proteomics of novel proteins associated with the peribacteroid membrane of soybean root nodules. Mol. Plant-Microbe Interact. 13, 325–333. [DOI] [PubMed] [Google Scholar]
- Preston, G.M., Carroll, T.P., Guggino, W.B., and Agre, P. (1992). Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256, 385–387. [DOI] [PubMed] [Google Scholar]
- Rivers, R.L., Dean, R.M., Chandy, G., Hall, J.E., Roberts, D.M., and Zeidel, M.L. (1997). Functional analysis of nodulin 26, an aquaporin in soybean root nodule symbiosomes. J. Biol. Chem. 272, 16256–16261. [DOI] [PubMed] [Google Scholar]
- Roth, E., Jeon, K., and Stacey, G. (1988). Homology in endosymbiotic systems: The term ‘symbiosome.’ In Molecular Genetics of Plant Microbe Interactions, R. Palcios and D.P.S. Verma, eds (St. Paul, MN: American Phytopathological Society Press), pp. 220–225.
- Saijo, Y., Hata, S., Kyozuka, J., Shimamoto, K., and Izui, K. (2000). Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 23, 319–327. [DOI] [PubMed] [Google Scholar]
- Sandal, N.N., and Marcker, K.A. (1988). Soybean nodulin 26 is homologous to the major intrinsic protein of the bovine lens fiber membrane. Nucleic Acids Res. 16, 9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serraj, R., Roy, G., and Drevon, J.J. (1994). Salt stress induces a decrease in the oxygen uptake of soybean nodules and in the permeability to oxygen diffusion. Physiol. Plant. 91, 161–163. [Google Scholar]
- Sheen, J. (1996). Ca2+-dependent protein kinases and stress signal transduction in plants. Science 274, 1900–1902. [DOI] [PubMed] [Google Scholar]
- Stougaard, J. (2000). Regulators and regulation of legume root nodule development. Plant Physiol. 124, 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui, H., Han, B.G., Lee, J.K., Walian, P., and Jap, B.K. (2001). Structural basis of water-specific transport through the AQP1 water channel. Nature 414, 872–878. [DOI] [PubMed] [Google Scholar]
- Sutton, W.D. (1983). Nodule development and senescence in nitrogen fixation. In Nitrogen Fixation, Vol. 3, W.J. Broughton, ed (Oxford, UK: Clarendon Press), pp. 144–212.
- Tyerman, S.D., Niemietz, C.M., and Bramley, H. (2002). Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ. 25, 173–194. [DOI] [PubMed] [Google Scholar]
- Udvardi, M.K., and Day, D.A. (1997). Metabolite transport across symbiotic membranes of legume nodules. Annu. Rev. Pant Physiol. Plant Mol. Biol. 48, 493–523. [DOI] [PubMed] [Google Scholar]
- Urao, T., Katagiri, T., Mizoguchi, T., Yamaguchi-Shinozaki, K., Hayashida, N., and Shinozaki, K. (1994). Two genes that encode Ca2+-dependent protein kinases are induced by drought and high-salt stresses in Arabidopsis thaliana. Mol. Gen. Genet. 244, 331–340. [DOI] [PubMed] [Google Scholar]
- Verma, D.P., and Hong, Z. (1996). Biogenesis of the peribacteroid membrane in root nodules. Trends Microbiol. 4, 364–368. [DOI] [PubMed] [Google Scholar]
- Wallace, I.S., Wills, D.M., Guenther, J.F., and Roberts, D.M. (2002). Functional selectivity for glycerol of the nodulin 26 subfamily of plant membrane intrinsic proteins. FEBS Lett. 523, 109–112. [DOI] [PubMed] [Google Scholar]
- Weaver, C.D., Crombie, B., Stacey, G., and Roberts, D.M. (1991). Calcium-dependent phosphorylation of symbiosome membrane proteins from nitrogen-fixing soybean nodules: Evidence for phosphorylation of nodulin 26. Plant Physiol. 95, 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver, C.D., and Roberts, D.M. (1992). Determination of the site of phosphorylation of nodulin 26 by the calcium-dependent protein kinase from soybean nodules. Biochemistry 31, 8954–8959. [DOI] [PubMed] [Google Scholar]
- Weig, A., Deswarte, C., and Chrispeels, M.J. (1997). The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiol. 114, 1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weig, A.R., and Jakob, C. (2000). Functional identification of the glycerol permease activity of Arabidopsis thaliana NLM1 and NLM2 proteins by heterologous expression in Saccharomyces cerevisiae. FEBS Lett. 481, 293–298. [DOI] [PubMed] [Google Scholar]
- Weisz, P.R., Denison, R.F., and Sinclair, T.R. (1985). Response to drought stress of nitrogen fixation (acetylene reduction) rates by field-grown soybeans. Plant Physiol. 78, 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi, G.A., Kreman, M., Boorer, K.J., Loo, D.D., Bezanilla, F., Chandy, G., Hall, J.E., and Wright, E.M. (1995). A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. J. Membr. Biol. 148, 65–78. [DOI] [PubMed] [Google Scholar]
- Zelenina, M., Zelenin, S., Bondar, A.A., Brismar, H., and Aperia, A. (2002). Water permeability of aquaporin-4 is decreased by protein kinase C and dopamine. Am. J. Physiol. Renal Physiol. 283, F309–F318. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., and Roberts, D.M. (1995). Expression of soybean nodulin 26 in transgenic tobacco: Targeting to the vacuolar membrane and effects on floral and seed development. Mol. Biol. Cell 6, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]