Abstract
Objectives To determine the best approach for live donor nephrectomy to minimise discomfort to the donor and to provide good graft function.
Design Single blind, randomised controlled trial.
Setting Two university medical centres, the Netherlands.
Participants 100 living kidney donors.
Interventions Participants were randomly assigned to either laparoscopic donor nephrectomy or to mini incision muscle splitting open donor nephrectomy.
Main outcome measures The primary outcome was physical fatigue using the multidimensional fatigue inventory 20 (MFI-20). Secondary outcomes were physical function using the SF-36, hospital stay after surgery, pain, operating times, recipient graft function, and graft survival.
Results Conversions did not occur. Compared with mini incision open donor nephrectomy, laparoscopic donor nephrectomy resulted in longer skin to skin time (median 221 v 164 minutes, P < 0.001), longer warm ischaemia time (6 v 3 minutes, P < 0.001), less blood loss (100 v 240 ml, P < 0.001), and a similar number of complications (intraoperatively 12% v 6%, P = 0.49, postoperatively both 6%). After laparoscopic nephrectomy, donors required less morphine (16 v 25 mg, P = 0.005) and shorter hospital stay (3 v 4 days, P = 0.003). During one year's follow-up mean physical fatigue was less (difference - 1.3, 95% confidence interval - 2.4 to - 0.1) and physical function was better (difference 6.2, 2.0 to 10.3) after laparoscopic nephrectomy. Function of the graft and graft survival rate of the recipient at one year censored for death did not differ (100% after laparoscopic nephrectomy and 98% after open nephrectomy).
Conclusions Laparoscopic donor nephrectomy results in a better quality of life compared with mini incision open donor nephrectomy but equal safety and graft function.
Introduction
Kidney transplantation is the best option for patients with end stage renal disease. As the number of patients requiring kidney replacement therapy is increasing, the recruitment of more kidney donors is important. Donation of a kidney from a live donor is the most realistic option to expand organ donation.1 From an ethical point of view, living donation becomes more acceptable if harm to the donor and the graft is limited. Therefore optimising the management of the living donor including screening, surgery, and anaesthesia is important.
Traditionally the kidney was removed through a flank incision, often including rib resection to allow sufficient access. This resulted in major postoperative pain, incisional hernias, and chronic neuralgia. Using small incisions has improved the comfort of the donor. In less than a decade, laparoscopic surgery has been adopted by most centres. Laparoscopic donor nephrectomy was first carried out in 1995.2 Concurrently the technique of open donor nephrectomy has been refined to a muscle sparing mini incision without resection of the ribs, which has improved convalescence of the donors.3-5 To date the best surgical technique within the multidisciplinary management of living donors is not defined.
We carried out a prospective randomised trial to compare laparoscopic donor nephrectomy with mini incision open donor nephrectomy for fatigue and quality of life of the donors and for clinical outcomes.
Participants and methods
We recruited to our study living kidney donors at the university medical centres in Rotterdam and Nijmegen. Eligible donors were informed about the surgical approaches and invited to participate in the study. Screening of donors included preoperative examination by a nephrologist, renal ultrasonography, and magnetic resonance angiography or computed tomography-angiography to evaluate the arterial and venous anatomy of the kidneys. If both kidneys were suitable for transplantation the right kidney was preferred.6 Exclusion criteria were bilateral abnormalities of the renal arteries (origin stenosis), previous operations of the kidney or adrenal gland, radiological abnormalities necessitating a modified approach (for example, solid tumours requiring frozen sections), and the inability to read Dutch. Patients were not excluded because of age, multiple arteries, obesity, or previous abdominal surgery other than adrenal or renal surgery. The day before surgery the surgeon confirmed that the patient had given informed consent.
Randomisation
The surgeon telephoned the study coordinator after informed consent had been confirmed, who opened the next numbered sealed opaque envelope provided by the trial statistician. Randomisation was carried out according to a computer generated list using a hidden block size of four. Stratification was not by centre. Donors were randomised less than 12 hours before surgery. All healthcare professionals except the surgical team were unaware of the allocated procedure. At the end of the operation the abdomen of all donors was covered with a standard pattern of dressings stained with one or two drops of blood to simulate real wound dressings. In case of an emergency, a sealed envelope detailing the procedure was left in the patient's notes.
Anaesthesia and analgesia
Donors were prehydrated with intravenous crystalloids. Antithrombotic stockings were used routinely. After endotracheal intubation, anaesthesia was carried out according to a strict protocol for drugs, ventilation, and fluid regimens. One hour after the start of surgery the donors received 20 mg mannitol. Except for one donor who required endocarditis prophylaxis, no antibiotics were given. At the end of surgery donors received patient controlled analgesia using intravenous morphine. They were also offered two 500 mg paracetamol tablets four times daily until discharge. The device for patient controlled analgesia was removed when morphine had not been required for at least six hours.
Surgical procedures
The surgical procedures were carried out by one of four surgeons skilled in both techniques. The trial coordinator in each centre attended the operations to document blood loss, operation time, use of instruments, and complications. Complications were defined as events necessitating intraoperative or postoperative interventions or that prolonged hospital stay.
Both techniques were carried out as described previously,5 with the donor in a lateral decubitus position. Briefly, during laparoscopic nephrectomy the camera and three or four additional trocars were introduced under vision. After dissection of the kidney, ureter, and vascular structures, an endobag (Endo-catch; US Surgical, Norwalk, CT, USA) was introduced. The renal artery and vein were divided with linear stapling devices (Endo GIA; US Surgical), and the kidney was extracted through a pfannenstiel incision. The skin wounds were sutured intracutaneously
To enable mini incision open nephrectomy a horizontal skin incision 10-12 cm long was made anterior to the 11th rib. The fascia and muscles of the abdominal wall were split using a mechanical retractor (Omnitract surgical, St Paul, USA). Gerota's fascia was opened on the lateral side of the kidney. After dissection of the kidney the surgeon clamped, cut, and ligated the ureter, renal artery, and vein. The kidney was extracted. The fascias of the abdominal muscles were closed, the subcutaneous fascia was approximated, and the skin was sutured intracutaneously.
Postoperative data and quality of life
The donor determined timing of discharge on the basis of tolerance to a normal diet and ability to use stairs. We calculated postoperative hospital stay with and without correction for time spent in hospital as a result of non-medical reasons (lack of care at home). Donors were seen at the outpatient clinic at three weeks, three months, and one year postoperatively. They were asked to complete forms related to pain, nausea, body image, fatigue, and quality of life. Preoperatively and at days 1, 3, 7, and 14 the donors scored pain and nausea on a visual analogue scale from 0 (none) to 10 (severe).
Body image was assessed at one year postoperatively using the body image questionnaire,7 which consists of two scales: the body image scale, which assesses attitudes to bodily appearance and consists of five questions (score 5-20), and the cosmetic scale, which assesses degree of satisfaction with the appearance of the scar and consists of three questions (score 3-24). Higher scores on both scales indicate greater satisfaction.
To assess whether laparoscopic nephrectomy and open nephrectomy differentially affected health related quality of life and fatigue, we administered the SF-36 and the multidimensional fatigue inventory 20 (MFI-20) preoperatively and at 1, 3, 6, and 12 months postoperatively. The SF-36 includes one multi-item scale measuring each of eight health concepts: physical function, role limitations due to physical health problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health.8 Scores per dimension of the SF-36 ranged from 0 to 100, with higher scores indicating better quality of life. We considered a five point difference between laparoscopic nephrectomy and open nephrectomy on a dimension as minimally clinically relevant.8
We determined levels of fatigue using the MFI-20,9 which consists of 20 items divided into five scales: general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. Scores per item ranged from 1 to 5: total score per scale ranged from 4 (no fatigue) to 20 (exhausted).
Recipients
Recipients were admitted to a surgical ward separated from that of the donor to minimise influence on the donor's recovery. Renal transplantation was carried out using the standard technique of preperitoneal placement in the iliac fossa. The immunosuppressive regimen included mycophenolate mofetil, tacrolimus, and prednisone. During the first year postoperatively we recorded survival rates of recipients and grafts, acute rejection rates (histologically proved), venous thrombosis, and ureteral complications as defined as the need for a percutaneous nephrostomy, ureter reconstructions, and renal function.
Statistical analysis
Fatigue and quality of life are closely related. However, fatigue as an outcome of the intervention might be more suitable for indicating the effect of a surgical approach than the quality of life test. The quality of life test may be influenced by other factors, such as satisfaction after donor nephrectomy. The primary outcome was therefore physical fatigue on the MFI-20 and the secondary outcome was physical function on the SF-36. Other secondary end points were postoperative hospital stay, pain, operating times, recipient graft function, and graft survival. Power calculations were based on physical fatigue. Fifty donors had to be included in each arm to establish a moderate significant difference of 0.6 standard deviations in physical fatigue with a power of 80% and an α of 0.05. We used the χ2 test to compare categorical variables and the Mann-Whitney U test to compare continuous variables. Repeated measurement of analysis of covariance was used to compare repeated continuous variables. We adjusted repeated measures for baseline values, donor's sex, and age. Analyses were carried out using SPSS version 11.0. We analysed data according to the intention to treat principle. P values less than 0.05 (two sided) were considered statistically significant.
Results
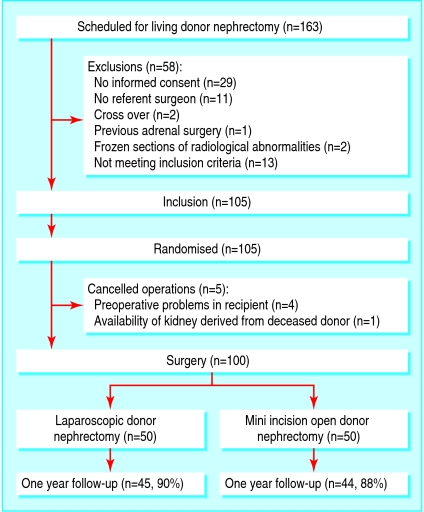
From November 2001 until February 2004 we recruited 105 of 163 living kidney donors to the study (fig 1). Two of the 163 donors were excluded because they were participating in a living donor kidney exchange programme.10 After randomisation one operation was cancelled and four were postponed because of clinical or radiological findings on the night before surgery. The Rotterdam centre carried out 34 laparoscopic donor nephrectomies and 38 mini incision open donor nephrectomies. The Nijmegen centre carried out 16 laparoscopic nephrectomies and 12 open nephrectomies. The number of participants was smaller in Nijmegen because recruitment was delayed while awaiting ethical approval. Table 1 shows the baseline characteristics of the donors and recipients.
Fig 1.
Flow of patients
Table 1.
Baseline characteristics of living kidney donors and recipients according to nephrectomy technique. Values are numbers (percentages) unless stated otherwise
| Variable | Laparoscopic donor nephrectomy (n=50) | Mini incision open donor nephrectomy (n=50) |
|---|---|---|
| Donor | ||
| Male | 29 (58) | 24 (48) |
| Female | 21 (42) | 26 (52) |
| Median (range) age (years) | 49 (20-77) | 48.5 (21-75) |
| Kidney removed: | ||
| Left | 30 (60) | 31 (62) |
| Right | 20 (40) | 19 (38) |
| Preoperative physical status: | ||
| I (healthy) | 38 (76.0) | 34 (68) |
| II | 12 (24.0) | 15 (30) |
| III | 1 (2) | |
| Median (range) body mass index | 25.9 (16.5-36.6) | 26.0 (17.7-33.2) |
| Renal arteries: | ||
| 1 | 37 (74) | 40 (80) |
| ≥2 | 13 (26) | 10 (20) |
| Renal veins: | ||
| 1 | 42 (84) | 46 (92) |
| ≥2 | 8 (16) | 4 (8) |
| Median (range) preoperative serum creatinine level (μmol/l) | 76 (49-105) | 79 (54-99) |
| Median (range) physical function† | 95 (35-100) | 100 (45-100) |
| Median (range) physical fatigue‡ | 4 (4-10) | 4.0 (4-20) |
| Recipient | ||
| Male | 32 (64) | 23 (46) |
| Female | 18 (36) | 27 (54) |
| Median (range) age (years) | 48 (13-68) | 44 (11-72) |
| Relation between donor and recipient: | ||
| Related | 39 (78) | 35 (70) |
| Unrelated | 11 (22) | 15 (30) |
| Median (range) preoperative serum creatinine level (μmol/l) | 799 (299-1793) | 783 (300-1777) |
American Society of Anesthesiologist classification.
SF-36.
Multidimensional fatigue inventory.
Surgery
The procedures were all carried out as planned without conversion to open or formal lumbotomy (table 2). In the laparoscopic group skin to skin time and warm ischaemia time were significantly longer and blood loss was less. Intraoperative complications occurred in six patients (12%) during laparoscopic nephrectomy, which were bleeding in three (total blood loss 400-860 ml), a serosal lesion of the colon, a bladder lesion, and a small capsular tear of the spleen. The lesions were recognised immediately and treated without conversion. Re-interventions were not indicated. The three (6%) complications during open nephrectomy involved bleeding (total blood loss 1000-1800 ml), which was controlled during surgery.
Table 2.
Surgical outcomes of living kidney donors and postoperative outcomes of donors and recipients. Values are numbers (percentages) unless stated otherwise
| Variable | Laparoscopic donor nephrectomy (n=50) | Mini incision open donor nephrectomy (n=50) | P value |
|---|---|---|---|
| Donor | |||
| Conversion to open donor nephrectomy | 0 | — | — |
| Median (range) time (min): | |||
| Kidney removal | 181 (107-307) | 118 (61-201) | <0.001 |
| Skin to skin | 221 (135-354) | 164 (92-298) | <0.001 |
| In operating theatre | 289.5 (180-420) | 226 (157-365) | <0.001 |
| Median (range) warm ischaemia time (min) | 6 (2-14) | 3 (1-6) | <0.001 |
| Blood loss (ml) | 100 (10-860) | 240 (20-1800) | <0.001 |
| Complications: | |||
| Intraoperative | 6 (12) | 3 (6) | 0.23 |
| Postoperative | 3 (6) | 3 (6) | 1.00 |
| Median (range) resumption of normal diet (h) | 19.5 (3-48) | 24 (16-72) | 0.01 |
| Median (range) morphine requirement (mg) | 16 (0-93) | 25 (1-107) | 0.005 |
| Median (range) length of hospital stay (days): | |||
| Unadjusted | 3 (1-6) | 4 (2-8) | 0.003 |
| Adjusted* | 3 (1-6) | 3 (2-8) | 0.002 |
| Median (range) serum creatinine level (μmol/l): | |||
| Day 1 | 112 (75-158) | 112.5 (68-183) | 0.81 |
| Day 2 | 118 (76-167) | 117.5 (74-222) | 0.99 |
| Month 3 | 107 (76-157) | 117 (79-191) | 0.31 |
| Year 1 | 107 (72-153) | 114 (75-169) | 0.17 |
| Median (range) nausea†: | |||
| Day 1 | 0 (0-9.2) | 0 (0-7.7) | 0.52 |
| Day 3 | 0 (0-4.6) | 0 (0-5.2) | 0.24 |
| Day 7 | 0 (0-3.2) | 0 (0-8.0) | 0.31 |
| Day 14 | 0 (0-2.2) | 0 (0-8.0) | 0.14 |
| Median (range) pain†: | |||
| Day 1 | 2.7 (0-6.2) | 3.5 (0-7.7) | 0.04 |
| Day 3 | 1.4 (0-6.6) | 1.8 (0-7.8) | 0.12 |
| Day 7 | 0.4 (0-6.1) | 1.7 (0-8.0) | 0.03 |
| Day 14 | 0 (0-4.8) | 0.4 (0-8.0) | 0.008 |
| Median (range) body image questionnaire: | |||
| Body image scale | 20 (13-20) | 20 (14-20) | 0.40 |
| Cosmetic scale | 20 (7-24) | 18 (12-24) | 0.14 |
| Recipient | |||
| Acute rejection | 9 (18) | 15 (30) | 0.24 |
| Ureteral complications | 6 (12) | 10 (20) | 0.41 |
| Graft survival at one year‡ | 48 (100) | 48 (98) | 1.00 |
| Patient survival at one year | 48 (96) | 49 (98) | 1.00 |
Adjusted for time spent in hospital for non-medical reasons.
Measured on visual analogue scale from 0 (none) to 10 (severe).
Censored for death.
Postoperative outcomes
Laparoscopic nephrectomy resulted in faster recovery as reflected by earlier resumption of a normal diet, less need for intravenous morphine, and earlier discharge (table 2). During the first two weeks donors in the laparoscopic group experienced significantly less pain. Postoperative complications after laparoscopic nephrectomy were wound infections at the extraction site in two donors, which were treated with oral antibiotics, and a blood transfusion. Complications after open nephrectomy were a urinary tract infection, a minor pulmonary infiltrate (both not requiring antibiotics), and an infected retroperitoneal haematoma, for which the patient required readmission for intravenous antibiotics. Other complications, such as incisional hernias, did not occur. Donors in both groups had similar serum creatinine levels. Scores on the body image scale did not differ significantly between the groups.
Recipients
One recipient (laparoscopic nephrectomy) died on the first postoperative day due to myocardial infarction. Two others (one in each group) died in the first year due to progressive infections related to an immunocompromised status. One recipient's graft (open nephrectomy) did not survive due to vascular rejection (table 2). She currently undergoes haemodialysis. Renal vein thrombosis did not occur in either group. Three recipients had ureteral complications after open nephrectomy, including ureteral stenosis and leaking, leading to ureteral reconstructions. Serum creatinine levels in recipients of kidneys from donors in both groups decreased in parallel without any significant differences over time.
Quality of life and fatigue
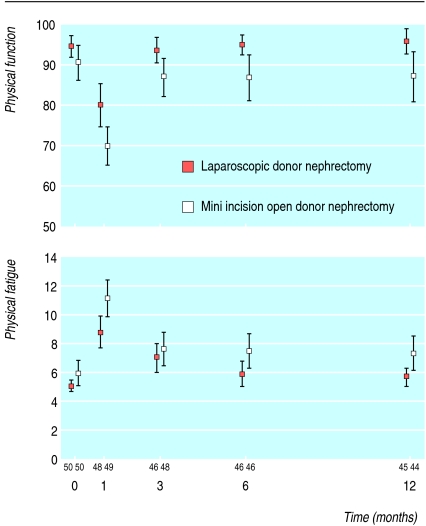
Response ranged from 97% at one month to 89% at 12 months, with an equal distribution at all times between laparoscopic and open nephrectomy. At baseline donors in both groups had excellent health status.8 Scores on the domains role physical and bodily pain were comparable at all time points (table 3). All other dimensions differed over time in favour of laparoscopic nephrectomy. Figure 2 shows scores for physical function over time in both groups. Patients in the laparoscopic group had higher mean scores for physical function during follow-up, indicating better quality of life (difference 6.2 points, 95% confidence interval 2.0 to 10.3, P = 0.004). Similar patterns were found for the other dimensions (see bmj.com).
Table 3.
Quality of life of living kidney donors after laparoscopic nephrectomy or mini incision open nephrectomy
| Dimension | Estimated difference (95% CI) (laparoscopic nephrectomy minus open nephrectomy) | P value |
|---|---|---|
| SF-36: | ||
| Physical function | 6.2 (2.0 to 10.3) | 0.004 |
| Role physical | 7.7 (−2.1 to 17.5) | 0.12 |
| Bodily pain | 4.1 (−0.3 to 8.5) | 0.07 |
| General health | 7.2 (2.2 to 12.1) | 0.005 |
| Vitality | 6.7 (1.1 to 12.2) | 0.02 |
| Social functioning | 5.9 (0.5 to 11.4) | 0.03 |
| Role emotional | 11.8 (4.1 to 19.5) | 0.003 |
| Mental health | 5.6 (1.8 to 9.4) | 0.005 |
| Multidimensional fatigue inventory: | ||
| General fatigue | −0.7 (−2.0 to 0.6) | 0.31 |
| Physical fatigue | −1.3 (−2.4 to -0.1) | 0.03 |
| Reduced activities | −0.8 (−2.0 to 0.3) | 0.16 |
| Reduced motivation | −1.0 (−2.1 to 0.1) | 0.07 |
| Mental fatigue | −0.2 (−1.7 to 0.3) | 0.70 |
Positive differences on SF-36 dimensions indicate better quality of life after laparoscopic donor nephrectomy. Negative differences on multidimensional fatigue inventory dimensions indicate less fatigue after laparoscopic donor nephrectomy.
Fig 2.
Physical function and physical fatigue (means with 95% confidence intervals) during follow-up of living kidney donors after laparoscopic nephrectomy or mini incision open donor nephrectomy. Numbers refer to donors evaluated at each time point
Physical fatigue scores were significantly lower for donors in the laparoscopic group, indicating less physical fatigue (fig 2): difference during one year's follow-up (- 1.3, 95% confidence interval - 2.4 to - 0.1, P = 0.03). Other dimensions of fatigue did not differ between the groups over time (see bmj.com).
Discussion
Laparoscopic donor nephrectomy results in faster recovery, less fatigue, and better quality of life of the donor compared with mini incision open donor nephrectomy but equal safety and graft function.
Most studies have investigated perioperative complications and recovery shortly after nephrectomy using different techniques. Three randomised trials compared hand assisted laparoscopic donor nephrectomy with mini incision open donor nephrectomy without blinding.11-13 Mini incision open donor nephrectomy has been proposed as an acceptable alternative to laparoscopic surgery,14 particularly if complications are expected.
We did not exclude donors for laparoscopic nephrectomy because of factors such as high body mass index. Unlike traditional lumbotomy, the applied open approach used a small incision and preserved continuity of abdominal wall muscles resulting in fewer complications, fast recovery,15,16 and cosmetic outcomes equivalent to laparoscopic nephrectomy. Despite modification of the open technique, laparoscopic nephrectomy was superior for recovery and, more importantly, fatigue and quality of life during follow up. The use of blood stained wound dressings blinded donors and medical staff in the immediate postoperative phase. In previous reports on laparoscopic cholecystectomy compared with open cholecystectomy this strategy avoided bias caused by medical staff.17,18 Although bias could have been present after discharge, this is the best possible blinding. The difference in variables measured after the operation, such as pain scores and length of hospital stay, was significantly in favour of laparoscopic nephrectomy, despite blinding.
Restoring quality of life is of utmost importance after living kidney donation. Other retrospective studies showed an improved quality of life after laparoscopic surgery compared with conventional open surgery.19,20 In our study laparoscopic surgery led to a better quality of life of donors. In studies of laparoscopic compared with open surgery for benign or malignant conditions, quality of life is at best considered a secondary outcome. Removal and permanent correction of the abnormality is the primary outcome and influences the feelings of patients postoperatively. Conversions from laparoscopic to open techniques often obscure the effect of the operation on quality of life. As donors are healthy individuals the benefits they achieve from laparoscopic surgery resemble the actual benefits for patients undergoing laparoscopic operations.
Although the benefits of laparoscopic nephrectomy were obvious in our study, extensive experience in laparoscopic surgery is necessary before implementation of a kidney donation programme using laparoscopic techniques. Complications, although rare, did occur in our study. Furthermore, the operation time was about an hour longer for laparoscopic nephrectomy. This was attributed to the time needed to set up for surgery and the inclusion of donors with more difficult anatomy. Alternatives closely related to laparoscopic nephrectomy need to be explored to tackle these issues. Retroperitoneoscopic donor nephrectomy may combine the advantage of a shorter operation time and a lower chance of complications from lesions of intraperitoneal organs.21 Laparoscopic donor nephrectomy may be advocated for donation programmes using living kidney donors.
What is already known on this topic
Both laparoscopic donor nephrectomy and minimally invasive open donor nephrectomy provide better outcomes than conventional open donor nephrectomy
What this study adds
Compared with mini incision open donor nephrectomy, laparoscopic donor nephrectomy was associated with faster recovery, less fatigue, and better quality of life of the donor but equal safety and graft function
Supplementary Material
 Table with data for additional dimensions is on bmj.com
Table with data for additional dimensions is on bmj.com
We thank J G van Duuren-van Pelt, data manager, and I P J Alwayn, surgeon, for their contributions to this study.
Contributors: WW, BMEH, GJvdW, EJH, IRAMMzB, HJB, JAvdV, and JNMI contributed to the design and initial planning of this study. BMEH, BCK, HJB, and JNMIJ attended all operations and controlled the carrying out of the surgical technique. NFMK, MYL, EJH, and DP collected the donors' data. WW and IMD collected the recipients' data. WCJH was the trial statistician. NFMK, EMMA, and GJvdW were responsible for collecting, analysing, interpreting, and writing up the quality of life data. IRAMMzB supervised and controlled the anaesthesiology protocol. NFMK, WW, JNMI, and EMMA coordinated the writing and drafting of the article. JNMI is guarantor.
Funding: This study was supported by unrestricted grants from the Society of American Gastrointestinal Endoscopic Surgeons and the Dutch Kidney Foundation.
Competing interest: None declared.
Ethical approval: This study was approved by the medical ethics committees of the university medical centres at Rotterdam and Nijmegen.
References
- 1.Ingelfinger JR. Risks and benefits to the living donor. N Engl J Med 2005;353: 447-9. [DOI] [PubMed] [Google Scholar]
- 2.Ratner LE, Ciseck LJ, Moore RG, Cigarroa FG, Kaufman HS, Kavoussi LR. Laparoscopic live donor nephrectomy. Transplantation 1995;60: 1047-9. [PubMed] [Google Scholar]
- 3.Srivastava A, Tripathi DM, Zaman W, Kumar A. Subcostal versus transcostal mini donor nephrectomy: is rib resection responsible for pain related donor morbidity. J Urol 2003;170: 738-40. [DOI] [PubMed] [Google Scholar]
- 4.Yang SL, Harkaway R, Badosa F, Ginsberg P, Greenstein MA. Minimal incision living donor nephrectomy: improvement in patient outcome. Urology 2002;59: 673-7. [DOI] [PubMed] [Google Scholar]
- 5.Kok NF, Alwayn IP, Lind MY, Tran KT, Weimar W, Ijzermans JN. Donor nephrectomy: mini-incision muscle-splitting open approach versus laparoscopy. Transplantation 2006;81: 881-7. [DOI] [PubMed] [Google Scholar]
- 6.Lind MY, Hazebroek EJ, Hop WC, Weimar W, Jaap Bonjer H, IJzermans JN. Right-sided laparoscopic live-donor nephrectomy: is reluctance still justified? Transplantation 2002;74: 1045-8. [DOI] [PubMed] [Google Scholar]
- 7.Dunker MS, Bemelman WA, Slors JF, van Duijvendijk P, Gouma DJ. Functional outcome, quality of life, body image, and cosmesis in patients after laparoscopic-assisted and conventional restorative proctocolectomy: a comparative study. Dis Colon Rectum 2001;44: 1800-7. [DOI] [PubMed] [Google Scholar]
- 8.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 health survey. Manual and interpretation guide. Boston, MA: Health Institute, New England Medical Center, 1993.
- 9.Smets EM, Garssen B, Cull A, de Haes JC. Application of the multidimensional fatigue inventory (MFI-20) in cancer patients receiving radiotherapy. Br J Cancer 1996;73: 241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Klerk M, Keizer KM, Claas FH, Witvliet M, Haase-Kromwijk BJ, Weimar W. The Dutch national living donor kidney exchange program. Am J Transplant 2005;5: 2302-5. [DOI] [PubMed] [Google Scholar]
- 11.Wolf JS Jr, Merion RM, Leichtman AB, Campbell DA Jr, Magee JC, Punch JD, et al. Randomized controlled trial of hand-assisted laparoscopic versus open surgical live donor nephrectomy. Transplantation 2001;72: 284-90. [DOI] [PubMed] [Google Scholar]
- 12.Simforoosh N, Basiri A, Tabibi A, Shakhssalim N, Hosseini Moghaddam SM. Comparison of laparoscopic and open donor nephrectomy: a randomized controlled trial. Br J Urolog Int 2005;95: 851-5. [DOI] [PubMed] [Google Scholar]
- 13.Oyen O, Andersen M, Mathisen L, Kvarstein G, Edwin B, Line PD, et al. Laparoscopic versus open living-donor nephrectomy: experiences from a prospective, randomized, single-center study focusing on donor safety. Transplantation 2005;79: 1236-40. [DOI] [PubMed] [Google Scholar]
- 14.Lewis GR, Brook NR, Waller JR, Bains JC, Veitch PS, Nicholson ML. A comparison of traditional open, minimal-incision donor nephrectomy and laparoscopic donor nephrectomy. Transpl Int 2004;17: 589-95. [DOI] [PubMed] [Google Scholar]
- 15.Berends FJ, den Hoed PT, Bonjer HJ, Kazemier G, van Riemsdijk I, Weimar W, et al. Technical considerations and pitfalls in laparoscopic live donornephrectomy. Surg Endosc 2002;16: 893-8. [DOI] [PubMed] [Google Scholar]
- 16.Lind MY, Liem YS, Bemelman WA, Dooper PM, Hop WC, Weimar W, et al. Live donor nephrectomy and return to work: does the operative technique matter? Surg Endosc 2003;17: 591-5. [DOI] [PubMed] [Google Scholar]
- 17.Majeed AW, Troy G, Nicholl JP, Smythe A, Reed MW, Stoddard CJ, et al. Randomised, prospective, single-blind comparison of laparoscopic versus small-incision cholecystectomy. Lancet 1996;347: 989-94. [DOI] [PubMed] [Google Scholar]
- 18.Johansson M, Thune A, Nelvin L, Stiernstam M, Westman B, Lundell L. Randomized clinical trial of open versus laparoscopic cholecystectomy in the treatment of acute cholecystitis. Br J Surg 2005;92: 44-9. [DOI] [PubMed] [Google Scholar]
- 19.Perry KT, Freedland SJ, Hu JC, Phelan MW, Kristo B, Gritsch AH, et al. Quality of life, pain and return to normal activities following laparoscopic donor nephrectomy versus open mini-incision donor nephrectomy. J Urol 2003;169: 2018-21. [DOI] [PubMed] [Google Scholar]
- 20.Buell JF, Lee L, Martin JE, Dake NA, Cavanaugh TM, Hanaway MJ, et al. Laparoscopic donor nephrectomy vs open live donor nephrectomy: a quality of life and functional study. Clin Transplant 2005;19: 102-9. [DOI] [PubMed] [Google Scholar]
- 21.Wadstrom J. Hand-assisted retroperitoneoscopic live donor nephrectomy: experience from the first 75 consecutive cases. Transplantation 2005;80: 1060-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.