Abstract
The inactivation of Salmonella enterica serovar Enteritidis by ultrasonic waves (20 kHz; 117-μm wavelength) under pressure (175 kPa) at nonlethal temperatures (manosonication [MS]) and lethal temperatures (manothermosonication [MTS]) in media of different water activities has been investigated. Heat decimal reduction time values increased 30 times when the water activity was decreased from nearly 1 to 0.96, but the MS resistance was increased only twofold. The inactivation of Salmonella serovar Enteritidis by ultrasound under pressure at low water activities was a phenomenon of the “all-or-nothing” type. A synergistic lethal effect was observed between heat and ultrasound in media with reduced water activity; the lower the water activity, the greater the synergistic effect. This work could be useful for improving sanitation and preservation treatments of foods, especially those which are sensitive to temperature and those in which components protect microorganisms to heat. It also contributes to our knowledge of microbial inactivation mechanisms by MS and MTS treatments.
During the last 3 decades, the number of food poisoning outbreaks in which different Salmonella enterica serotypes were involved has increased in industrialized countries (34, 35). In the late 1980s, the frequency of isolation of Salmonella enterica serotype Enteritidis from eggs and egg products has increased steadily (25, 34). The influence of different environmental factors on the survival of other Salmonella serotypes has been investigated repeatedly (12, 13, 15, 37), but little is known about their effect on Salmonella serovar Enteritidis.
It is well-known that the heat resistance of microorganisms is influenced by many environmental factors. The water activity (aw) of the heating medium is one of the most important factors (5, 7, 10, 16, 36). The thermal protective effect of media with reduced aw is very high (100-fold) (14, 33). The use of high-intensity heat treatments to obtain the required microbial inactivation would impair food quality. Therefore, perhaps the new nonthermal methods of bacterial inactivation would be good alternative methods for the preservation and sanitation of products with reduced aw.
Microbial inactivation by high-power ultrasound under pressure at nonlethal temperatures (manosonication [MS]) and lethal temperatures (manothermosonication [MTS]) was first reported by Sala et al. in 1995 (29). Recently, Raso et al. (27) reported the influence of temperature, pressure, and amplitude of ultrasonic waves on the lethal effect of ultrasound. Pagán et al. (24) studied the effects of MS and MTS on gram-positive and gram-negative bacterial species, and Mañas et al. (20) studied the lethal effect of MS and MTS on several Salmonella serotypes suspended in buffer and in liquid whole egg. However, no specific investigations have been performed on Salmonella bacterial inactivation by ultrasonic waves under pressure in media with reduced water activity. From published data (20), it could be deduced that the eventual advantages of MS or MTS for sanitation and/or food preservation purposes will be higher in temperature-sensitive foods (e.g., when raw materials are contaminated with very heat-resistant bacterial species or when food components protect microorganisms against heat).
The purpose of this work was to investigate the inactivation of Salmonella serovar Enteritidis by ultrasonic waves under pressure at different temperatures in media of different water activities. The heat resistance of this microorganism in the same medium was also studied as a control.
Bacterial culture and media.
The Salmonella serovar Enteritidis strain (ATCC 13076) used in this investigation was supplied by the Spanish Type Culture Collection. Erlenmeyer flasks containing 50 ml of sterile nutrient broth (Biolife, Milan, Italy) were inoculated to a final concentration of 106 cells ml−1 and incubated at 37°C under agitation (130 rpm) (Selecta; Rotabit, Barcelona, Spain). When the stationary growth phase was reached (after 24 h of incubation), suspensions were stored at 4°C until use. This storage did not change cell resistance to heat or ultrasonic waves for the time in which this investigation was performed (P < 0.05) (data not shown).
Heat, MS, and MTS treatments.
Heat, MS, and MTS treatments were performed in a specially designed resistometer as described previously (27). The resistometer, a mixing method that avoids the heating lag phases, allowed us to obtain survival curves to heat and ultrasound treatments at different temperatures, pressures, and ultrasonic wave amplitudes. Once the temperature, pressure, and amplitude of ultrasonic waves were stabilized, the cell suspension (0.2 ml) was injected into the 23-ml treatment chamber containing the treatment medium. Before the injection, cells were allowed to adapt to a solution with the same aw as the treatment medium for 5 min. Longer adaptation times did not change the survival curve profiles (data not shown). After injection and at preset intervals, 0.1-ml samples for each treatment time were directly collected into test tubes of melted sterile nutrient agar (NA) (Biolife) and immediately plated. Survival curves were plotted, with 6 to 15 separate samples collected over time. NA plates were incubated for 24 h at 37°C. Previous experiments showed that longer incubation times did not influence survivor counts. When damage and repair mechanisms were investigated, NA with 3% (wt/vol) sodium chloride (Panreac, Barcelona, Spain) added (NA-SC) was used as the recovery medium. This medium did not affect the viability of undamaged cells (data not shown). The NA-SC plates were incubated for 48 h at 37°C. After incubation, the CFU were counted with an Image Analyzer Automatic Counter (Protos; Analytical Measuring Systems, Cambridge, United Kingdom) as described elsewhere (3).
McIlvaine citrate phosphate buffer (pH 7; aw > 0.99) (6) or the same medium with different amounts of sucrose (Azucarera Española, Madrid, Spain) added (according to the data of Robinson and Stokes [28]) was prepared and used as the treatment media. The final aw of the treatment media (0.98 and 0.96) was measured at room temperature with a specially designed instrument (Water Activity System model CX-1; Decagon Devices, Inc., Pullman, Wash.).
Heat, MS, and MTS resistance parameters.
The inactivation rate was measured by determining the decimal reduction time value (Dt for heat, DMS for MS, and DMTS for MTS treatments) from the slope of the regression line of the survival curves. Decimal reduction time curves (DRTC) were obtained by plotting log10 D values versus their corresponding heating temperatures, and z values (increase in temperature [in degrees Celsius] for the D value to decrease by one log10 cycle) were calculated. The coefficient of determination (r2) of survival curves and the 95% confidence limits of D and z values were calculated by using the Excel package (Microsoft, Seattle, Wash.).
The individual contributions of heat and ultrasound under pressure to the lethal effect of MTS treatments at different temperatures were evaluated by determining how well experimental values matched the theoretical DRTC values. Theoretical DMTS values were calculated, as Raso et al. (27) described, with the equation DMTS = (Dt × DMS)/(Dt + DMS).
Heat resistance.
The survival curves obtained in this investigation followed first-order inactivation kinetics, at least for 99.9% of the cell population. Neither temperature adaptation phenomena nor subpopulations resistant to high heat or ultrasound under pressure were detected. As a consequence, D values were a useful parameter for resistance comparison purposes.
Table 1 shows the decimal reduction time values at different temperatures of Salmonella serovar Enteritidis heated in media with different aws. The 95% confidence limits and the r2 values of the corresponding survival curves have also been included to illustrate the precision of the results. Table 1 also includes the z values, their 95% confidence limits, and the corresponding r2 values. The temperature resistance values for Salmonella serovar Enteritidis treated in McIlvaine buffer of pH 7 (aw > 0.99) found in this study were consistent with the little data published (2, 20) on this serotype and were similar to those obtained for other temperature-sensitive serotypes of Salmonella (8, 9, 20). Also, the z value was similar to that reported for most vegetative cells investigated (17, 20, 22, 30). From these data, it could be deduced that current heat pasteurization treatments for liquid food with high aws (close to 1) were sufficient to avoid a food poisoning outbreak caused by Salmonella serovar Enteritidis.
TABLE 1.
Heat resistance of Salmonella serovar Enteritidis in media with different aws
| aw | T (°C)a | Dt (min)b | 95% CLc | r2 |
|---|---|---|---|---|
| >0.99 | 53 | 2.60 | 2.47-2.73 | 0.99 |
| 54 | 1.73 | 1.64-1.82 | 0.99 | |
| 56 | 0.78 | 0.74-0.82 | 0.99 | |
| 58 | 0.22 | 0.21-0.23 | 0.98 | |
| 60 | 0.10 | 0.09-0.10 | 0.99 | |
| 63 | 0.03 | 0.03-0.03 | 0.99 | |
| z(°C)d | 4.89 | 4.64-5.13 | 0.99 | |
| 0.98 | 56 | 3.68 | 3.50-3.86 | 0.99 |
| 58 | 1.53 | 1.45-1.61 | 0.99 | |
| 60 | 0.88 | 0.84-0.93 | 0.99 | |
| 62 | 0.38 | 0.36-0.40 | 0.98 | |
| 65 | 0.12 | 0.11-0.12 | 0.99 | |
| 68 | 0.04 | 0.03-0.04 | 0.99 | |
| z(°C) | 6.03 | 5.73-6.33 | 0.99 | |
| 0.96 | 58 | 4.56 | 4.34-4.79 | 0.98 |
| 60 | 2.70 | 2.57-2.84 | 0.99 | |
| 62 | 1.33 | 1.26-1.40 | 0.99 | |
| 64 | 0.68 | 0.64-0.71 | 0.99 | |
| 67 | 0.16 | 0.16-0.17 | 0.99 | |
| 71 | 0.05 | 0.05-0.05 | 0.99 | |
| z(°C) | 6.38 | 6.06-6.70 | 0.99 |
Temperature of the treatment.
Decimal reduction time values.
95% CL, 95% confidence limits.
Increase in temperature for the Dt value to decrease by one log10 cycle. The z values are shown in bold type.
The aw of the heating media had a great influence on the heat resistance of this serotype. The D value at 60 min increased from 0.10 to 0.88 and 2.70 min when the aw was reduced from >0.99 to 0.98 and 0.96, respectively. The z value significantly increased (P > 0.05) from 4.9 to 6.4°C when the aw was decreased from >0.99 to 0.96. Therefore, the thermal protective effect of media with reduced aw increased with increases in the treatment temperature. Comparing our data with those obtained by other investigators is difficult, since the effect of aw on microbial inactivation depends on the solute (1, 4, 9, 16, 31). From published data obtained for solutions of sucrose and water, it could be deduced that the effect of aw on the thermal tolerance of Salmonella serovar Enteritidis was among the highest published for Salmonella serotypes. It was similar to that observed for serovars Alachua, Anatum, and Montevideo (9) but greater than those reported for serovars Infantis, Typhimurium, and Tennessee (8, 9, 33). For Salmonella serovar Senftenberg, the effect of aw of the heating media was lower (Dt × 2 or Dt × 3) (8, 9, 19). It is generally believed that the thermal protective effect of reduced aws is higher for the most heat-sensitive species (1, 8, 9). Our results are in accordance with this hypothesis.
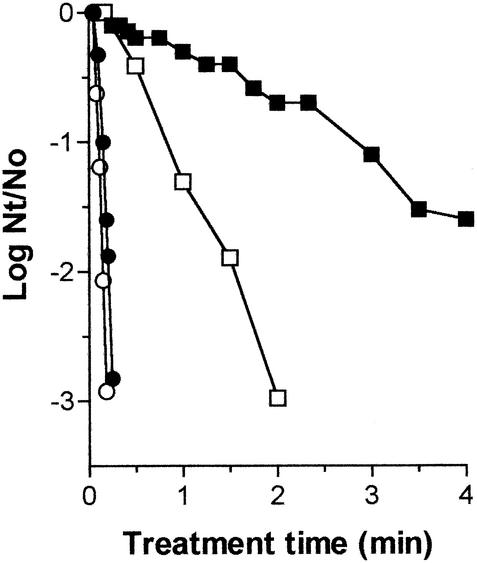
Figure 1 shows the survival curves of Salmonella serovar Enteritidis heated at 60°C in McIlvaine buffer (pH 7) with an aw of >0.99 and in the same medium with 41% (wt/vol) sucrose added (aw = 0.96), obtained by growing cells after treatment in NA and NA-SC. As can be observed in Fig. 1, the percentage of damaged cells increased with the treatment time and was greater at reduced aws. The medium with reduced aw protected Salmonella serovar Enteritidis by increasing the thermal stability of some bacterial structures to heat. However, a higher injury repair capability also contributed to the high thermal tolerance of cells heated in media with lower aws. The Dt value obtained in NA-SC was 1.4 times lower than that obtained in NA after heating in citrate phosphate buffer (0.07 and 0.10 min, respectively) but 4 times lower after heating in medium with an aw of 0.96 (0.62 and 2.46 min, respectively).
FIG. 1.
Survival curves of Salmonella serovar Enteritidis treated in media with different aws at 60°C. The bacteria were treated in media with an aw of >0.99 (•, ○) or 0.96 (▪, □) and recovered in NA (•, ▪) or NA-SC (○, □). Nt/No, number of cells treated/number of original cells.
Overall, the thermal inactivation of Salmonella serovar Enteritidis in liquid foods with an aw of 0.96 would require an increase, by approximately 30 times, of the intensity of current treatments designed for the pasteurization of liquid foods with an aw close to 1. These thermal treatments would probably impair the quality of most liquid foods. Therefore, alternative sanitation processes would be very useful.
MS or MTS resistance.
Table 2 shows the decimal reduction time values for ultrasonic waves (117-μm wavelength) under pressure (175 kPa) at different temperatures of Salmonella serovar Enteritidis treated in media with different aws. The DMS value in citrate phosphate buffer of pH 7 (0.89 min) was similar to that previously reported for this serotype by different researchers (20, 24). While the heat resistance of Salmonella serovar Enteritidis increased 30 times when the aw of the treatment media decreased from >0.99 to 0.96 (Table 1), the DMS value (Table 2) hardly doubled (0.89 and 1.37 min, respectively). This seemed to confirm that the mechanisms by which heat and ultrasound inactivate microorganisms are different. Furthermore, comparison of the survival curves obtained by growing cells in NA and in NA-SC after MS treatments in citrate phosphate buffer of pH 7 showed the lack of any damaged cells (data not shown), which contrasted with the results observed after heat treatments (Fig. 1). This would also indicate that the bacterial inactivation by MS is a phenomenon of the “all-or-nothing” type, probably due to the mechanical disruption of the cell envelopes, as has previously been observed by several researchers (20, 21, 23, 26) in media with high aws.
TABLE 2.
Resistance to ultrasonic waves (117-μm wavelength; 20 kHz) under pressure (175 kPa) at several temperatures of Salmonella serovar Enteritidis suspended in media with different aws
| aw | T (°C)a | D (min)b | 95% CLc | r2 |
|---|---|---|---|---|
| >0.99 | 35 | 0.89 | 0.84-0.93 | 0.99 |
| 50 | 0.77 | 0.73-0.81 | 0.99 | |
| 53 | 0.42 | 0.40-0.44 | 0.99 | |
| 56 | 0.25 | 0.24-0.26 | 0.99 | |
| 59 | 0.12 | 0.12-0.13 | 0.99 | |
| 63 | 0.02 | 0.02-0.02 | 0.98 | |
| 0.98 | 35 | 0.85 | 0.81-0.89 | 0.98 |
| 50 | 0.46 | 0.44-0.48 | 0.99 | |
| 60 | 0.16 | 0.15-0.17 | 0.99 | |
| 65 | 0.08 | 0.07-0.08 | 0.99 | |
| 68 | 0.04 | 0.03-0.04 | 0.99 | |
| 0.96 | 35 | 1.37 | 1.30-1.44 | 0.99 |
| 50 | 0.87 | 0.83-0.92 | 0.99 | |
| 56 | 0.32 | 0.30-0.33 | 0.99 | |
| 60 | 0.25 | 0.24-0.26 | 0.99 | |
| 67 | 0.09 | 0.09-0.09 | 0.99 | |
| 71 | 0.04 | 0.04-0.04 | 0.98 |
Temperature of the treatment.
Decimal reduction time values.
95% CL, 95% confidence limits.
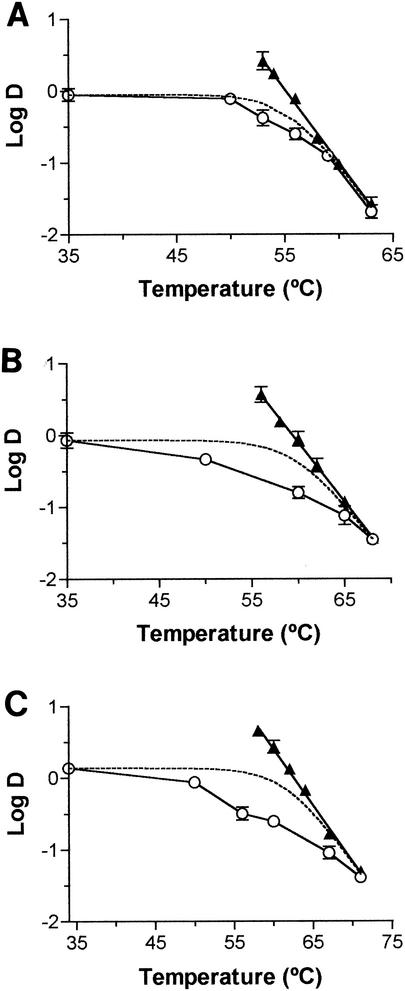
Figure 2 shows the relationship between the temperature and decimal reduction time values to MS and MTS treatments of Salmonella serovar Enteritidis obtained in media with aws of >0.99 (Fig. 2A), 0.98 (Fig. 2B), and 0.96 (Fig. 2C). The DRTC corresponding to heat treatments and the theoretical DRTC corresponding to MS and MTS treatments have also been included. The theoretical DRTC has been calculated, as proposed by Raso et al. (27), by assuming that heat and ultrasonic waves acted independently and that heat, MS, and MTS destruction of bacterial cells were single reactions ruled by first-order kinetics. As shown in Fig. 2A, experimental data obtained in McIlvaine buffer (pH 7) fitted the theoretical decimal reduction time values with a r2 of ≥0.98. The DMS value was the same until 50°C. From 50 to 60°C, the rapid decrease in the D value would be due to the exponential increase in the lethality of heat by linear increases in temperature, making the lethality of ultrasound negligible at 60°C. Over this temperature, the DMTS and Dt became equal. The same behavior has been observed in several bacterial species treated at high aws (20, 23, 24, 27). These data indicated that the lethality of MTS was the result of adding the inactivation rate due to heat to that due to ultrasound. The individual contributions of heat and ultrasound to the whole lethal effect depended on the temperature.
FIG. 2.
Resistance of Salmonella serovar Enteritidis to ultrasound (117-μm wavelength) under pressure (175 kPa) at different temperatures in media with aws of >0.99 (A), 0.98 (B), and 0.96 (C). Decimal reduction time values to ultrasonic waves under pressure (○) and to heat (▴) are shown. The broken line represents the theoretical DRTC to ultrasonic waves under pressure calculated by the equation DMTS = (Dt × DMS)/(Dt + DMS).
In contrast to the values observed when Salmonella serovar Enteritidis was subjected to MT or MTS treatment in citrate phosphate buffer (pH 7) (Fig. 2A), the theoretical DMS and DMTS values did not fit the experimental values obtained in media with reduced aws (Fig. 2B and C). The lower the aw was, the greater the differences were. The DMTS value at 60°C in medium with an aw of 0.96 was three times lower than was expected. This indicated the existence of a synergistic lethal effect between heat and ultrasound. The existence of a synergistic effect in which the whole lethal effect was higher than the lethal effect of heat added to the lethal effect of ultrasound has been previously reported for bacterial cells resistant to very high heat (21, 24).
Conclusions.
The addition of sucrose to the treatment media strongly protected Salmonella serovar Enteritidis cells to heat but hardly changed their MS resistance. Salmonella serovar Enteritidis inactivation by ultrasonic waves under pressure was a phenomenon of the all-or-nothing type. No sodium chloride-sensitive cells could be detected after MS treatments. The whole lethal effect of MTS in phosphate citrate buffer (pH 7) with an aw of >0.99 was the result of the lethal effect of heat added to that of ultrasonic waves under pressure. When Salmonella serovar Enteritidis cells were treated by MTS in media with reduced aws, a synergistic effect was observed. The lower the aw was, the higher the synergism. The synergistic effect was due to the sensitizing effect of heat, and ultrasound was the ultimate cause of bacterial inactivation.
Acknowledgments
This work was supported in part by a scholarship granted to I.A. by the “Ministerio de Educación, Cultura y Deporte.”
We thank E. Pickett for help correcting the English in the manuscript.
REFERENCES
- 1.Baird-Parker, A. C., M. Boothroyd, and E. Jones. 1970. The effect of water activity on the heat resistance of heat sensitive and heat resistant strains of Salmonellae. J. Appl. Bacteriol. 33:515-522. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn, C. W., L. M. Curtis, L. Humpheson, C. Billon, and P. J. McClure. 1997. Development of thermal inactivation models for Salmonella enteritidis and Escherichia coli O157:H7 with temperature, pH and NaCl as controlling factors. Int. J. Food Microbiol. 38:31-44. [DOI] [PubMed] [Google Scholar]
- 3.Condón, S., A. Palop, J. Raso, and F. J. Sala. 1996. Influence of the incubation temperature after heat treatment upon the estimated heat resistance values of spores of Bacillus subtilis. Lett. Appl. Microbiol. 22:149-152. [Google Scholar]
- 4.Corry, J. E. L. 1974. The effect of sugars and polyols on the heat resistance of Salmonellae. J. Appl. Bacteriol. 37:31-43. [DOI] [PubMed] [Google Scholar]
- 5.Cotterill, O. J., and J. Glauert. 1968. Thermal resistance of Salmonella in egg yolk products containing sugar or salt, p. 1156-1168. In Proceedings of the 56th Annual Poultry Science Meeting. University of New Hampshire, Durham, N.H. [DOI] [PubMed]
- 6.Dawson, R. M. C., D. C. Elliot, W. H. Elliot, and K. M. Jones (ed.). 1974. pH and buffers, p. 475-508. In pH and buffers. Data for biochemical research. Clarendon Press, New York, N.Y.
- 7.Fletcher, S. A., and L. N. Csonka. 1998. Characterization of the induction of increased thermotolerance by high osmolarity in Salmonella. Food Microbiol. 15:307-317. [Google Scholar]
- 8.Gibson, B. 1973. The effect of high sugar concentrations on the heat resistance of vegetative microorganisms. J. Appl. Bacteriol. 36:365-376. [DOI] [PubMed] [Google Scholar]
- 9.Goepfert, J. M., I. K. Iskander, and C. H. Amundson. 1970. Relation of the heat resistance of Salmonella to the water activity of the environment. Appl. Microbiol. 19:429-433. [DOI] [PMC free article] [PubMed]
- 10.Hansen, N. H., and H. Rieman. 1963. Factors affecting the heat resistance of nonsporing organisms. J. Appl. Bacteriol. 26:314-333. [Google Scholar]
- 11.Helander, I. M., A. von Wright, and T. M. Mattila-Sandholm. 1997. Potential of lactic acid bacteria and novel antimicrobial against Gram-negative bacteria. Trends Food Sci. Technol. 8:146-150. [Google Scholar]
- 12.Hills, B. P., C. E. Manning, Y. Ridge, and B. Brocklehurst. 1997. Water availability and the survival of Salmonella typhimurium in porous systems. Int. J. Food Microbiol. 36:187-198. [DOI] [PubMed] [Google Scholar]
- 13.Jung, Y. S., and L. R. Beuchat. 1999. Survival of multidrug-resistant Salmonella typhimurium DT104 in egg powders as affected by water activity and temperature. Int. J. Food Microbiol. 49:1-8. [DOI] [PubMed] [Google Scholar]
- 14.Kwast, R. H., and C. T. Verrips. 1982. Heat resistance of Salmonella senftenberg 775W at various sucrose concentrations in distilled water. Eur. J. Appl. Microbiol. Biotechnol. 14:193-201. [Google Scholar]
- 15.Kwon, Y. M., and S. C. Ricke. 1998. Survival of a Salmonella typhimurium poultry isolate in the presence of propionic acid under aerobic and anaerobic conditions. Anaerobe 4:251-256. [DOI] [PubMed] [Google Scholar]
- 16.Lee, A. C., and J. M. Goepfert. 1975. Influence of selected solutes on thermally induced death and injury of Salmonella typhimurium. J. Milk Food Technol. 38:195-200.
- 17.Lovett, J., J. G. Bradshaw, and J. T. Peeler. 1982. Thermal inactivation of Yersinia enterocolitica in milk. Appl. Environ. Microbiol. 44:517-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mañas, P., R. Pagán, and J. Raso. 2000. Predicting lethal effect of ultrasonic waves under pressure treatments on Listeria monocytogenes ATCC 15313 by power measurements. JFS Food Eng. Phys. Prop. 65:663-667. [Google Scholar]
- 19.Mañas, P., R. Pagán, F. J. Sala, and S. Condón. 2001. Low molecular weight milk whey components protect Salmonella senftenberg 775W against heat by a mechanism involving divalent cations. J. Appl. Microbiol. 91:871-877. [DOI] [PubMed] [Google Scholar]
- 20.Mañas, P., R. Pagán, J. Raso, F. J. Sala, and S. Condón. 2000. Inactivation of Salmonella Enteritidis, Salmonella Typhimurium, and Salmonella Senftenberg by ultrasonic waves under pressure. J. Food Prot. 63:451-456. [DOI] [PubMed] [Google Scholar]
- 21.Pagán, R., P. Mañas, A. Palop, and F. J. Sala. 1999. Resistance of heat-shock cells of Listeria monocytogenes to mano-sonication and mano-thermo-sonication. Lett. Appl. Microbiol. 28:71-75. [DOI] [PubMed] [Google Scholar]
- 22.Pagán, R., P. Mañas, I. Álvarez, and F. J. Sala. 1998. Heat resistance in different heating media of Listeria monocytogenes ATCC 15313 grown at different temperatures. J. Food Saf. 18:205-219. [Google Scholar]
- 23.Pagán, R., P. Mañas, I. Álvarez, and S. Condón. 1999. Resistance of Listeria monocytogenes to ultrasonic waves under pressure at sublethal (manosonication) and lethal (manothermosonication) temperatures. Food Microbiol. 16:139-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagán, R., P. Mañas, J. Raso, and S. Condón. 1999. Bacterial resistance to ultrasonic waves under pressure at nonlethal (manosonication) and lethal (manothermosonication) temperatures. Appl. Environ. Microbiol. 65:297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabsch, W., H. Tschäpe, and A. J. Bäumler. 2001. Non-typhoidal salmonellosis: emerging problems. Microb. Infect. 3:237-247. [DOI] [PubMed] [Google Scholar]
- 26.Raso, J., A. Palop, R. Pagán, and S. Condón. 1998. Inactivation of Bacillus subtilis spores by combining ultrasonic waves under pressure and mild heat treatment. J. Appl. Microbiol. 85:849-854. [DOI] [PubMed] [Google Scholar]
- 27.Raso, J., R. Pagán, S. Condón, and F. J. Sala. 1998. Influence of temperature and pressure on the lethality of ultrasound. Appl. Environ. Microbiol. 64:465-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson, R. A., and R. M. Stokes. 1965. Electrolyte solutions. Butterworths, London, United Kingdom.
- 29.Sala, F. J., J. Brugos, S. Condón, P. López, and J. Raso. 1995. Effect of heat and ultrasound on microorganisms and enzymes, p. 176-204. In G. W. Gould (ed.), New methods of food preservation. Blackie Academic & Professional, London, United Kingdom.
- 30.Schuman, J. D., B. W. Sheldon, and P. M. Foegeding. 1997. Thermal resistance of Aeromonas hydrophila in liquid whole egg. J. Food Prot. 60:231-236. [DOI] [PubMed] [Google Scholar]
- 31.Smith, J. L., R. C. Benedict, and A. Palumbo. 1982. Protection against heat-injury in Staphylococcus aureus by solutes. J. Food Prot. 45:54-58. [DOI] [PubMed] [Google Scholar]
- 32.Stevens, K. A., B. W. Sheldon, N. A. Klapes, and T. R. Klaenhammer. 1992. Effect of the treatment conditions on nisin inactivation of gram-negative bacteria. J. Food Prot. 55:763-766. [DOI] [PubMed] [Google Scholar]
- 33.Sumner, S. S., T. M. Sandros, M. C. Harmon, V. N. Scott, and D. T. Bernard. 1991. Heat resistance of Salmonella typhimurium and Listeria monocytogenes in sucrose solutions of various water activities. J. Food Sci. 56:1741-1743. [Google Scholar]
- 34.Tauxe, R. T. 1991. Salmonella: a postmortem pathogen. J. Food Prot. 54:563-568. [DOI] [PubMed] [Google Scholar]
- 35.Tood, E. C. D. 1996. Worldwide surveillance of foodborne disease: the need to improve. J. Food Prot. 59:82-92. [DOI] [PubMed] [Google Scholar]
- 36.Tuncan, E. U., and E. M. Scott. 1989. Combined effect of sucrose and heat treatment temperature in the thermal resistance of Staphylococcus aureus MF-31. J. Food Sci. 54:936-939. [Google Scholar]
- 37.Weissinger, W. R., W. Chantarapanont, and L. R. Beuchat. 2000. Survival and growth of Salmonella baildon in shredded lettuce and diced tomatoes, and effectiveness of chlorinated water as a sanitizer. Int. J. Food Microbiol. 62:123-131. [DOI] [PubMed] [Google Scholar]