Abstract
Trisodium phosphate (TSP) is now widely used during the processing of poultry and red meats, but the mechanism whereby it inactivates gram-negative bacteria such Salmonella spp. remains unclear. Thus, Salmonella enterica serovar Enteritidis (ATCC 4931) cells were treated with different concentrations of TSP (1.5, 2.0, and 2.5% [wt/vol]) and compared with (i) cells treated with the same pH as the TSP treatments (pH 10.0, 10.5, and 11.0, respectively) and (ii) cells treated with different concentrations of TSP (1.5, 2.0, and 2.5% [wt/vol]) adjusted to a pH of 7.0 ± 0.2 (mean ± standard deviation). Cell viability, loss of membrane integrity, cellular leakage, release of lipopolysaccharides, and cell morphology were accordingly examined and quantified under the above treatment conditions. Exposure of serovar Enteritidis cells to TSP or equivalent alkaline pH resulted in the loss of cell viability and membrane integrity in a TSP concentration- or alkaline pH-dependent manner. In contrast, cells treated with different concentrations of TSP whose pH was adjusted to 7.0 did not show any loss of cell viability or membrane integrity. A 30-min pretreatment with 1.0 mM EDTA significantly enhanced the loss of membrane integrity only when followed by TSP or alkaline pH treatments. Measuring the absorbance at 260 nm, agarose gel electrophoresis, Bradford assay, and Tricine-sodium dodecyl sulfate gel electrophoresis of filtrates of treated cell suspensions revealed considerable release of DNA, proteins, and lipopolysaccharides compared to controls and pH 7.0 TSP treatments. Electron microscopic examination of TSP- or alkaline pH-treated cells showed disfigured cell surface topology and wrinkled appearance and showed evidence of a TSP concentration- and pH-dependent disruption of the cytoplasmic and outer membranes. These results demonstrate that TSP treatment permeabilizes and disrupts the cytoplasmic and outer membranes of serovar Enteritidis cells because of the alkaline pH, which in turn leads to release of intracellular contents and eventual cell death.
Salmonella species continue to be commonly associated with cases of food-borne disease in developed countries. In the United States in 2001, the incidence per 100,000 people was highest for salmonellosis (15.1), followed by campylobacteriosis (13.8) and shigellosis (6.4) (4). Enteric pathogens usually contaminate the surface of raw animal products during slaughter and primary processing (scalding, defeathering or dehiding, rinsing, cutting, mixing, grinding, etc.) and can attach and/or reside in the regular and irregular surfaces of the skin, multiply and, thereafter, contaminate food preparation surfaces, hands, and utensils. Food spoilage and illness can result from this carryover of bacteria if these contaminated products are undercooked (5) or handled improperly. Due to the wide range of potential handling abuses, it is highly desirable to significantly reduce the numbers of pathogenic bacteria attached to the surfaces of these products.
An array of methods for reducing the load of potential pathogens on the surfaces of meat products has been developed. These methods use ionizing radiation (11), organic acid sprays (8, 35), and phosphate (e.g., trisodium phosphate [TSP] and polyphosphates) dips or sprays (8) to reduce the numbers of bacterial pathogens present on raw animal products following processing. TSP is generally recognized as safe by the Food and Drug Administration and has been approved by the U.S. Department of Agriculture for use as a food ingredient (12) and for the reduction of Salmonella contamination during poultry processing (13). The process involves immersing postchill whole birds for 15 s in a 10% solution of Av-Gard TSP, allowing the excess TSP solution to drip from the bird, presumably leaving minimal amounts of phosphate on the carcass (30). Taste tests showed that TSP treatment at these relatively high concentrations had no effect on flavor, texture, or appearance of treated poultry (3, 17). At 8 to 15% (wt/vol), TSP has been demonstrated to reduce the number of artificially inoculated gram-negative pathogens surviving on surfaces of foodstuffs (9, 31, 38, 40). Log reductions in viable counts of 1.4 and 0.9 were obtained for Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium, respectively, on beef adipose tissue, although significantly lower reductions (0.9 and 0.5, respectively) were observed on fascia (19). On chicken carcasses, Slavik et al. (34) and Kim et al. (20) showed that TSP treatment at 10 or 50°C reduced Salmonella counts by 1.6 to 1.8 log units per carcass, and in a scanning electron microscopic study Kim and Slavik (18) showed TSP effectively removed attached Salmonella organisms from chicken skin.
Very little scientific evidence is available which identifies the actual mechanisms of TSP antimicrobial activity. Possible modes of action of TSP include (i) exposing microorganisms to high pH, which might particularly affect cell membrane components (27); (ii) enhancing detachment of bacteria from food surfaces by sequestration of metal ions (22); and (iii) removing fat from the skin surface, thereby allowing bacteria to be washed from the food surfaces more effectively (1, 14, 18). To date, studies involving TSP have mainly focused on evaluating the efficiency of Salmonella removal by the process (6, 8, 40-42), whereas there is no published information that documents possible mechanisms of TSP antimicrobial action. We were interested in the role of the high pH generated by dissolved TSP, which during TSP treatment of Salmonella enterica serovar Enteritidis could be responsible for lethal action. This hypothesis was examined using a series of comparative studies involving treatment solutions containing different concentrations of TSP, treatment solutions adjusted to the equivalent pH as in each of the TSP treatments, and TSP solutions pH adjusted to 7.0. Direct and indirect indices of cell survival, membrane damage, and cellular leakage were also employed to examine specific antimicrobial effects.
MATERIALS AND METHODS
Bacteria and culture conditions.
S. enterica serovar Enteritidis (ATCC 4931) was obtained from the American Type Culture Collection (Rockville, Md.). The stock culture was grown on tryptic soy agar (TSA; Sigma Chemical Co., St. Louis, Mo.) at 37°C for 18 h and maintained for a maximum period of 1 month at 4°C, after which a new stock culture was prepared from the frozen stock. A loopful of this stock culture was used to prepare the inoculum.
Preparation of cell suspensions for antimicrobial studies.
The inoculum culture was prepared by transferring a loopful of colony material from TSA plates and incubating the resulting liquid culture on a gyratory shaker (100 rpm) at 37°C for 24 h. Cells in the stationary phase of growth were prepared by transferring 1.0 ml of this batch culture into 100 ml of fresh tryptic soy broth (TSB; Sigma Chemical Co.) and incubating this culture for 16 h under the same conditions.
Exposure of cells to TSP and high pH.
Stationary-phase cells were harvested by centrifugation at 12,000 × g for 10 min at 4°C. The pellet was then washed once with phosphate-buffered saline (PBS) (pH 7.0). Washed cells were harvested again by centrifugation and resuspended in 100 ml of treatment solution at room temperature (RT; 25 ± 0.2°C) containing either (i) TSB (control), (ii) TSB with 1.5, 2.0, or 2.5% (wt/vol) TSP, (iii) 1.5, 2.0, or 2.5% (wt/vol) TSP (in a TSB solution) that was pH adjusted to 7.0 ± 0.2 with 6 N HCl, or (iv) TSB that was pH adjusted to 10.0 ± 0.2, 10.5 ± 0.2, or 11.0 ± 0.2 with NaOH (these solutions correspond to the pH values of 1.5, 2.0, and 2.5% TSP treatment solutions above, respectively).
Cell viability.
One-milliliter aliquots of each treated cell suspension were removed at predetermined time intervals (0, 5, 10, 20, 30, 40, 50, and 60 min) and serially diluted in 0.1% peptone water. Appropriate dilutions were then plated on TSA. The plates were counted for CFU following incubation at 37°C for 24 h and plotted against time (survival curves). The decimal reduction time (D value), defined as the time required to destroy 90% of the organisms, was calculated from these survival curves. All cell viability experiments were repeated three separate times.
PI uptake.
Washed stationary-phase cells resuspended in different treatment solutions (as described above) were incubated in 250-ml Erlenmeyer flasks on a gyratory shaker (100 rpm) at RT for 30 min. Propidium iodide (PI; Sigma Chemical Co.) was then added to each flask (final concentration of 5.0 μM) and incubated for an additional 30 min under the same conditions. Following incubation with PI, cells were centrifuged and washed twice with PBS. Washed cells were diluted to an optical density at 680 nm (OD680) of 0.5 ± 0.2 with PBS. The fluorescence of the resultant cell suspensions was then measured with a fluorometer (G. K. Turner Associates, Palo Alto, Calif.); the excitation wavelength was set at 495 nm, and the emission wavelength was set at >595 nm. Fluorescence values obtained from untreated control cells were subtracted from all treatment values.
Experiments were also performed to delineate the effects of TSP on the outer membrane; cells were pretreated with EDTA, which removes stabilizing divalent cations from the outer membrane by chelation, before exposure to TSP. Washed stationary-phase cells were resuspended in 100 ml of 0.1 M Tris-HCl (pH 7.5) containing 1.0 mM EDTA and incubated on a gyratory shaker (100 rpm) at RT for 30 min. After incubation, cells were centrifuged and washed once with PBS. Washed cells were resuspended in treatment solutions containing either (i) TSB (control), (ii) TSB with 1.5, 2.0, or 2.5% TSP, (iii) 1.5, 2.0, or 2.5% (wt/vol) TSP (in a TSB solution) that was pH adjusted to 7.0 ± 0.2 with 6 N HCl, or (iv) TSB that was pH adjusted to 10.0 ± 0.2, 10.5 ± 0.2, or 11.0 ± 0.2 with NaOH and then measured for PI uptake as described above.
To isolate the effect of Mg2+ on the outer membrane of cells pretreated with EDTA, washed stationary-phase cells were resuspended in 100 ml of 0.1 M Tris-HCl (pH 7.5) containing different concentrations of MgCl2 (0.5, 2.5, 5.0, 7.5, and 10.0 mM) along with 1.0 mM EDTA. The resuspended cells were incubated on a gyratory shaker (100 rpm) at RT for 30 min before exposure to 2.0% TSP for 1 h and then measured for PI uptake, as described above. Simultaneously, washed stationary-phase cells were incubated on a gyratory shaker (100 rpm) at RT for 1 h in 100 ml of TSB containing 2.0% TSP along with different concentrations of MgCl2 (5.0, 10.0, 15.0, 20.0, and 25.0 mM) and measured for PI uptake.
Measurement of osmotic response.
Approximately 1.0 ml of cells was exposed for 1 h to either (i) 1.5, 2.0, or 2.5% TSP (in a TSB solution), (ii) 1.5, 2.0, or 2.5% TSP (in a TSB solution) that was pH adjusted to 7.0 ± 0.2 with 6 N HCl, or (iii) TSB that was pH adjusted to 10.0 ± 0.2, 10.5 ± 0.2, or 11.0 ± 0.2 with NaOH. The cells from each treatment were then centrifuged and washed once with PBS. Washed cells were resuspended in 1.0 ml of PBS, after which 100 μl of this suspension was added in triplicate to (i) 1.0 ml of PBS and (ii) 1.0 ml of PBS containing 0.75 M NaCl. The OD680 of these suspensions was measured 4 min after mixing by using a Spectronic 601 spectrophotometer (Milton Roy, N.Y.). The increase in OD680 was calculated by subtracting the mean value of the three measurements in PBS from the mean value of the three measurements in PBS containing 0.75 M NaCl. These OD increases were expressed as a percentage of the mean value obtained with PBS alone.
Measurement of cellular leakage.
Leakage of cytoplasmic contents was determined by measuring the absorbance at 260 nm (Spectronic 601) of the cell filtrates following various treatments. Washed stationary-phase cells were resuspended in 100 ml of sterile distilled water containing different concentrations of TSP (0 [control], 0.5, 1.0, 1.5, 2.0, and 2.5%) and distilled water containing different concentrations of TSP (0.5, 1.0, 1.5, 2.0, and 2.5%) with pH adjusted to 7.0 and incubated on a gyratory shaker at RT for 1 h. The pHs of the treatment solutions containing 0, 0.5, 1.0, 1.5, 2.0, or 2.5% TSP were 7.2, 12.10, 12.28, 12.35, 12.40, and 12.44, respectively. The treatment solutions were not prepared in TSB as in previous studies, as the absorbance of TSB at 260 nm was unreadable by the spectrophotometer. After incubation, 3.0 ml of each treated cell suspension was filtered through a 25-mm-diameter, 0.2-μm-pore-size Nalgene syringe filter (VWR CanLab, Mississauga, Ontario, Canada). The filtrates were then examined for the presence of nucleic acids by measuring the absorbance at 260 nm (A260) and also running 1.0-ml aliquots of the phenol-chloroform-isoamyl alcohol (25:24:1 [vol/vol]; Life Technologies, Grand Island, N.Y.) concentrated supernatants on a 0.75% agarose gel. The Bradford assay (2) was also performed on the filtrates to quantify release of proteins by the various treatments. The possible release of outer membrane components like lipopolysaccharides (LPS) following various treatments was examined as described by Vaara (39).
Electron microscopy.
Cells exposed to TSP or alkaline pH were harvested by centrifugation, washed twice in 1× PBS, and pelleted by centrifugation at 12,000 × g for 10 min. Pelleted cells were resuspended in fresh fixative containing 3.0% glutaraldehyde in 0.1 M sodium cacodylate buffer (SCB). The cells were pelleted by centrifugation, washed three times with 0.1 M SCB, and fixed with 1.0% osmium tetroxide (OsO4) in 0.1 M SCB for 1 h at RT. The cells were then stained en bloc in saturated uranyl acetate and dehydrated in a gradient series of ethanol (50, 70, 95, and 100% [vol/vol]) for 5.0 min per immersion. The cells were then washed three times for 5 min each with propylene oxide and perfused with Epon-Ardite. Preparations were thin sectioned and viewed using a Phillips 410 LS electron microscope. All chemicals used in the preparation of specimens for electron microscopy were purchased from Electron Microscopy Supply (Fort Washington, Pa.).
RESULTS AND DISCUSSION
Effect of TSP on cell viability.
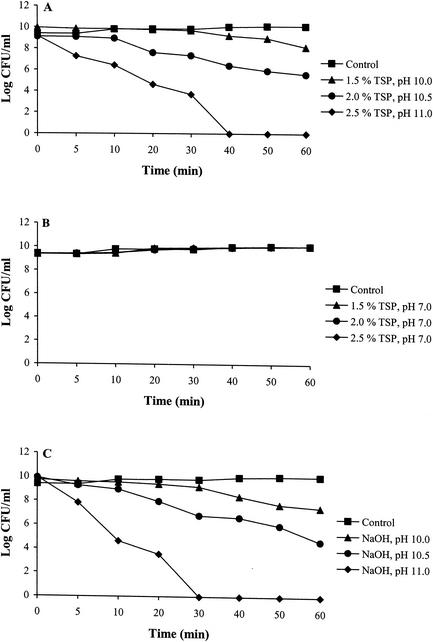
The results of this study showed that TSP reduces the viability of serovar Enteritidis cells in a concentration-dependent manner (Fig. 1A). This effect was lost, however, if cells were exposed to TSB solutions containing 1.5, 2.0, or 2.5% (wt/vol) TSP adjusted to pH 7.0 (Fig. 1B). Serovar Enteritidis cells were only slightly inhibited when treated with 1.5% TSP (D value, 36.90 min) or equivalent alkaline pH (10.0) TSB (D value, 25.12 min) solutions for 1 h (Fig. 1A and C). However, increasing the concentration of TSP in the treatment solution from 1.5 to 2.5% resulted in a rapid loss of viability (D values for 1.5, 2.0, and 2.5% TSP were 36.90, 15.17, and 6.27 min, respectively), with no detectable survivors after 1 h. These decreases in survival were similar to those observed when cells were exposed to high pH TSB solutions (Fig. 1C). Cells of serovar Enteritidis better survived treatment with 1.5% TSP, or equivalent alkaline pH (pH 10.0) TSB solutions, and showed only a 2- or 3-log reduction in CFU, respectively, after a 1-h exposure (Fig. 1A and C) compared to a 5- or 6-log reduction in CFU within 20 min of exposure to 2.5% TSP or equivalent alkaline pH (pH 11.0), respectively. It is noteworthy that when the pH of the various TSP treatment solutions was adjusted to 7.0, no loss in bacterial survival was observed (Fig. 1B), demonstrating that TSP had no effect on cell viability at pH 7.0. Thus, the effectiveness of TSP appeared to directly correlate with the alkaline pH of TSP solutions.
FIG. 1.
Effect of different concentrations of TSP (A), TSP with pH adjusted to 7.0 (B), or alkaline pH (C) on the viability of S. enterica serovar Enteritidis ATCC 4931 cells over time. Following treatment, cell suspensions were diluted and plated on TSA, and CFU were enumerated after incubation at 37°C for 24 h. Log CFU were plotted against time. Data from each treatment are the mean of three separate replications. Standard deviations were in the range of 10 to 15% of the given values.
PI uptake.
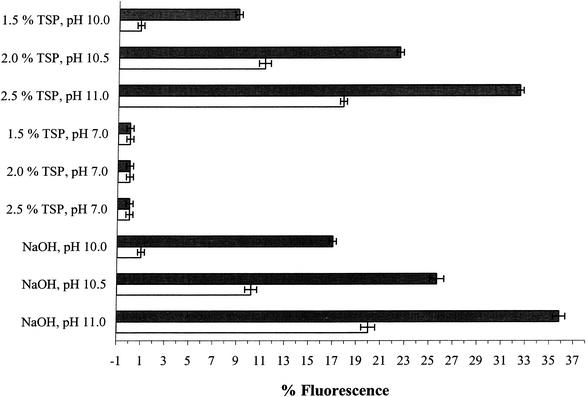
When serovar Enteritidis cells were exposed to increasing concentrations of TSP, an increase in the uptake of the membrane-impermeant fluorescent dye PI was seen, suggesting that TSP treatment permeabilized both the outer and cytoplasmic membrane (Fig. 2). However, when cells were exposed to treatment solutions containing different concentrations of TSP where the pH had been adjusted to 7.0, the PI uptake by cells was similar to that of the control (Fig. 2).
FIG. 2.
PI uptake of S. enterica serovar Enteritidis ATCC 4931 cells exposed to different concentrations of TSP, TSP with pH adjusted to 7.0, or alkaline pH for 1 h following pretreatment with (solid bars) or without (open bars) 1.0 mM EDTA in 0.1 M Tris-HCl for 30 min. The percent fluorescence value for each treatment is the mean of three separate replications. The fluorescence value obtained for untreated control cells was subtracted from all experimental values. Error bars represent ± 1 standard deviation.
Gram-negative bacteria have previously been shown to be more susceptible to high pH because of their very thin (2 to 3 nm) peptidoglycan layer (28). The peptidoglycan layer stabilizes the cytoplasmic membrane of intact bacterial cells against turgor pressure (7). The peptidoglycan layer of gram-negative bacteria, once weakened by high pH, may be less capable of preventing the cytoplasmic membrane from bursting, as shown by Mendonca et al. (27) for serovar Enteritidis and E. coli cells suspended in NaHCO3-NaOH buffer solution at pH 10.0.
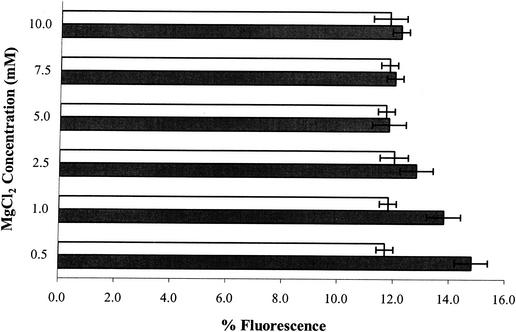
To understand the effect of TSP or equivalent alkaline pH on the outer membrane of serovar Enteritidis cells, stationary-phase cells were pretreated with EDTA to sequester stabilizing divalent cations from the outer membrane. EDTA pretreatment of serovar Enteritidis cells significantly (P < 0.05) enhanced PI uptake following TSP or equivalent alkaline pH exposure, compared to the PI uptake of cells treated similarly without EDTA pretreatment (Fig. 2). EDTA is known to have a significant effect on the outer membrane permeability of gram-negative bacteria (23) and removes, by chelation, stabilizing divalent cations like Mg2+ and to a lesser extent Ca2+ from their binding sites in LPS. As a consequence of EDTA treatment, the outer membrane becomes more permeable to agents that otherwise would not cross the cell membrane (15, 23, 24, 29). Short-term EDTA treatments of bacteria have been used to introduce macromolecules and hydrophobic compounds through the outer membrane without affecting cell viability (16, 23, 33, 36). In this study, short-term (30-min) treatment with EDTA alone did not affect cell viability or induce PI uptake (data not shown). The addition of MgCl2 during pretreatment of cells with EDTA before exposure to 2.0% TSP inhibited the PI uptake-enhancing activity seen in EDTA-pretreated cells, resulting in fluorescence levels similar to those seen in cells exposed to 2.0% TSP without any pretreatment (Fig. 3). This effect is likely the consequence of the chelation of excess Mg2+ with EDTA, thus making EDTA unavailable for reaction with Mg2+ in the outer membrane. Pretreatment of serovar Enteritidis cells with MgCl2 at 0.5 to 10.0 mM (in 0.1 M Tris-HCl, pH 7.5) before exposure to 2.0% TSP did not have any effect on the PI uptake (data not shown). However, the addition of MgCl2 along with 2.0% TSP in the treatment solution resulted in a reduction in PI uptake compared to that in cells exposed to 2.0% TSP alone. This reduction in PI uptake was observed at a 10 mM MgCl2 concentration, and at concentrations of 25 mM MgCl2 PI uptake was completely inhibited, possibly due to direct complex formation between TSP and MgCl2 making TSP unavailable to act on the outer membrane. Alternatively, MgCl2 stabilizes the outer membrane of the cells, making it resistant to the effects of TSP. The former explanation seems most likely, as pretreatment with MgCl2, unlike EDTA, had no effect on the uptake of PI (data not shown) following TSP exposure (i.e., TSP resulted in an increase in PI uptake with or without MgCl2 pretreatment). These observations suggest that TSP, like EDTA, forms chelates with divalent cations in the outer membrane, leading to increased permeability of the outer membrane.
FIG. 3.
PI uptake by S. enterica serovar Enteritidis ATCC 4931 cells pretreated with different concentrations of MgCl2 (open bars) or MgCl2 in combination with 1.0 mM EDTA (solid bars) for 30 min prior to 1-h treatment with 2.0% TSP. The percent fluorescence value for each treatment is the mean of three separate replications. The fluorescence value obtained for untreated control cells was subtracted from all experimental values. Error bars represent ± 1 standard deviation.
Cells exposed to either 2.5% TSP or equivalent alkaline pH (11.0) TSB solution rapidly lost viability and accordingly exhibited the greatest PI uptake (Fig. 2). This suggested that the cells had sustained membrane damage and become permeable to the membrane-impermeant dye PI. In contrast, cells exposed to either 1.5% TSP or equivalent alkaline pH (10.0) TSB solution showed very little loss of viability and the least uptake of PI.
Osmotic response.
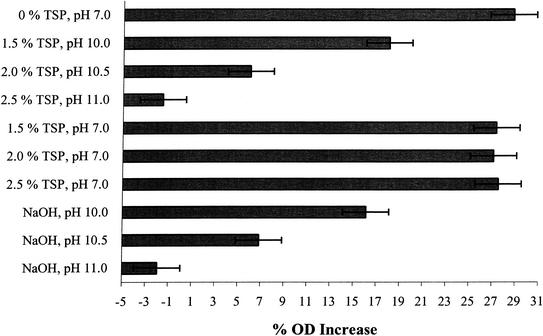
When cell suspensions are placed in hypertonic salt solutions, an increase in OD results as a consequence of light scattering and has been shown to be related to the extent of plasmolysis (26). This ability to plasmolyse in hypertonic salt solutions is referred to as the osmotic response. In cold-shocked Klebsiella aerogenes cells, a good correlation between this loss of osmotic response and cell death was found to exist (37). Korber et al. (21) developed a microscopic method for the direct measurement of plasmolysis in single cells, demonstrating that loss of osmotic response (i.e., loss of membrane integrity) correlated with the death of individual cells. During the present study, serovar Enteritidis cells exposed to increasing TSP concentrations lost their ability to plasmolyse in 0.75 M NaCl in a TSP concentration-dependent manner (Fig. 4), suggesting that the cells had sustained membrane damage. A similar response was observed when cells were exposed to high pH. Results from PI uptake and osmotic response studies confirmed that a negative relationship existed between cell viability and membrane damage when cells were exposed to TSP or equivalent alkaline pH TSB solutions.
FIG. 4.
Osmotic response of S. enterica serovar Enteritidis ATCC 4931 cells following 1 h of treatment with different concentrations of TSP, TSP with pH adjusted to 7.0, or alkaline pH. The OD680 of cell suspensions was measured in both PBS and PBS containing 0.75 M NaCl. The osmotic response of treated cells was measured as the increase in OD680 of the cell suspension placed in 0.75 M NaCl divided by the OD680 in PBS. The OD680 increase was then expressed as a percentage of the value obtained with PBS alone. The percent OD increase for each treatment is the mean of three separate experiments. Error bars represent ± 1 standard deviation.
Leakage of cellular contents.
While the A260 of filtrates of cells exposed to TSP was significantly (P < 0.05) higher than that for untreated control values, no significant (P < 0.05) difference in the A260 was observed in filtrates of cells exposed to different concentrations of TSP (Table 1). Thus, increasing the time of exposure to different concentrations of TSP did not have any significant effect on A260 values. The presence of nucleic acids in the cell-free filtrates of TSP- or alkaline pH-treated cells was confirmed by agarose gel electrophoresis, whereas none could be detected in untreated control and pH 7.0-adjusted TSP treatments (data not shown). Bradford assays performed on filtrates of cells exposed to TSP also revealed the presence of a very high concentration of protein; no protein was detected in filtrates of untreated control (Table 1) and pH 7.0 TSP-treated cell suspensions (data not shown). Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of cell-free supernatants of TSP- or alkaline pH-treated serovar Enteritidis cells revealed a prominent ladder pattern characteristic of smooth-type LPS, indicating that these treatments facilitated the release of LPS from the outer membrane (data not shown). Very little LPS was present in the supernatant of control or pH 7.0 TSP-treated cell suspensions.
TABLE 1.
Absorbance at 260 nm and concentration of proteins in cell-free filtrates of S. enterica serovar Enteritidis ATCC 4931 cells exposed to different concentrations of TSP
| TSP concn (% wt/vol) | A260a,b | Protein concn (μg/μl)b,c |
|---|---|---|
| 0.0 | 0.12 | 0.00 |
| 0.5 | 1.96 | 1.57 |
| 1.0 | 1.98 | 1.99 |
| 1.5 | 1.98 | 2.82 |
| 2.0 | 1.98 | 3.11 |
| 2.5 | 1.98 | 3.12 |
Absorbance values from the control treatment (0% TSP) were subtracted from the treatment values.
Data are means of three separate replications.
Protein content was estimated by Bradford assay.
Thus, membrane damage due to TSP treatment was in fact found to be consistent with the increased release of 260-nm-absorbing material to the medium (Table 1) and the presence of DNA, proteins (Table 1), and LPS in the filtrates of TSP-treated cell suspensions.
Electron microscopy.
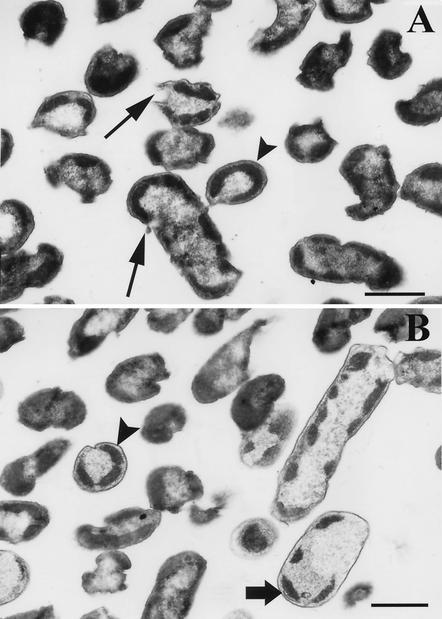
The cells used for this study were stationary-phase washed cells. As a result, the electron micrographs of the untreated control (cells suspended in fresh TSB for 1 h) consisted mostly of single or dividing “doublet” cells. The dimensions and sizes of these cells were 0.75 to 1.0 by 0.5 μm in length and diameter. Internal ultrastructures of these cells show a centrally situated genome surrounded by the cytoplasmic area with tightly packed ribosomes. The cell membranes and walls and the periplasmic spaces of these cells can be clearly distinguished. Cells treated with TSP (Fig. 5A) showed that cells had lost their general shape. The genome area was disorganized and appeared as loosely packed DNA and polyribosomes. These effects are expected if DNA degradation and release are associated with TSP treatment. TSP-treated cells also showed distortions and collapse in the membrane-wall continuity. In many instances, and especially with 2.5% TSP treatments, breaks in the outer membranes were evident. Finally, distinct condensation and beading of cytoplasmic material (Fig. 5) preferentially towards the inner membrane or the polar regions of the cells and some vacuolization were seen. Cells exposed to alkaline pH (Fig. 5B) showed a number of similarities to TSP-treated cells. Disappearance of the outer membrane and breaks between the cell wall and outer membranes observed in TSP-treated cells were also evident during alkaline pH treatment. Vacuolization, exaggerated condensation, and beading in the cytoplasmic area were observed, similar to TSP treatments. In most cases, disrupted cytoplasmic membranes could be observed in close association with the condensed cytoplasm (Fig. 5B). The condensation of the cytoplasm in cells exposed to TSP or alkaline pH could, in part, be explained by the fact that proteins and other cytoplasmic constituents would denature and precipitate at high pH. DNA, being highly hydrophilic (32), would remain water soluble and separate in the liquid phase from the rest of the cytoplasm and would be released out of the cell once the outer and cytoplasmic membranes were disrupted (Fig. 5A). This was confirmed by the increased A260 values of filtrates of cells exposed to TSP or high pH and also detection of DNA on agarose gels in these filtrates (Table 1). Mendonca et al. (27) reported similar membrane damage and discharge of intracellular material in serovar Enteritidis and E. coli cells exposed to pH 12 at 45°C. Membrane damage is not restricted to exposure to high pH alone. Electron microscopic studies have shown similar effects in Salmonella spp. and E. coli following starvation, freezing, and exposure to lysozyme (10, 25) or high pressure (250 MPa), and in Listeria monocytogenes after freezing and prolonged frozen storage or exposure to high pressure (500 MPa). A similar response was observed when L. monocytogenes cells were exposed to pH 12.0 (27).
FIG. 5.
Electron micrograph thin sections of S. enterica serovar Enteritidis ATCC 4931 cells exposed to 2.5% TSP (A) or pH 11.0 TSB solutions (B). In panel A, long arrows show disruption and holes in the outer and cytoplasmic membranes, whereas the arrowheads (without shafts) in panels A and B show what appeared to be compacted regions of residual ribosomes and amorphous bodies situated close to the disrupted membrane. In panel B, the wide arrow with shaft shows an example of the disrupted cytoplasmic membrane closely associated with the condensed cytoplasm. Bars, 0.5 μm.
The results presented in this study indicate that TSP is a potent membrane-acting agent, as indirectly evidenced by its ability to induce the uptake of the membrane-impermeant dye PI and release of LPS, DNA and RNA, and intracellular proteins from treated cells. The TSP action on membranes occurs at both functional and structural levels, as directly evidenced by electron microscopic examination. The inhibition of PI uptake by MgCl2 added in excess during TSP treatment suggests that the mode of action of TSP could involve chelation of divalent cations in the outer membrane. However, when the pH of the treatment solutions containing TSP was adjusted to 7.0, TSP had no effect on cell viability and there was no evidence of membrane damage. Overall, these results provide compelling evidence that the high pH during TSP treatment, and to a lesser extent sequestration of metal ions in the outer membrane by TSP, lead to membrane damage, release of intracellular contents, and cell death.
Acknowledgments
Saskatchewan Agriculture Development and Food, Alberta Agriculture Research Institute, and NSERC Canada are acknowledged for financial support.
REFERENCES
- 1.Bender, F. G., and E. Brotsky. September 1992. Process for treating poultry carcasses to control salmonella growth. U.S. patent 5,143,739.
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Capita, R., C. C. Alonso, M. Sierra, B. Moreno, and M. d.-C. Garcia-Fernandez. 2000. Effect of trisodium phosphate washing on the sensory evaluation of poultry meat. Meat Sci. 55:471-474. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2002. Preliminary FoodNet data on the incidence of foodborne illnesses-selected sites, United States, 2001. Morb. Mortal. Wkly. Rep. 51:325-329. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1993. Update: multistate outbreak of Escherichia coli O157:H7 infections from hamburgers, western United States, 1992-1993. Morb. Mortal. Wkly. Rep. 42:258-263. [PubMed] [Google Scholar]
- 6.Coppen, P., S. Fenner, and G. Salvant. 1998. Antimicrobial efficacy of AvGard carcass wash under industrial processing conditions. Br. Poultry Sci. 39:229-234. [DOI] [PubMed] [Google Scholar]
- 7.Csonka, J. S. 1989. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 53:121-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delmore, R. J., J. N. Sofos, G. R. Schmidt, K. E. Belk, W. R. Lloyd, and G. C. Smith. 2000. Interventions to reduce microbiological contamination of beef meats. J. Food Prot. 63:44-50. [DOI] [PubMed] [Google Scholar]
- 9.Dorsa, W. J., C. N. Cutter, and G. R. Siragusa. 1997. Effects of acetic acid, lactic acid and trisodium phosphate on the microflora of refrigerated beef carcass surface tissue inoculated with Escherichia coli O157:H7, Listeria innocua and Clostridium sporogenes. J. Food Prot. 60:619-624. [DOI] [PubMed] [Google Scholar]
- 10.El-Kest, S. E., and E. H. Marth. 1992. Transmission electron microscopy of unfrozen and frozen/thawed cells of Listeria monocytogenes treated with lipase and lysozyme. J. Food Prot. 55:687-696. [DOI] [PubMed] [Google Scholar]
- 11.Farkas, J. 1998. Irradiation as a method for decontaminating food: a review. Int. J. Food Microbiol. 44:189-204. [DOI] [PubMed] [Google Scholar]
- 12.Federal Register. 1982. Meat and poultry products: phosphates and sodium hydroxide. Fed. Regis. 47:10779. [Google Scholar]
- 13.Federal Register. 1994. Use of trisodium phosphate on raw, chilled poultry carcasses. Fed. Regist. 59:551-554. [Google Scholar]
- 14.Giesse, J. 1992. Experimental process reduces salmonella on poultry. Food Technol. 46:112. [Google Scholar]
- 15.Helander, I., and M. Vaara. 1987. Reversible binding of Salmonella typhimurium lipopolysaccharides by immobilized protamine. Eur. J. Biochem. 163:51-55. [DOI] [PubMed] [Google Scholar]
- 16.Helander, I. M., P. H. Mäkelä, O. Westphal, and E. T. Rietschel. 1996. Lipopolysaccharides, p. 462-471. In R. A. Meyers (ed.), Encyclopaedia of molecular biology and molecular medicine, vol. 3. VCH, Weinheim, Germany.
- 17.Kim, C. R., and D. L. Marshall. 1999. Microbiological, color and sensory changes of refrigerated chicken legs treated with selected phosphates. Food Res. Int. 32:209-215. [Google Scholar]
- 18.Kim, J., and M. F. Slavik. 1994. Removal of Salmonella typhimurium attached to chicken skin by rinsing with trisodium phosphate solution: scanning electron microscopic examination. J. Food Safety 14:77-84. [Google Scholar]
- 19.Kim, J., and M. F. Slavik. 1994. Trisodium phosphate treatment of beef surfaces to reduce Escherichia coli O157:H7 and Salmonella typhimurium. J. Food Sci. 59:20-22. [Google Scholar]
- 20.Kim, J., M. F. Slavik, M. D. Pharr, D. P. Raben, C. M. Lobsinger, and S. Tsai. 1994. Reduction of Salmonella on post-chill chicken carcasses by trisodium phosphate treatment. J. Food Safety 14:9-17. [DOI] [PubMed] [Google Scholar]
- 21.Korber, D. R., A. Choi, G. M. Wolfaardt, and D. E. Caldwell. 1996. Bacterial plasmolysis as a physical indicator of viability. Appl. Environ. Microbiol. 62:3939-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, R. M., P. A. Hartman, D. G. Olson, and F. D. Williams. 1994. Metal ions reverse the inhibitory effects of selected food-grade phosphates in Staphylococcus aureus. J. Food Prot. 57:284-288. [DOI] [PubMed] [Google Scholar]
- 23.Leive, L. 1974. The barrier function of gram-negative envelope. Ann. N. Y. Acad. Sci. 235:109-127. [DOI] [PubMed] [Google Scholar]
- 24.Lugtenberg, B., and L. van Alpen. 1983. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochem. Biophys. Acta 737:51-115. [DOI] [PubMed] [Google Scholar]
- 25.Mackey, B. M., K. Forestière, N. S. Isaacs, R. Stenning, and B. Brooker. 1994. The effect of high hydrostatic pressure on Salmonella thompson and Listeria monocytogenes examined by electron microscopy. Lett. Appl. Microbiol. 19:429-432. [Google Scholar]
- 26.Mager, J., M. Kuczynski, G. Schatzberg, and Y. Avi-Dor. 1956. Turbidity changes in bacterial suspensions in relation to osmotic pressure. J. Gen. Microbiol. 14:69-75. [DOI] [PubMed] [Google Scholar]
- 27.Mendonca, A. F., T. L. Amoroso, and S. J. Knabel. 1994. Destruction of gram-negative food-borne pathogens by high pH involves disruption of the cytoplasmic membrane. Appl. Environ. Microbiol. 60:4009-4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray, R. G. E., P. Steed, and H. E. Elson. 1965. The location of the mucopeptide in sections of the cell wall of Escherichia coli and other gram-negative bacteria. Can. J. Microbiol. 11:547-560. [DOI] [PubMed] [Google Scholar]
- 29.Nikaido, H., and M. Vaara. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 49:1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhône-Poulenc. 1992. AvGard™ TSP Salmonella reduction process. Rhône-Poulenc, Princeton, N.J.
- 31.Salvant, G., P. Coppen, J. C. Allo, S. Fenner, M. J. Laisney, M. T. Toquin, F. Humbert, and P. Colin. 1997. Effects of Av-Gard treatment on the microbiological flora of poultry carcasses. Br. Poultry Sci. 38:489-498. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 33.Scudamore, R. A., T. J. Beveridge, and M. Goldner. 1979. Outer-membrane penetration barriers as components of intrinsic resistance to beta-lactam and other antibiotics in Escherichia coli K-12. Antimicrob. Agents Chemother. 15:182-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slavik, M. F., J. Kim, M. D. Pharr, D. P. Raben, S. Tsai, and C. M. Lobsinger. 1994. Effect of trisodium phosphate on Campylobacter attached to post-chill chicken carcasses. J. Food Prot. 57:324-326. [DOI] [PubMed] [Google Scholar]
- 35.Smulders, F. J. M., and G. G. Greer. 1998. Integrating microbial decontamination with organic acids in HACCP programmes for muscle foods: prospects and controversies. Int. J. Food Microbiol. 44:149-169. [DOI] [PubMed] [Google Scholar]
- 36.Stevens, K. A., N. A. Klapes, B. W. Sheldon, and T. R. Klaenhammer. 1992. Effect of treatment conditions on nisin inactivation of gram-negative bacteria. J. Food Prot. 55:763-766. [DOI] [PubMed] [Google Scholar]
- 37.Strange, R. E. 1964. Effect of magnesium on permeability control in chilled bacteria. Nature 203:1304-1305. [DOI] [PubMed] [Google Scholar]
- 38.Taormina, P. J., and L. R. Beuchat. 1999. Comparison of chemical treatments to eliminate enterohemorrhagic Escherichia coli O157:H7 on alfalfa seeds. J. Food Prot. 62:318-324. [DOI] [PubMed] [Google Scholar]
- 39.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong, H., Y. Li, M. F. Slavik, and J. Walker. 1998. Chemical spray conditions for reducing bacteria on chicken skins. J. Food Sci. 63:699-701. [Google Scholar]
- 41.Xiong, H., Y. Li, M. F. Slavik, and J. T. Walker. 1998. Spraying chicken skin with selected chemicals to reduce attached Salmonella typhimurium. J. Food Prot. 61:272-275. [DOI] [PubMed] [Google Scholar]
- 42.Yang, Z., Y. Li, and M. F. Slavik. 1998. Use of antimicrobial spray applied with an inside-outside birdwasher to reduce bacterial contamination on prechilled chicken carcasses. J. Food Prot. 61:829-832. [DOI] [PubMed] [Google Scholar]