Abstract
Compound-specific isotope analysis has the potential to distinguish physical from biological attenuation processes in the subsurface. In this study, carbon and hydrogen isotopic fractionation effects during biodegradation of benzene under anaerobic conditions with different terminal-electron-accepting processes are reported for the first time. Different enrichment factors (ɛ) for carbon (range of −1.9 to −3.6‰) and hydrogen (range of −29 to −79‰) fractionation were observed during biodegradation of benzene under nitrate-reducing, sulfate-reducing, and methanogenic conditions. These differences are not related to differences in initial biomass or in rates of biodegradation. Carbon isotopic enrichment factors for anaerobic benzene biodegradation in this study are comparable to those previously published for aerobic benzene biodegradation. In contrast, hydrogen enrichment factors determined for anaerobic benzene biodegradation are significantly larger than those previously published for benzene biodegradation under aerobic conditions. A fundamental difference in the previously proposed initial step of aerobic versus proposed anaerobic biodegradation pathways may account for these differences in hydrogen isotopic fractionation. Potentially, C-H bond breakage in the initial step of the anaerobic benzene biodegradation pathway may account for the large fractionation observed compared to that in aerobic benzene biodegradation. Despite some differences in reported enrichment factors between cultures with different terminal-electron-accepting processes, carbon and hydrogen isotope analysis has the potential to provide direct evidence of anaerobic biodegradation of benzene in the field.
Contamination of groundwater systems by petroleum hydrocarbons is widespread due to leaking underground storage tanks, accidental spills, and inappropriate disposal methods. Aromatic hydrocarbons, such as benzene, toluene, ethylbenzene, and m-, o-, and p-xylenes (BTEX compounds), are significant constituents of petroleum products. Groundwater contamination by benzene is of particular concern, since benzene is the most water-soluble BTEX compound and a human carcinogen (13, 52).
Microbial degradation of benzene is an important mechanism for natural attenuation and may be a major control of the migration of dissolved benzene in the subsurface (8, 12, 19). Although benzene is susceptible to other mechanisms of natural attenuation, such as sorption, volatilization, and dilution, biodegradation by indigenous microorganisms is the only attenuation mechanism whereby the contaminant undergoes degradation to benign end products. For example, biodegradation of benzene under aerobic conditions involves complete mineralization to carbon dioxide (18). Biodegradation of benzene also occurs under anaerobic conditions through various terminal-electron-accepting processes, such as methanogenic (22, 30), nitrate-reducing (6, 10, 42), iron-reducing (30, 42), and sulfate-reducing (30, 34, 42) conditions, although details of the biochemical pathways are still unknown. Anaerobic biodegradation is of particular significance, since BTEX-contaminated groundwater is often anaerobic (2, 32) or quickly becomes anaerobic as oxygen is depleted in early stages of biodegradation. Biostimulation of aerobic benzene biodegradation is often unfavorable for heavily contaminated groundwater because of the large oxygen demand required to degrade benzene aerobically and plugging problems related to the formation of biomass and insoluble oxides (33, 36). A study on sulfate-enhanced biodegradation of benzene in petroleum-contaminated groundwater suggested that anaerobic remediation of benzene may be more applicable than aerobic remediation due to the higher solubility and bioavailability of sulfate versus oxygen in groundwater (56).
Traditional methods used to confirm bioremediation in the field have involved monitoring decreases in contaminant concentrations, electron acceptors, and increases in microbial by-products (4, 9). However, it can be a challenge to prove biodegradation in the field, since other processes such as volatilization, dispersion, and sorption can cause contaminant attenuation, and accurate mass balances are difficult to obtain (11).
Compound-specific isotope analysis (CSIA) can be used to directly monitor biodegradation of aromatic hydrocarbons in groundwater by measuring the isotopic fractionation of the remaining contaminant as degradation proceeds. Isotopes of elements such as carbon (12C and 13C) and hydrogen (1H and 2H) react at slightly dissimilar rates during mass-differentiating reactions. During biodegradation, bonds containing the lighter isotopes are preferentially broken, causing the remaining contaminant to be enriched in the heavier isotopes compared to the original isotopic value (1, 3, 21, 27-29, 39, 47, 50, 55). A large isotopic fractionation effect (primary isotope effect) can be observed if a bond containing the element of interest is broken or formed in the rate-limiting step (57). In contrast, for petroleum and chlorinated hydrocarbons, carbon isotopic fractionation is smaller than the total analytical error (±0.5‰) associated with CSIA during volatilization (23, 25, 44), dissolution (14, 49), and adsorption (23, 48). Similarly, Ward et al. (55) determined that for toluene, hydrogen isotopic fractionation is smaller than the total analytical error (±5‰) during volatilization and dissolution. Thus, CSIA has the ability to identify biodegradation of petroleum hydrocarbons in the field and to distinguish contaminant mass loss due to biodegradation versus that due to physical processes.
Laboratory studies have investigated the extent of isotopic fractionation during biodegradation of organic contaminants for different compounds, degradation pathways, microbial consortia, and terminal-electron-accepting processes. For example, biodegradation of chlorinated compounds such as perchloroethene (3, 50), trichloroethene (47, 50), and 1,2-dichloroethane (27) results in carbon isotopic fractionation an order of magnitude larger than does biodegradation of petroleum hydrocarbons such as toluene (1, 39) or methyl tert-butyl ether (21, 29). During aerobic degradation of toluene using two different pathways, a carbon enrichment factor of −2.6‰ ± 0.2‰ was observed for toluene degradation via the methyl oxidation pathway (Pseudomonas putida mt-2) (39), whereas no carbon isotopic fractionation of toluene occurred during degradation via ring oxidation by P. putida F1 (41). In a study examining hydrogen isotopic fractionation during biodegradation of toluene in pure cultures with various terminal-electron-accepting processes, a small range of hydrogen enrichment factors was reported (40). For aerobic biodegradation of benzene, carbon (26, 53) and hydrogen (26) isotopic fractionation has been documented in the literature. However, to date there have been no studies of carbon and hydrogen isotopic fractionation during anaerobic benzene biodegradation. Anaerobic biodegradation processes are key for intrinsic bioremediation and natural attenuation of benzene, and methods to monitor these processes are critical to the use of intrinsic bioremediation as a remedial alternative. The objective of this study was to characterize carbon and hydrogen isotopic fractionation during anaerobic biodegradation of benzene under nitrate-reducing, sulfate-reducing, and methanogenic conditions in order to potentially use CSIA to monitor anaerobic biodegradation of benzene in the field.
MATERIALS AND METHODS
Enrichment cultures.
Methanogenic and sulfate-reducing enrichment cultures were derived from microcosms originally prepared with soil and groundwater from a gasoline-contaminated site (42). A nitrate-reducing enrichment culture was derived from microcosms prepared with soil and groundwater from a pristine freshwater swamp (6). Over the past 6 years, these microcosms have been successively transferred into defined mineral medium as described by Edwards et al. (16) and amended repeatedly with benzene ranging in concentration from 130 μM to 260, 460, and 1,100 μM for nitrate-reducing, sulfate-reducing, and methanogenic cultures, respectively (54).
Experimental design.
To prepare culture bottles for measurement of isotopic fractionation during biodegradation, 50-ml aliquots from the various enrichment cultures were centrifuged at 2,000 × g for 10 min, and the cell pellet was resuspended in 50 ml of fresh defined mineral medium (16) in 250-ml glass bottles sealed with Mininert (Supelco Inc.) valves according to the technique of Slater et al. (49). Three replicate bottles were prepared for each enrichment culture. Each methanogenic replicate bottle was amended with 770 μM benzene (ca. 6.6 μl per bottle). Each sulfate-reducing replicate bottle was amended with 190 μM benzene (ca. 1.6 μl per bottle) and 20 mM Na2SO4 (from a 500 mM anaerobic stock). Each nitrate-reducing replicate bottle was amended with 260 μM benzene (ca. 2.2 μl per bottle) and 5 mM NaNO3 (from a 500 mM anaerobic stock). The sulfate-reducing and nitrate-reducing cultures were spiked to lower benzene concentrations than the methanogenic cultures to ensure that biodegradation would proceed without the buildup of excessive nitrite (6) or sulfide (16, 30). Benzene concentrations and isotopic compositions were measured frequently in the various enrichment cultures during biodegradation. Once biodegradation of benzene was complete, the three methanogenic and three nitrate-reducing cultures were reamended and degradation was monitored a second time. Experiment 1 refers to the observations from degradation of the initial benzene added for each terminal-electron-accepting culture. Experiment 2 refers to the second set of observations during benzene degradation after cultures were reamended with neat benzene. Degradation was inhibited in one of the three nitrate-reducing replicates, so a second set of data was collected for only two of three replicates. In the sulfate-reducing culture bottles, concentrations and isotopic compositions were measured only during degradation of the initial benzene added because the rates of degradation were low. All culture bottles were incubated statically in the dark inside an anaerobic chamber (Coy Laboratories Products Inc., Ann Arbor, Mich.) filled with a gas mixture containing 80% N2, 10% H2, and 10% CO2.
To examine the effects of different initial biomass concentrations on carbon and hydrogen isotopic fractionation during biodegradation, a methanogenic culture with 10-fold protein concentration was prepared by combining the volumes from two culture bottles (total volume, 100 ml), centrifuging the combined cultures, and resuspending the pellet in 10 ml of fresh medium.
To verify that the isotopic composition was unaffected by the experimental setup, sampling, or analytical methods, a series of control bottles was also prepared and analyzed at the same time as the culture bottles. Four sterile aqueous control bottles were prepared with 50 ml of double-distilled water in 250-ml glass bottles capped with Mininert valves. Two were amended with 6.6 μl of neat benzene, and two were amended with 2.2 μl of neat benzene. Four additional control bottles were prepared in the same way, but 50 ml of defined mineral medium was used instead of water. Twenty millimolar Na2SO4 was also added to the two control bottles containing medium and 2.2 μl of benzene to mimic the experimental setup for the culture bottles as closely as possible.
Analytical procedures.
Benzene and methane concentrations were measured by removing a 300-μl headspace sample from a culture or control bottle with a 500-μl Pressure-Lok gas syringe (Precision Sampling Corp., Baton Rouge, La.) and injecting the sample into a Hewlett-Packard 5890 Series II gas chromatograph (GC) equipped with a J&W Scientific GSQ 30-m by 0.53-mm (inner diameter) PLOT column and a flame ionization detector. The injector temperature was set at 200°C, the oven temperature was held isothermal at 190°C, and the detector temperature was set at 250°C. The carrier gas was helium at a flow rate of 11 ml/min. The error for benzene concentrations was ±5%. Calibration was done with external standards. Nitrate, nitrite, and sulfate concentrations were analyzed by injecting 500 μl of diluted liquid samples onto a Dionex 300 Series ion chromatograph with an IonPac AS11 4-mm column. The eluent flow rate was 1.5 ml/min with the following concentration gradient: 0.5 mM NaOH for 3.6 min, increased linearly to 5 mM NaOH over 5.4 min, and increased further to 27 mM NaOH over the next 4 min. The errors for nitrite, nitrate, and sulfate concentrations were ±0.5, ±2.4, and ±5.5%, respectively.
The biomass concentration in each culture was determined before and after the biodegradation experiments by measuring the protein concentration, which was assumed to comprise about 55% of a cell's dry weight (35). Protein was measured by the Bradford method (5) with a colorimetric microassay kit (Bio-Rad, Hercules, Calif.) and bovine serum albumin as a standard. Using a sterile syringe, 0.25 ml of culture was removed from each bottle. The culture was centrifuged, and the cell pellet was resuspended in 700 μl of 0.66 N NaOH for 48 h at 35°C to solubilize protein. The sample was then centrifuged, and the supernatant was removed, neutralized with 200 μl 2 N HCl, mixed with dye reagent (200 μl), and measured spectrophotometrically (Milton Roy Company Spectronic 21) at 595 nm. Means and standard deviations of protein concentration measurements are reported in Table 1.
TABLE 1.
Maximum benzene degradation rates and initial protein concentrations
| Culture | Initial maximum ratea (μM/day) | Initial protein concns (mg/liter) | ΔG°′b (kJ/mol of benzene) |
|---|---|---|---|
| Nitrate reducing | 18 (5.7) | 55 (30) | −2,000 |
| Sulfate reducing | 0.37 (0.02) | 100 (4.22) | −200 |
| Methanogenic | 55 (7.4) | 20 (0.7) | −116 |
Values are means (standard deviations) of triplicates
Standard free energy change for the oxidation of benzene to CO2 coupled to the reduction of CO2 to CH4, SO42− to H2S, and NO3− to NO2− (6).
Carbon isotope values for benzene were analyzed by GC-combustion-isotope ratio mass spectrometry. The system was equipped with a Varian 3400 GC interfaced with a combustion oven in line with a Finnigan Mat 252 isotope ratio mass spectrometer. The combustion oven temperature was set at 980°C. The GC was equipped with a J&W Scientific DB-624 column (30 m by 0.25 mm [inner diameter]). The GC temperature program was held at 80°C isothermal. For carbon isotopic compositions, 100 to 1,000 μl of headspace was withdrawn in a gas-tight syringe according to the method of Slater et al. (49). The detection limit for headspace injection of benzene, for this study, was approximately 500 μg/liter. At benzene concentrations of lower than 500 μg/liter, baseline separation of methane and benzene peaks was difficult to obtain due to tailing on the increasingly large methane peaks as degradation proceeded. The split injector was set at 6:1 to 3:1. Throughout the experiment, aqueous and medium controls were run for δ13C on alternate days and had a carbon isotopic reproducibility of ±0.2‰ of the mean of all controls. Daily reproducibility for replicate injections of a single control bottle was always <0.3‰; however, the total error reported for each sample is ±0.5‰ to incorporate both accuracy and reproducibility (14, 37, 49).
Benzene δ2H values were determined with a continuous flow mass spectrometry system consisting of a Hewlett-Packard 6890 GC interfaced with a pyrolysis oven in line with a Finnigan Mat Delta plus XL isotope ratio mass spectrometer. The GC was equipped with a J&W Scientific DB-624 column (30 m by 0.25 mm [inner diameter]), and the oven was set at 60°C for 2 min, ramped to 160°C at 10°C/min, and held for 2 min. For hydrogen isotopic compositions, 500 to 2000 μl of headspace was withdrawn with gas-tight syringes according to the method of Ward et al. (55) and injected into the continuous-flow mass spectrometer. The injector split setting decreased from 1 to 0.1 and injection volumes increased from 500 to 2,000 μl as the benzene concentrations decreased in the sample bottles. Throughout the experiment, aqueous and medium controls were run on alternate days and had a hydrogen isotopic reproducibility of ± 3‰ of the mean of all controls. Daily reproducibility of replicate injections of a single control bottle was always <3‰. However, the total error reported for each sample is ±5 ‰, incorporating both accuracy and reproducibility (21, 37, 55).
RESULTS
Benzene biodegradation.
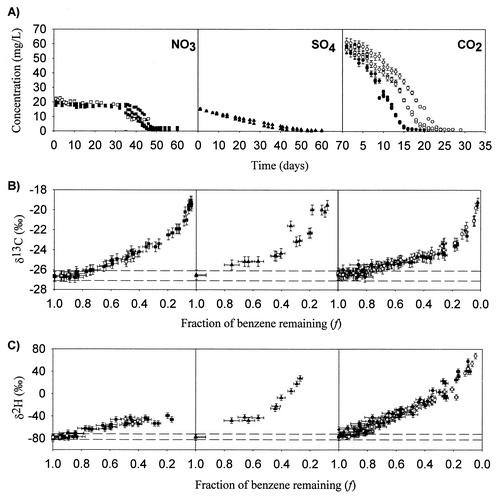
Benzene concentrations for biodegradation experiments with different electron acceptors are plotted in Fig. 1A. The benzene concentrations in the aqueous and medium controls for the higher concentration range had mean values of 60.2 ± 2.6 mg/liter (n = 22) and 56.3 ±2.0 mg/liter (n = 27), respectively, and those for the lower concentration range had mean values of 22.9 ± 0.8 mg/liter (n = 5) and 24.7 ± 0.6 mg/liter (n = 3), respectively, with no change in concentration outside of error (±5%) over the course of the study. The methanogenic and nitrate-reducing experiments had mean initial concentrations of 58 ± 2.3 and 20 ± 1.4 mg/liter, respectively. The sulfate-reducing cultures had a mean initial concentration of 15 ± 0.2 mg/liter. All replicate bottles for the methanogenic, nitrate-reducing, and sulfate-reducing cultures degraded benzene from initial concentrations to completion within 30 days for the methanogenic cultures and within 60 days for the sulfate-reducing and nitrate-reducing cultures (Fig. 1A). The production of methane and the depletion of sulfate or nitrate in the cultures confirmed that biodegradation of benzene proceeded under methanogenic, sulfate-reducing, and nitrate-reducing conditions. Specifically, a ratio of 11.4 ± 1.05 mol of nitrate consumed per mol of benzene consumed was measured, which exceeds the theoretical stoichiometric ratio of 9.45 and confirms that biodegradation proceeded via nitrate reduction. For the sulfate-reducing cultures, a ratio of 4.0 ± 0.17 mol of sulfate consumed per mol of benzene consumed was measured, which is close to the theoretical ratio of 3.5 mol, indicating that benzene biodegradation proceeded via sulfate reduction in these cultures. Protein concentrations increased over the course of biodegradation in all cases (data not shown).
FIG. 1.
Biodegradation of benzene in enriched cultures under nitrate-reducing (closed squares, replicates from experiment 1; open squares, replicates from experiment 2), sulfate-reducing (closed triangles, replicates from one experiment), and methanogenic (closed circles, replicates from experiment 1; open circles, replicates from experiment 2) conditions. (A) Concentration of benzene versus time during biodegradation of benzene in enriched cultures. Error bars represent ±5% error on individual benzene concentrations. Note that the scale on the x axis is different for the methanogenic experiment. (B) δ13C of remaining benzene versus fraction of benzene remaining during biodegradation of benzene. The error on the x axis is ±7%, based on the propagation of ±5% error through the equation for the fraction of benzene remaining (concentration at time t/initial concentration). All error bars on δ13C values represent ±0.5‰ (incorporating both accuracy and reproducibility [see text]). The dashed lines represent ±0.5‰ error on the mean δ13C values for all controls. (C) δ2H of remaining benzene versus fraction of benzene remaining during biodegradation of benzene. The error on the x axis is ±7%, based on the propagation of ±5% error through the equation for fraction of benzene remaining (concentration at time t/initial concentration). All error bars on δ2H values represent ±5‰ (incorporating both accuracy and reproducibility [see text]). The dashed lines represent ±5‰ error on the mean δ2H values for all controls.
Table 1 lists the maximum benzene degradation rates and initial protein concentrations for the three culture types. Degradation rates (micromolar per day) were highest in the methanogenic cultures, followed by the nitrate-reducing cultures and the sulfate-reducing cultures (Table 1). The same result was obtained for rates normalized by initial protein concentration. Based on standard free energy calculations, the nitrate-reducing culture is expected to have a significantly higher yield than either of the other two cultures (45). The low rates of degradation observed in the nitrate-reducing and sulfate-reducing cultures relative to the methanogenic cultures may be related to the accumulation of nitrite (6), sulfide (15), or other inhibitory products during degradation (34). In all nitrate-reducing culture bottles, nitrite concentrations rose significantly during the course of biodegradation and ranged from approximately 0.50 to a maximum 5.1 mM. Sulfide concentrations were not measured.
Isotopic fractionation.
The δ13C value of the Stable Isotope Laboratory benzene working standard is −26.6‰ ± 0.2‰ (n = 42), measured by headspace extraction over an aqueous solution of benzene according to the method of Slater et al. (49). The mean benzene δ13C values of all aqueous and medium controls measured throughout the experiment were −26.6‰ ± 0.2‰ (n = 26) and −26.5‰ ± 0.1‰ (n = 28), respectively. Similarly, all culture bottles had initial δ13C values of benzene well within the error of the benzene working standard and controls, with initial δ13C values of −26.6‰ ± 0.1‰ (n = 6), −26.6‰ ± 0.1‰ (n = 6), and −26.5‰ ± 0.1‰ (n = 5) for the nitrate-reducing, sulfate-reducing, and methanogenic experiments, respectively.
Figure 1B shows the δ13C values of residual benzene during biodegradation under nitrate-reducing, sulfate-reducing, and methanogenic conditions. All culture bottles exhibited progressive enrichment in 13C of the remaining benzene as biodegradation proceeded. The δ13C value of benzene in the nitrate-reducing culture increased to a maximum value of −19.1‰ ± 0.5‰ at 96% degradation, an absolute enrichment of +7.5‰. Similarly, the δ13C value of benzene in the sulfate-reducing culture increased to a maximum value of −19.5‰ ± 0.5‰ at 92% degradation, an absolute enrichment of +7.1‰. For the methanogenic experiments, the δ13C value of benzene increased to a maximum value of −18.4‰ ± 0.5‰ at 99% degradation, representing an absolute enrichment of +8.2‰. Since all controls remained within error of the standard benzene value (−26.6‰ ± 0.2‰), the progressive enrichment in 13C of the remaining benzene in all culture bottles was a result of biodegradation of benzene and not physical processes (23, 48, 49), the sampling procedure, or benzene sorption to medium particles.
The δ2H value of the Stable Isotope Laboratory benzene working standard is −77‰ ± 3‰ (n = 36), measured by headspace extraction over an aqueous solution of benzene according to the method of Ward et al. (55). The mean benzene δ2H values of all aqueous and medium controls throughout the experiments were −76‰ ± 3‰ (n = 20) and −77‰ ± 3‰ (n = 25), respectively. Similarly, initial δ2H values of benzene in the culture bottles were within the error of both the working standard and controls, with initial δ2H values of −78‰ ± 2‰ (n = 6), −77‰ ± 1‰ (n = 6), and −75‰ ± 5‰ (n = 5) for nitrate-reducing, sulfate-reducing, and methanogenic experiments, respectively.
Figure 1C shows the δ2H values of remaining benzene during biodegradation under the three terminal-electron-accepting processes. Substantial hydrogen isotopic fractionation of benzene was observed in all culture bottles over the course of each experiment. As biodegradation proceeded, the δ2H value of remaining benzene increased to maximum values of −39‰ ± 0.5‰ (at 80% degradation), +28‰ ± 0.5‰ (at 70% degradation), and +68‰ ± 0.5‰ (at 95% degradation) for the nitrate-reducing, sulfate-reducing, and methanogenic cultures, respectively (Fig. 1C). These shifts represent absolute enrichments in 2H of +39‰, +106‰, and +146‰ for the respective cultures. Since all controls remained within the error of the standard benzene value (−77‰ ± 3‰), the substantial hydrogen isotopic fractionation observed in all culture bottles was a result of biodegradation of benzene.
Quantification of isotopic fractionation.
To compare the reproducibility of isotopic fractionation between replicate bottles and experiments, carbon and hydrogen enrichment factors were calculated for each bottle, and mean enrichment factors were then calculated for each experiment (Table 2). Enrichment factors were determined by using a Rayleigh isotopic model for closed systems (38), expressed as ln (Rt/R0) = (α − 1)ln f, where Rt is the ratio of the isotopic composition of the substrate at a given time, R0 is the ratio of the isotopic composition of the initial substrate, α is the fractionation factor, and f is the fraction of substrate (i.e., benzene) remaining. The fractionation factor (α) was determined by plotting ln f versus ln (Rt/R0) and determining the slope of this linear regression (m), which is related to the fractionation factor (α) by m = (α − 1). Fractionation factors were then converted into enrichment factors (ɛ) by using the following relationship to the fractionation factor: ɛ = (α − 1) × 1,000.
TABLE 2.
Carbon and hydrogen enrichment factors for all replicate cultures and repeat experiments per electron acceptor culture
| Culture and expt | Bottle no. | ɛC
|
ɛH
|
||
|---|---|---|---|---|---|
| Mean ± 95% CIa (‰) | r2 | Mean ± 95% CI (‰) | r2 | ||
| Nitrate reducing | |||||
| 1 | 1 | −2.4 ± 0.1 | 0.99 | −31 ± 10 | 0.70 |
| 2 | −2.4 ± 0.1 | 0.99 | −26 ± 7 | 0.72 | |
| 3 | −2.3 ± 0.1 | 0.98 | −39 ± 11 | 0.88 | |
| All datab | −2.4 ± 0.1 | 0.99 | −29 ± 4 | 0.73 | |
| 2 | 1 | −2.0 ± 2.4 | 0.98 | −41 ± 13 | 0.93 |
| 2 | −2.4 ± 1.9 | 0.99 | −32 ± 1 | 0.99 | |
| All data | −2.2 ± 0.4 | 0.95 | −35 ± 6 | 0.91 | |
| Sulfate reducing | 1 | −3.6 ± 1.4 | 0.82 | −79 ± 11 | 0.87 |
| 2 | −3.4 ± 0.9 | 0.92 | −78 ± 13 | 0.91 | |
| 3 | −2.7 ± 0.3 | 0.92 | −79 ± 6 | 0.76 | |
| All data | −3.6 ± 0.3 | 0.92 | −79 ± 4 | 0.79 | |
| Methanogenic | |||||
| 1 | 1 | −1.9 ± 0.1 | 0.98 | −58 ± 6 | 0.94 |
| 2 | −1.9 ± 0.1 | 0.97 | −64 ± 8 | 0.89 | |
| 3 | −1.8 ± 0.1 | 0.99 | −58 ± 4 | 0.97 | |
| All data | −1.9 ± 0.1 | 0.98 | −60 ± 3 | 0.92 | |
| 2 | 1 | −2.0 ± 0.1 | 0.99 | −54 ± 6 | 0.90 |
| 2 | −2.3 ± 0.1 | 0.98 | −72 ± 7 | 0.90 | |
| 3 | −2.0 ± 0.1 | 0.97 | −58 ± 6 | 0.89 | |
| All data | −2.1 ± 0.1 | 0.98 | −59 ± 4 | 0.86 | |
| With 10-fold-higher initial biomass | −2.0 ± 0.1 | 0.98 | −59 ± 3 | 0.91 | |
Ninety-five percent confidence intervals determined on the slope of the linear regression of In f versus In (Rt/R0) and converted into per mille units as described in Results.
Mean of enrichment factors calculated on the basis of data from all three bottles in each experiment.
Table 2 lists the carbon and hydrogen enrichment factors (ɛ) determined for each bottle as well as the mean for each experiment. All cultures exhibited a strong linear relationship between ln f and ln (Rt/R0) for both carbon (r2 > 0.92 for all but one bottle) and hydrogen (r2 > 0.86 for all but three bottles) isotope data (Table 2). The high values of r2 indicate that fractionation in these experiments fit a Rayleigh model with a constant isotopic fractionation regardless of the extent of benzene remaining (f). For the methanogenic cultures, the enrichment factors calculated for all but one bottle fall within 95% confidence intervals of each other, indicating a high degree of reproducibility in fractionation within and between experiments (Table 2). Similar results were observed for the nitrate-reducing cultures (Table 2). The sulfate-reducing bottles exhibited slight variability in the carbon enrichment factors, from −2.7‰ ± 0.3‰ to −3.6‰ ± 1.4‰, although all three replicate bottles were within 95% confidence intervals of each other. It is interesting that the sulfate-reducing cultures exhibited both the highest carbon and the highest hydrogen enrichment factors.
Carbon and hydrogen enrichment factors were used to compare isotopic fractionation during biodegradation of benzene between cultures with different terminal-electron-accepting processes. The carbon enrichment factors determined for the methanogenic experiments were very similar to those determined for the nitrate-reducing experiments (Fig. 2A). In contrast, the mean carbon enrichment factor for the sulfate-reducing experiment (−3.6‰ ± 0.3‰) was significantly different from those for both the methanogenic and nitrate-reducing cultures. The hydrogen enrichment factors determined for the nitrate-reducing, sulfate-reducing, and methanogenic cultures were all significantly different, with the sulfate-reducing cultures exhibiting the largest enrichment factor (Table 2). The hydrogen enrichment factors for the different electron-accepting cultures ranged from −26‰ to −79‰.
FIG. 2.
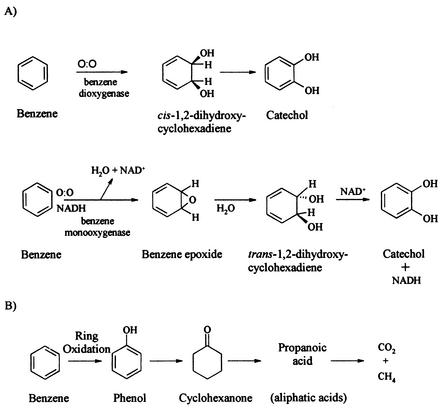
(A) Two proposed reaction sequences for aerobic biodegradation of benzene to form catechol via cis-1,2-dihydroxycyclohexadiene (modified from reference 17) and a benzene epoxide intermediate (modified from reference 35). The reaction illustrates oxygen addition to benzene ring in the initial transformation steps. (B) Proposed pathway for anaerobic biodegradation of benzene to form the first intermediate phenol in the initial transformation step (reprinted from reference 22).
Effect of initial biomass concentration.
The initial biomass concentrations (measured as protein concentration in milligrams per liter) for the three different types of cultures were significantly different (Table 1). To determine if the larger enrichment factors observed for the sulfate-reducing cultures were a function of the higher protein concentrations in these cultures, the protein concentration in the methanogenic cultures was increased 10-fold (to 200 mg/liter) as described in Materials and Methods. The biodegradation experiment was then repeated, and carbon and hydrogen isotope values measured. While the maximum rate of biodegradation was much higher (140 μM/day), the carbon and hydrogen enrichment factors for the methanogenic culture with a 10-fold-higher initial biomass were within 95% confidence intervals of the methanogenic cultures, with enrichment factors of −2.0‰ ± 0.1‰ and −59‰ ± 3‰, respectively (Table 2).
DISCUSSION
The main objective of this study was to determine the carbon and hydrogen enrichment factors of several different anaerobic cultures known to use different terminal-electron-accepting processes, in order to assess the applicability of CSIA to monitor anaerobic biodegradation of benzene in the environment under a variety of redox conditions. Rates of benzene biodegradation showed no major influence on the carbon and hydrogen enrichment factors for the methanogenic cultures despite the significantly higher rate of biodegradation in the culture with a 10-fold-higher initial biomass. Although Rudnicki et al. (46) and Goldhaber and Kaplan (20) observed a reasonable inverse correlation between rates of sulfate reduction and the extent of sulfur isotopic fractionation in bacterial studies, no such correlation was observed in this study with respect to the methanogenic cultures. Rudnicki et al. (46) suggested that large sulfur isotope fractionation can be attributed to lower rates of supply of sulfate in natural versus pure sulfate-reducing cultures.
This study also indicates that differences in initial biomass (measured as protein concentration in milligrams per liter) did not influence carbon and hydrogen isotopic fractionation during biodegradation. Ten-fold-higher initial protein concentrations of methanogenic biomass did not produce different carbon and hydrogen enrichment factors (Table 2). This finding is consistent with the similarity in carbon enrichment factors reported by Meckenstock et al. (39) for two bacterial strains despite differing initial biomass concentrations (measured by optical density).
There is a growing body of literature on carbon and hydrogen isotopic fractionation during aerobic biodegradation of benzene and during both aerobic and anaerobic biodegradation of toluene. For anaerobic toluene-degrading strains, Meckenstock et al. (39) reported constant carbon enrichment factors of −1.7 to −1.8‰ for pure cultures, using three different terminal-electron-accepting processes. Ahad et al. (1) also reported relatively small carbon enrichment factors of −0.5 to −0.8‰ during methanogenic and sulfate-reducing toluene degradation in mixed cultures. While the difference in carbon enrichment factors between mixed and pure cultures is not understood, anaerobic biodegradation of toluene to date shows no significant change in enrichment factors related to cultures using different terminal-electron-accepting processes. In contrast, the carbon enrichment factors observed during anaerobic biodegradation of benzene in this study show more variability between different cultures (−1.9 to −2.4‰ for methanogenic and nitrate-reducing cultures versus −3.6 for sulfate-reducing cultures) (Table 2). In similar natural-abundance hydrogen isotopic fractionation experiments, Morasch et al. (40) showed some variability in hydrogen enrichment factors during anaerobic biodegradation of toluene by two bacterial strains. Similarly, for benzene, hydrogen enrichment factors determined during anaerobic biodegradation in this study showed variability (−29 to −79‰) with respect to the three different cultures (Table 2).
While no other study has, to date, reported values for isotopic enrichment factors for anaerobic biodegradation of benzene, carbon (26, 53) and hydrogen (26) isotopic enrichment factors have been published for aerobic biodegradation of benzene. For aerobic biodegradation by two bacterial isolates, Hunkeler et al. (26) reported a range in carbon enrichment factors (−1.46 to −3.53‰) similar to that obtained for anaerobic biodegradation of benzene in this study (−1.9 to −3.6‰). In contrast, the hydrogen enrichment factors determined in the two studies are significantly different. The enrichment factors obtained for anaerobic biodegradation of benzene in this study are significantly larger (−29‰ ± 4‰ to −79‰ ± 4‰) than those reported for aerobic biodegradation of benzene (−12.8‰ ± 0.7‰ and −11.2‰ ± 1.8‰) (26). Aerobic biodegradation of benzene is known to proceed via initial hydroxylation of benzene by monooxygenase (18, 31, 35) or dioxygenase (17) enzymes to form a benzene epoxide or cis-1,2-dihydroxycyclohexadiene intermediate, respectively (Fig. 2A). The benzene epoxide or the cis-1,2-dihydroxycyclohexadiene is then transformed to catechol and degraded via the ortho- or meta-cleavage pathway (17). All known aerobic benzene-degrading bacteria share these initial transformation steps to catechol (51). Experiments using an atmosphere of known oxygen isotope composition showed that the initial transformation step to the intermediate cis-1,2-dihydroxycyclohexadiene occurs via oxygen addition to the benzene ring and involves no C-H cleavage (17) (Fig. 2A). Although the exact enzymatic mechanisms for the two bacterial isolates were unknown and may have involved monooxygenases or diooxygenases, Hunkeler et al. (26) proposed that the lack of a C-H cleavage in the initial transformation step may explain the small hydrogen isotopic fractionation observed during aerobic biodegradation of benzene. This conclusion is supported by another study in which a small hydrogen isotope effect was measured during benzene oxidation by mammalian monooxygenase and explained by direct oxygen atom addition to benzene rather than C-H atom abstraction during the initial transformation step to an arene oxide (57). Conversely, during oxidation of toluene by methane monooxygenase catalysis, a large primary hydrogen isotope effect is observed due to a C-H bond breakage in the methyl group of toluene (57). Similarly, a large primary isotope effect was observed during methane oxidation catalyzed by methane monooxygenase due to a complete C-H bond cleavage in the oxygenation step of the reaction (43).
In this study, anaerobic biodegradation of benzene produced substantially larger hydrogen isotopic fractionation in the three culture types than that observed in aerobic biodegradation. Using natural abundance toluene, Morasch et al. (40) documented significant hydrogen isotopic fractionation (ɛ = −728 and −198) during anaerobic toluene degradation by two bacterial isolates and confirmed that this isotopic fractionation was caused by a C-H bond breakage during the initial enzymatic attack on the methyl group to form a benzylsuccinate intermediate. Although the biochemical pathways utilized during anaerobic biodegradation of benzene are still unknown, it is hypothesized that benzene undergoes ring hydroxylation to form a phenol intermediate, as detected in methanogenic (22), iron-reducing, and sulfate-reducing enrichment cultures (7) (Fig. 2B). The large hydrogen enrichment factors observed during anaerobic biodegradation of benzene under nitrate-reducing, sulfate-reducing, and methanogenic conditions may be consistent with C-H bond cleavage to form phenol during the initial transformation of benzene. These findings suggest that the initial step in the biodegradation of benzene potentially determines the magnitude of hydrogen isotopic fractionation. Whether or not a C-H bond breakage occurs in the initial step may explain the differences in fractionation observed in aerobic versus anaerobic benzene biodegradation. At this point, no speculation can be made with respect to the observed differences in enrichment factors between the different anaerobic cultures investigated in this study. It is known that anaerobic toluene biodegradation proceeds via the same initial transformation step regardless of terminal-electron-accepting process with the addition of fumarate to toluene to form a benzylsuccinate intermediate (24). Further investigation into the biochemical pathways during anaerobic biodegradation of benzene is required.
Acknowledgments
Funding for this project was provided by the Environmental Science and Technology Alliance of Canada and the Natural Science and Engineering Research Council of Canada Strategic Program.
We thank Charles Whang for his assistance with culture maintenance, and we thank the members of the Stable Isotope Laboratory, especially J. Gray and N. VanStone, for valuable discussions.
REFERENCES
- 1.Ahad, J. M. E., B. Sherwood Lollar, E. A. Edwards, G. F. Slater, and B. E. Sleep. 2000. Carbon isotope fractionation during anaerobic biodegradation of toluene: implications for intrinsic bioremediation. Environ. Sci. Technol. 34:892-896. [Google Scholar]
- 2.Berry, D. F., A. J. Francis, and J. M. Bollag. 1987. Microbial metabolism of homocyclic and heterocyclic aromatic compounds under anaerobic conditions. Microbiol. Rev. 51:43-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom, Y., R. Aravena, D. Hunkeler, E. Edwards, and S. K. Frape. 2000. Carbon isotope fractionation during microbial dechlorination of trichloroethene, cis-1,2-dichloroethene, and vinyl chloride: implications for assessment of natural attenuation. Environ. Sci. Technol. 34:2768-2772. [Google Scholar]
- 4.Borden, R. C., C. A. Gomez, and M. T. Becker. 1995. Geochemical indicators of intrinsic bioremediation. Ground Water 33:180-189. [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Burland, S. M., and E. A. Edwards. 1999. Benzene biodegradation linked to nitrate reduction. Appl. Environ. Microbiol. 65:529-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldwell, M. E., and J. M. Sulflita. 2000. Detection of phenol and benzoate as intermediates of anaerobic benzene biodegradation under different terminal electron-accepting conditions. Environ. Sci. Technol. 34:1216-1220. [Google Scholar]
- 8.Chiang, C. Y., J. P. Salanitro, E. Y. Chai, J. D. Colthart, and C. L. Klein. 1989. Aerobic biodegradation of benzene, toluene, and xylene in a sandy aquifer—data analysis and computer modeling. Ground Water 27:823-834. [Google Scholar]
- 9.Cho, J. S., J. T. Wilson, D. C. DiGiulio, J. A. Vardy, and W. Choi. 1997. Implementation of natural attenuation at a JP-4 jet fuel release after active remediation. Biodegradation 8:265-273. [DOI] [PubMed] [Google Scholar]
- 10.Coates, J. D., R. Chakraborty, J. G. Lack, S. M. O'Connor, K. A. Cole, K. S. Bender, and L. A. Achenbach. 2001. Anaerobic benzene oxidation coupled to nitrate reduction in pure culture by two strains of Dechloromonas. Nature 411:1039-1043. [DOI] [PubMed] [Google Scholar]
- 11.Davis, G. B., C. Barber, T. R. Power, J. Thierrin, B. M. Patterson, J. L. Rayner, and Q. Wu. 1999. The variability and intrinsic remediation of a BTEX plume in anaerobic sulphate-rich groundwater. J. Contam. Hydrol. 36:265-290. [Google Scholar]
- 12.Davis, J. W., N. J. Klier, and C. L. Carpenter. 1994. Natural biological attenuation of benzene in ground water beneath a manufacturing facility. Ground Water 32:215-226. [Google Scholar]
- 13.Dean, B. J. 1985. Recent findings on the genetic toxicology of benzene, toluene, xylenes and phenols. Mutat. Res. 154:153-181. [DOI] [PubMed] [Google Scholar]
- 14.Dempster, H. S., B. Sherwood Lollar, and S. Feenstra. 1997. Tracing organic contaminants in groundwater: a new methodology using compound-specific isotopic analysis. Environ. Sci. Technol. 31:3193-3197. [Google Scholar]
- 15.Edwards, E. A., and D. Grbic-Galic. 1992. Complete mineralization of benzene by aquifer microorganisms under strictly anaerobic conditions. Appl. Environ. Microbiol. 58:2663-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards, E. A., L. E. Wills, M. Reinhard, and D. Grbic-Galic. 1992. Anaerobic degradation of toluene and xylene by aquifer microorganisms under sulfate-reducing conditions. Appl. Environ. Microbiol. 58:794-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson, D. T., M. Hensley, H. Yoshioka, and T. J. Mabry. 1970. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry 9:1626-1630. [DOI] [PubMed] [Google Scholar]
- 18.Gibson, D. T., J. R. Koch, and R. E. Kallio. 1968. Oxidative degradation of aromatic hydrocarbons by microorganisms. I. Enzymatic formation of catechol from benzene. Biochemistry 7:2653-2662. [DOI] [PubMed] [Google Scholar]
- 19.Gieg, L. M., R. V. Kolhatkar, M. J. McInerney, R. S. Tanner, S. H. Harris Jr., K. L. Sublette, and J. M. Suflita. 1999. Intrinsic bioremediation of petroleum hydrocarbons in a gas condensate-contaminated aquifer. Environ. Sci. Technol. 33:2550-2560. [Google Scholar]
- 20.Goldhaber, M. B., and I. R. Kaplan. 1975. Controls and consequences of sulfate reduction rates in recent marine sediments. Soil Sci. 119:42-55. [Google Scholar]
- 21.Gray, J. R., G. Lacrampe-Couloume, D. Gandhi, K. M. Scow, R. D. Wilson, D. M. Mackay, and B. Sherwood Lollar. 2002. Carbon and hydrogen isotopic fractionation during biodegradation of methyl tert-butyl ether. Environ. Sci. Technol. 36:1931-1938. [DOI] [PubMed] [Google Scholar]
- 22.Grbic-Galic, D., and T. M. Vogel. 1987. Transformation of toluene and benzene by mixed methanogenic cultures. Appl. Environ. Microbiol. 53:254-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrington, R. R., S. R. Poulson, J. I. Drever, P. J. S. Colberg, and E. F. Kelly. 1999. Carbon isotope systematics of monoaromatic hydrocarbons: vaporization and adsorption experiments. Org. Geochem. 30:765-775. [Google Scholar]
- 24.Heider, J., A. M. Spormann, H. R. Beller, and F. Widdel. 1999. Anaerobic bacterial metabolism of hydrocarbons. FEMS Microbiol. Rev. 22:459-473. [Google Scholar]
- 25.Huang, L., N. C. Sturchio, T. Abrajano, Jr., L. J. Heraty, and B. D. Holt. 1999. Carbon and chlorine isotope fractionation of chlorinated aliphatic hydrocarbons by evaporation. Org. Geochem. 30:777-785. [Google Scholar]
- 26.Hunkeler, D., N. Andersen, R. Aravena, S. M. Bernasconi, and B. J. Butler. 2001. Hydrogen and carbon isotope fractionation during aerobic biodegradation of benzene. Environ. Sci. Technol. 35:3462-3467. [DOI] [PubMed] [Google Scholar]
- 27.Hunkeler, D., and R. Aravena. 2000. Evidence of substantial carbon isotope fractionation among substrate, inorganic carbon, and biomass during aerobic mineralization of 1,2-dichloroethane by Xanthobacter autotrophicus. Appl. Environ. Microbiol. 66:4870-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunkeler, D., R. Aravena, and B. J. Butler. 1999. Monitoring microbial dechlorination of tetrachloroethene (PCE) in groundwater using compound-specific stable carbon isotope ratios: microcosm and field studies. Environ. Sci. Technol. 33:2733-2738. [Google Scholar]
- 29.Hunkeler, D., B. J. Butler, R. Aravena, and J. F. Barker. 2001. Monitoring biodegradation of methyl tert-butyl ether (MTBE) using compound-specific carbon isotope analysis. Environ. Sci. Technol. 35:676-681. [DOI] [PubMed] [Google Scholar]
- 30.Kazumi, J., M. E. Caldwell, J. M. Sulflita, D. R. Lovley, and L. Y. Young. 1997. Anaerobic degradation of benzene in diverse anoxic environments. Environ. Sci. Technol. 31:813-818. [Google Scholar]
- 31.Kitayama, A., E. Suzuki, Y. Kawakami, and T. Nagamune. 1996. Gene organization and low regiospecifity in aromatic-ring hydroxylation of a benzene monooxygenase of Pseudomonas aeruginosa JI104. J. Ferment. Bioeng. 82:421-425. [Google Scholar]
- 32.Krumholz, L. R., M. E. Caldwell, and J. M. Suflita. 1996. Biodegradation of ‘BTEX’ hydrocarbons under anaerobic conditions, p. 61-99. In R. L. Crawford and D. L. Crawford (ed.), Bioremediation: principles and applications. Cambridge University Press, Cambridge, United Kingdom
- 33.Lee, M. D., J. M. Thomas, J. C. Borden, P. B. Bedient, C. H. Ward, and J. T. Wilson. 1988. Biorestoration of aquifers contaminated with organic compounds. Crit. Rev. Environ. Control 18:29-89. [Google Scholar]
- 34.Lovley, D. R., J. D. Coates, J. C. Woodward, and E. J. P. Phillips. 1995. Benzene oxidation coupled to sulfate reduction. Appl. Environ. Microbiol. 61:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madigan, M., J. M. Martinko, and J. Parker. 2000. Brock biology of microorganisms, 9th ed., Prentice Hall, Upper Saddle River, N.Y.
- 36.Major, D. W., C. I. Mayfield, and J. F. Barker. 1988. Biotransformation of benzene by denitrification in aquifer sand. Ground Water 26:8-14. [Google Scholar]
- 37.Mancini, S. A., G. Lacrampe-Couloume, H. Jonker, B. M. van Breukelen, J. Groen, F. Volkering, and B. Sherwood Lollar. 2002. Hydrogen isotopic enrichment: an indicator of biodegradation at a petroleum hydrocarbon contaminated field site. Environ. Sci. Technol. 36:2464-2470. [DOI] [PubMed] [Google Scholar]
- 38.Mariotti, A., J. C. Germon, P. Hubert, P. Kaiser, R. Letolle, A. Tardieux, and P. Tardieux. 1981. Experimental determination of nitrogen kinetic isotope fractionation: some principles; illustration for the denitrification and nitrification processes. Plant Soil 62:413-430. [Google Scholar]
- 39.Meckenstock, R. U., B. Morasch, R. Warthmann, B. Schink, E. Annweiler, W. Michaelis, and H. H. Richnow. 1999. 13C/12C isotope fractionation of aromatic hydrocarbons during microbial degradation. Environ. Microbiol. 1:409-414. [DOI] [PubMed] [Google Scholar]
- 40.Morasch, B., H. H. Richnow, B. Schink, and R. U. Meckenstock. 2001. Stable hydrogen and carbon isotope fractionation during microbial toluene degradation: mechanistic and environmental aspects. Appl. Environ. Microbiol. 67:4842-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morasch, B., H. H. Richnow, B. Schink, A. Vieth, and R. U. Meckenstock. 2002. Carbon and hydrogen stable isotope fractionation during aerobic bacterial degradation of aromatic hydrocarbons. Appl. Environ. Microbiol. 68:5191-5194. [DOI] [PMC free article] [PubMed]
- 42.Nales, M., B. J. Butler, and E. A. Edwards. 1998. Anaerobic benzene biodegradation: a microcosm survey. Bioremediation J. 2:125-144. [Google Scholar]
- 43.Nesheim, J. C., and J. D. Lipscomb. 1996. Large kinetic isotope effects in methane oxidation catalyzed by methane monooxygenase: evidence for C-H bond cleavage in a reaction cycle intermediate. Biochemistry 35:10240-10247. [DOI] [PubMed] [Google Scholar]
- 44.Poulson, S. R., and J. I. Drever. 1999. Stable isotope (C, Cl, and H) fractionation during vaporization of trichloroethylene. Environ. Sci. Technol. 33:3689-3694. [Google Scholar]
- 45.Rittman, B. E., and P. L. McCarty. 2001. Environmental bio/technology: principles and applications. McGraw-Hill Inc., New York, N.Y.
- 46.Rudnicki, M. D., H. Elderfield, and B. Spiro. 2001. Fractionation of sulfur isotopes during bacterial sulfate reduction in deep ocean sediments at elevated temperatures. Geochim. Cosmochim. Acta 65:777-789. [Google Scholar]
- 47.Sherwood Lollar, B., G. F. Slater, J. Ahad, B. Sleep, J. Spivack, M. Brennan, and P. MacKenzie. 1999. Contrasting carbon isotope fractionation during biodegradation of trichloroethylene and toluene: implications for intrinsic bioremediation. Org. Geochem. 30:813-820. [Google Scholar]
- 48.Slater, G. F., J. M. E. Ahad, B. Sherwood Lollar, R. Allen-King, and B. Sleep. 2000. Carbon isotope effects resulting from equilibrium sorption of dissolved VOCs. Anal. Chem. 72:5669-5672. [DOI] [PubMed] [Google Scholar]
- 49.Slater, G. F., H. S. Dempster, B. Sherwood Lollar, and J. Ahad. 1999. Headspace analysis: a new application for isotopic characterization of dissolved organic contaminants. Environ. Sci. Technol. 33:190-194. [Google Scholar]
- 50.Slater, G. F., B. Sherwood Lollar, B. E. Sleep, and E. A. Edwards. 2001. Variability in carbon isotopic fractionation during biodegradation of chlorinated ethenes: implications for field applications. Environ. Sci. Technol. 35:901-907. [DOI] [PubMed] [Google Scholar]
- 51.Smith, M. R. 1990. The biodegradation of aromatic hydrocarbons by bacteria. Biodegradation 1:191-206. [DOI] [PubMed] [Google Scholar]
- 52.Snyder, R. 2000. Overview of the toxicology of benzene. J. Toxicol. Environ. Health 61:339-346. [DOI] [PubMed] [Google Scholar]
- 53.Stehmeier, L. G., M. M. Francis, T. R. Jack, E. Diegor, L. Winsor, and T. A. Abrajano. 1999. Field and in vitro evidence for in-situ bioremediation using compound-specific 13C/12C ratio monitoring. Org. Geochem. 30:821-833. [Google Scholar]
- 54.Ulrich, A. C., and E. A. Edwards. 2002. Physiological and molecular characterization of anaerobic benzene-degrading mixed cultures. Environ. Microbiol., in press. [DOI] [PubMed]
- 55.Ward, J. A. M., J. M. E. Ahad, G. Lacrampe-Couloume, G. F. Slater, E. A. Edwards, and B. Sherwood Lollar. 2000. Hydrogen isotope fractionation during methanogenic degradation of toluene: potential for direct verification of bioremediation. Environ. Sci. Technol. 34:4577-4581. [Google Scholar]
- 56.Weiner, J. M., T. S. Lauck, and D. R. Lovley. 1998. Enhanced anaerobic benzene degradation with the addition of sulfate. Bioremediation J. 2:159-173. [Google Scholar]
- 57.Wilkins, P. C., H. Dalton, C. J. Samuel, and J. Green. 1994. Further evidence for multiple pathways in soluble methane-monooxygenase-catalysed oxidations from the measurement of deuterium kinetic isotope effects. Eur. J. Biochem. 226:555-560. [DOI] [PubMed] [Google Scholar]