Abstract
The 4-hydroxyacetophenone monooxygenase (HAPMO) from Pseudomonas fluorescens ACB catalyzes NADPH- and oxygen-dependent Baeyer-Villiger oxidation of 4-hydroxyacetophenone to the corresponding acetate ester. Using the purified enzyme from recombinant Escherichia coli, we found that a broad range of carbonylic compounds that are structurally more or less similar to 4-hydroxyacetophenone are also substrates for this flavin-containing monooxygenase. On the other hand, several carbonyl compounds that are substrates for other Baeyer-Villiger monooxygenases (BVMOs) are not converted by HAPMO. In addition to performing Baeyer-Villiger reactions with aromatic ketones and aldehydes, the enzyme was also able to catalyze sulfoxidation reactions by using aromatic sulfides. Furthermore, several heterocyclic and aliphatic carbonyl compounds were also readily converted by this BVMO. To probe the enantioselectivity of HAPMO, the conversion of bicyclohept-2-en-6-one and two aryl alkyl sulfides was studied. The monooxygenase preferably converted (1R,5S)-bicyclohept-2-en-6-one, with an enantiomeric ratio (E) of 20, thus enabling kinetic resolution to obtain the (1S,5R) enantiomer. Complete conversion of both enantiomers resulted in the accumulation of two regioisomeric lactones with moderate enantiomeric excess (ee) for the two lactones obtained [77% ee for (1S,5R)-2 and 34% ee for (1R,5S)-3]. Using methyl 4-tolyl sulfide and methylphenyl sulfide, we found that HAPMO is efficient and highly selective in the asymmetric formation of the corresponding (S)-sulfoxides (ee > 99%). The biocatalytic properties of HAPMO described here show the potential of this enzyme for biotechnological applications.
In nature, many oxygenation reactions are carried out by flavin-dependent monooxygenases (24). The diversity of conversions that can be catalyzed is large; the reactions include Baeyer-Villiger reactions, aromatic hydroxylations, sulfoxidations, amine oxidations, and epoxidations. Many of these conversions occur with high enantio- and/or regioselectivity. The variety of reactivity is also reflected in the diverse physiological processes in which these enzymes play a prominent role, including xenobiotic compound metabolism in humans (49), biosynthesis of toxins (21), and pollutant degradation by bacteria (46).
Enantio- and regioselective oxygenations are often difficult to achieve by chemical means, while these types of reactions can lead to valuable optically active compounds. Due to their exquisite regio- and/or enantioselectivity and catalytic efficiency, flavin-dependent monooxygenases are useful biocatalysts for the synthesis of a variety of fine chemicals (12, 35, 41, 47). However, so far, only a limited number of flavin-dependent monooxygenase genes have been cloned and overexpressed, which has limited the application of these biocatalysts for synthetic processes.
The Baeyer-Villiger reaction, i.e., the oxidation of ketones or aldehydes by peroxides resulting in oxygen insertion adjacent to the carbonyl group (2), has many applications in organic chemistry (23). Its biological counterpart has been recognized in the oxygenation by flavoprotein monooxygenases of various ketones, including steroids (19), terpenes (32, 45), cyclopentanone (16), cyclohexanone (30), cyclododecanone (22), 2-tridecanone (5), and acetophenone derivatives (28, 44). So far, cyclohexanone monooxygenase (CHMO) is the only Baeyer-Villiger monooxygenase (BVMO) that has been extensively studied with regard to its catalytic and mechanistic properties. In the catalytic mechanism of this enzyme, the nucleophilic species that attacks the carbonyl group (38) is a peroxyflavin intermediate that is generated by reaction of the enzyme-bound flavin cofactor with NAD(P)H and oxygen. CHMO is able to catalyze a wide range of oxidative reactions, including enantioselective Baeyer-Villiger reactions (40), sulfoxidations (9), amine oxidations (31), and epoxidations (10). CHMO has been tested for several biocatalytic applications (37).
Recently, a BVMO called 4-hydroxyacetophenone monooxygenase (HAPMO) was isolated from Pseudomonas fluorescens ACB and its gene was cloned (20). This organism uses HAPMO for growth on 4-hydroxyacetophenone (18). The flavin-dependent monooxygenase was shown to be active on several acetophenone derivatives. In this study we examined the catalytic abilities of HAPMO in further detail. Our results show that this enzyme has a remarkably broad substrate range and is capable of enantioselective formation of lactones from ketones and stereoselective sulfoxidation.
MATERIALS AND METHODS
Chemicals.
Acetophenone, 4-hydroxyacetophenone, and 4-hydroxypropiophenone were obtained from Sigma. 4-Aminoacetophenone, 3-hydroxyacetophenone, methylphenyl sulfide, rac-methylphenyl sulfoxide, methyl 4-tolyl sulfide, rac-methyl 4-tolyl sulfoxide, (R)-methyl 4-tolyl sulfoxide, 2-acetylpyridine, 3-acetylpyridine, 4-acetylpyridine, acetol (hydroxyacetone), 1-indanone, 4-hydroxy 1-indanone, 4-chromanone, progesterone, and dihydrocarvone were obtained from Aldrich, and 2′-acetonaphthone was provided by Syncom B.V., Groningen, The Netherlands. Butyrophenone, 2-hydroxyacetophenone, and 2-acetylpyrrole were obtained from Acros. 4-Heptanone, 3-chloro-2-butanone, and 2,4-pentanedione were obtained from Fluka. rac-Bicyclo[3.2.0]hept-2-en-6-one and 4-hydroxybenzoate were obtained from Merck.
Enzyme expression and purification.
HAPMO was purified from Escherichia coli TOP10 carrying the cloned gene (hapE) from P. fluorescens ACB (20). For efficient expression the hapE gene was cloned into a pBAD/myc-HisA vector. Optimal expression was achieved when cells were grown at 25°C with 0.002% (wt/vol) arabinose.
Enzyme activity measurements.
Unless indicated otherwise, all activity measurements were performed in air-saturated 50 mM potassium phosphate buffer (pH 7.5). Enzyme concentrations were determined spectrophotometrically by using a molar extinction coefficient of 12.4 mM−1 cm−1 at 440 nm for protein-bound flavin adenine dinucleotide (20). Enzyme activities were determined spectrophotometrically at 30°C by monitoring the decrease in absorbance at 370 nm due to NADPH oxidation (ɛ370= 2.7 mM−1 cm−1). This wavelength was chosen since 4-hydroxyacetophenone displays significant absorbance at 340 nm, the λmax of NADPH. The standard assay mixture (1 ml) contained 250 μM NADPH and 1 mM substrate. Stock solutions of substrates (1 M) with poor solubility in water were made in dimethyl sulfoxide or dimethyl formamide.
Steady-state kinetic parameters of the different substrates were determined by using 8 to 10 substrate concentrations ranging from 0 to 10 times the Km, with a maximum of 20 mM. Data were fitted by using the Michaelis-Menten equation in Origin 6.0 (Microcal Software, Inc.). When low Km values were observed (with 4-aminoacetophenone, 4-hydroxyacetophenone, and 4-hydroxypropiophenone), apparent kinetic parameters were obtained from progress curves. To do this, the decrease in absorbance at 370 nm was monitored throughout the conversion of the limiting aromatic substrate while excess amounts of the nonlimiting reagents (NADPH and oxygen) were present.
Formation of hydrogen peroxide due to nonproductive NADPH oxidation was measured indirectly by measuring oxygen concentrations. After completion of a HAPMO-catalyzed conversion in air-saturated buffer, as shown by depletion of oxygen, a catalytic amount of catalase (50 U) was added, which converted any hydrogen peroxide formed into water and oxygen. Oxygen consumption and formation were monitored in a 1-ml stainless steel stirred vessel by using an optical MOPS-1 oxygen sensor (Comte, Hannover, Germany). By using this method hydrogen peroxide concentrations higher than 25 μM, corresponding to a detection limit of 10% uncoupling, could be measured.
Product identification.
For gas chromatography (GC)-mass spectrometry analysis, samples (typically 0.2 or 0.4 ml) were extracted with an equal volume of ethyl acetate containing dodecane or hexadecane as an internal standard. The extracts were dried over anhydrous magnesium sulfate. The analysis was performed with a Hewlett-Packard HP5890 gas chromatograph equipped with an HP-5 column and an HP5971 mass spectrometer. The products formed when 4-hydroxyacetophenone, acetophenone, 2-hydroxyacetophenone, 2-acetylpyrrole, and 2-acetylfuran were the substrates were identified as the corresponding acetate esters.
Products formed upon conversion of 4-hydroxybenzaldehyde were identified by nuclear magnetic resonance (NMR). To do this, a mixture containing 5 mM 4-hydroxybenzaldehyde, 5 mM NADPH, and 0.53 μM enzyme was incubated in O2-saturated phosphate buffer (in D2O). NMR spectra were obtained with a Varian VXR 300 instrument. Substrate and product peaks could be assigned from spectra of the reference compounds, as follows: 4-hydroxybenzaldehyde 1H NMR (D2O) δ 6.99 (d, J = 8.8 Hz, 2 H), 7.90 (d, J = 8.8 Hz, 2 H), 9.74 (s, 1 H); hydroquinone 1H NMR (D2O) δ 6.82 (s, 4 H); 4-hydroxybenzoate 1H NMR (D2O) δ 6.91 (d, J = 8.4 Hz, 2 H), 7.81 (d, J = 8.4 Hz, 2 H).
To determine the enantioselectivity of HAPMO with bicyclo[3.2.0]hept-2-en-6-one, the racemic substrate at a concentration of 5 mM was incubated under air with continuous stirring at 25°C with 7 mM NADPH and 1 μM HAPMO. Samples were extracted with an equal volume of ethyl acetate containing dodecane as an internal standard, and extracts were analyzed by chiral GC by using a Hewlett-Packard HP6890 gas chromatograph equipped with a ChromPack CP-cyclodextrin-β-2,3,6-M-19 column. Products were identified by comparing retention times and elution orders with retention times and elution orders described previously (48). The retention times (tr) were as follows: (1R,5S)-1, 5.41 min; (1S,5R)-1, 5.10 min; (1R,5S)-2, 12.62 min; (1S,5R)-2, 13.05 min; (1R,5S)-3, 12.36 min; and (1S,5R)-3, 12.85 min.
To monitor the asymmetric sulfoxidation of methyl 4-tolyl sulfide, the reaction mixture (1 ml) contained 5 mM methyl 4-tolyl sulfide, 2 mM NADPH, and 0.1 μM HAPMO. After 30 min of incubation the mixture was extracted with diethyl ether and dried over anhydrous sodium sulfate. Samples were injected into a Chiraldesc G-TA column (30 m by 0.25 mm). The temperature gradient was as follows: increase from 150 to 170°C at a rate of 10°C min−1; 13 min at 170°C; and decrease from 170 to 150°C at a rate of 10°C min−1. The retention times for the remaining substrate and the (R)- and (S)-sulfoxide isomers formed were 3.2, 10.5, and 11.0 min, respectively.
To determine the enantioselectivity of HAPMO with methylphenyl sulfide, a high-performance liquid chromatography (HPLC) method was employed. To do this, the reaction mixture (1 ml) contained 1 mM methylphenyl sulfide, 1 mM NADPH, and 0.1 μM HAPMO. After 30 min of incubation at room temperature, the mixture was extracted with 1.5 ml of diethyl ether. The enantiomeric excess was determined by chiral HPLC analysis by using a Chiralcel OD column. The elution order of the (R)- and (S)-methylphenyl sulfoxides formed [tr(R) = 11.7 min; tr(S) = 14.2 min] was adapted from Brunel et al. (6).
The enantiomeric ratios (E values) for kinetic resolution of substrates were calculated as follows: E = ln[(1 − c)(1 − eeS)]/ln[(1 − c)(1 + eeS)], where c is the extent of conversion calculated from c = 1 − [substrate]/[substratet = 0] and eeS is the enantiomeric excess of the substrate calculated from eeS = |([sR] − [sS])/([sR] + [sS])|. The formulas were obtained from reference 42.
RESULTS AND DISCUSSION
Optimization of the expression system.
To improve the level of expression of HAPMO in E. coli, the hapE gene was cloned into a pBAD vector under control of the PBAD promoter. Expression was induced by adding arabinose to the media. As with the previous construct with a pET5-a vector, production of soluble and active enzyme was observed only when the cells were grown at temperatures below 30°C. Therefore, the cells were routinely cultivated at 25°C. Expression was most efficient when 0.002% (wt/vol) arabinose was used. Lower concentrations decreased the level of expression, while higher concentrations decreased the yield of biomass. When the optimized conditions were used, the cellular levels of HAPMO typically accounted for 30% of the total protein in a cell extract. Enzyme could be purified to homogeneity in a two-step procedure as described previously, which yielded fully flavinylated and active enzyme (20).
HAPMO displays broad substrate specificity.
In the absence of a suitable substrate, HAPMO displays weak NADPH oxidase activity with a maximal turnover rate of 0.11 s−1 (20). A similar low rate of nonproductive oxidation of NADPH has been found for CHMO (34). However, when both NADPH and a suitable ketone substrate were present, no formation of hydrogen peroxide was detected (Table 1). This indicates that nonproductive oxidation of NADPH does not play a significant role during catalysis. The direct coupling of substrate conversion with NADPH consumption prevents nonproductive oxidation of NADPH. This coupling has also been observed with other flavin-dependent monooxygenases (13, 43).
TABLE 1.
Steady-state kinetic parameters for HAPMO
| Substrate | Structure | Km (μM) | kcat (s−1) | kcat/Km (103 s−1 · M−1) |
|---|---|---|---|---|
| Aromatic compounds | ||||
 |
||||
| 4-Hydroxyacetophenoneb,d | 9.2 (0.5)c | 12.6 (0.8) | 1,400 | |
| 4-Hydroxypropiophenonea,b | 2.4 (0.2) | 11.9 (1.2) | 5,000 | |
| 4-Hydroxybenzaldehydea | 100 (20) | 7.6 (0.3) | 75 | |
| 4-Aminoacetophenoneb | 0.82 (0.1) | 12.7 (0.7) | 15,000 | |
| 4-Fluoroacetophenonea | 1,000 (300) | 0.60 (0.1) | 0.60 | |
| 4-Methylacetophenonea | 160 (40) | 6.3 (0.5) | 39 | |
| 4-Methoxyacetophenonea | 540 (120) | 1.7 (0.2) | 3.1 | |
| 2-Hydroxyacetophenone | 610 (120) | 6.7 (0.3) | 20 | |
| 3-Hydroxyacetophenone | 1,400 (200) | 4.8 (0.3) | 3.4 | |
| Benzaldehyded | 1,600 (200) | 2.2 (0.2) | 1.4 | |
| Acetophenoned | 2,300 (900) | 13.2 (1.7) | 5.7 | |
| Propiophenone | 530 (100) | 11.0 (0.2) | 21 | |
| Butyrophenone | 2,000 (700) | 1.2 (0.3) | 0.60 | |
| Isobutyrophenone | 540 (40) | 2.5 (0.1) | 4.6 | |
| Methylphenyl sulfide | 1,400 (400) | 4.7 (0.6) | 3.4 | |
| Methyl 4-tolyl sulfided | 370 (50) | 7.3 (0.6) | 20 | |
| Heteroaromatic compounds | ||||
| 2-Acetylpyridine |  |
1,200 (90) | 7.8 (0.2) | 6.5 |
| 4-Acetylpyridine | 1,900 (100) | 0.5 (0.2) | 0.3 | |
| 2-Acetylpyrrole | 330 (20) | 9.4 (0.14) | 28 | |
| 2-Pyrrole carboxaldehyded | 410 (30) | 8.6 (0.2) | 21 | |
| Aliphatic compounds | ||||
| Acetylcyclohexane |  |
4,800 (900) | 3.9 (0.3) | 0.81 |
| Cyclohexane carboxaldehyded | 3,000 (500) | 5.0 (0.4) | 1.7 | |
| Hydroxyacetone (acetol) | 29,000 (3,000) | 4.1 (0.3) | 0.14 | |
| 3-Chloro-2-butanone | 23,000 (4,000) | 7.4 (1.0) | 0.32 | |
| 2,4-Pentanedione | 4,900 (900) | 2.1 (0.5) | 0.43 | |
| rac-Bicyclo[3.2.0]hept-2-en-6-one | 3,300 (300) | 6.7 (0.3) | 2.0 |
The values for these compounds were obtained from reference 20 and were determined at pH 8.0. All other values were determined at pH 7.5.
There are data for these compounds in reference 20, but values were determined again by progress curve analysis at pH 7.5 (see Materials and Methods).
The values in parentheses are standard errors.
These substrates were checked for hydrogen peroxide generation.
To explore the catalytic capacity of HAPMO, we tested a range of potential substrates. The enzyme activity with all compounds was assayed by measuring consumption of NADPH. The turnover rates for all substrates identified were well above the rate of nonproductive NADPH oxidation. The compounds that were shown to be converted could be grouped into three classes: (i) aromatic compounds resembling the physiological substrate, (ii) heteroaromatic compounds, and (iii) aliphatic compounds (Table 1). The compounds that did not show significant activity (<0.1 μmol of NADPH/min/mg of protein) at a concentration of 1.0 mM were 2-nitroacetophenone, 4-nitroacetophenone, benzophenone, benzoin, 2′-acetonaphthone, 3-acetylpyridine, benzoic acid, methyl 4-hydroxybenzoate, benzamide, N,N-dimethylaniline, phenylacetone, 1-indanone, 4-hydroxy-1-indanone, 1,3-indanone, 4-chromanone, cyclopentanone, cyclohexanone, progesterone, dihydrocarvone, acetone, butanone, and 4-heptanone.
Conversion of aromatic substrates.
It was found that the enzyme was quite relaxed in converting acetophenone derivatives. Both substitutions on the phenyl ring and modifications of the aceto function were accepted. However, depending on the nature and the position of the substituents, these substrate modifications could have effects on both Km and kcat values (Table 1). Conversion of aromatic ketones into the corresponding esters by an isoenzyme of HAPMO was reported previously by Tanner and Hopper (44). Our data only allow comparison of the Km values for acetophenone, 4-hydroxyacetophenone, and 4-hydroxypropiophenone, which are in the same range as the values obtained for HAPMO.
If Km is used as a parameter for substrate affinity, substituents at the para position of acetophenone derivatives appear to be critical for substrate recognition. A hydroxy or amino substituent at this position results in relatively low Km values, whereas hydrophobic or bulky substituents decrease the affinity. The presence of a nitro substituent at the para position prevents activity, as 4-nitroacetophenone is not converted by HAPMO. 2-Hydroxyacetophenone and 3-hydroxyacetophenone display similar kinetic parameters when they are compared with acetophenone, indicating that substitutions at the meta and ortho positions do not result in significant effects on the kinetic parameters. Except for low activity with 4-fluoroacetophenone, the kcat values for all the acetophenone derivatives tested are in the same range (1.7 to 13.2 s−1), indicating that substitutions on the ring do not significantly influence the maximal turnover rate. As a consequence, the catalytic efficiency (kcat/Km) for most acetophenones is determined mainly by the Km.
Modification of the aceto function influences both the affinity and the rate of catalysis. Whereas benzaldehyde displays a lower kcat than acetophenone and a similar Km, an additional methyl group (propiophenone) results in increased affinity but a similar kcat. More bulky substituents (butyrophenone, isobutyrophenone) result in decreased kcat values, suggesting that a propionyl function is optimal for catalysis.
The kinetic behavior of HAPMO with its physiological substrate, 4-hydroxyacetophenone, is significantly affected by pH. In the pH range from 7 to 9, a strong increase in the apparent Km was observed, whereas the apparent kcat appeared to be hardly affected (Table 2). The effect on the Km appears to be related to the (de)protonation of the para-hydroxyl group of the substrate (pKa = 7.99 [39]). This was confirmed by the finding that the kinetic parameters for the analogous compound 4-aminoacetophenone, which remains neutral in the pH range tested (pKa = 2.75), do not display pH dependency (Table 2). The latter compound is converted with a similar kcat and, on the basis of the kcat/Km ratio, is an even better substrate for HAPMO than 4-hydroxyacetophenone. The observed values may be accounted for by the assumption that the deprotonated form of 4-hydroxyacetophenone has less affinity for the active site, while the protonated form has an apparent Km of about 6 μM.
TABLE 2.
Influence of pH on the steady-state kinetic parameters of HAPMO for 4-hydroxyacetophenone and 4-aminoacetophenone
| pH | 4-Hydroxyacetophenone
|
4-Aminoacetophenone
|
||
|---|---|---|---|---|
| Km (μM) | kcat (s−1) | Km (μM) | kcat (s−1) | |
| 7.0 | 5.1 | 10.4 | 0.87 | 11.0 |
| 8.0 | 12 | 12.8 | 0.82 | 12.7 |
| 9.0 | 70 | 14.5 | 0.99 | 11.1 |
As HAPMO readily converted 4-hydroxybenzaldehyde, we determined the products formed upon conversion of this aldehyde. For CHMO it has been shown that aromatic aldehydes are converted to the corresponding esters and acids, implying that carbon migration and hydrogen migration, respectively, occur (4). To test the regioselectivity of oxygenation by HAPMO, we determined the formation of the ester and/or the acid upon conversion of 4-hydroxybenzaldehyde by using 1H NMR. During conversion of the aromatic aldehyde, formation of the corresponding 4-hydroxyphenol formate ester was observed (doublets at 7.1 and 7.3 ppm). No formation of 4-hydroxybenzoate was detected. In addition to formation of the ester, formation of hydroquinone was also observed. This indicates that the ester formed was readily hydrolyzed to hydroquinone. In line with this, prolonged incubation of the reaction mixture resulted in the formation of only one product, hydroquinone. Thus, during oxygenation of 4-hydroxybenzaldehyde, HAPMO exclusively directs the aromatic ring to migrate, resulting in a typical Baeyer-Villiger reaction.
HAPMO catalyzes enantioselective sulfoxidations.
In addition to converting ketones and aldehydes, BVMOs are also known to catalyze sulfoxidation reactions. In fact, CHMO has been extensively studied as a biocatalyst for enantioselective sulfoxidations of a variety of sulfides (7, 11), which is important since enantiomerically pure sulfoxides are valuable building blocks for the pharmaceutical industry (8). We tested the enantioselectivity of HAPMO towards two alkyl aryl sulfides, methylphenyl sulfide and methyl 4-tolyl sulfide. Both sulfides were converted at reasonable rates (kcat= 4.7 and 7.3 s−1), while the Km values were relatively high (1.4 and 0.37 mM). However, when these kinetic parameters of the sulfides were compared with those of the carbonylic counterparts (acetophenone and 4-methylacetophenone, respectively), we concluded that the enzyme does not show preference towards sulfides or Baeyer-Villiger substrates as the kinetic parameters were very similar.
Product analysis with chiral GC revealed that methyl 4-tolyl sulfide was oxidized to the (S)-oxide with a high ee (>99%). No formation of (R)-oxide was observed (Fig. 1). CHMO also has a preference for the (S)-oxide to be formed (7). However, while with CHMO an ee of only 37% is observed, HAPMO is highly enantioselective. Oxidation of methylphenyl sulfide by HAPMO also leads to formation of the (S)-sulfoxide, again displaying outstanding enantioselectivity (ee, >99%). Interestingly, oxidation of methylphenyl sulfide by CHMO was shown to lead to formation of the opposite (R) enantiomer, with an ee of 99% (7). This difference in enantioselectivity was not observed for HAPMO.
FIG. 1.
Enantioselective oxidation of methyl 4-tolyl sulfide by HAPMO. (A) Gas chromatogram of the two isomeric sulfoxide products [tr(R) = 10.5 min and tr(S) = 11.0 min]. The retention times of the (R) and (S) enantiomers were assigned by analyzing a sample containing only (R)-methyl 4-tolyl sulfoxide. (B) Chromatogram after reaction of HAPMO with the substrate for 30 min.
Conversion of heteroaromatic compounds.
In addition to aromatic compounds, some heteroaromatic compounds were tested as substrates. To our knowledge, such compounds have not been identified before as BVMO substrates. Most heteroaromatic compounds tested were efficiently converted, indicating that HAPMO is active not only with benzylic compounds (Table 1). The kinetic parameters of these substrates were in the same range as the values obtained for acetophenone derivatives. Of the two ring systems tested, the pyrrole ring is preferred over pyridine. Furthermore, it is interesting that while 2-acetyl- and 4-acetylpyridines are converted, 3-acetylpyridine is not a substrate for HAPMO. Product analysis by GC-mass spectrometry revealed that 2-acetylpyrrole was converted to the ester, indicating that the regioselectivity is similar to that of the acetophenone derivatives.
Conversion of aliphatic compounds.
In addition to the compounds mentioned above, we found that HAPMO is also able to convert aliphatic compounds, such as acetylcyclohexane, cyclohexane carboxyaldehyde, and 2,4-pentanedione. Steady-state kinetic analysis with these substrates showed that catalysis was not very efficient. This was mainly due to the relatively high Km values (1 to 30 mM). For most of these substrates, conversion by a BVMO has not been reported previously, and thus our findings complement the biocatalytic potential of BVMOs.
Several other aliphatic ketones have been shown to be substrates for other microbial BVMOs; these compounds include acetol (17), cyclohexanone (29), cyclopentanone (16), progesterone (19, 26), and dihydrocarvone (45). Of these compounds, only acetol was converted by HAPMO. However, the Km for this relatively small substrate was found to be exceptionally high, which resulted in low catalytic efficiency. All other ketones tested were not converted by HAPMO, indicating that different BVMOs have evolved to act on specific types of ketones.
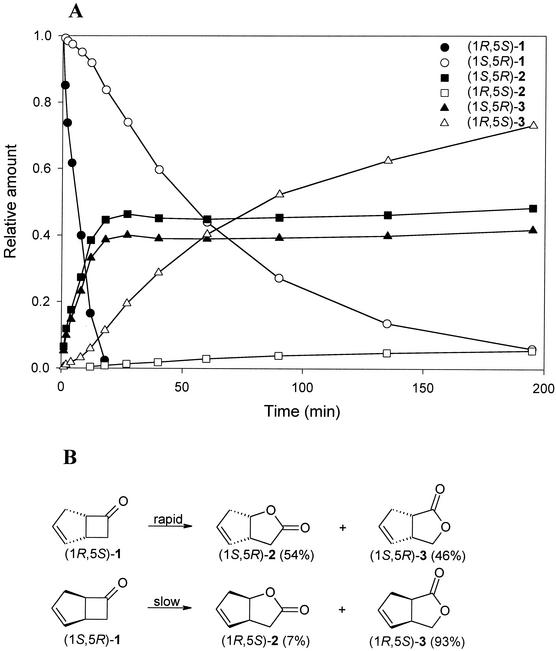
Enantioselective conversion of bicyclohept-2-en-6-one.
Enantioselective conversions of various bicyclic ketones have been studied with CHMO (33). In these conversions enantioselectivity and regioselectivity are intertwined, as oxygen insertion may occur on either side of the carbonyl group. For example, with bicyclohept-2-en-6-one (Fig. 2), CHMO displays enantiodivergent conversion, which yields both 2-oxabicyclo[3.3.0]oct-6-en-3-one (1S,5R)-2 from (1R,5R)-1 and 3-oxabicyclo[3.3.0]oct-6-en-2-one (1R,5S)-3 from (1S,5R)-1, each with an ee of >95% (1, 48). These chiral lactones are valuable building blocks for the chemical synthesis of prostaglandins (3). HAPMO is also able to convert the bicyclic ketone (Table 1). HAPMO displays a significant preference for the (1R,5S) enantiomer, as reflected in an E value of about 20 (Fig. 2). In contrast to the results obtained with CHMO, the conversion of this enantiomer by HAPMO proceeds with hardly any regioselectivity, as reflected by the formation of the two possible lactone products, (1S,5R)-2 and (1S,5R)-3, in almost equal amounts. Even though much more regioselectivity is encountered in the conversion of the other enantiomer, the ee values of the product mixture resulting from complete conversion are too low to be of practical significance, viz., 77% for (1S,5R)-2 and 34% for (1R,5S)-3. Nonetheless, HAPMO may be suitable for kinetic resolution of 1 to isolate the (1S,5R) enantiomer, with concomitant formation of (1S,5R)-2 and (1S,5R)-3 in fair enantiomeric excess.
FIG. 2.
(A) Time course of HAPMO-catalyzed oxidation of racemic bicyclo[3.2.0]hept-2-en-6-one (1) to 2-oxabicyclo[3.3.0]oct-6-en-3-one (2) and 3-oxabicyclo[3.3.0]oct-6-en-2-one (3). The reaction was performed at 25°C, and samples were extracted with ethyl acetate and analyzed by GC with a chiral column. (B) Structures of bicyclo[3.2.0]hept-2-en-6-one isomers and lactones formed by HAPMO activity.
HAPMO can catalyze a broad range of oxygenation reactions.
When the kinetic parameters of all the substrates are compared, it is apparent that the observed kcat values are within a limited range (typically 1 to 10 s−1). This indicates that the chemical reactivities of the different substrates have only marginal effects on the rate of catalysis. In contrast to the rather invariable kcat values, the Km values are more sensitive to substrate modification. The Km values range from 0.82 μM to 29 mM. The variability observed for the Km values suggests that although the enzyme is able to accommodate a wide range of different molecules, the substrate specificity is strongly dictated by the architecture of the active site. When the Km values for different types of substrates are compared, it appears that a suitable substrate for HAPMO must have a certain degree of hydrophobicity. Also, the pH dependency of the Km for 4-hydroxyacetophenone indicates that binding of uncharged acetophenone is preferred. The fact that 4-nitroacetophenone is not converted by HAPMO is in agreement with this.
Industrial synthesis of a phenol- or catechol-containing compound often requires protection of the hydroxyl group(s) to prevent oxidation reactions. Ethers are the most widely used protective groups, but esterification is also an important alternative (15). Production of acylated phenols and catechols by using HAPMO, starting from (ring-substituted) aromatic ketones, might provide an attractive biocatalytic alternative for the synthesis of protected phenols and partially protected catechols (27).
At present, only a small number of flavin-dependent monooxygenases have been produced and successfully used for industrial purposes. However, recent studies have indicated that these types of biocatalysts are very valuable synthetic tools (25, 36, 37). Furthermore, a newly recognized BVMO-specific sequence motif has revealed a large number of BVMO genes in sequenced microbial genomes (14). This indicates that this specific class of monooxygenases is a promising source for novel biocatalysts.
The work described here shows that HAPMO is a new and attractive novel biocatalyst as it is able to catalyze a large variety of monooxygenation reactions while exhibiting remarkable selectivity. With the identification of the substrate range of HAPMO, the scope of reactions that can be catalyzed by a flavin-dependent monooxygenase is greatly expanded.
Acknowledgments
This research was funded through the Biotechnology Programme of the European Commission (project BIO4980267; Biotransformations Using Baeyer-Villiger Monooxygenases) and by the Council for Chemical Sciences of The Netherlands Organization for Scientific Research (CW-NWO) through the program Procesvernieuwing voor een schoner milieu.
We thank Marc van Gelder for his assistance with the GC and HPLC analyses. Erik de Vries is acknowledged for help with the NMR measurements.
REFERENCES
- 1.Alphand, V., A. A. Archelas, and R. Furstoss. 1989. Microbial transformations. 16. One-step synthesis of a pivotal prostaglandin chiral synthon via a highly enantioselective microbiological Baeyer-Villiger type reaction. Tetrahedron Lett. 30:3663-3664. [Google Scholar]
- 2.Baeyer, A., and V. Villiger. 1899. Einwirkung des Caro'schen Reagens auf Ketone. Ber. Dtsch. Chem. Ges. 32:3625-3633.
- 3.Banerjee, A. 2000. Stereoselective microbial Baeyer-Villiger oxidations, p. 867-876. In R. N. Patel (ed.), Stereoselective biocatalysis. Marcel Dekker, Inc., New York, N.Y.
- 4.Branchaud, B. P., and C. T. Walsh. 1985. Functional group diversity in enzymatic oxygenation reactions catalyzed by bacterial flavin-containing cyclohexanone monooxygenase. J. Am. Chem. Soc. 107:2153-2161. [Google Scholar]
- 5.Britton, L. N., and A. J. Markovetz. 1977. A novel ketone monooxygenase from Pseudomonas cepacia. Purification and properties. J. Biol. Chem. 252:8561-8566. [PubMed] [Google Scholar]
- 6.Brunel, J.-M., P. Diter, M. Duetsch, and H. B. Kagan. 1995. Highly enantioselective oxidation of sulfides mediated by a chiral titanium complex. J. Org. Chem. 60:8086-8088. [Google Scholar]
- 7.Carrea, G., B. Redigolo, S. Riva, S. Colonna, N. Gaggero, E. Battistel, and D. Bianchi. 1992. Effects of substrate structure on the enantioselective and stereochemical course of sulfoxidation catalyzed by cyclohexanone monooxygenase. Tetrahedron Asymm. 3:1063-1068. [Google Scholar]
- 8.Carreno, M. C. 1995. Application of sulfoxides to asymmetric synthesis of biologically active compounds. Chem. Rev. 95:1717-1760. [Google Scholar]
- 9.Chen, G., M. M. Kayser, M. D. Mihovilovic, M. E. Mrstik, C. A. Martinez, and J. D. Stewart. 1999. Asymmetric oxidations at sulfur catalyzed by engineered strains that overexpress cyclohexanone monooxygenase. New J. Chem. 23:827-832. [Google Scholar]
- 10.Colonna, S., N. Gaggero, G. Carrea, G. Ottolina, P. Pasta, and F. Zambianchi. 2002. First asymmetric epoxidation catalysed by cyclohexanone monooxygenase. Tetrahedron Lett. 43:1797-1799. [Google Scholar]
- 11.Colonna, S., N. Gaggero, P. Pasta, and G. Ottolina. 1996. Enantioselective oxidation of sulfides to sulfoxides catalysed by bacterial cyclohexanone monooxygenase. Chem. Commun. 20:2303-2307. [Google Scholar]
- 12.Duetz, W. A., J. B. van Beilen, and B. Witholt. 2001. Using proteins in their natural environment: potential and limitations of microbial whole-cell hydroxylations in applied biocatalysis. Curr. Opin. Biotechnol. 12:419-425. [DOI] [PubMed] [Google Scholar]
- 13.Entsch, B., and W. J. H. van Berkel. 1995. Structure and mechanism of para-hydroxybenzoate hydroxylase. FASEB J. 9:476-483. [DOI] [PubMed] [Google Scholar]
- 14.Fraaije, M. W., N. M. Kamerbeek, W. J. H. van Berkel, and D. B. Janssen. 2002. Identification of a Baeyer-Villiger monooxygenase sequence motif. FEBS Lett. 518:43-47. [DOI] [PubMed] [Google Scholar]
- 15.Greene, T. W., and P. G. M. Wuts. 1999. Protective groups in organic synthesis, 3rd ed. Wiley, New York, N.Y.
- 16.Griffin, M., and P. W. Trudgill. 1976. Purification and properties of cyclopentanone oxygenase of Pseudomonas NCIB 9872. Eur. J. Biochem. 63:199-209. [DOI] [PubMed] [Google Scholar]
- 17.Hartmans, S., and J. A. M. de Bont. 1986. Acetol monooxygenase from Mycobacterium Py1 cleaves acetol into acetate and formaldehyde. FEMS Microbiol. Lett. 36:155-158. [Google Scholar]
- 18.Higson, F. K., and D. D. Focht. 1990. Bacterial degradation of ring-chlorinated acetophenones. Appl. Environ. Microbiol. 56:3678-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itagaki, E. 1986. Studies on steroid monooxygenase from Cylindrocarpon radicicola ATCC 11011. Purification and characterization. J. Biochem. 99:815-824. [DOI] [PubMed] [Google Scholar]
- 20.Kamerbeek, N. M., M. J. Moonen, J. G. Van Der Ven, W. J. H. Van Berkel, M. W. Fraaije, and D. B. Janssen. 2001. 4-Hydroxyacetophenone monooxygenase from Pseudomonas fluorescens ACB. A novel flavoprotein catalyzing Baeyer-Villiger oxidation of aromatic compounds. Eur. J. Biochem. 268:2547-2557. [DOI] [PubMed] [Google Scholar]
- 21.Keller, N. P., C. M. Watanabe, H. S. Kelkar, T. H. Adams, and C. A. Townsend. 2000. Requirement of monooxygenase-mediated steps for sterigmatocystin biosynthesis by Aspergillus nidulans. Appl. Environ. Microbiol. 66:359-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostichka, K., S. M. Thomas, K. J. Gibson, V. Nagarajan, and Q. Cheng. 2001. Cloning and characterization of a gene cluster for cyclododecanone oxidation in Rhodococcus ruber SC1. J. Bacteriol. 183:6478-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krow, G. R. 1993. The Baeyer-Villiger oxidation of ketones and aldehydes. Org. React. 43:251-798.
- 24.Massey, V. 2000. The chemical and biological versatility of riboflavin. Biochem. Soc. Trans. 28:283-296. [PubMed] [Google Scholar]
- 25.Mihovilovic, M. D., B. Muller, M. M. Kayser, J. D. Stewart, J. Frohlich, P. Stanetty, and H. Spreitzer. 2001. Baeyer-Villiger oxidations of representative heterocyclic ketones by whole cells of engineered Escherichia coli expressing cyclohexanone monooxygenase. J. Mol. Catal. B Enzymatic 11:349-353. [Google Scholar]
- 26.Miyamoto, M., J. Matsumoto, T. Iwaya, and E. Itagaki. 1995. Bacterial steroid monooxygenase catalyzing the Baeyer-Villiger oxidation of C21-ketosteroids from Rhodococcus rhodochrous: the isolation and characterization. Biochim. Biophys. Acta 1251:115-124. [DOI] [PubMed] [Google Scholar]
- 27.Moonen, M. J., I. M. Rietjens, and W. J. van Berkel. 2001. 19F NMR study on the biological Baeyer-Villiger oxidation of acetophenones. J. Ind. Microbiol. Biotechnol. 26:35-42. [DOI] [PubMed] [Google Scholar]
- 28.Moonen, M. J. H., I. M. C. M. Rietjens, and W. J. H. van Berkel. 1999. Purification and some properties of acetophenone monooxygenase, p. 375-378. In S. Ghisla, P. Kroneck, P. Macheroux, and H. Sund (ed.), Flavins and flavoproteins. 13th International Congress on Flavins and Flavoproteins. Agency for Scientific Publication, Berlin, Germany.
- 29.Norris, D. B., and P. W. Trudgill. 1971. The metabolism of cyclohexanol by Nocardia globerula CL1. Biochem. J. 121:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norris, D. B., and P. W. Trudgill. 1972. The purification and properties of cyclohexanone oxygenase from Nocardia globerula CL 1. Biochem. J. 130:30P. [DOI] [PMC free article] [PubMed]
- 31.Ottolina, G., S. Bianchi, B. Belloni, G. Carrea, and B. Danieli. 1999. First asymmetric oxidation of tertiary amines by cyclohexanone monooxygenase. Tetrahedron Lett. 40:8483-8486. [Google Scholar]
- 32.Ougham, H. J., D. G. Taylor, and P. W. Trudgill. 1983. Camphor revisited: involvement of a unique monooxygenase in metabolism of 2-oxo-delta 3-4,5,5-trimethylcyclopentenylacetic acid by Pseudomonas putida. J. Bacteriol. 153:140-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts, S. M., and P. W. H. Wan. 1998. Enzyme-catalysed Baeyer-Villiger oxidations. J. Mol. Catal. B Enzymatic 4:111-136. [Google Scholar]
- 34.Ryerson, C. C., D. P. Ballou, and C. Walsh. 1982. Mechanistic studies on cyclohexanone oxygenase. Biochemistry 21:2644-2655. [DOI] [PubMed] [Google Scholar]
- 35.Schmid, A., J. S. Dordick, B. Hauer, A. Kiener, M. Wubbolts, and B. Witholt. 2001. Industrial biocatalysis today and tomorrow. Nature 409:258-268. [DOI] [PubMed] [Google Scholar]
- 36.Schmid, A., I. Vereyken, M. Held, and B. Witholt. 2001. Preparative regio- and chemoselective functionalization of hydrocarbons catalyzed by cell free preparations of 2-hydroxybiphenyl 3-monooxygenase. J. Mol. Catal. B Enzymatic 11:455-462. [Google Scholar]
- 37.Schwarz-Linek, U., A. Krodel, F.-A. Ludwig, A. Schulze, S. Rissom, U. Kragl, V. I. Tishkov, and M. Vogel. 2001. Synthesis of natural product precursors by Baeyer-Villiger oxidation with cyclohexanone monooxygenase from Acinetobacter. Synthesis 6:947-951.
- 38.Sheng, D., D. P. Ballou, and V. Massey. 2001. Mechanistic studies of cyclohexanone monooxygenase: chemical properties of intermediates involved in catalysis. Biochemistry 40:11156-11167. [DOI] [PubMed] [Google Scholar]
- 39.Stahl, N., and W. P. Jencks. 1986. Hydrogen bonding between solutes in aqueous solution. J. Am. Chem. Soc. 108:4196-4205. [Google Scholar]
- 40.Stewart, J. D. 1998. Cyclohexanone monooxygenase: a useful reagent for asymmetric Baeyer-Villiger reactions. Curr. Org. Chem. 2:195-216. [Google Scholar]
- 41.Stewart, J. D. 2000. Organic transformations catalyzed by engineered yeast cells and related systems. Curr. Opin. Biotechnol. 11:363-368. [DOI] [PubMed] [Google Scholar]
- 42.Straathof, A. J. J., and J. A. Jongejan. 1997. The enantiomeric ratio: origin, determination and prediction. Enzyme Microb. Technol. 21:559-571. [Google Scholar]
- 43.Suske, W. A., W. J. H. van Berkel, and H. P. E. Kohler. 1999. Catalytic mechanism of 2-hydroxybiphenyl 3-monooxygenase, a flavoprotein from Pseudomonas azelaica HBP1. J. Biol. Chem. 274:33355-33365. [DOI] [PubMed] [Google Scholar]
- 44.Tanner, A., and D. J. Hopper. 2000. Conversion of 4-hydroxyacetophenone into 4-phenyl acetate by a flavin adenine dinucleotide-containing Baeyer-Villiger-type monooxygenase. J. Bacteriol. 182:6565-6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Werf, M. J. 2000. Purification and characterization of a Baeyer-Villiger mono-oxygenase from Rhodococcus erythropolis DCL14 involved in three different monocyclic monoterpene degradation pathways. Biochem. J. 3:693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wieser, M., B. Wagner, J. Eberspacher, and F. Lingens. 1997. Purification and characterization of 2,4,6-trichlorophenol-4-monooxygenase, a dehalogenating enzyme from Azotobacter sp. strain GP1. J. Bacteriol. 179:202-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willetts, A. 1997. Structural studies and synthetic applications of Baeyer-Villiger monooxygenases. Trends Biotechnol. 15:55-62. [DOI] [PubMed] [Google Scholar]
- 48.Zambianchi, F., P. Pasta, G. Ottolina, G. Carrea, S. Colonna, N. Gaggero, and J. M. Ward. 2000. Effect of substrate concentration on the enantioselectivity of cyclohexanone monooxygenase from Acinetobacter calcoaceticus and its rationalization. Tetrahedron Asymm. 11:3653-3657. (Author's correction, 11:4817.)
- 49.Ziegler, D. M. 1990. Flavin-containing monooxygenases: enzymes adapted for multisubstrate specificity. Trends Pharmacol. Sci. 11:321-324. [DOI] [PubMed] [Google Scholar]