Abstract
The pathway from averufin (AVR) to versiconal hemiacetal acetate (VHA) in aflatoxin biosynthesis was investigated by using cell-free enzyme systems prepared from Aspergillus parasiticus. When (1′S,5′S)-AVR was incubated with a cell extract of this fungus in the presence of NADPH, versicolorin A and versicolorin B (VB), as well as other aflatoxin pathway intermediates, were formed. When the same substrate was incubated with the microsome fraction and NADPH, hydroxyversicolorone (HVN) and VHA were formed. However, (1′R,5′R)-AVR did not serve as the substrate. In cell-free experiments performed with the cytosol fraction and NADPH, VHA, versicolorone (VONE), and versiconol acetate (VOAc) were transiently produced from HVN in the early phase, and then VB and versiconol (VOH) accumulated later. Addition of dichlorvos (dimethyl 2,2-dichlorovinylphosphate) to the same reaction mixture caused transient formation of VHA and VONE, followed by accumulation of VOAc, but neither VB nor VOH was formed. When VONE was incubated with the cytosol fraction in the presence of NADPH, VOAc and VOH were newly formed, whereas the conversion of VOAc to VOH was inhibited by dichlorvos. The purified VHA reductase, which was previously reported to catalyze the reaction from VHA to VOAc, also catalyzed conversion of HVN to VONE. Separate feeding experiments performed with A. parasiticus NIAH-26 along with HVN, VONE, and versicolorol (VOROL) demonstrated that each of these substances could serve as a precursor of aflatoxins. Remarkably, we found that VONE and VOROL had ring-opened structures. Their molecular masses were 386 and 388 Da, respectively, which were 18 Da greater than the molecular masses previously reported. These data demonstrated that two kinds of reactions are involved in the pathway from AVR to VHA in aflatoxin biosynthesis: (i) a reaction from (1′S,5′S)-AVR to HVN, catalyzed by the microsomal enzyme, and (ii) a new metabolic grid, catalyzed by a new cytosol monooxygenase enzyme and the previously reported VHA reductase enzyme, composed of HVN, VONE, VOAc, and VHA. A novel hydrogenation-dehydrogenation reaction between VONE and VOROL was also discovered.
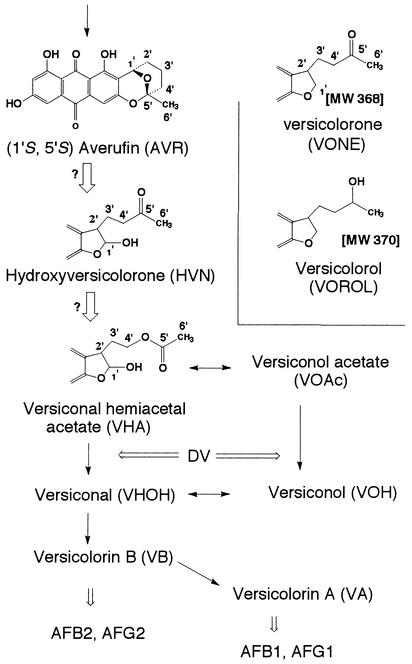
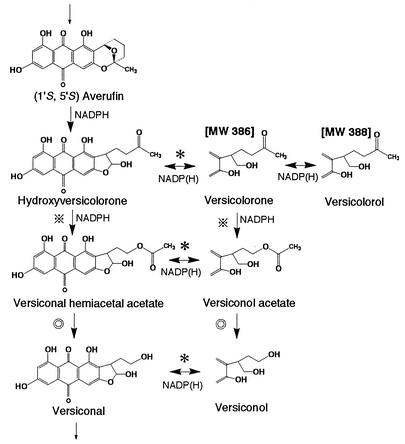
Aflatoxins are extremely potent carcinogenic substances produced by some strains of the common molds Aspergillus flavus, Aspergillus parasiticus, Aspergillus tamarii, and Aspergillus nomius. Although the biosynthetic pathways of aflatoxins have been well clarified (1, 3, 17, 20, 22, 23, 26-35), there are a few unknown steps, including the steps from averufin (AVR) to versiconal hemiacetal acetate (VHA) (Fig. 1). The metabolites and reactions of the portion of the aflatoxin pathway from AVR to versicolorin A (VA) are shown in Fig. 1. Only (1′S)-AVR, not (1′R)-AVR, is produced in the early phase of aflatoxin biosynthesis (15, 32). It has been reported previously that only one (1′S,5′R)-AVR enantiomer, not the other enantiomer, serves as a precursor of aflatoxins in feeding experiments (32). Note that although the absolute configurations of the bioactive AVR and the inactive AVR were written as (1′S,5′R) and (1′R,5′S), respectively, in a previous a paper (32), the correct forms are (1′S,5′S) and (1′R,5′R), respectively, which are used in this paper.
FIG. 1.
Pathways and metabolites analyzed in this study. The solid arrows indicate previously confirmed reactions; the open arrows indicate expected but unconfirmed reactions. Structures of VONE and VOROL are shown based on previous reports (2, 25). Reactions inhibited by dichlorovos (DV) are also indicated. AFB1, AFB2, AFG1, and AFG2, aflatoxins B1, B2, G1, and G2, respectively; MW, molecular weight.
Versicolorone (VONE) was first isolated from Aspergillus versicolor by Berger-Deguee and Bergar (2). On the basis of a comparison of the structure of VONE with the structures of related aflatoxin precursors, these authors suggested that another intermediate, which was later called hydroxyversicolorone (HVN), might serve as a precursor just before versiconal hemiacetal acetate (VHA). It was suggested that VONE arose from HVN by dehydration and dehydrogenation, and this compound was generally accepted to be a dehydrated dead-end product of HVN. Bennett's group isolated mutant WE-47 (hvn-1) from an aflatoxigenic strain of A. parasiticus by UV irradiation. Townsend et al. then determined that this mutant accumulated HVN, VONE, and an alcohol derivative of VONE (25), which we named versicolorol (VOROL). Aflatoxigenic A. parasiticus incorporated [1′-2H]HVN into aflatoxin B1 (23), and rearrangement of AVR to HVN was assumed (24). However, the enzyme step(s) in which HVN was involved in aflatoxin biosynthesis was not determined.
Sterigmatocystin is a late intermediate in aflatoxin biosynthesis and is the final metabolite in the cognate polyketide pathway in Aspergillus nidulans. The genes and enzymes required for sterigmatocystin production in A. nidulans are homologs of the genes and enzymes required for aflatoxin production in A. flavus and A. parasiticus (5). Moreover, Keller et al. reported that disruption of either the stcB or stcW gene led to accumulation of AVR in A. nidulans (14) Subsequently, the cypX and moxY genes of A. parasiticus were shown to be homologous to stcB and stcW, respectively (37). Recently, it was suggested that the avfA gene is involved in the step from AVR to VHA (38). However, the exact function of these genes in aflatoxin or sterigmatocystin biosynthesis is still unknown.
VHA is another intermediate in the aflatoxin/sterigmatocystin biosynthesis pathway. Dichlorvos (dimethyl 2,2-dichlorovinylphosphate) is an inhibitor of the esterase that catalyzes both reactions from VHA to versiconal (VHOH) (21, 22, 36). It has previously been reported that versiconol acetate (VOAc) and VHA accumulate after dichlorvos treatment and that the aflatoxin precursors VHA, VOAc, versiconol (VOH), and VHOH form a metabolic grid that is catalyzed by two cytosol enzymes, a reductase and an esterase (29). The VHA reductase that catalyzes the pathway from VHA to VOAc was purified (16), and the gene coding for this enzyme was isolated (M. Motomura, N. Chihaya, T. Shinozawa, T. Hamasaki, and K. Yabe, unpublished data). The enzyme activity of VHA reductase and expression of the VHA reductase gene were dependent on the aflatoxin-inducible medium, indicating that this enzyme may be specifically involved in aflatoxin biosynthesis. However, it could not be determined which precursor, VHA or VOAc, was the first substance at the entrance of the grid from the earlier precursor, AVR, because the order of accumulation of VHA and VOAc could not be distinguished in the dichlorvos-treated cultures (29).
Our objectives in this study were (i) to establish a cell-free system for reactions from AVR to VHA, (ii) to investigate the relationship of HVN, VONE, and VOROL to aflatoxin biosynthesis, and (iii) to clarify the involvement of a novel metabolic grid in aflatoxin biosynthesis. Finally, we describe a scheme for the conversion of AVR to VHOH in aflatoxin biosynthesis.
MATERIALS AND METHODS
Microorganisms.
A. parasiticus WE-47 (hvn-1) (25), a UV-derived mutant of A. parasiticus SU-7, was used for preparation of HVN, VONE, and VOROL. A. parasiticus NIAH-26, a UV-derived mutant of A. parasiticus NRRL 2999, was used for biosynthetic studies. The NIAH-26 mutant induced all enzymes during the conversion of norsolorinic acid to aflatoxins in an aflatoxin-inducing YES liquid culture medium (2% yeast extract, 20% sucrose), although it produced neither aflatoxins nor anthraquinone or xanthone precursors (28-32, 34, 35). A. parasiticus NIAH-9, another UV-derived mutant of A. parasiticus NRRL 2999, accumulated VA but did not produce aflatoxins (31).
Preparation of metabolites.
HVN, VONE, and VOROL were isolated from mycelia of A. parasiticus mutant WE-47 (hvn-1). For preparation of HVN, this mutant was cultured in YES medium for 5 days; mycelial pigments were extracted with acetone, and the extract was then dried. The dried residue was solubilized in methanol; HVN was purified by high-performance liquid chromatography (HPLC) by using an octadecyl silane (ODS) HPLC column and was identified by comparing its retention time with that of synthetic [1′-2H]HVN, which was kindly supplied by C. A. Townsend (23). The molecular mass of the sample was confirmed to be 384 Da by using atmospheric pressure chemical ionization-liquid chromatography-mass spectrometry (APCI-LC/MS) with methanol as the solvent. For preparation of VONE and VOROL, the same mutant was cultured for 16 days, and pigments in the mycelia were extracted with acetone and then purified by extraction with ethyl acetate and separation by Daisogel IR-60, Sephadex LH-20, and preparative thin-layer chromatography procedures. The purified VONE and VOROL were identified based on the chemical data obtained from 1H nuclear magnetic resonance (NMR), mass spectrometry, and infrared analyses, which were compared with previously reported data (2, 25).
VHA and VOAc were prepared as previously described (29) from mycelia of A. parasiticus NIAH-9 cultured in YES medium supplemented with 10 ppm of dichlorvos (0.14 mg ml−1). AVR, VA, versicolorin B (VB), and VOH were prepared from mycelia of A. versicolor (Vuillemin) Tiraboschi (9-11, 13). Each enantiomer of AVR was prepared from racemic AVR by using a Chiralcel OD column (0.46 by 25 cm; Daisel Chemical Industry) and a solvent system consisting of n-hexane, ethanol, and trifluoroacetic acid (96:6:0.2, vol/vol/vol) (32). The concentrations of the metabolites in methanol were determined from UV absorption spectra by using the following molar absorption coefficients (6): AVR, 6,700 M−1 cm−1 (454 nm); HVN, 5,300 M−1 cm−1 (476 nm); VONE, 6,000 M−1 cm−1 (468 nm); VHA, 7,000 M−1 cm−1 (453 nm); VOAc, 8,500 M−1 cm−1 (453 nm); VOH, 7,400 M−1 cm−1 (455 nm); VB, 8,700 M−1 cm−1 (450 nm); VA, 8,166 M−1 cm−1 (452 nm); and VOROL, 5,500 M−1 cm−1 (579 nm).
Feeding experiments.
By using the tip culture method (28, 33), A. parasiticus NIAH-26 was cultured in YES medium supplemented with HVN, YES medium supplemented with VONE, and YES medium supplemented with VOROL for 4 days at 28°C. Aflatoxin formation was measured by extraction of the medium with chloroform, followed by HPLC analysis with a silica gel HPLC column as described previously (30).
Preparation of cell extract, cytosol, and microsome fractions and VHA reductase.
Cell extract, cytosol, and microsome fractions were prepared from the mycelia of A. parasiticus NIAH-26 (34). To remove contaminating pigments, the cytosol protein fraction was further purified with a Sephadex G-25 M column (PD-10; Pharmacia LKB Biotechnology, Uppsala, Sweden). The VHA reductase, which corresponded to VHA reductase II (16), was purified from A. parasiticus NIAH-26. The VHA reductase was more than 80% pure, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining of the proteins. Each fraction or enzyme was stored at −80°C. Protein concentrations were determined by using a protein assay solution (Bio-Rad, Richmond, Calif.).
Enzyme assay and HPLC analyses.
The cell extract (0.4 mg of protein ml−1), the cytosol fraction (1 mg ml−1), or the microsome fraction (1.3 mg ml−1) of A. parasiticus NIAH-26 was incubated in a reaction mixture consisting of solution A (90 mM potassium phosphate [pH 7.5], 10% [vol/vol] glycerol, 0.9 mg of bovine serum albumin per ml) supplemented with each substrate and 4 mM NADPH, as indicated below. The total volume of each reaction mixture was 20 μl in a 0.5-ml microtube. After incubation at 30°C, the reaction was terminated by adding 35 μl of ethyl acetate and mixing with a Vortex mixer. Following centrifugation at 10,000 × g for 2 min, an aliquot of the upper ethyl acetate layer was transferred to a new microtube and dried by keeping the tube open at room temperature in the dark. The residue was then solubilized with methanol for HPLC analysis. HPLC was performed at 35°C at a flow rate of 1 ml min−1 by using a Shimadzu HPLC apparatus (LC-6A) equipped with an ODS HPLC column (0.46 by 25 cm; Shimadzu Co. Ltd.), and absorbance at 290 nm was monitored. The solvent system was acetonitrile-tetrahydrofuran-water (25:20:55, vol/vol/vol). The retention times of standard samples were as follows: VOH, 3.2 min; VHOH or VOROL, 3.8 min; VONE, 4.5 min; VOAc, 5.0 min; HVN, 5.5 min; VHA, 6.8 min; and VB, 11.8 min. The total amounts of each compound produced in 20 μl of the reaction mixture are shown below.
To investigate the reaction from HVN to VONE, purified VHA reductase (29 μg ml−1) was incubated with 10 μM HVN in the presence of 4 mM NADPH for various times. The reaction was terminated by adding 35 μl of ethyl acetate and mixing with a Vortex mixer; the ethyl acetate extract was then analyzed by HPLC after drying as described above. To calculate the amounts of the metabolites, the standard curve for VHA was used.
Each result shown below was confirmed by repeating the same experiments at least two times.
Determination of in vivo structures of VONE and VOROL.
Fast atom bombardment mass spectrometry (FABMS) spectra were obtained by using a JEOL AX505HA spectrometer and 3-nitrobenzyl alcohol as the matrix. The APCI-LC/MS spectrum was routinely determined by direct injection of the substance dissolved in methanol into an APCI-LC/MS apparatus (Hitachi M-1200) under the following conditions: desolvator temperature, 400°C; multiplier voltage, 1,800 V; needle voltage, 3,000 V; and flow rate, 1 ml min−1. Three solutions were used as the moving solvent; the A solution contained acetonitrile, tetrahydrofuran, and water (18:17:65), the B solution contained 100% methanol, and the C solution contained methanol and water (1:1, vol/vol). The nebulizer temperature and drift voltage were changed as described below.
For acetylation of VONE, 1 mg of VONE in 0.5 ml of acetone was supplemented with 0.3 ml of water and then with 0.1 ml of acetic anhydride and then mixed well for 2 h at room temperature. The reaction was then quenched by adding 0.5 ml of methanol and mixing for 30 min. The solution was concentrated by evaporation, spotted onto a silica gel thin-layer chromatography plate, and then developed three times with n-hexane-ethyl acetate (1:1, vol/vol). Four pigments (Rfs, 0.77, 0.73, 0.55, and 0.48) were extracted from the thin-layer chromatography plate, and then the 1H-NMR spectrum of each pigment was determined in deuterated chloroform with a JEOL JNM Delta 500 FTNMR spectrometer. The molecular masses of the material recovered from thin-layer chromatography were also determined by electron ionization mass spectrometry (EIMS) and chemical ionization mass spectrometry (CIMS) (with isobutane as the reagent gas) with a JEOL AX505HA spectrometer.
RESULTS
Formation of VB and VA from (1′S,5′S)-AVR by cell extract.
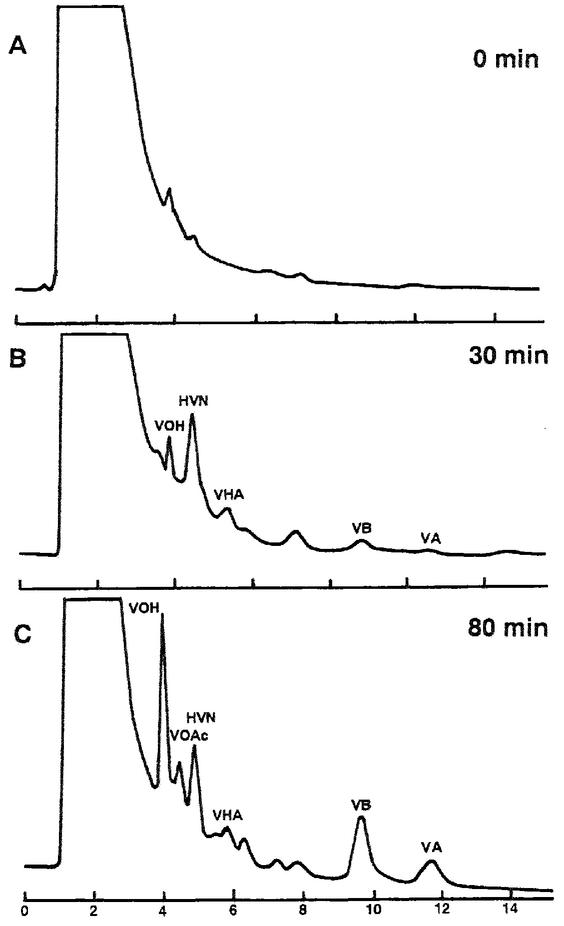
Cell extract of A. parasiticus NIAH-26 mycelium cultured in YES medium was incubated with (1′S,5′S)-AVR in the presence of NADPH (Fig. 2). Several peaks were detected by HPLC, and their elution times corresponded to those of VA, VB, VHA, HVN, VOAc, and VOH (Fig. 2B and C). Although the amounts of HVN and VHA did not change after 30 min of incubation, the amounts of VA, VB, and VOH kept increasing for at least 80 min. These reactions required NADPH. None of these products was detected when (1′R,5′R)-AVR was used instead of (1′S,5′S)-AVR. The results of these cell-free experiments support the findings of the previous report in which feeding experiments were performed (32). Furthermore, involvement of HVN as an intermediate in the pathway from AVR to aflatoxins was detected for the first time.
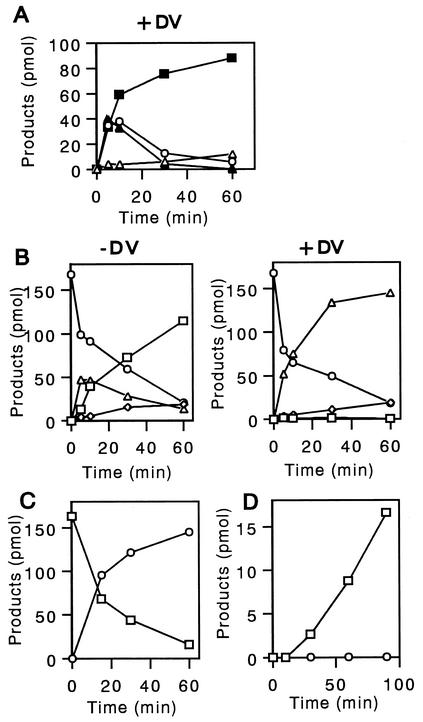
FIG. 2.
Conversion of AVR to VA through formation of various intermediates. (1′S,5′S)-AVR was incubated with cell extract of A. parasiticus NIAH-26 in the presence of NADPH for 0 min (A), 30 min (B), and 80 min (C). The ethyl acetate extract of each reaction mixture was analyzed as described in the text. When NADPH was omitted from the reaction mixture, no substance was newly formed.
When dichlorvos was added to the same reaction mixture, neither VB nor VA was detected. However, large amounts of VOAc accumulated (data not shown), indicating that the reactions observed here may reflect in vivo aflatoxin biosynthesis.
Conversion of (1′S,5′S)-AVR to HVN by the microsome fraction.
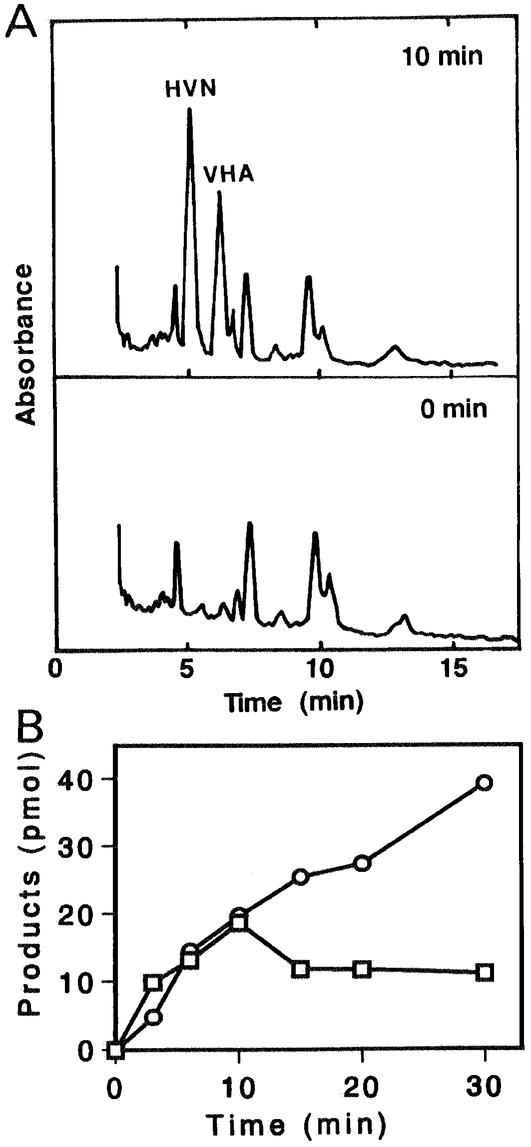
The enzyme reactions following AVR formation were investigated by using either the microsome or cytosol fraction of A. parasiticus NIAH-26. When the microsome fraction was incubated with (1′S,5′S)-AVR, HVN and VHA were formed (Fig. 3A). This reaction did not occur when the cytosol fraction was used, when NADPH was omitted from the reaction mixture, or when the other enantiomer, (1′R,5′R)-AVR, was used instead of (1′S,5′S)-AVR. Neither HVN nor VHA was formed when 1 mM flavin adenine dinucleotide (FAD) was used instead of NADPH (data not shown), and FAD did not enhance the reaction in the presence of NADPH. The rates of production of HVN and VHA were similar until 10 min in the enzyme reaction. The amount of HVN reached a maximum value after 10 min and then remained constant (Fig. 3B). In contrast, the amount of VHA continued to increase until 30 min. These results indicate that (1′S,5′S)-AVR is first converted to HVN and then HVN is converted to VHA. The microsome enzyme may catalyze conversion of AVR to HVN, because the conversion of HVN to VHA was catalyzed by the cytosol enzyme, as described below.
FIG. 3.
Conversion of AVR to HVN and VHA by the microsome fraction. (A) (1′S,5′S)-AVR was incubated with the microsome fraction of A. parasiticus NIAH-26 in the presence of NADPH for 0 min (lower panel) and 10 min (upper panel), and the metabolites were analyzed by HPLC. (B) Amounts of HVN (□) and VHA (○) produced after various incubation times, determined as described in Materials and Methods. Neither HVN nor VHA was formed in the absence of NADPH.
Formation of VHA from HVN by the cytosol fraction.
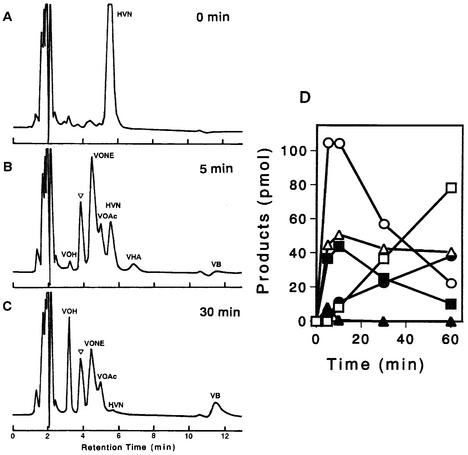
The cytosol fraction was incubated with HVN in the presence of NADPH (Fig. 4). VOH, VHOH, VONE, VHA, and VB (Fig. 4C) were newly formed after 5 min of incubation, indicating that VHA was produced from HVN by the cytosol enzyme. VHA, VHOH, and VONE were transiently produced in the early phase; that is, the amount of each substance reached the maximum value after 10 min of incubation and then decreased (Fig. 4D). Although the peaks of VHOH and VOROL overlapped each other in the HPLC spectrum, the large part of the peak seemed to correspond to VHOH, especially in the early phase, because the activity of VOROL formation from VONE appeared to be very low (Fig. 5A and B). In contrast, VOH and VB accumulated after 5 min of incubation, and then the amounts kept increasing (Fig. 4D). Formation of all substances depended on the presence of NADPH.
FIG. 4.
Formation of various intermediates from HVN by the cytosol fraction. (A to C) HVN incubated with the cytosol fraction in the presence of NADPH for 0 min (A), 5 min (B), and 30 min (C). An inverted open triangle indicates the overlapping VHOH and VOROL peaks. (D) Amounts of the reaction products VONE (○), VOAc (▪), VHA (▴), VB (•), VOROL-VHOH (▵), and VOH (□), as determined after various incubation times. No metabolite was newly produced in the absence of NADPH.
FIG. 5.
Conversions among HVN, VONE, VOROL, VOAc, VHA, and VOH. (A) Formation of various intermediates from HVN by the cytosol fraction in the presence of dichlorvos. HVN was incubated with the cytosol fraction in the presence of NADPH for various times by using the conditions described in the legend to Fig. 4, except that dichlorvos (DV) was added. The amounts of the reaction products VHA (▴), VOAc (▪), VONE (○), and VOROL (▵) were measured. No metabolite was produced in the absence of NADPH. (B) Formation of various intermediates from VONE by the cytosol fraction. VONE was incubated with the cytosol fraction in the presence of NADPH for various times (left panel). Dichlorvos was also added to the reaction mixture (right panel). The amounts of VONE (○), VOAc (▵), VOH (□), and VOROL (⋄) were then measured. No metabolite was produced in the absence of NADPH. (C) Formation of VONE from HVN by the purified VHA reductase. HVN was incubated with the purified VHA reductase in the presence of NADPH for various times, and the amounts of HVN (□) and VONE (○) were measured by HPLC. The same reaction did not occur in the absence of NADPH. (D) Formation of VOAc from VOROL by the cytosol fraction. VOROL was incubated with the cytosol fraction in the presence (□) or in the absence (○) of NADP. The amount of VOAc was measured by HPLC analysis.
When dichlorvos was added to the same reaction mixture, HVN was first converted to VHA, VONE, and VOAc after 5 min of incubation (Fig. 5A). However, the amounts of VHA and VONE then began to decrease, and these compounds were almost undetectable at 30 min. In contrast, the amount of VOAc kept increasing for at least 60 min. At 60 min, almost all of the HVN was converted to VOAc. In summary, cell-free conversion of HVN to VHA and cell-free conversion of HVN to VONE were detected in this study for the first time. Furthermore, with time, significant amounts of VOROL were also formed from HVN.
Formation of VOAc, VOH, and VOROL from VONE by the cytosol fraction.
When VONE was incubated with the cytosol fraction in the presence of NADPH (Fig. 5B, left panel), VOAc was transiently produced, and then VOH accumulated. Production of a small amount of VOROL was also observed. All transformations were NADPH dependent.
In the presence of dichlorvos, VOAc and VOROL accumulated, whereas VOH was not formed (Fig. 5B, right panel). Since dichlorvos inhibits an esterase enzyme that catalyzes conversion of VOAc to VOH, these results indicate that VONE was converted to VOAc by the cytosol enzyme and then VOAc was finally converted to VOH by the esterase (29). Conversion of VONE to VOROL occurred irrespective of the presence of dichlorvos.
Formation of VONE from HVN by the VHA reductase.
We previously purified the VHA reductase from A. parasiticus NIAH-26, which catalyzes the reaction from VHA to VOAc in a metabolic grid composed of VHA, VOAc, VOH, and VHOH (16). When HVN was incubated with purified VHA reductase, only VONE was produced, and the presence of NADPH was required (Fig. 5C).
Formation of VOAc from VOROL by the cytosol fraction.
When VOROL was incubated with the cytosol fraction in the presence of NADP, VOAc, but not VONE, was formed after 10 min (Fig. 5D). This reaction required the presence of NADP. However, when the purified VHA reductase was incubated with VONE, VOROL was not formed (data not shown).
Formation of aflatoxins from HVN, VONE, and VOROL in feeding experiments.
The results obtained in the cell-free experiments strongly indicated that VONE and VOROL are precursors of aflatoxins. Therefore, we repeated the feeding experiments using each substance separately. When HVN was added to the culture medium, four aflatoxins, aflatoxin B1, aflatoxin B2, aflatoxin G1, and aflatoxin G2, were produced (Table 1). Conversion of HVN to aflatoxins was determined previously by performing tracer experiments with [1′-2H]HVN (23). Here, for the first time, we found that small but significant amounts of aflatoxins were produced from either VONE or VOROL, although the amounts of the aflatoxins were much smaller than the amounts produced from HVN. These results demonstrated that VONE and VOROL could serve as precursors of aflatoxins.
TABLE 1.
Aflatoxin production from exogenous substances in feeding experimentsa
| Substance added | Concn (μM) | Mycelial wet wt (mg) | Concn (pmol/250 μl of medium) of:
|
|||
|---|---|---|---|---|---|---|
| Aflatoxin B1 | Aflatoxin B2 | Aflatoxin G1 | Aflatoxin G2 | |||
| Expt 1 | ||||||
| None | 0 | 17.1 ± 0.9 | ||||
| HVN | 10 | 14.6 ± 0.6 | 25.2 ± 0.1 | 10.4 ± 1.1 | 141.6 ± 2.9 | 47.1 ± 2.2 |
| VONE | 10 | 18.7 ± 0.1 | 8.3 ± 0.2 | 16.9 ± 0.4 | 5.7 ± 0.5 | |
| VOROL | 10 | 17.7 ± 0.6 | 8.0 ± 0.1 | 10.9 ± 0.1 | 3.6 ± 0.4 | |
| Expt 2 | ||||||
| None | 0 | 16.9 ± 0.2 | ||||
| VONE | 40 | 16.8 ± 0.1 | 8.7 ± 0.2 | 38.7 ± 3.5 | 9.9 ± 0.01 | |
| VOROL | 40 | 17.1 ± 0.1 | 4.4 ± 4.4 | 8.3 ± 0.2 | 10.5 ± 0.2 | 3.4 ± 0.3 |
A. parasiticus NIAH-26 was cultured with HVN, VONE, or VOROL by performing feeding experiments for 4 days. The amount of each aflatoxin excreted into the medium was determined by HPLC. The values are the means ± standard errors of duplicate experiments.
Determination of molecular masses of VONE and VOROL.
The fragmentation pattern for VONE as determined by FABMS (positive, with 3-nitrobenzyl alcohol as the matrix) was as follows: m/z (relative intensity) 409 [M+Na]+ (7%), 387 [M+H]+ (6), 369 [M − H2O+H]+ (30), 329 (26), 307 (71), 289 (38), 176 (80), 155 (94), 154 (100), 149 (52), 138 (100), 137 (100), 136 (100), 120 (40), 107 (73), 77 (63), 55 (46). Similarly, the fragmentation pattern for VOROL as determined by FABMS (positive, with 3-nitrobenzyl alcohol as the matrix) was as follows: m/z 460 (1%), 411 [M+Na]+ (1), 389 [M+H]+ (5), 371 [M − H2O+H]+ (4), 329 (4), 307 (12), 289 (9), 176 (16), 154 (100), 136 (87), 120 (15), 107 (29), 89 (30), 77 (29), 55 (28).
The APCI-LC/MS data obtained for VONE were as follows: [nebulizer temperature, drift voltage, solvent] m/z (relative intensity), [160°C, 60 V, B solution] 385 [M − H]− (32%), 367 [M − H2O − H]− (100); [160°C, 60 V, C solution] 385 [M − H]− (100), 367 [M − H2O − H]− (48); [160°C, 30 V, A solution] 385 [M − H]− (94), 367 [M − H2O − H]− (100). Negative ionization was appropriate for observing a clear signal, whereas a weak signal of 387 [M+H]+ also was significantly observed at [160°C, 20 V, A solution]. The APCI-LC/MS results obtained for VOROL were as follows: [200°C, 60 V, A solution] 389 [M+H]+ (100), 371 [M − H2O +H]+ (0); [160°C, 30 V, C solution] 389 [M+H]+ (100), 371 [M − H2O+H]+ (0); [160°C, 40 V, B solution] 389 [M+H]+ (100), 371 [M − H2O+H]+ (20); [160°C, 60 V, B solution] 389 [M+H]+ (47), 371 [M − H2O+H]+ (100); [160°C, 80 V, B solution] 389 [M+H]+ (14), 371 [M − H2O+H]+ (100). These results indicate that the molecular masses of VONE and VOROL were 386 and 388 Da, respectively, which were 18 Da greater than the molecular masses published previously (2, 25), when relatively milder conditions were used in FABMS and APCI-LC/MS analyses.
Acetylation of VONE.
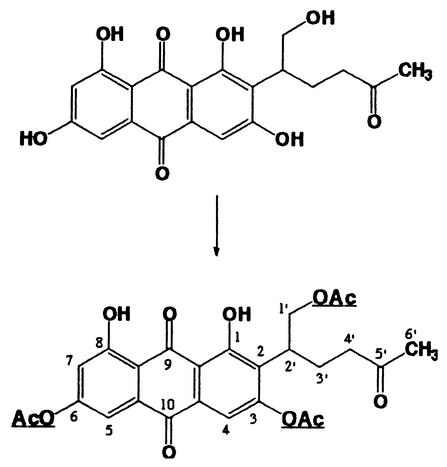
To confirm that VONE has a structure with the dihydrofuran ring open, VONE was treated with acetic anhydride in aqueous acetone; four products were obtained. The physicochemical properties of the major product were as follows: CIMS (isobutane): m/z (relative intensity) 513 (47%), [M+H]+), 471 (16), 453 (37), 411 (100); 1H-NMR (CDCl3) δ12.70 (1H, s), 12.15 (1H, s), 7.43 (1H, d, J = 2.5 Hz), 7.20 (1H, s), 6.97 (1H, d, J = 2.5 Hz), 4.62 (1H, dd, J = 10.5, ′7.0 Hz), 4.38 (1H, dd, J = 10.5, 7.0 Hz), 3.58 (1H, m), 2.42-1.88 (4H, m), 2.29 (6H, s), 2.07 (3H, s), 1.96 (3H, s). When the molecular mass of natural VONE was assumed to be 386 Da, the CIMS data indicated that three hydroxyls in the molecule were acetylated. Two resonances at δ 12.70 and 12.15 in the NMR spectrum indicated that two chelated phenolic hydroxyls (1-OH, 8-OH) (Fig. 6 shows numbering of the molecule) were not acetylated. There were resonances due to four acetyl methyls (δ 2.29, 2.07, and 1.96), one of which (presumably δ 1.96) was assigned to the methyl in —CH2COCH3. Two protons of the methylene in —CH2OCOCH3 showed different chemical shifts (δ 4.62 and 4.38); this is because the methylene is adjacent to a chiral carbon (2′-C) (Fig. 6) and different spatial environments influence the chemical shifts of these protons. These results indicate that in vivo VONE exists as dihydrofuran ring-opened form, as shown in Fig. 6.
FIG. 6.
Structure of the reaction product of VONE obtained by acetylation. The acetylated sites are underlined.
DISCUSSION
In this work we focused on the proposed overall pathway from AVR to VHOH (Fig. 7), with particular emphasis on the formation of VHA. Moreover, a complex metabolic grid involving multiple metabolites was explored. This pathway develops following HVN synthesis. An interesting feature is that two metabolites, VONE and VOROL, occur in vivo as structures in which the dihydrofuran ring has undergone ring opening.
FIG. 7.
Reaction scheme suggested in this study and previous work (28). The open structures of VONE and VOROL were confirmed in this study. The microsome enzyme catalyzes the conversion of (1′S,5′S)-AVR to HVN. Cytosolic enzymes catalyzed all reactions except this reaction. Involvement of the same enzyme is indicated by identical symbols. Also, except for the esterase (double circles), which catalyzes both conversion from VHA to VHOH and conversion from VOAc to VOH, all enzymes require NADPH or NADP as a coenzyme. MW, molecular weight.
For a long time we were puzzled about the structures of VONE and VOROL. Previously (2, 25), the molecular masses of VONE and VOROL were reported to be 368 and 370 Da, respectively, suggesting that there is a dihydrofuran ring in the side chain (Fig. 1). Since an ether bond in the dihydrofuran ring is chemically stable, both VONE and VOROL appeared to be dead-end products (2, 25). However, there were the following discrepancies. (i) In the cell-free and feeding experiments, both VONE and VOROL could be transformed to VOAc, VOH, and aflatoxins (Fig. 5B and D and Table 1). (ii) In the ODS HPLC analysis, the retention times of VONE (4.5 min) and VOROL (3.8 min) were close to those of other polar intermediates, such as VOH (3.2 min), VHOH (3.8 min), and VOAc (5.0 min). Since the polarities of VONE and VOROL deduced from the reported closed structures were expected to be much lower than the polarities of the polar substances, this discrepancy could not be explained. (iii) With purified VHA reductase, only one product, VONE, was produced from HVN without any additional product (Fig. 5C). If VONE had a closed form, two steps, enzymatic reduction of HVN to an open form of VONE, which could be catalyzed by VHA reductase, and spontaneous dehydration of the open form to the closed form of VONE, would be required. However, we did not find any certain intermediate between HVN and VONE.
From these discrepancies, we predicted that the in vivo structures of VONE and VOROL are ring-opened forms containing two more OH groups than previously reported. We assumed that cyclization leading to the formation of a dihydrofuran ring may occur by dehydration under extreme conditions (high temperature for vaporization, high voltage for ionization, and nonpolar solvent) in mass spectrometry analyses. Therefore, we used FABMS and APCI-LC/MS to determine the presumed in vivo structures of these substances, because milder conditions could be used in these analyses than in other EIMS and CIMS mass analyses. We decreased the temperature of the nebulizer and drift voltage and changed the constituents of the solvent. In this way, molecular masses that were 18 Da greater than those previously reported were determined. The structure of VONE was further confirmed by acetylation; the acetyl derivative was analyzed by CIMS and 1H-NMR. Finally, we found that the true molecular masses of VONE and VOROL were 386 and 388 Da, respectively. Therefore, in vivo VONE and VOROL have furan ring-opened forms, which contain two OH groups in the side chains, similar to VOAc and VOH. For the first time, in this work VONE and VOROL were confirmed to be intermediates in aflatoxin biosynthesis.
In the pathway for aflatoxin biosynthesis, a microsome enzyme(s) catalyzes the reaction from (1′S,5′S)-AVR to HVN. This preparation showed strict stereospecificity for (1′S,5′S)-AVR and required NADPH for activity. In this assay, a low concentration of the microsome fraction and a short incubation time were used, because HVN and VHA were easily changed to other substrates, such as VB and VOAc, by the subsequent cytosol enzymes contained in the microsome fraction. Detection of the low level of HVN or VHA that was transiently formed was hindered by the substances contaminating the microsome, as shown in Fig. 3A. The difficulty of detecting transient substances may explain why enzymatic conversion of AVR to HVN has not been reported previously. Although VHA and HVN were formed from AVR in the microsome systems, VHA is likely produced from HVN by the cytosol enzyme that contaminated the microsome fraction. In our preliminary experiments, we found that the Km of NADPH in HVN formation was larger than that in VHA formation (data not shown). The difference in the apparent Km values may explain why both VHA and HVN could be detected.
The reactions following HVN were very complicated. The pathway from HVN to VHA to VHOH may be the main pathway in aflatoxin biosynthesis, because these compounds were the main metabolites observed when AVR was incubated with the cell extract composed of the microsome and cytosol fractions (Fig. 2). Also, the amounts of aflatoxins produced from HVN were much larger than the amounts produced from VONE or VOROL in the feeding experiments. However, a metabolic grid also operates among HVN, VONE, VOAc, and VHA in the fungal cell, which is obvious when fungus cultures become old or when a certain step in the pathway from HVN to VHOH is blocked. In conjunction with previous work that demonstrated the presence of a metabolic grid among VHA, VOAc, VOH, and VHOH (29), the findings presented here suggest the presence of a big metabolic grid from HVN to VHOH (Fig. 7).
Given the similarities of the structures of HVN and VONE and the similarities of the structures of VHA and VOAc, the same cytosol enzyme is likely to catalyze the reactions from HVN to VHA and from VONE to VOAc. The observation that the A. parasiticus mutant WE-47 (hvn-1) accumulates VONE together with HVN also indicates that both conversion of HVN to VHA and conversion of VONE to VOAc were blocked by mutation of a single enzyme. The enzyme reaction involved in these steps is a unique reaction, and it resembles other microbial monooxygenase reactions in catalytic properties. All of the enzymes equally catalyzed insertion of an oxygen atom into a C—C bond adjacent to the carbonyl group of an aliphatic or alicyclic ketone (i.e., a Baeyer-Villiger reaction) and contained FAD in their molecules (4, 7, 8, 12, 18, 19). Similarly, the enzyme involved in the reaction from HVN to VHA may contain FAD in its molecule, because neither a requirement for FAD nor enhancement by FAD was observed. In the reactions from HVN to VONE and from VHA to VOAc, the same enzyme (VHA reductase) was involved (Fig. 5C). Therefore, this enzyme may recognize the hemiacetal structure in HVN and VHA.
Interestingly, we found a novel enzyme activity that catalyzed the reversible reaction between VONE and VOROL. Accumulation of VONE and VOROL, as well as HVN, in the A. parasiticus hvn-1 mutant indicates that the pathway from HVN to VONE to VOROL functions in vivo. Also, the time lag before the formation of VOAc from VOROL in the cell-free experiment (Fig. 5D) may indicate that accumulation of a sufficient amount of an intermediate, probably VONE, may be necessary for subsequent formation of VOAc.
All enzymes except the esterase in the pathway from AVR to VB required NADPH as a cofactor. The results of our cell-free experiments suggested that a high concentration of intracellular NADPH tended to accelerate the formation of more reduced substances, such as VONE, VOROL, and VOAc, in the side pathway. The accumulation of VONE and VOROL in aged A. parasiticus hvn-1 mutant cultures (16 days) suggests that the intracellular concentration of NADPH increases with time. It is possible that the concentration of NADPH may regulate metabolite formation in these pathways. Moreover, the metabolites in the side pathways may function as reservoirs of aflatoxin precursors. Motomura et al. did not find that the VHA reductase gene is in the aflatoxin gene cluster region (unpublished data). However, expression of the VHA reductase gene was dependent on the culture medium; the gene was expressed in GY medium (2% glucose, 0.5% yeast extract) but not in PY medium (2% peptone, 0.5% yeast extract). Also, expression of the gene was repressed by a culture temperature of 37°C (Motomura et al., unpublished data). Similar effects of the culture conditions on gene expression have been reported for genes involved in the aflatoxin gene cluster. Therefore, the whole metabolic grid may specifically function under the aflatoxin-inducible conditions. We are now investigating the physiological meaning of this metabolic grid and the genes involved in the reactions from AVR to VHA.
Acknowledgments
We thank C. A. Townsend, John Hopkins University, for a gift of synthetic [1′-2H]HVN, Ronald Bentley, University of Pittsburgh, for helpful discussions and for critically reviewing the manuscript, S. Hoshino of our laboratory for her technical help with LC/MS measurements, and M. Kameyama, Molecular Structure and Dynamics Laboratory, for useful advice about LC/MS measurements.
This work was supported in part by grant-in-aid BDP-VI-1-4 (Bio Design Project) from the Ministry of Agriculture, Forestry and Fisheries.
REFERENCES
- 1.Bennett, J. W., P.-K. Chang, and D. Bhatnagar. 1997. One gene to whole pathway: the role of norsolorinic acid in aflatoxin research. Adv. Appl. Microbiol 45:1-15. [DOI] [PubMed] [Google Scholar]
- 2.Berger-Deguee, M., and Y. Bergar. 1982. Structure of versicolorone isolated from Aspergillus versicolor. Phytochemistry 21:1449-1451. [Google Scholar]
- 3.Bhatnagar, D., T. E. Cleveland, and P. J. Cotty. 1994. Mycological aspects of aflatoxin formation, p. 327-346. In D. L. Eaton and J. D. Groopman (ed.), Toxicology of aflatoxins. Human health, veterinary, and agricultural significance. Academic Press, Inc., New York, N.Y.
- 4.Britton, L. N., and A. J. Markovetz. 1977. A novel ketone monooxygenase from Pseudomonas cepacia. Purification and properties. J. Biol. Chem. 252:8561-8566. [PubMed] [Google Scholar]
- 5.Brown, D. W., J. H. Yu, H. S. Kelkar, M. Fernandes, T. C. Nesbitt, N. P. Keller, T. H. Adams, and T. J. Leonard. 1996. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 93:1418-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole, R. J., and R. H. Cox (ed.). 1981. Handbook of toxic fungal metabolites, p. 1-127, Academic Press, Inc., New York, N.Y.
- 7.Donoghue, N. A., D. A. Norris, and P. W. Trudgill. 1976. The purification and properties of cyclohexanone oxygenase from Nocardia globerula CL1 and Acinetobacter NCIB 9871. Eur. J. Biochem. 63:175-192. [DOI] [PubMed] [Google Scholar]
- 8.Griffin, M., and P. W. Trudgill. 1976. Purification and properties of cyclopentanone oxygenase of Pseudomonas NCIB 9872. Biochemistry 63:199-209. [DOI] [PubMed] [Google Scholar]
- 9.Hamasaki, T., Y. Hatsuda, N. Terashima, and M. Renbutsu. 1965. The structure of a new metabolite of Aspergillus versicolor. Agric. Biol. Chem. 29:696-697. [Google Scholar]
- 10.Hamasaki, T., Y. Hatsuda, N. Terashima, and M. Renbutsu. 1967. Studies on the metabolites of Aspergillus versicolor (Vuillemin) Traboschi. Part V. Isolation and structures of three new metabolites, versicolorin A, B and C. Agric. Biol. Chem. 31:11-17. [Google Scholar]
- 11.Hatsuda, Y., T. Hamasaki, M. Ishida, and S. Yoshikawa. 1969. The structure of a new metabolite from Aspergillus versicolor. Agric. Biol. Chem. 33:131-133. [Google Scholar]
- 12.Itagaki, E. 1986. Studies on steroid monooxygenase from Cylindrocarpon radicicola ATCC 11011. Purification and characterization. J. Biochem. 99:815-824. [DOI] [PubMed]
- 13.Katsube, Y., T. Tsukihara, N. Tanaka, K. Ando, T. Hamasaki, and Y. Hatsuda. 1972. The crystal and molecular structure of averufin. Bull. Chem. Soc. Jpn. 45:2091-2096. [Google Scholar]
- 14.Keller, N. P., C. M. H. Watanabe, H. S. Kelker, T. H. Adams, and C. A. Townsend. 2000. Requirement of monooxygenase-mediated steps for sterigmatocystin biosynthesis by Aspergillus nidulans. Appl. Environ. Microbiol. 66:359-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koreeda, M., B. Hulin, M. Yoshihara, C. A. Townsend, and S. B. Christensen. 1985. Synthesis and absolute configuration of (+)-averufin. J. Org. Chem. 50:5426-5428. [Google Scholar]
- 16.Matsushima, K., Y. Ando, T. Hamasaki, and K. Yabe. 1994. Purification and characterization of two versiconal hemiacetal acetate reductases involved in aflatoxin biosynthesis. Appl. Environ. Microbiol. 60:2561-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minto, R. E., and C. A. Townsend. 1997. Enzymology and molecular biology of aflatoxin biosynthesis. Chem. Rev. 97:2537-2555. [DOI] [PubMed] [Google Scholar]
- 18.Norris, D. B., and P. W. Trudgill. 1976. Multiple forms of cyclohexanone oxygenase from Nocardia globerula CL1. Eur. J. Biochem. 63:193-198. [DOI] [PubMed] [Google Scholar]
- 19.Ougham, H. J., D. G. Taylor, and P. W. Trudgill. 1983. Camphor revisited: involvement of a unique monooxygenase in metabolism of 2-oxo-Δ3-4,5,5-trimethylcyclopentenylacetic acid by Pseudomonas putida. J. Bacteriol. 153:140-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payne, G., and M. P. Brown. 1998. Genetics and physiology of aflatoxin biosynthesis. Annu. Rev. Plant Pathol. 36:329-362. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder, H. W., R. J. Cole, R. D. Grisby, and H. Hein, Jr. 1974. Inhibition of aflatoxin production and tentative identification of an aflatoxin intermediate “versiconol acetate” from treatment with dichlorvos. Appl. Microbiol. 27:394-399. [DOI] [PMC free article] [PubMed]
- 22.Steyn, P. S., R. Vlegaar, and P. L. Wessels. 1979. Structure and carbon-13 nuclear magnetic resonance assignments of versiconal acetate, versiconol acetate, and versiconol, metabolites from cultures of Aspergillus parasiticus treated with dichlorvos. J. Chem. Soc. Perkin Trans. I 1979:451-459.
- 23.Townsend, C., P. R. O. Whilamore, and S. W. Brobst. 1988. Hydroxyversicolorone: synthesis and incorporation of a new intermediate in aflatoxin biosynthesis. J. Chem. Soc. Chem. Commun. 1988:726-728. [Google Scholar]
- 24.Townsend, C. A., Y. Isomura, S. G. Davis, and J. A. Hodge. 1989. Reaction models of the oxidative rearrangement of averufin to 1′-hydroxyversicolorone: the first step in dihydrobisfuran formation in aflatoxin biosynthesis. Tetrahedron 45:2263-2276. [Google Scholar]
- 25.Townsend, C. A., K. A. Plavcan, K. Pal, S. W. Brobst, M. S. Irish, E. W. Ely, and J. W. Bennett. 1988. Hydroxyversicolorone: isolation and characterization of a potential intermediate in aflatoxin biosynthesis. J. Org. Chem. 53:2472-2477. [Google Scholar]
- 26.Woloshuk, C. P., and R. Prieto. 1998. Genetic organization and function of the aflatoxin B1 biosynthetic genes. FEMS Microbiol. Lett. 160:169-176. [DOI] [PubMed] [Google Scholar]
- 27.Yabe, K. Pathway and genes of aflatoxin biosynthesis. In F. Fierro and J. Francisco (ed.), Microbial secondary metabolites: biosynthesis, genetics and regulation, in press. Research Signpost, Kerala, India.
- 28.Yabe, K., Y. Ando, and T. Hamasaki. 1988. Biosynthetic relationship among aflatoxins B1, B2, G1, and G2. Appl. Environ. Microbiol. 54:2101-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yabe, K., Y. Ando, and T. Hamasaki. 1991. A metabolic grid among versiconal hemiacetal acetate, versiconol acetate, versiconol and versiconal during aflatoxin biosynthesis. J. Gen. Microbiol. 137:2469-2475. [DOI] [PubMed] [Google Scholar]
- 30.Yabe, K., Y. Ando, and T. Hamasaki. 1991. Desaturase activity in the branching step between aflatoxins B1 and G1 and aflatoxins B2 and G2, Agric. Biol. Chem. 55:1907-1911. [Google Scholar]
- 31.Yabe. K., and T. Hamasaki. 1993. Stereochemistry during aflatoxin biosynthesis: cyclase reaction in the conversion of versiconal to versicolorin B and racemization of versiconal hemiacetal acetate. Appl. Environ. Microbiol. 59:2493-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yabe, K., Y. Matsuyama, Y. Ando, H. Nakajima, and T. Hamasaki. 1993. Stereochemistry during aflatoxin biosynthesis: conversion of norsolorinic acid to averufin. Appl. Environ. Microbiol. 59:2486-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yabe, K., H. Nakamura, Y. Ando, N. Terakado, and H. Nakajima, and T. Hamasaki. 1988. Isolation and characterization of Aspergillus parasiticus mutants with impaired aflatoxin production by a novel tip culture method. Appl. Environ. Microbiol. 54:2096-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yabe, K., M. Nakamura, and T. Hamasaki. 1999. Enzymatic formation of G-group aflatoxins and biosynthetic relationship between G- and B-group aflatoxins. Appl. Environ. Microbiol. 65:3867-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yabe, K., Y. Nakamura, H. Nakajima, Y. Ando, and T. Hamasaki. 1991. Enzymatic conversion of norsolorinic acid to averufin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 57:1340-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao, R. C., and D. P. H. Hsieh. 1974. Step of dichlorvos inhibition in the pathway of aflatoxin biosynthesis. Appl. Microbiol. 28:52-57. [DOI] [PMC free article] [PubMed]
- 37.Yu, J., P.-K. Chang, D. Bhatnagar, and T. E. Cleveland. 2000. Genes encoding cytochrome P450 and monooxygenase enzymes define one end of the aflatoxin pathway gene cluster in Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 53:583-590. [DOI] [PubMed] [Google Scholar]
- 38.Yu, J., C. P. Woloshuk, D. Bhatnagar, and T. E. Cleveland. 2000. Cloning and characterization of avfA and omtB genes involved in aflatoxin biosynthesis in three Aspergillus species. Gene 248:157-167. [DOI] [PubMed] [Google Scholar]