Patients with spontaneous (non-traumatic) subarachnoid haemorrhage usually present first to their general practitioner. As general practitioners may see only a few cases during their career, subarachnoid haemorrhage can be a diagnostic and management challenge1; the incidence is about 8 per 100 000 per year.2 The condition accounts for 3% of patients presenting to emergency departments with headache3 and around 20 admissions per year to a general hospital covering 300 000 people. The prognosis remains poor: up to half of patients die and one third of survivors are left dependent.4 Early treatment can improve outcome5-8; therefore prompt diagnosis and referral to a neuroscience unit is important.
How subarachnoid haemorrhage presents
Patients with subarachnoid haemorrhage usually present with a characteristic combination of symptoms (box 1). Sudden severe headache is the cardinal symptom, but it may be the only symptom in up to one third of patients with aneurysmal subarachnoid haemorrhage.12 When patients were asked how long it took for their headache to reach its maximum severity half of those with subarachnoid haemorrhage described it as instantaneous, one fifth said it developed over 1-5 minutes, and the rest said it escalated over more than five minutes.12 The headache usually persists for several days but may occasionally be much shorter. Even in the emergency department the positive predictive value of instantaneous severe headache for aneurysmal subarachnoid haemorrhage is only 39% (95% confidence interval 29% to 50%),12 so the speed of onset cannot be relied on to identify all cases of subarachnoid haemorrhage.
Although some believe that “sentinel bleeds” or “warning leaks” precede aneurysmal subarachnoid haemorrhage, the evidence is that headaches preceding the haemorrhage are rare and do not help in its diagnosis.14 Overestimation of the importance of sentinel bleeds arose from recall bias in hospital based studies.1 We recommend that the terms sentinel bleeds and warning leaks should be abandoned: people either have had a subarachnoid haemorrhage or not and the important task is to recognise when they have.1,15
Summary points
Subarachnoid haemorrhage should be suspected in someone with sudden severe headache that peaks within minutes and lasts more than an hour
Subarachnoid haemorrhage cannot be excluded by unenhanced computed tomography of the brain because the sensitivity of the procedure declines rapidly within hours of symptom onset
When an unenhanced computed tomogram of the brain is normal, specialist advice should be sought about subsequent lumbar puncture
When patients are referred to a neuroscience unit, the referring clinician should specify time from onset of symptoms, age, comorbidities, conscious level, and any neurological deficit
Oral nimodipine 60 mg every four hours reduces poor outcome, but good evidence for other medical interventions is limited
Endovascular coiling is superseding neurosurgical clipping for the occlusion of many ruptured aneurysms
Multidisciplinary follow-up clinics help manage complications of subarachnoid haemorrhage
Other causes of sudden severe headache
Only about one in four people presenting with sudden severe headache will have had a subarachnoid haemorrhage,14 a further 40% will have benign thunderclap headache,12 and the rest will have other primary and secondary headache syndromes (box 2). When sudden severe headache is the only symptom, one in 10 cases turns out to be subarachnoid haemorrhage,14 so the absence of other symptoms cannot be used to rule out the condition. Conversely, other symptoms of subarachnoid haemorrhage (box 1) may accompany other causes of sudden severe headache (box 2), so they cannot reliably distinguish subarachnoid haemorrhage.
Sources and selection criteria
We searched for relevant articles in Ovid Medline between 1966 and November 2005 using the search term subarachnoid haemorrhage. We also searched the Cochrane Database of Systematic Reviews.5-7,9-11 We have classified the evidence for our treatment recommendations.w1
Investigations for suspected subarachnoid haemorrhage
Because no clinical feature is sufficiently reliable to make the diagnosis,12 subarachnoid haemorrhage must be excluded in anyone presenting with sudden severe headache that is maximal within minutes, lasts for more than an hour, and has no alternative explanation.16 Box 3 lists the initial investigations for suspected subarachnoid haemorrhage.
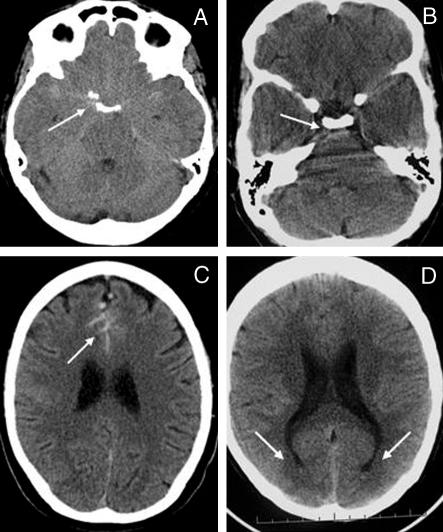
The ideal investigation for subarachnoid haemorrhage is unenhanced computed tomography of the brain, which needs to be interpreted by an experienced radiologist and carried out as soon as possible after the onset of headache (immediately if consciousness is impaired). Delays in scanning allow subarachnoid blood time to degrade and increase the possibility of a tomogram appearing normal. Third generation computed tomography scanners miss about 2% of cases of subarachnoid haemorrhage within 12 hoursw5 and about 7% by 24 hours.w6 Subarachnoid blood is almost completely reabsorbed within 10 days. Changes can be subtle and interpretation requires expertise (fig 1).
Fig 1.
(A) Subarachnoid haemorrhage from right posterior communicating artery aneurysm with hyperdense or isodense blood in anterior and posterior basal cisterns (arrow). (B) Blood in prepontine cistern (arrow). (C) Blood in anterior interhemispheric fissure (arrow). (D) Blood sedimenting in occipital horns of lateral ventricles (arrows)
When and how to examine cerebrospinal fluid
Anyone with suspected subarachnoid haemorrhage and a normal computed tomogram requires a lumbar puncture.17 Because expertise is required in both the conduct and the timing of lumbar puncture, as well as in the interpretation of cerebrospinal fluid results,1,18 we recommend that cases should at least be discussed with a specialist at a neuroscience unit before the procedure.
Box 1: Presenting symptoms
Sudden death—occurs in 10% of cases of spontaneous subarachnoid haemorrhage13
Sudden severe headache—maximal immediately or within minutes, and lasting an hour or more (usually days)14
Other symptoms of meningism—nausea, vomiting, photophobia, neck stiffness
Loss of consciousness—transient at onset (around 50% of cases) or persisting
Epileptic seizures—about 6% of cases
Focal neurological symptoms—dysphasia, hemisensory or motor symptoms
Box 2: Common causes of sudden severe headache1,14
Spontaneous (non-traumatic) subarachnoid haemorrhage
-
Other secondary headache syndromes
Vascular—intracranial venous thrombosis; intracerebral, intraventricular, extradural, or subdural haemorrhage; ischaemic stroke; arterial dissection; vasculitides
Infection—for example, meningitis, encephalitis
Acute hydrocephalus—for example, colloid cyst of the third ventricle)
Intracranial tumour—including pituitary apoplexy
Intracranial hypotension—spontaneous or after dural puncture
Metabolic—for example, phaeochromocytoma, tyramine ingestion with monoamine oxidase inhibitors
-
Primary headache syndromesw2
Thunderclap headache
Migraine
Cluster headache
Headache associated with sexual activity or exertion
Unless there is any suspicion of an alternative diagnosis such as meningitis, we delay lumbar puncture for at least six hours—preferably 12 hours—after headache onset. This allows sufficient time for haemoglobin to degrade into oxyhaemoglobin and bilirubin.19 Bilirubin signifies subarachnoid haemorrhage because it is only synthesised in vivo, unlike oxyhaemoglobin, which may result from a traumatic tap or prolonged storage or agitation of bloodstained cerebrospinal fluid in vitro.20
Box 3: Initial investigations
Full blood count—anaemia (sickle cell disease), leucocytosis (after a seizure, systemic infection)
Coagulation screen—underlying coagulopathy
Urea and electrolytes—hyponatraemia is common after subarachnoid haemorrhage due to salt wasting, not inappropriate antidiuretic hormone secretion
Serum glucose—hypoglycaemia needs correction, hyperglycaemia is associated with poor outcome after subarachnoid haemorrhagew3
Serum magnesium—hypomagnesaemia is common and associated with poor outcome after subarachnoid haemorrhagew4
Chest radiography—pulmonary oedema, aspiration
12. lead electrocardiography—cardiac arrhythmia, ST segment changes, myocardial “stunning”
Unenhanced computed tomography of the brain—as soon as possible after onset of headache (< 24 hours)
Lumbar puncture—if an unenhanced computed tomogram of the brain is normal
Computed tomography angiography—if subarachnoid haemorrhage is confirmed by computed tomography or lumbar puncture
The opening pressure of cerebrospinal fluid must be recorded and samples analysed for protein, cells, and glucose (paired with a serum sample). To minimise the risk of oxyhaemoglobin arising in vitro, the last (least bloodstained) sample of cerebrospinal fluid should be transported to the laboratory by hand and centrifuged immediately.21 If there are delays the fluid should be protected from light to prevent degradation of bilirubin. Bilirubin causes yellow pigmentation of the supernatant after centrifugation of cerebrospinal fluid, known as xanthochromia. Clinicians should use the naked eye to compare the colour of the cerebrospinal fluid supernatant with water against a white background,22 but every sample should be analysed for bilirubin using spectrophotometry.21 The estimation of erythrocyte counts in three consecutive samples of cerebrospinal fluid does not reliably distinguish subarachnoid haemorrhage from a traumatic tap.19
Box 4: Complications of aneurysmal subarachnoid haemorrhage
Rebleeding (without aneurysm occlusion27)
On day 1: 15%
By one month: 40%
After six months: 3% per year
Immediate cerebral ischaemia—due to raised intracranial pressure and hence reduced cerebral perfusion pressure
Delayed cerebral ischaemia—peaks between days 4 and 14 after subarachnoid haemorrhage
Hydrocephalus
Seizures
Cardiopulmonary dysfunction—predicted by elevated cardiac troponin Iw7
Hyponatraemia or hypomagnesaemia—caused by salt wasting
Normal computed tomograms and cerebrospinal fluid samples within days of presentation
If a computed tomogram and cerebrospinal fluid (including spectrophotometry) are normal within two weeks of a sudden severe headache, then subarachnoid haemorrhage is excluded and an alternative diagnosis must be considered (box 2).
Suspicion of subarachnoid haemorrhage more than two weeks after onset
The sensitivity of computed tomography for subarachnoid haemorrhage declines rapidly after 10 days. Xanthochromia is only detected in 70% of cases after three weeks and 40% after four weeks.23 People who present two or more weeks after a suspected subarachnoid haemorrhage therefore should be referred urgently to a neuroscience unit for consideration of further investigations.
Investigations for the cause of subarachnoid haemorrhage
Possible causes of subarachnoid haemorrhage determine further investigations. Three quarters of spontaneous cases are due to a ruptured aneurysm, 20% have no identifiable cause (of which at least half are due to idiopathic or non-aneurysmal perimesencephalic subarachnoid haemorrhage), and the remainder are caused by a variety of rare disorders such as arteriovenous malformations of the brain or spine, arterial dissection, sympathomimetic drugs, tumours, or vasculitis.
The pattern of blood on computed tomograms may suggest the cause. Ruptured aneurysms are most commonly found on the anterior communicating artery complex (suggested by blood in the interhemispheric fissure, fig 1C), followed by the internal carotid artery, middle cerebral artery (blood in the sylvian fissure), and vertebrobasilar circulation. About 20% of people with subarachnoid haemorrhage have multiple aneurysms, so the pattern on the computed tomogram may be crucial to identify which aneurysm has ruptured. Idiopathic perimesencephalic subarachnoid haemorrhage is restricted to the perimesencephalic cistern anterior to the midbrain, but rupture of an aneurysm in the posterior circulation underlies 5% of this pattern of subarachnoid haemorrhage, so further investigation is always required.
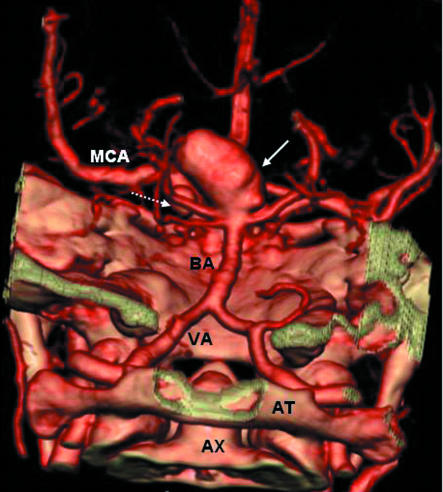
Multislice CTA (computed tomography angiography) is our preferred investigation for underlying causes of spontaneous subarachnoid haemorrhage because of its speed, tolerability, convenience, and the ability to provide three dimensional reconstructions (fig 2). The sensitivity of the procedure for identifying aneurysms greater than 3 mm diameter is about 96% but poorer for smaller aneurysms.24,25 We proceed to four vessel catheter angiography if aneurysmal subarachnoid haemorrhage is strongly suspected but the computed tomography angiogram is normal. Repeated cerebral catheter angiography, spinal catheter angiography, or magnetic resonance imaging may be necessary to identify alternative causes in some cases. For people with a purely perimesencephalic distribution, a normal, good quality computed tomography angiogram allows a diagnosis of idiopathic perimesencephalic subarachnoid haemorrhage without the need for further investigation.19
Fig 2.
Volume rendered computed tomography angiogram, viewed from posterior to anterior, showing a 20 mm diameter aneurysm (solid arrow) at tip of basilar artery (BA) anda6mm diameter aneurysm (dashed arrow) on the left posterior communicating artery. MCA=left middle cerebral artery; VA=left vertebral artery; AT=atlas (C1); AX=odontoid process of axis (C2)
Outcome after aneurysmal subarachnoid haemorrhage
Case fatality after aneurysmal subarachnoid haemorrhage in the community is 12% and a further 10% of those who reach hospital alive die within 24 hours. In population based studies the overall case fatality was about 50%, which has reduced marginally between 1960 and the mid-1990s.4 Almost all deaths due to subarachnoid haemorrhage occur within the first three weeks, most due to rebleeding (box 4). Around one third of survivors are dependent,4 often with cognitive impairment,26 and two thirds have a reduced quality of life.19,26
The three strongest predictors of death or dependence are impaired conscious level on admission, increasing age, and large volume of blood on initial computed tomography of the brain. The World Federation of Neurological Surgeons grading scale standardises clinical evaluation over time and helps estimate prognosis (table 1).
Table 1.
World Federation of Neurological Surgeons grading scheme for aneurysmal subarachnoid haemorrhagew8
| Grade | Glasgow coma score | Motor deficit* |
|---|---|---|
| I | 15 | Absent |
| II | 13 or 14 | Absent |
| III | 13 or 14 | Present |
| IV | 7 to 12 | — |
| V | 3 to 6 | — |
Excludes cranial neuropathies but includes dysphasia.
Management of aneurysmal subarachnoid haemorrhage
People with confirmed subarachnoid haemorrhage should be referred to a neuroscience unit for supportive management and interventions directed at preventing complications (box 4). The specific treatment of complications such as hydrocephalus, delayed ischaemia, or rebleeding is covered elsewhere.28
Best medical management
Frequent checking of the Glasgow coma score and pupillary responses as well as vigilance for focal neurological deficits is essential. A drop of just one point in the Glasgow coma score may be the first sign of a complication. Close monitoring of blood pressure, fluid balance, heart rhythm, and respiratory function are crucial because of the risk of cardiac arrhythmia and pulmonary oedema. Table 2 lists the medical treatments for aneurysmal subarachnoid haemorrhage. Several drugs could plausibly improve outcome, including magnesium, antiplatelet agents, statins, and endothelin receptor antagonists (see bmj.com).
Table 2.
Medical interventions for aneurysmal subarachnoid haemorrhage
| Treatment | Indication or benefit | Evidencew1 |
|---|---|---|
| Standard practice: | ||
| Nimodipine (oral) 60 mg every four hours for three weeks | Prevention—reduces risk (absolute 5%, relative 18%) of poor outcome and delayed cerebral ischaemia | Grade A,5 level 1+ |
| ≥3 litres intravenous 0.9% saline daily | Prevention—sodium depletion and hypovolaemia contribute to delayed cerebral ischaemia.29 Monitor fluid balance and cardiac function | Grade C, level 2+ |
| Analgesia (paracetamol 1 g every six hours or dihydrocodeine 30 to 60 mg every four hours, or both | Pain relief | — |
| Graduated compression stockings | Prophylaxis against deep vein thrombosis | Grade B,30 level 1++ |
| Antiemetics | As required | — |
| Stool softeners | As required | — |
| Variation in practice: | ||
| Intermittent pneumatic compression | Prophylaxis against deep vein thrombosis | Grade B,31 level 1+ |
| Plasma volume expanders | Poor evidence for prevention or treatment of delayed cerebral ischaemia, and increases complications | Grade B,10 level 1- |
| Antihypertensives | No proved benefit in preventing rebleeding, and may cause cerebral ischaemiaw9 | Grade B,w10 level 1- |
| Antifibrinolytics | No overall benefit because reduction of rebleeding is offset by more delayed cerebral ischaemia. Use before aneurysm occlusion seems more promising, but evidence is still lackingw11 | Grade A,11 level 1++ |
| Antipyretics | Temperature >37.5°C | Fever associated with poor outcomew12 |
| Insulin sliding scale or infusion | Plasma glucose >11 mmol/l | Hyperglycaemia associated with poor outcomew3 |
| Magnesium supplementation | Plasma magnesium <0.7 mmol/l | Hypomagnesaemia associated with poor outcomew4, w13 |
| Antiepileptic drugs | For treatment (not prophylaxis) of seizures | — |
When and how should ruptured aneurysms be secured?
The main purpose of occluding an aneurysm is to prevent rebleeding. Age, World Federation of Neurological Surgeons grade, comorbidity, time from onset of subarachnoid haemorrhage, and the aneurysm's vascular anatomy help determine whether intervention is worthwhile and possible.
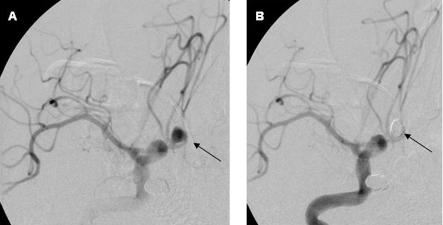
Although neurosurgical “clipping” became the standard treatment of aneurysms in the 20th century, endovascular occlusion of ruptured aneurysms using detachable coils (“coiling,” fig 3) is now superseding clipping (grade A, level 1+).6 For aneurysms suitable for either treatment, coiling confers an absolute risk reduction over clipping of about 7% (25% relative risk reduction) for dependency or death at one year, with benefit sustained to seven years32; the number needed to coil to prevent one poor outcome is 14 (95% confidence interval 10 to 25).6
Fig 3.
Digital subtraction catheter angiography (internal carotid artery injection) showing a ruptured anterior communicating artery aneurysm (A, arrow) treated with endovascular coiling (B, arrow)
The evidence for coiling over clipping is influenced by one large trial, the international subarachnoid aneurysm trial.32 Those included in the trial were mostly young and had good World Federation of Neurological Surgeons grades and small anterior circulation aneurysms: this represents half to three quarters of people with aneurysmal subarachnoid haemorrhage33 and reflects the lack of equipoise about most posterior circulation aneurysms that had already developed in clinical practice at the time of the trial. Despite the overall superiority of coiling, complete aneurysm occlusion was more likely to be achieved with clipping. Evidence for the durability of coiling beyond seven years is awaited from the trial.
Aneurysm occlusion tends to be attempted as soon as practicable (within 3-4 days) after onset of subarachnoid haemorrhage to prevent rebleeding. Morbidity seems to occur more often after clipping in the second week after haemorrhage when the risk of delayed cerebral ischaemia is greatest (grade B, level 1+),7,8 although the timing of coiling does not seem to influence morbidity related to it (grade C, level 2+).34 Immediate evacuation of some intracerebral haematomas caused by aneurysm rupture, with concomitant aneurysm clipping, is supported by one small randomised trial (grade B, level 1 -).35
What to do after aneurysmal subarachnoid haemorrhage
Survivors of subarachnoid haemorrhage need good advice and information on support groups. Multidisciplinary follow-up clinics involving a neurologist, neurosurgeon, neuroradiologist, and professions allied to medicine are likely to help identify and manage the physical and cognitive complications, which often delay or preclude return to work.26 Tackling modifiable risk factors for aneurysmal subarachnoid haemorrhage is likely to be beneficial in preventing its recurrence: the most important are smoking, hypertension, and excessive alcohol intake (> 150 g (19 units) of ethanol per week), each individually conferring a twofold to threefold increase in risk.36
Only 2% of relatives of people with aneurysmal subarachnoid haemorrhage are similarly affected, but the risk is higher when two or more first degree relatives are affected.37 Familial aggregation is only partly explained by classic risk factorsw14 and monogenic conditions such as adult polycystic kidney disease. Screening family members for the presence of unruptured aneurysms may be justifiable when two or more first degree relatives are affected, taking into account age, comorbidities, and other risk factors.37
This approach is not supported by evidence from clinical trials.
Information for patients
The Brain and Spine Foundation (www.brainandspine.org.uk/)—provides information, advice, and booklets (for example, recovering from a subarachnoid haemorrhage, a guide for patients and carers, coiling of brain aneurysms)
The Brain Aneurysm Foundation (www.bafound.org/)—provides information and support
Conclusion
People with subarachnoid haemorrhage should be managed by multidisciplinary teams in specialist neuroscience units. Future improvements in treatment of aneurysms and the management of medical complications of subarachnoid haemorrhage should be tested in randomised controlled trials. Such research should improve outcome, which should be monitored in population based studies or by comprehensive national audits.33
Supplementary Material
 References w1-w14 and ongoing research studies are on bmj.com
References w1-w14 and ongoing research studies are on bmj.com
Contributors: RAS searched the literature and drafted the article. He is guarantor. All authors revised the article critically for intellectual content. PMW produced the figures.
Funding: RAS is funded by the UK Medical Research Council.
Competing interests: RJD and KWL have acted as expert witnesses in cases involving subarachnoid haemorrhage. PMW has received reimbursement for expenses in attending international conferences from Siemens, Cordis, Boston Scientific, UK Medical, and Microvention; has been reimbursed by Pyramed UK for running an educational programme; and holds a research grant from Microvention funding a randomised controlled trial (hydrocoil endovascular aneurysm occlusion and packing study). PMW has received consulting fees from Boston Scientific, Cordis, UK Medical, and Microvention.
References
- 1.Davenport RJ. Sudden headache in the emergency department. Pract Neurol 2005;5: 132-43. [Google Scholar]
- 2.Linn FH, Rinkel GJ, Algra A, van Gijn J. Incidence of subarachnoid hemorrhage: role of region, year, and rate of computed tomography: a metaanalysis. Stroke 1996;27: 625-9. [DOI] [PubMed] [Google Scholar]
- 3.Morgenstern LB, Huber JC, Luna-Gonzales H, Saldin KR, Grotta JC, Shaw SG, et al. Headache in the emergency department. Headache 2001;41: 537-41. [DOI] [PubMed] [Google Scholar]
- 4.Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 1997;28: 660-4. [DOI] [PubMed] [Google Scholar]
- 5.Rinkel GJE, Feigin VL, Algra A, van den Bergh WM, Vermeulen M, van Gijn J. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2005;(1):CD000277. [DOI] [PubMed]
- 6.Van der Schaaf I, Algra A, Wermer M, Molyneux A, Clarke M, van Gijn J, et al. Endovascular coiling versus neurosurgical clipping for patients with aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2005;(4):CD003085. [DOI] [PubMed]
- 7.Whitfield PC, Kirkpatrick PJ. Timing of surgery for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2001;(2):CD001697. [DOI] [PubMed]
- 8.De Gans K, Nieuwkamp DJ, Rinkel GJ, Algra A. Timing of aneurysm surgery in subarachnoid hemorrhage: a systematic review of the literature. Neurosurgery 2002;50: 336-40. [DOI] [PubMed] [Google Scholar]
- 9.Feigin VL, Anderson N, Rinkel GJE, Algra A, van Gijn J, Bennett DA. Corticosteroids for aneurysmal subarachnoid haemorrhage and primary intracerebral haemorrhage. Cochrane Database Syst Rev 2005;(3):CD004583. [DOI] [PubMed]
- 10.Rinkel GJE, Feigin VL, Algra A, van Gijn J. Circulatory volume expansion therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2004;(4):CD000483. [DOI] [PMC free article] [PubMed]
- 11.Roos YBWEM, Rinkel GJE, Vermeulen M, Algra A, van Gijn J. Anti-fibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2003;(2):CD001245. [DOI] [PubMed]
- 12.Linn FH, Rinkel GJ, Algra A, van Gijn J. Headache characteristics in subarachnoid haemorrhage and benign thunderclap headache. J Neurol Neurosurg Psychiatry 1998;65: 791-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, van Gelder JM. The probability of sudden death from rupture of intracranial aneurysms: a meta-analysis. Neurosurgery 2002;51: 1101-5. [DOI] [PubMed] [Google Scholar]
- 14.Linn FH, Wijdicks EF, van der Graaf Y, Weerdesteyn-van Vliet FA, Bartelds AI, van Gijn J. Prospective study of sentinel headache in aneurysmal subarachnoid haemorrhage. Lancet 1994;344: 590-3. [DOI] [PubMed] [Google Scholar]
- 15.Polmear A. Sentinel headaches in aneurysmal subarachnoid haemorrhage: what is the true incidence? A systematic review. Cephalalgia 2003;23: 935-41. [DOI] [PubMed] [Google Scholar]
- 16.Warlow CP, Dennis MS, van Gijn J, Hankey GJ, Sandercock PAG, Bamford JM, et al. What caused this subarachnoid haemorrhage? Stroke. A practical guide to management. Oxford: Blackwell Science, 2001: 376-413.
- 17.Van der Wee N, Rinkel GJ, Hasan D, van Gijn J. Detection of subarachnoid haemorrhage on early CT: is lumbar puncture still needed after a negative scan? J Neurol Neurosurg Psychiatry 1995;58: 357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Gijn J, Rinkel GJE. How to do it: investigate the CSF in a patient with sudden headache and a normal CT brain scan. Pract Neurol 2005;5: 362-5. [Google Scholar]
- 19.Van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001;124: 249-78. [DOI] [PubMed] [Google Scholar]
- 20.Williams A. Xanthochromia in the cerebrospinal fluid. Pract Neurol 2004;4: 174-5. [Google Scholar]
- 21.UK National External Quality Assessment Scheme for Immunochemistry Working Group. National guidelines for analysis of cerebrospinal fluid for bilirubin in suspected subarachnoid haemorrhage. Ann Clin Biochem 2003;40: 481-8. [DOI] [PubMed] [Google Scholar]
- 22.Linn FH, Voorbij HA, Rinkel GJ, Algra A, van Gijn J. Visual inspection versus spectrophotometry in detecting bilirubin in cerebrospinal fluid. J Neurol Neurosurg Psychiatry 2005;76: 1452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vermeulen M, van Gijn J. The diagnosis of subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 1990;53: 365-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White PM, Wardlaw JM, Easton V. Can non-invasive imaging accurately depict intracranial aneurysms? A systematic review. Radiology 2000;217: 361-70. [DOI] [PubMed] [Google Scholar]
- 25.Van Gelder JM. Computed tomographic angiography for detecting cerebral aneurysms: implications of aneurysm size distribution for the sensitivity, specificity, and likelihood ratios. Neurosurgery 2003;53: 597-605. [DOI] [PubMed] [Google Scholar]
- 26.Hackett ML, Anderson CS, for the Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS) Group. Health outcomes 1 year after subarachnoid hemorrhage: an international population-based study. The Australian Cooperative Research on Subarachnoid Hemorrhage Study Group. Neurology 2000;55: 658-62. [DOI] [PubMed] [Google Scholar]
- 27.Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. Based on 6368 cases in the cooperative study. J Neurosurg 1966;25: 321-68. [DOI] [PubMed] [Google Scholar]
- 28.Warlow CP, Dennis MS, van Gijn J, Hankey GJ, Sandercock PAG, Bamford JM, et al. Specific treatment of aneurysmal subarachnoid haemorrhage. Stroke. A practical guide to management. Oxford: Blackwell Science, 2001: 518-59.
- 29.Vermeij FH, Hasan D, Bijvoet HW, Avezaat CJ. Impact of medical treatment on the outcome of patients after aneurysmal subarachnoid hemorrhage. Stroke 1998;29: 924-30. [DOI] [PubMed] [Google Scholar]
- 30.Amaragiri SV, Lees TA. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev 2000;(3):CD001484. [DOI] [PubMed]
- 31.Lacut K, Bressollette L, Le Gal G, Etienne E, De Tinteniac A, Renault A, et al. Prevention of venous thrombosis in patients with acute intracerebral hemorrhage. Neurology 2005;65: 865-9. [DOI] [PubMed] [Google Scholar]
- 32.Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005;366: 809-17. [DOI] [PubMed] [Google Scholar]
- 33.Society of British Neurological Surgeons, the British Society of Neuroradiologists, and the Royal College of Surgeons of England, Clinical Effectiveness Unit. National comparative outcomes study of subarachnoid haemorrhage. London: Royal College of Surgeons of England, Clinical Effectiveness Unit, 2006.
- 34.Baltsavias GS, Byrne JV, Halsey J, Coley SC, Sohn MJ, Molyneux AJ. Effects of timing of coil embolization after aneurysmal subarachnoid hemorrhage on procedural morbidity and outcomes. Neurosurgery 2000;47: 1320-9. [PubMed] [Google Scholar]
- 35.Heiskanen O, Poranen A, Kuurne T, Valtonen S, Kaste M. Acute surgery for intracerebral haematomas caused by rupture of an intracranial arterial aneurysm. A prospective randomized study. Acta Neurochir (Wien) 1988;90: 81-3. [DOI] [PubMed] [Google Scholar]
- 36.Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 2005;36: 2773-80. [DOI] [PubMed] [Google Scholar]
- 37.Teasdale GM, Wardlaw JM, White PM, Murray G, Teasdale EM, Easton V, et al. The familial risk of subarachnoid haemorrhage. Brain 2005;128: 1677-85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.