Abstract
Mung bean nuclease treatment of 16S-23S ribosomal DNA intergenic transcribed spacers (ITS) amplified from several strains of the six species of the Bacillus cereus group showed that B. anthracis Davis TE702 and B. mycoides G2 have other intermediate fragments in addition to the 220- and 550-bp homoduplex fragments typical of the B. cereus group. Long and intermediate homoduplex ITS fragments from strains Davis TE702 and G2 and from another 19 strains of the six species were sequenced. Two main types of ITS were found, either with two tRNA genes (tRNAIle and tRNAAla) or without any at all. Strain Davis TE702 harbors an additional ITS with a single tRNA gene, a hybrid between the tRNAIle and tRNAAla genes, suggesting that a recombination event rather than a deletion generated the single tDNA-containing ITS. Strain G2 showed an additional ITS of intermediate length with no tDNA and no similarity to other known sequences. Neighbor-joining analysis of tDNA-containing long ITS indicated that B. cereus and B. thuringiensis represent a single clade. Three signature sequences discriminated B. anthracis from B. cereus and B. thuringiensis, indicating that the anthrax agent started evolving separately from the related clades of the B. cereus group. B. mycoides and B. weienstephanensis were very closely related, while B. pseudomycoides appeared the most distant species.
Bacillus anthracis, B. cereus, B. mycoides, B. pseudomycoides, B. thuringiensis, and B. weihenstephanensis are six related species, five of which have an important impact on human activity (16, 37, 45, 46, 51): B. anthracis is the active agent of anthrax (48); B. cereus causes food-borne disease syndromes associated with enterotoxin and emetic toxin (16, 25); B. thuringiensis is an insect pathogen (45) and is widely used for the biological control of insects in crop protection; B. mycoides has been recognized as a plant growth-promoting bacterium associated with conifer roots (38); B. weihenstephanensis, a psychrotolerant species frequently found in pasteurized milk, is a potential cause of spoilage problems (32). The six species are not easily distinguished on the basis of phenotypic or genetic traits (48). Recently, B. anthracis, B. cereus, and B. thuringiensis were found to be very closely related, and it has been proposed that they belong to a single species (24, 33). This proposal has been based on multilocus enzyme electrophoresis data and sequencing of discrete genetic loci (24) and on the presence of an S-layer on the cell surface (33). The model considering B. anthracis, B. cereus, and B. thuringiensis as subspecies of a phylogenetically monomorphic group, differing mainly in characters linked to mobile genetic elements such as plasmids, is supported by very high sequence homology in the conserved molecular chronometers of the ribosomal operons, the 16S and 23S ribosomal DNA (rDNA), and the short intergenic spacer between them (3-5, 7, 21, 30). Considering the dangerousness of B. anthracis and the wide in-field application of B. thuringiensis as a biological insecticide, it would be opportune to further evaluate the phylogenetic relationship between the different clades of the B. cereus group. Whole-genome sequence-based analysis could give a definitive view of the genetic relationship between these species (29). However, an approach that is economically feasible, given current technology, is possible for few strains in a given species (42). Hence, for phylogenetic surveys based on a relatively large number of isolates of each species, permitting an assessment of the amount of variability and overlap within a species, the best means of approach remains the use of highly conserved molecules with no, or a low, horizontal gene transfer rate such as the ribosomal operon.
In the prokaryote genome, the ribosomal operon can be present in multiple copies, up to 15 copies in Clostridium paradoxum (41). The 16S-23S rDNA intergenic transcribed spacers (ITS) are the most variable regions of the ribosomal operon, and, apart from interoperonic nucleotide substitutions, insertions, and deletions, such ITS can be differentiated, given the presence of the different numbers and types of tRNA genes (9, 10, 31, 49). Since the ITS have fewer functional constraints than the adjacent ribosomal genes, which undergo concerted evolution (17-19), their sequences can contain traces of ribosomal operon rearrangements and species-specific or even strain-specific traits that are useful for strain typing.
An analysis of ITS homoduplex-heteroduplex polymorphisms has shown that wide variability exists in the strains of the six species of the B. cereus group (14), indicating widely different length and sequence polymorphisms among the 8 to 12 ribosomal operons (26). In the present study, we examined the sequence heterogeneity of the ITS of several strains of the six species showing different ITS homoduplex-heteroduplex polymorphism haplotypes (14). It has been shown that four different types of ribosomal spacers can be found and that genetic structural variation (22-24) is not restricted to variable species like B. cereus and B. thuringiensis; in fact, major variation can also be found in the very monomorphic species B. anthracis (27). Since no sequences of the tDNA-containing ITS of strains of the B. cereus group have been reported until now, we focused our attention on this region, which has been reported to allow the separation of closely related strains of different ecotypes in other species (see, for example, reference 43). On the basis of the tDNA-containing ITS sequences, the phylogenetic relationship between the species was evaluated, and it has been shown that B. anthracis represents a phylogenetic clade diverging from B. cereus and B. thuringiensis.
MATERIALS AND METHODS
Strains, PCR amplifications, and 16S-23S rDNA ITS fingerprinting.
The strains used in this study (Table 1) (14) were grown routinely, as previously described (6, 11, 13), and the DNA suitable for amplification was obtained through lysis by boiling (15) or by sodium dodecyl sulfate-proteinase K treatment (44), as already described (2, 6, 11, 13). The ITS homoduplex-heteroduplex polymorphism profiles were obtained by PCR followed by electrophoresis in MDE gel (BioWhittaker Molecular Applications, Milan, Italy) and silver staining using the procedures described previously (14). MDE is a separation matrix made of a polyacrylamide specifically designed to separate nucleic acid fragments on the basis of their secondary structure. It can be used to highlight nucleotidic polymorphisms on the basis of differential single-strand or heteroduplex conformations.
TABLE 1.
Signature nucleotides in long ITS containing tDNA typical of the species and clades of the B. cereus group
| Species or cladea | Strainsa,b | Nucleotide positionc | Signature sequence |
|---|---|---|---|
| BA | BA: 7700, 663, 282, Davis TE702 | 75-80 | AAAAAG |
| 121 | A | ||
| 213-214 | AT | ||
| BC/BT | BC: 31T, 345, 626, V65SP | 75-76 | TC |
| BT: 2046T, HD1, Bt14, BMG1.7, Ht39 | 117-121 | CCTGC | |
| 213-220 | TTTTGG | ||
| BM/BW | BM: 2048T, 299, 309 | 117-121 | TCCGC |
| BW: 10204T, 10202, 10208 | 213-218 | TTTTAC | |
| BP | BP: BD10 | 75-81 | AAATAT |
| 117-121 | TCTGC | ||
| 213-214 | AC |
BA, B. anthracis; BC, B. cereus; BM, B. mycoides; BP, B. pseudomycoides; BT, B. thuringiensis; BW, B. weihenstephanensis.
Strains that harbor the signature sequences.
Nucleotide positions based on the sequence coordinates of B. anthracis 7700 reported in Fig. 3.
The homoduplex fragments in the ITS homoduplex-heteroduplex polymorphism profiles were highlighted by subjecting the ITS amplified products to mung bean nuclease treatment, which eliminates single strands in heteroduplex products, permitting only homoduplex products to be detected in the gel (14). The mung bean nuclease reaction was performed as previously described (14), the resulting sample was electrophoresed in MDE gel, and the DNA bands were revealed by silver staining.
Cloning and sequencing of the 16S-23S rDNA ITS.
The homoduplex fragments separated in the MDE gel after mung bean nuclease treatment were excised from the gel (46), reamplified using the same forward (S-D-Bact-1494-a-S-20 on the 16S rDNA) and reverse (L-D-Bact-0035-a-A-15 on the 23S rDNA) primers (13) used for the generation of the original ITS homoduplex-heteroduplex polymorphism profiles, and sequenced in an ABI Prism 310 sequence analyzer (Applied Biosystems, Milan, Italy). Besides direct sequencing of the PCR product, the long ITS of B. anthracis 282 was sequenced after being cloned in the pMOS cloning kit (Amersham Pharmacia Biotech, Milan, Italy), as specified by the supplier. Cloned ITS were sequenced using the T7 and U19 primers on the vector. For all the strains, the sequences were confirmed by sequencing the DNA fragments obtained by amplifying the 5′- and 3′-end stretches of the long ITS from the genomic DNA. The primers targeting 16S rDNA (ITS-A-f, [5′-CCTTGTACACACCGCCCGT-3′]) and 23S rDNA (ITS-B-r [5′-GTGGGTTTCCCCATTCGG-3′]) were designed in a more internal position on the respective gene than were the original primers used to amplify ITS, and they were used in combination with two ITS internal primers (ITS-A-r [5′-AAAATAGCTTTTTGGTGGAG-3′]) and (ITS-B-f [5′-AAATTTGTATGGGCCTATAG-3′]), designed from tDNAAla and tDNAIle, respectively (see Fig. 2A). The short spacer of B. anthracis strain Cepanzo was sequenced after being cloned in the pMOS kit by the same procedure that was used for the long ITS of strain 282.
FIG. 2.
(A) Position of the tDNA-targeting primers designed for sequencing the long 16S-23S rDNA ITS of B. cereus group. S-D-Bact-1494-a-S-20 plus L-D-Bact-0035-a-A-15 (13) is the universal primer set initially used for ITS homoduplex-heteroduplex polymorphism analysis. ITS-A-f plus ITS-A-r and ITS-B-f plus ITS-B-r are primer sets used to amplify and sequence the 5′- and 3′-end regions, respectively, of the long ITS containing tRNA genes. (B) Schematic structure of the 16S-23S rDNA ITS types found in the B. cereus group. The genes encoding tRNA are indicated by open arrows. Conserved regions flanking 16S or 23S rDNA are indicated by bold bars. The other conserved regions are underlined and labeled i, ii, and iii. The broken lines are gaps for the alignment. (C) Sequence alignment of different types of 16S-23S rDNA ITS found in the B. cereus group. ITS B, long ITS of B. anthracis 7700; ITS C1, intermediate ITS of B. anthracis Davis TE702; ITS C2, intermediate ITS of B. mycoides G2; ITS A, short ITS of B. anthracis Cepanzo. Dots indicate nucleotides identical to those of ITS-B. Sequence gaps for the alignment are shown by hyphens. The genes encoding tRNA are indicated by a continuous black line. Conserved regions flanking 16S and 23S rDNA are shaded in grey.
Phylogenetic relationship in the B. cereus group based on sequences of ITS-containing tDNA.
The sequences of the long ITS, determined for 21 strains, were used for neighbor-joining analysis to assess the phylogenetic relationship between the species of the B. cereus group. The sequences were aligned and the alignment was checked manually. Similarity values and a neighbor-joining tree were determined using Jalview software (http://circinus.ebi.ac.uk:6543/jalview).
RESULTS AND DISCUSSION
ITS length variation in the B. cereus group.
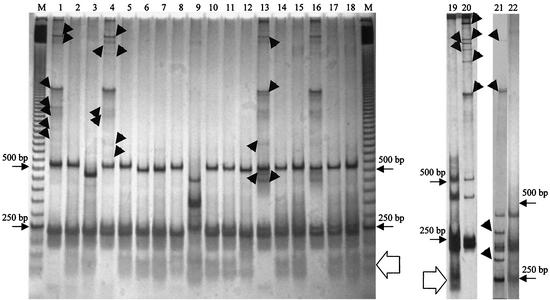
During the PCR for amplification of the ITS of strains of the B. cereus group, heteroduplex cross-hybridization products may be formed between the different ITS (14). The heteroduplex products are visualized in electrophoretic gels as discrete bands and may cause overestimation of the ITS size types in a given strain. To avoid this artifact and to obtain a realistic description of the ITS in a strain, we treated the PCR products with mung bean nuclease, which cleaves the unpaired DNA strands of the heteroduplex products, releasing the original homoduplex fragments and the digestion products. This resulted in two major ITS size classes: one of 220 to 250 bp (short ITS; size measurement including the 16S and 23S rDNA stretches obtained with amplification) and the other of 450 to 550 bp (long ITS). In Fig. 1, the ITS homoduplex-heteroduplex polymorphism profiles after mung bean nuclease treatment are illustrated for some strains of the B. cereus group. On comparing the profiles in Fig. 1, it can be seen that B. anthracis Davis TE702 and B. mycoides G2, besides the short and the long ITS, show additional fragments of 350 and 320 bp (intermediate ITS), respectively.
FIG. 1.
Identification of the homoduplex bands in the ITS homoduplex-heteroduplex polymorphism patterns separated in a MDE gel after mung bean nuclease digestion of the PCR products. Lanes M contain a 50-bp ladder. Normal ITS homoduplex-heteroduplex polymorphism profiles: lanes 1, 4, 13, 16, 20, and 21, B. weihenstephanensis 10204T, B. thuringiensis Ht39, B. anthracis 376, B. anthracis Cepanzo, B. anthracis Davis TE 702, and B. mycoides G2. ITS homoduplex-heteroduplex polymorphism profiles after treatment with mung bean nuclease: lanes 2 and 3, B. weihenstephanensis 10204T and B. pseudomycoides 617T; lanes 5 to 12, B. thuringiensis Ht39, B. thuringiensis 2046T, B. cereus V65SP, B. mycoides 2048T, B. mycoides G2, B. anthracis 256, B. anthracis 282, and B. cereus 31T; lanes 14 and 15, B. anthracis 376 and B. anthracis 779; lanes 17 and 18, B. anthracis Cepanzo and B. anthracis 663; lanes 19 and 22, B. anthracis Davis TE 702 and B. mycoides G2. The 250- and 500-bp bands of the ladder are indicated. Arrowheads indicate the heteroduplex products removed by the mung bean nuclease. The open arrows indicate degradation products of the heteroduplex bands.
ITS size classes and organization in the B. cereus group.
To compare the structure between the different types of ITS in the B. cereus group, differently sized ITS, i.e., short, intermediate, and long ITS, were studied. The sequences of the short ITS of B. anthracis Cepanzo, intermediate ITS of B. anthracis Davis TE702, intermediate ITS of B. mycoides G2, and long ITS of B. anthracis Cepanzo, determined in this study, were compared. Figure 2B depicts the schematic organization of the four ITS types found in the B. cereus group. The first type was a classical 144-bp ITS without tDNA genes, already reported for almost all the species of the B. cereus group (7, 21, 30). The second main ITS type was 359 to 371 bp long and contained two tRNA genes, tRNAIle and tRNAAla, as in B. subtilis (29). The third ITS type, typical of B. anthracis strain Davis TE702, was 286 bp long and contained single putative tRNAAla gene. The 5′-end stretch (19 bp) of the tRNAAla gene could be identified with the corresponding stretch of the tRNAIle gene (Fig. 2B). This hybrid sequence suggests that a recombination event, rather than a deletion, of the tRNAIle gene generated the single tDNA-containing ITS.
A fourth type of ITS, 242 bp long, was found in B. mycoides strain G2. In this ITS, no tRNA genes were found.
The nucleotide sequence of the end regions flanking both 16S and 23S rDNA was mostly identical in all of the spacers (Fig. 2C). The conserved regions at the 5′ and 3′ ends of the ITS were 67 to 68 and 51 to 56 bp long, respectively. Several other conserved DNA sequence regions were found in the noncoding regions flanked by tRNA genes (regions i, ii, and iii). Regions i (81 bp) and ii (24 bp) were identical in the different tDNA-containing ITS. Region iii (13 bp) was identical in the short ITS without tRNA genes and the intermediate ITS of strain G2.
The 5′-end (67-bp) and 3′-end (51-bp) regions of the intermediate ITS of strain G2 were homologous to the corresponding regions of the short and long ITS in the B. cereus group. The remaining sequence of 127 bp did not show significant homology to any sequence in the databases (data not shown).
Signature nucleotides for the B. cereus group species in ITS.
To evaluate the presence of species-specific signature nucleotides, the sequences of long ITS containing tRNA genes were determined for another 20 strains belonging to the six species of the B. cereus group. Seven polymorphic regions were found. Figure 3 shows the sequence alignment of the polymorphic regions of the long ITS of all the strains analyzed. Based on these polymorphic regions, Table 1 shows the signature nucleotides for the different species of the B. cereus group as deduced from Fig. 3. By referring to the sequence coordinates of long ITS of B. anthracis 7700 (Fig. 3), B. anthracis can be discriminated from the other species at positions 75 to 80 (AAAAAG), 121 (A), and 213 to 214 (AT). In particular, the AT positions appear useful discriminants, since they are located in the intergenic region between the two tRNA genes (Fig. 2); this region is absent in the short ITS.
FIG. 3.
Alignment of DNA traits showing sequence variability in the long 16S-23S rDNA ITS containing tRNA genes of 21 strains of the B. cereus group. BA, B. anthracis; BC, B. cereus; BM, B. mycoides; BP, B. pseudomycoides; BT, B. thuringiensis; BW, B. weihenstephanensis.
B. cereus and B. thuringiensis could not be discriminated from each other, but together they could be discriminated from all the other species at positions 75 to 76 (TC), 117 to 121 (CCTGC), and 213 to 220 (TTTTGG).
B. mycoides and B. weihenstephanensis could not be discriminated, confirming the close genotypic and phenotypic relationship already described for these two species (14, 30, 40, 50). B. mycoides and B. weihenstephanensis could be discriminated from all the other species at positions 117 to 121 (TCCGC) and 213 to 218 (TTTTAC) (Table 1). The only exception was strain G2, which showed nucleotide variations in these sites.
B. pseudomycoides could be discriminated from all the other species at positions 75 to 81 (AAATAT), 117 to 121 (TCTGC), and 213 to 214 (AC) (Table 1).
The signatures in Table 1 appear useful for designing species-specific probes or primers for the rapid identification of B. cereus group isolates (12).
Phylogenetic relationship in the B. cereus group based on sequences of ITS containing tDNA.
To evaluate the phylogenetic relationship between the species of the B. cereus group on the basis of the long ITS containing tDNA, a phylogenetic tree including all the sequences determined in this study was established by using Jalview software (Fig. 4). The phylogenetic relationship based on the long ITS resulted in the identification of four groups of strains, the first containing B. cereus and B. thuringiensis, the second containing B. anthracis, the third containing B. mycoides and B. weihenstephanensis, and the fourth containing B. pseudomycoides.
FIG. 4.
Phylogenetic relationship between strains of the species of the B. cereus group determined by neighbor-joining analysis of the sequences of the long 16S-23S rDNA ITS containing tRNA genes, as determined in this study. Symbols indicate the different species of the B. cereus group. Sequences without symbols are from B. subtilis strain 168. Bar, 2% of the phylogenetic distance.
B. anthracis was grouped in a branch separated from B. cereus and B. thuringiensis, indicating that B. anthracis, even though related, represents an independent phylogenetic lineage that diverges from B. cereus and B. thuringiensis. It is probable that the particular niche occupied by B. anthracis led to a divergence that also began to show differences at the intraspecies level. These differences can be observed in the ribosomal operon organization (35, 36, 39) or at the level of the ITS, as in strain Davis TE702 (14, 24, 35) or the strains isolated from the Pyrenees and Alps (37) during the 1994 and 1997 outbreaks (14). The few variations observed in B. anthracis by ITS homoduplex-heteroduplex polymorphism analysis (14) and sequencing of ITS containing tDNA mirror the whole-genome polymorphisms identified by amplified fragment length polymorphism (27, 28), long-range repetitive elements PCR (8), and, recently, whole-genome sequencing (42).
These data on ITS sequences confirm the observation that B. cereus and B. thuringiensis cannot be distinguished easily, apart from the entomocidal genotype and phenotype. The sequence similarity of ITS containing tDNA supports the proposal that B. cereus and B. thuringiensis are monophyletic and represent a single clade (14, 24, 34, 47). From the practical point of view, species separation can be useful in distinguishing B. thuringiensis from B. cereus, with the former generally being regarded as safe and being used throughout the world for the biological control of insect pests, while the latter is pathogenic. Although genes and proteins toxic to mammals have been found in B. thuringiensis (reference 20 and references therein), B. thuringiensis strains have been associated with human disease in only a very few cases (reference 20 and references therein; 25). It has been suggested that besides mammalian and insecticidal toxins, other genetic determinants, such as PlcR, a pleiotropic regulator of extracellular virulence, drive the pathogenic activity in B. cereus or activate entomocidal patterns in B. thuringiensis (1). These determinants could permit a genetic and functional distinction between these two clades in the B. cereus group (1).
The close relation of B. mycoides to B. weihenstephanensis was confirmed by the sequence of the tDNA-containing ITS. It has been proposed that the only characteristic that can distinguish these two species is colony morphology, which shows typical rhizoid growth in B. mycoides (30). This characteristic is ambiguous since B. mycoides strains lacking the rhizoid phenotype have been isolated from soils (52). On the basis of all the characteristics and genetic markers analyzed until now, the relatedness of these species appears very high, but DNA markers that clearly discriminate the two species still remain to be identified. (21, 30, 40, 50). Strain G2 harbors a relatively low sequence homology of 91.3% to B. pseudomycoides, the closest species. This confirms that wide genetic variability exists in the B. cereus group (14).
From the sequences of the ITS containing tDNA, it was confirmed that B. pseudomycoides is a clearly separate species from B. mycoides. Further studies need to be performed on B. pseudomycoides to evaluate its degree of activity in the soil and rhizosphere environment.
Summary.
This study reports, for the first time, sequences of ITS containing tDNA from strains of the six species of the B. cereus group. It is shown that these sequences are more informative for species discrimination that are those of short ITS, which were shown by Bourque et al. (7) to be highly conserved among the different species of the B. cereus group. The use of the long ITS containing tDNA allowed us to discriminate B. anthracis from the related clades B. cereus and B. thuringiensis. From these data, it is proposed that when inferring the genetic relationship between closely related clades on the basis of the sequences of the ITS, the use of ITS containing tDNA is more informative and appropriate than ITS without tDNA for the discrimination of clades of different ecotype.
ITS nucleotide sequence accession numbers.
The nucleotide sequences of the short and the tDNA-containing intermediate and long ITS analyzed in this study have been deposited in the EMBL nucleotide sequence database (GenBank/EMBL/DDBJ) under the following accession numbers (the corresponding strains are given in parentheses): B. anthracis long ITS: AJ420048 (7700), AJ420049 (663), AJ420050 (282) and AJ420051 (Davis TE702); B. anthracis intermediate ITS: AJ420069 (Davis TE702); B. anthracis short ITS: AJ420071 (Cepanzo); B. cereus long ITS: AJ420052 (31T), AJ420053 (345), AJ420054 (626), and AJ420055 (V65SP); B. thuringiensis long ITS: AJ420056 (2046T), AJ420057 (HD1), AJ420058 (Bt14), AJ420059 (BMG1.7), and AJ420060 (Ht39); B. weihenstephanensis long ITS: AJ420061 (10204T), AJ420062 (10202), and AJ420063 (10208); B. mycoides long ITS: AJ420064 (2048T), AJ420065 (299), AJ420066 (309), and AJ420067 (G2); B. mycoides intermediate ITS: AJ420070 (G2); B. pseudomycoides long ITS: AJ420068 (BD10).
Acknowledgments
Partial support came from the Italian Ministry for University and Scientific Research within the project “Risposta della comunità microbica del suolo a differenti pressioni antropiche: effetti su struttura, dinamica e diversità della microflora” (Cofin 2000) and from the INTAS-International Association for the promotion of cooperation with scientists from the New Independent States of the former Soviet Union within the project “An epidemiological study of outbreaks of B. anthracis in Georgia” (INTAS-01-0725). A.C. was supported by a grant from the Direction Generale de Recherche Scientifique et Technologique of the Ministere de l'Education Superieure of Tunisia.
We thank Michèle Mock, Guy Patra, Samir Jaoua, Hala Khyami-Horani, Lawrence K. Nakamura, Siegfried Scherer, Ralf Mayr, and Daniel R. Zeigler for kindly giving us Bacillus strains and/or DNA, and we acknowledge three anonymous reviewers for their helpful discussion and suggestions. The manuscript was edited by Barbara Carey.
REFERENCES
- 1.Agaisse, H., M. Gominet, O. A. Okstad, A.-B. Kolsto, and D. Lereclus. 1999. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol. Microbiol. 32:1043-1053. [DOI] [PubMed] [Google Scholar]
- 2.Andreoni, V., M. Moro Luischi, L. Cavalca, D. Erba, and S. Ciappellano. 2000. Selenite tolerance and accumulation in the Lactobacillus species. Ann. Microbiol. 50:77-88. [Google Scholar]
- 3.Ash, C., and M. D. Collins. 1992. Comparative analysis of 23S ribosomal RNA gene sequences of Bacillus anthracis and emetic Bacillus cereus determined by PCR-direct sequencing. FEMS Microbiol. Lett. 94:75-80. [DOI] [PubMed] [Google Scholar]
- 4.Ash, C., J. A. E. Farrow, M. Dorsch, E. Stackebrandt, and M. D. Collins. 1991. Comparative analysis of Bacillus anthracis, Bacillus cereus, and related species on the basis of reverse transcriptase sequencing of 16S rRNA. Int. J. Syst. Bacteriol. 41:343-346. [DOI] [PubMed] [Google Scholar]
- 5.Ash, C., J. A. E. Farrow, S. Wallbanks, and M. D. Collins. 1991. Phylogenetic heterogeneity of the genus Bacillus revealed by comparative analysis of small-subunit-ribosomal RNA sequences. Lett. Appl. Microbiol. 13:202-206. [Google Scholar]
- 6.Borin, S., D. Daffonchio, and C. Sorlini 1997. Single strand conformation polymorphism analysis of PCR-tDNA fingerprinting to address the identification of Bacillus species. FEMS Microbiol. Lett. 157:87-93. [DOI] [PubMed] [Google Scholar]
- 7.Bourque, S. N., J. R. Valéro, M. C. Lavoie, and R. C. Levesque. 1995. Comparative analysis of the 16S to 23S ribosomal intergenic spacer sequences of Bacillus thuringiensis strains and subspecies and of closely related species. Appl. Environ. Microbiol. 61:1623-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brumlik, M. J., U. Szymajda, D. Zakowska, X. Liang, R. J. Redkar, and V. G. Del Vecchio. 2001. Use of long-range repetitive element polymorphism-PCR to differentiate Bacillus anthracis strains. Appl. Environ. Microbiol. 67:3021-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casanova, A., J. C. Obreque, A. M. G. Sandino, and M. Jashés. 2001. tRNA genes were found in Piscirickettsia salmonis 16S-23S rDNA spacer region (ITS). FEMS Microbiol. Lett. 197:19-22. [DOI] [PubMed] [Google Scholar]
- 10.Condon, C., J. Philips, Z. Fu, C. Squires, and C. L. Squires. 1992. Comparison of the expression of seven ribosomal RNA operons in Escherichia coli. EMBO J. 11:4175-4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daffonchio, D., S. Borin, A. Consolandi, D. Mora, P. L. Manachini, and C. Sorlini. 1998. 16S-23S rRNA internal transcribed spacers as molecular markers for the species of the 16S rRNA group 1 of the genus Bacillus. FEMS Microbiol. Lett. 163:229-236. [DOI] [PubMed] [Google Scholar]
- 12.Daffonchio, D., S. Borin, A. Consolandi, and C. Sorlini. 1999. Restriction site insertion-PCR (RSI-PCR) for rapid discrimination and typing of closely related microbial strains. FEMS Microbiol. Lett. 180:77-83. [DOI] [PubMed] [Google Scholar]
- 13.Daffonchio, D., S. Borin, G. Frova, P. L. Manachini, and C. Sorlini. 1998. PCR fingerprinting of whole genomes, the spacers between the 16S and 23S rRNA genes and of intergenic tRNA gene regions reveals a different intraspecific genomic variability of Bacillus cereus and Bacillus licheniformis. Int. J. Syst. Bacteriol. 48:107-116. [DOI] [PubMed] [Google Scholar]
- 14.Daffonchio, D., A. Cherif, and S. Borin. 2000. Homoduplex and heteroduplex polymorphisms of the amplified ribosomal 16S-23S internal transcribed spacers describe genetic relationships in the “Bacillus cereus group.” Appl. Environ. Microbiol. 66:5460-5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Lamballerie, X., C. Zandotti, C. Vignoli, C. Bollet, and P. De Micco. 1992. A one-step microbial DNA extraction method using “Chelex 100” suitable for gene amplification. Res. Microbiol. 142:793-796. [DOI] [PubMed] [Google Scholar]
- 16.Drobniewski, F. A. 1993. Bacillus cereus and related species. Clin. Microbiol. Rev. 6:324-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gürtler, V. 1999. The role of recombination and mutation in 16S-23S rDNA spacer rearrangement. Gene 238:241-252. [DOI] [PubMed] [Google Scholar]
- 18.Gürtler, V., Y. Rao, S. R. Pearson, S. M. Bates, and B. C. Mayall. 1999. DNA sequence heterogeneicity in three copies of the long 16S-23S rDNA spacer of Enterococcus faecalis isolates. Microbiology 145:1785-1796. [DOI] [PubMed] [Google Scholar]
- 19.Gürtler, V., and V. A. Stanisich. 1996. New approaches to typing and identification of bacteria using the 16S-23S rDNA spacer region. Microbiology 142:3-16. [DOI] [PubMed] [Google Scholar]
- 20.Hansen, B. M., and N. B. Hendriksen. 2001. Detection of enterotoxic Bacillus cereus and Bacillus thuringiensis strains by PCR analysis. Appl. Environ. Microbiol. 67:185-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrell, L. J., G. L. Andersen, and K. H. Wilson. 1995. Genetic variability of Bacillus anthracis and related species. J. Clin. Microbiol. 33:1847-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helgason, E., D. A. Caugant, M. M. Lecadet, Y. Chen, J. Mahillon, A. Lövgren, I. Hegna, K. Kvaloy, and A.-B. Kolstø. 1998. Genetic diversity of Bacillus cereus/Bacillus thuringiensis isolates from natural sources. Curr. Microbiol. 37:80-87. [DOI] [PubMed] [Google Scholar]
- 23.Helgason, E., D. A. Caugant, I. Olsen, and A.-B. Kolstø. 2000. Genetic structure of population of Bacillus cereus and B. thuringiensis isolates associated with periodontitis and other human infections. J. Clin. Microbiol. 38:1615-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helgason, E., O. A. Økstad, D. A. Caugant, H. A. Johansen, A. Fouet, M. Mock, I. Hegna, and A.-B. Kolstø. 2000. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis — one species on the basis of genetic evidence. Appl. Environ. Microbiol. 66:2627-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson, S. G., R. B. Goodbrand, R. Ahmed, and S. Kasatiya. 1995. Bacillus cereus and Bacillus thuringiensis isolated in a gastroenteritis outbreak investigation. Lett. Appl. Microbiol. 21:103-105. [DOI] [PubMed] [Google Scholar]
- 26.Johansen, T., C. R. Carlson, and A.-B. Kolstø. 1996. Variable numbers of rRNA operons in Bacillus cereus strains. FEMS Microbiol. Lett. 136:325-328. [DOI] [PubMed] [Google Scholar]
- 27.Keim, P., A. Kalif, J. Schupp, K. Hill, S. E. Travis, K. Richmond, D. M. Adair, M. Hugh-Jones, C. R. Kuske, and P. Jackson. 1997. Molecular evolution and diversity in Bacillus anthracis as detected by amplified fragment length polymorphism markers. J. Bacteriol. 179:818-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keim, P., L. B. Price, A. M. Klevytska, K. L. Smith, J. M. Schupp, R. Okinaka, P. J. Jackson, and M. E. Hugh-Jones. 2000. Multiple-locus variable-number tandem repeat analysis reveals genetic relationships within Bacillus anthracis. J. Bacteriol. 182:2928-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, and A. Danchin. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 30.Lechner, S., R. Mayr, K. P. Francis, B. M. Pruss, T. Kaplan, E. Wiessner-Gunkel, G. S. Stewart, and S. Scherer. 1998. Bacillus weihenstephanensis sp. nov. is a new psychrotolerant species of the Bacillus cereus group. Int. J. Syst. Bacteriol. 48:1373-1382. [DOI] [PubMed] [Google Scholar]
- 31.Maeda, T., N. Takada, M. Furushita, and T. Shiba. 2000. Structural variation in the 16S-23S rRNA intergenic spacers of Vibrio parahaemolyticus. FEMS Microbiol. Lett. 192:73-77. [DOI] [PubMed] [Google Scholar]
- 32.Mayr, R., I. Eppert, and S. Scherer. 1999. Incidence and identification of psychrotrophic (7°C-tolerant) Bacillus spp. in German HTST pasteurized milk. Milchwissenschaft 54:26-30. [Google Scholar]
- 33.Mignot, T., B. Denis, E. Couture-Tosi, A.-B. Kolste, M. Mock, and A. Fouet. 2001. Distribution of S-layers on the surface of Bacillus cereus strains: phylogenetic origin and ecological pressure. Environ. Microbiol. 3:493-501. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura, L. K. 1994. DNA relatedness among Bacillus thuringiensis serovars. Int. J. Syst. Bacteriol. 44:125-129. [DOI] [PubMed] [Google Scholar]
- 35.Patra, G., A. Fouet, J. Vaissaire, J.-L. Guesdon, and M. Mock. 2002. Variation in rRNA operon number as revealed by ribotyping of Bacillus anthracis strains. Res. Microbiol. 153:139-148. [DOI] [PubMed] [Google Scholar]
- 36.Patra, G., P. Sylvestre, V. Ramisse, J. Therasse, and J.-L. Guesdon. 1995. DNA fingerprinting of Bacillus anthracis strains, p. 59. In P. C. B. Turnbull (ed.), Salisbury medical bulletin. Salisbury Medical Society, Salisbury, United Kingdom.
- 37.Patra, G., J. Vaissaire, M. Weber-Levy, C. Le Doujet, and M. Mock. 1998. Molecular characterization of Bacillus strains involved in outbreaks of anthrax in France in 1997. J. Clin. Microbiol. 36:3412-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen, D. J., M. Shishido, F. B. Holl, and C. P. Chanway. 1995. Use of species- and strain-specific PCR primers for identification of conifer root-associated Bacillus spp. FEMS Microbiol. Lett. 133:71-76. [DOI] [PubMed] [Google Scholar]
- 39.Priest, F. G., D. A. Kaji, Y. B. Rosato, and V. P. Canhos. 1994. Characterization of Bacillus thuringiensis and related bacteria by ribosomal RNA gene restriction fragment polymorphisms. Microbiology 140:1015-1022. [DOI] [PubMed] [Google Scholar]
- 40.Pruss, B. M., K. P. Francis, F. von Stetten, and S. Scherer. 1999. Correlation of 16S ribosomal DNA signature sequences with temperature-dependent growth rates of mesophyllic and psychrotolerant strains of the Bacillus cereus group. J. Bacteriol. 181:2624-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rainey, F. A., N. L. Ward-Rainey, P. H. Janssen, H. Hippe, and E. Stackebrandt. 1996. Clostridium paradoxum DSM 7308T contains multiple 16S rRNA genes with heterogeneous intervening sequences. Microbiology 142:2087-2095. [DOI] [PubMed] [Google Scholar]
- 42.Read, T. D., S. L. Salzberg, M. Pop, M. Shumway, L. Umayam, L. Jiang, E. Holtzapple, J. D. Busch, K. L. Smith, J. M. Schupp, D. Solomon, P. Keim, and C. M. Fraser. 2002. Comparative genome sequencing for discovery of novel polymorphisms in Bacillus anthracis. Science 296:2028-2033. [DOI] [PubMed] [Google Scholar]
- 43.Rocap, G., D. L. Distel, J. B. Waterbury, and S. W. Chisholm. 2002. Resolution of Prochlorococcus and Synecococcus ecotypes by using 16S-23S ribosomal DNA internal transcribed spacer sequences. Appl. Environ. Microbiol. 68:1180-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwieger, F., and C. Tebbe. 1998. A new approach to utilise PCR-single-strand conformation polymorphism for 16S rRNA gene-based microbial community analysis. Appl. Environ. Microbiol. 64:4870-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ticknor, L. O., A.-B. Kolstø, K. K. Hill, P. Keim, M. T. Laker, M. Tonks, and P. J. Jackson. 2001. Fluorescent amplified fragment length polymorphism analysis of Norwegian Bacillus cereus and Bacillus thuringiensis soil isolates. Appl. Environ. Microbiol. 67:4863-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turnbull, P. C., R. A. Hutson, M. J. Ward, M. N. Jones, C. P. Quinn, N. J. Finnie, C. J. Duggleby, J. M. Kramer, and J. Melling. 1992. Bacillus anthracis but not always anthrax. J. Appl. Bacteriol. 72:21-28. [DOI] [PubMed] [Google Scholar]
- 49.Vold, B. S. 1985. Structure and organization of genes for transfer ribonucleic acid in Bacillus subtilis. Microbiol. Rev. 49:71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Stetten, F., K. P. Francis, S. Lechner, K. Neuhaus, and S. Scherer. 1998. Rapid discrimination of psychrotolerant and mesophilic strains of the Bacillus cereus group by PCR targeting of 16S rDNA. J. Microbiol. Methods 34:99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Stetten, F., R. Mayr, and S. Scherer. 1999. Climatic influence on mesophilic Bacillus cereus and psychrotolerant Bacillus weihenstephanensis populations in tropical, temperate and alpine soil. Environ. Microbiol. 1:503-515. [DOI] [PubMed] [Google Scholar]
- 52.von Witzingerode, F., F. A. Rainey, R. M. Kroppenstedt, and E. Stackebrandt. 1997. Identification of environmental strains of Bacillus mycoides by fatty acid analysis and species-specific 16S rDNA oligonucleotide probe. FEMS Microbiol. Ecol. 24:201-209. [Google Scholar]